Abstract
Collagenous colitis is characterized by the deposition of a superficial subepithelial collagenous layer, the pathogenesis of which is unknown. Because the excess matrix deposition is potentially reversible, a labile imbalance between fibrogenesis and fibrolysis may be suspected. Expression of procollagen α1(I) and α1(IV), matrix-metalloproteinase (MMP)-1 and -13, and tissue inhibitor of metalloproteinase (TIMP)-1 genes was semiquantitated by in situ hybridization on serial biopsies of 12 patients with collagenous colitis and compared to controls. Collagen types I, III, IV, and VI, tenascin, undulin/collagen XIV, and α-actin were localized by immunohistology. The superficial collagen layer stained strongly for collagen types I, III, and VI, and particularly for tenascin, but not for undulin. Elevated procollagen α1(I), procollagen α1(IV), and TIMP-1 transcript levels were found in α-actin-positive cells with linear distribution underneath the superficial collagenous layer, whereas MMP-1 RNA expression was variable and restricted to cell clusters. MMP-13 expression was undetectable. The patterns of procollagen α1(I)- and α1(IV)-specific labeling, combined with an intense tenascin- but absent undulin-specific staining, indicate deposition of an immature interstitial matrix that may be susceptible to degradation. The restricted MMP-1 RNA expression, counteracted by increased TIMP-1 expression, suggests locally impaired fibrolysis as a relevant factor in the pathogenesis of collagenous colitis.
Collagenous colitis (CC), initially described by Lindström in 1976, is clinically characterized by watery diarrhea in the absence of significant endoscopic and radiological findings. 1 Histologically, CC typically displays a band-like linear deposition of extracellular matrix (ECM) underneath the surface epithelium, with a minimal thickness of 10 μm required for diagnosis. 2-5 This ECM layer does not extend along crypts and is mainly composed of collagens type I and III as well as fibronectin. 6,7 The surface epithelium is often flattened and atrophic and may be infiltrated by lymphocytes, similar to the condition described as lymphocytic colitis, which, however, lacks the subepithelial collagen band. 3,8,9 The etiology of CC is still unknown and has been related to autoimmune phenomena, to the use of nonsteroidal anti-inflammatory drugs, 10 and to other exogenous, possibly bacterial, agents. 11 Today many authors favor a combination of inflammatory and autoimmune mechanisms as cause of the disease. 9 The pathogenic events leading to the formation of the subepithelial collagenous band are similarly enigmatic.
Recent studies on patients subjected to surgical fecal stream diversion 12 as well as anecdotal evidence 13 point to a potentially rapid reversibility of the excess collagenous deposits. Thus, these deposits seem to reflect a local disturbance of ECM turnover resulting in the formation of a provisional ECM. Moreover, this suggests that not only the formation, but also the degradation of excess ECM may be relevant in the pathogenesis of CC.
Among ECM degrading enzymes, matrix-matalloproteinases (MMPs) have a central role. MMPs are a family of zinc-dependent neutral proteinases with overlapping but distinct substrate spectra. Four subfamilies of MMPs are known: collagenases, gelatinases, stromelysins, and membrane-MMPs. 14-16 Together with MMP-8 (collagenase-2, neutrophil collagenase), MMP-1 (collagenase-1, interstitial collagenase) and MMP-13 (collagenase-3) form the MMP-subfamily of collagenases, which are instrumental for the degradation of native interstitial collagens, in particular collagen types I, II, and III. 14-16 Because of their destructive potential, MMPs are regulated at three levels, namely transcription, zymogen activation, and inhibition of the active enzyme. In vitro, stromelysin-1 (MMP-3) may enhance the activity of MMP-1 by a factor of 5 to 12 by additional N-terminal proteolysis, whereas MMP-13 may be activated by gelatinase A (MMP-2) and the membrane type MT1-MMP (MMP-14). 16-18 The details of MMP-1 and -13 activation in vivo, however, are largely unknown. Inhibition of active MMP-1 is a major function of tissue inhibitor of metalloproteinases (TIMP)-1. 14-16 In body fluids, MMP-1 may also be inhibited by α2-macroglobulin. 19
CC is diagnosed by histology and, unlike ulcerative colitis, does not require continuous histological re-evaluation. Thus, in a common clinical setting, CC biopsies are usually available only as single time-point formalin-fixed specimens. Because of their size, extractive procedures cannot be performed, making analysis of enzyme activities impossible. The study of ECM and MMPs in CC biopsies depends, therefore, on localizing a limited number of relevant gene products.
These considerations and restrictions led us to use immunohistology and semiquantitative in situ hybridization to establish the expression patterns of procollagens type I and IV as representative molecules for interstitial and basement membrane ECM, respectively. Furthermore, immunostaining for tenascin and undulin/collagen XIV was performed to assess the maturity of the matrix deposits. Expression of MMP-1, MMP-13, and TIMP-1 as pivotal elements in collagen type I degradation was assessed by in situ hybridization to obtain indicators for the local fibrolytic potential.
Materials and Methods
Tissues
Paraffin blocks of mucosal biopsies from 12 patients with a clinical history of chronic watery diarrhea were drawn from the files of the University Institutes of Pathology in Berlin, Hamburg, and Freiburg. Biopsies had been immersed in formalin immediately after removal and submitted for histopathological diagnosis. Criteria for the diagnosis of CC were as recently described. 7 The thickness of the superficial collagen layer was measured with an ocular micrometer on well-oriented tissue sections. For each biopsy the minimal, maximal, and mean thickness of the superficial ECM layer were recorded along a range of 5 to 10 consecutive intercryptal spaces. The patient data, the biopsy sites, and the histopathological characteristics are listed in Table 1 ▶ . As controls, we used colonic tissues with regular histology (at a distance of >10 cm from the focal lesions, n = 7), with ulcerative colitis (n = 4), and with Crohn’s disease (n = 3), and squamous cell carcinomas of the head and neck region (n = 9).
Table 1.
Summary of Clinical Information, Histopathological Diagnoses, and Morphometric Data
| Case | Age/sex | Diagnosis | Location | Collagenous band (μm) | Inflammation | IEL |
|---|---|---|---|---|---|---|
| 1 | 43 F | CC | 1 Ascending | 30.3 (7.5–50.0) | 1 | <5 |
| 2 Hepatic flexure | 38.0 (12.5–42.5) | 1 | <5 | |||
| 3 Transverse | 29.3 (7.5–40.0) | 1 | <5 | |||
| 4 Splenic flexure | 27.3 (15.0–40.0) | 1 | <5 | |||
| 5 Descending | 24.5 (7.5–50.0) | 1 | <5 | |||
| 6 Sigmoid | 36.7 (17.5–42.5) | 1 | <5 | |||
| 7 Rectum | 26.7 (10.0–50.0) | 1 | <5 | |||
| 2 | 81 F | CC | Splenic flexure | 12.7 (5.0–37.5) | 0–1 | <5 |
| 3 | 52 F | CC | Sigmoid | 18.8 (7.5–57.5) | 1 | <5 |
| 4 | 56 M | CC | Rectum | 16.7 (5.0–37.5) | 1 | <5 |
| 5 | 52 F | CC | 1 Appendix | 16.9 (10.0–75.0) | 1 | <5 |
| 2 Sigmoid | 11.1 (5.0–50.0) | 1 | <5 | |||
| 3 Rectum | 11.3 (5.0–12.5) | 1 | <5 | |||
| 6 | 51 F | CC | 1 Colon | 36.3 (5.0–50.0) | 1 | <5 |
| 2 Colon | 35.3 (12.5–42.5) | 1 | <5 | |||
| 3 Colon | 22.9 (10.0–45.0) | 1 | 5–10 | |||
| 4 Colon | 33.8 (17.5–50.0) | 1 | 5–10 | |||
| 7 | 55 F | CC | 1 Cecum | 38.3 (2.5–82.5) | 1 | <5 |
| 2 Ascending | 23.8 (2.5–50.0) | 1 | <5 | |||
| 3 Transverse | 44.2 (20.0–65.0) | 1 | <5 | |||
| 4 Descending | 37.7 (12.5–50.0) | 1 | <5 | |||
| 5 Sigmoid | 36.2 (5.0–55.0) | 1 | <5 | |||
| 6 Rectum | 17.1 (10.0–52.5) | 1 | <5 | |||
| 8 | 55 F | CC | 1 Descending | 16.8 (5.0–27.5) | 1 | 5–10 |
| 2 Sigmoid | 14.5 (7.5–37.5) | 1 | <5 | |||
| 3 Rectum | 15.4 (5.0–35.0) | 1 | <5 | |||
| 9 | 68 M | CC | Cecum | 26.3 (5.0–82.5) | 1 | <5 |
| 10 | 38 F | CC | 1 Ascending | 38.2 (7.5–100.0) | 1 | <5 |
| 2 Transverse | 25.8 (5.0–42.5) | 1 | <5 | |||
| 11 | 85 F | CC | 1 Colon | 35.0 (12.5–75.0) | 1 | <5 |
| 2 Colon | 24.3 (7.5–65.0) | 1 | <5 | |||
| 12 | 75 F | CC | 1 Rectosigmoid | 30.5 (12.5–137.5) | 1 | <5 |
| 2 Transverse | 20.8 (5.0–75.0) | 1 | <5 | |||
| 13 | 60 M | Adenoma | Colon* | 6.0 (2.5–12.5) | 0 | <5 |
| 14 | 68 M | Carcinoma | Colon* | 7.8 (2.5–12.5) | 0 | <5 |
| 15 | 84 M | Carcinoma | Colon* | 8.0 (2.5–12.5) | 0 | <5 |
| 16 | 74 M | Carcinoma | Colon* | 4.0 (1.5–7.5) | 0 | <5 |
| 17 | 64 M | Carcinoma | Colon* | 3.8 (1.5–5.0) | 0 | <5 |
| 18 | 40 F | Carcinoma | Colon* | 6.5 (2.5–10.0) | 0 | <5 |
| 19 | 73 M | Diverticulitis | Colon* | 7.5 (1.5–12.5) | 0 | <5 |
| 20 | 30 F | UC | Colon | 2.5 (1.5–5.0) | 2 | <5 |
| 21 | 40 F | UC | Colon | 3.0 (1.5–5.0) | 2–3 | <5 |
| 22 | 70 F | UC (rem.) | Colon | 1.5 (0.0–5.0) | 1 | <5 |
| 23 | 50 M | UC (rem.) | Colon | 2.0 (0.0–2.5) | 1 | <5 |
| 24 | 38 F | CD | Colon | 5.5 (1.5–7.5) | 2 | <5 |
| 25 | 26 F | CD | Colon | 2.5 (1.5–4.0) | 2 | <5 |
| 26 | 31 F | CD | Colon | 2.0 (1.5–2.5) | 2 | <5 |
| 27 | 64 M | CD | Colon | 2.2 (2.0–2.4) | 3 | <5 |
CC, collagenous colitis; UC, ulcerative colitis; UC (rem.), ulcerative colitis in remission; CD, Crohn’s disease; Colon*, mucosa distant to focal lesion; IEL, intraepithelial lymphocytes per 100 epithelial cells.
Inflammation: density of lymphoplasmocytic infiltrate graded as absent (0), mild (1), moderate (2), or severe (3).
Tissue Sectioning
Sections (5 μm) were collected onto 3-aminopropyl triethoxysilane-coated slides, dried for several hours at 37°C, dewaxed in xylene, rehydrated in graded alcohols, and immediately processed.
Immunohistology
Collagens type I, III, and VI were detected using affinity-purified rabbit antibodies specific for monkey collagen type I and monkey procollagen type III aminoterminal propeptide 20 as well as a rabbit antibody against human collagen type VI. 21 Tenascin was localized with a monoclonal antibody specific for human tenascin (MAB1927, Chemikon, Temecula, CA). Immunohistology for undulin was performed with a monoclonal antibody against human undulin (clone 15/III/D6). 22 Collagen type IV and smooth-muscle α-actin were detected with the antibodies CIV22 and 1A4, respectively (Dako, Glostrup, Denmark). The monoclonal antibodies required pretreatment of paraffin sections with pronase (Boehringer Mannheim, Mannheim, Germany) at a concentration of 0.5 mg/ml in phosphate buffered saline, pH 7.2, for 10 minutes at 37°C. The immunohistochemical detection of MMP-1 was performed with affinity-purified polyclonal rabbit antibodies specific for human MMP-1 (Quartett, Berlin, Germany). The alkaline phosphatase anti-alkaline phosphatase (APAAP) method was used for the detection of immobilized antibodies, using affinity-purified mouse anti-rabbit immunoglobulin serum (Dianova, Hamburg, Germany), affinity-purified rabbit anti-mouse immunoglobulin serum (Dako), and APAAP complex (1:20 dilution, Dako). Alkaline phosphatase was developed with new fuchsin.
Preparation and Labeling of Probes
pGEM1 (Promega, Madison, WI)-based run-off transcription expression vectors were constructed by subcloning of the 1300-bp Pst fragment of pα1R1 corresponding to the triple helical region of rat α1(I) procollagen, 23 the 500-bp BamHI fragment of pHT21 corresponding to the triple helical region of human α1(IV) procollagen, 24 as well as the 735 bp SstI-EcoRI and the 730 bp EcoRI-SstI fragments of pCllaseI (#57685, American Type Culture Collection, Manassas, VA) harboring a human MMP-1 cDNA. 25 The TIMP-1 probe was a reverse transcription-polymerase chain reaction-derived cDNA fragment. 26 All of these probes had been extensively characterized in previous studies. 26-29 The MMP-13 probe was generated by reverse transcription and subsequent amplification of positions 24 to 495 in the reported sequence 30 using placental RNA as well as the primers 5′-CTG CCT TCC TCT TCT TGA GCT GG and 5′-ATG ATG TCA GCA ATG CCA TCG. All constructs were subjected to nucleic acid sequence analysis and conformed to the published sequences. After linearization of the plasmids with either HindIII or EcoRI restriction endonuclease, T7 or SP6 RNA-polymerase (Gibco-BRL, Eggenstein, Germany), respectively, were employed to obtain run-off transcripts of either the anti-sense (complementary to mRNA), or sense (anti-complementary, negative control) strands. Transcription and labeling of RNA probes were performed as described previously using [35S]-uridine-5′-(α-thio)-triphosphate (1250 Ci/mmol, New England Nuclear, Dreieich, Germany). 26,27 The specific activity routinely obtained was 1.2–1.4 × 10 9 cpm/μg.
In Situ Hybridization
Pre-hybridization, hybridization, washing procedures including removal of non-specifically bound probe by ribonuclease A digestion, and autoradiography of slides, as well as the procedure for combined immunohistology and radioactive in situ hybridization, were as described in detail. 26,27
Statistical Evaluation
Autoradiographic signals were evaluated by counting silver grains attributable to individual cells. The background labeling was determined on adjacent serial sections subjected to sense control hybridization. In each tissue section the number of positively labeled cells and the relative transcript levels, ie, grains per cell, were determined in 10 microscopic fields at a magnification of 1:400. For this purpose the lamina propria was divided into three compartments: the superficial subepithelial myofibroblast (sSEMF) sheet, the pericryptal subepithelial myofibroblast (pcSEMF) sheet, and the remainder of the lamina propria (LP). The sSEMF sheet was defined as the double cell layer immediately under the superficial collagenous band extending to the crypt opening. The pcSEMF sheet consisted of the double cell layer immediately adjacent to the necks and bases of crypts. The standard error of the mean (SE) was calculated and statistical evaluation was performed using the Mann-Whitney U-test.
Results
Conventional Histology
Colon biopsies diagnosed as CC displayed a characteristic sharply delineated, band-like linear deposition of ECM immediately underneath the surface epithelium without extension along the crypts. The subepithelial layer showed an average thickness between 11.1 and 44.2 μm. In many cases, the surface epithelium appeared flattened. The number of intraepithelial lymphocytes was small. These findings were accompanied by a mild, uncharacteristic inflammatory infiltrate of the lamina propria, composed mainly of small lymphocytes, plasma cells, and few eosinophils (Table 1) ▶ .
Immunohistology
In all cases immunostaining for procollagen type III (Figure 1, A and B) ▶ , and collagens type I and VI was found in the extracellular space of the lamina propria, around blood vessels, and under the surface and crypt epithelium. In CC, staining with these antibodies was most intense in the superficial collagenous layer. In ulcerative colitis and Crohn’s disease, a moderate and partially intense staining for procollagen type III and collagens type I and VI was seen in the submucosal stroma. Collagen type IV was localized in basement membranes of epithelium and blood vessels but did not accumulate in the superficial collagenous layer. Tenascin-specific staining was largely restricted to a thin layer underneath the surface epithelium in normal mucosa (Figure 1C) ▶ . In CC biopsies, tenascin-specific staining was most prominent within the subepithelial layer in its entire thickness (Figure 1D) ▶ . In ulcerative colitis, staining with this antibody was seen in areas with a dense inflammatory infiltrate of the lamina propria, particularly around blood vessels. The undulin-specific antibody displayed a weak staining in the lamina propria, decorating densely packed fiber bundles in the submucosal stroma (Figure 1E) ▶ . The subepithelial ECM in normal controls and, remarkably, in CC specimens did not display undulin-specific staining (Figure 1F) ▶ . In contrast, areas of transmural fibrosis in Crohn’s disease showed intense staining for undulin but not for tenascin. Some of the stromal cells beneath the superficial collagenous layer stained for smooth-muscle α-actin, indicating a myofibroblastic phenotype of these cells. Immunostaining for MMP-1 displayed a weak diffuse staining within the lamina propria with accentuation around scattered mononuclear cells.
Figure 1.
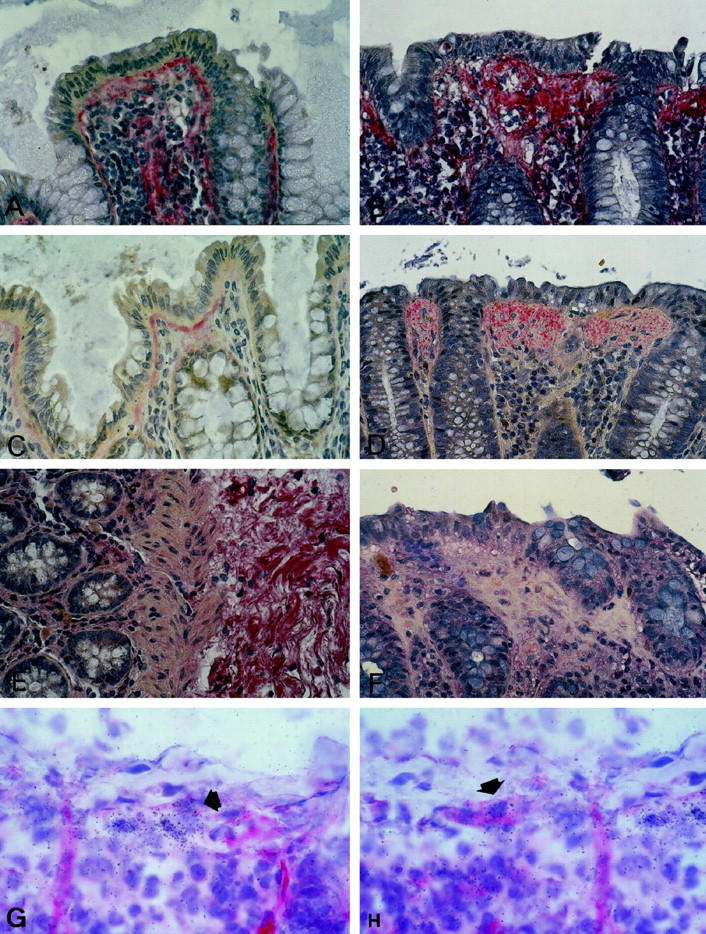
Immunostaining specific for procollagen type III (A, B), tenascin (C, D), and undulin (E, F). Procollagen type III specific staining is present in the lamina propria of normal mucosa, particularly underneath the superficial and crypt epithelium (A), and accumulates in the superficial collagenous layer of CC (B). Tenascin-specific staining is largely localized to a thin subepithelial layer in beneath the surface epithelium of normal colon mucosa (C) and stains the subepithelial matrix deposits of CC in their full thickness (D). Tenascin staining is virtually absent from the pericryptal area in CC and normal mucosa. In normal mucosa, undulin is found in small amounts along vessels in the lamina propria and decorating dense fibrillar bundles of the submucosal stroma (E). At variance with tenascin, undulin is absent from the subepithelial collagenous layer of CC (F). Combined immunohistology and in situ hybridization for smooth-muscle α-actin and procollagen α1(I) RNA in CC (cryostat sections; G, H). A proportion of the procollagen α1(I) RNA-positive cells underneath the superficial collagenous band is decorated by α-actin (arrows, CC case no. 8, Table 1 ▶ ). APAAP technique, original magnification, ×100.
In Situ Hybridization
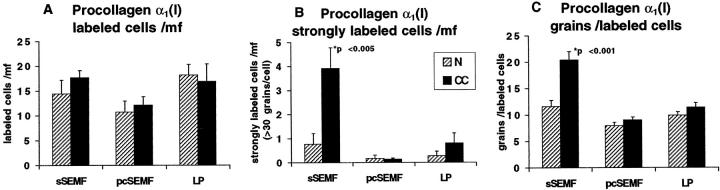
Procollagen α1(I) transcripts were detectable within the lamina propria of all specimens. In normal colonic tissue, the signal intensity, ie, the number of procollagen α1(I)-specific silver grains attributable to individual cells, did not differ significantly between sSEMF, pcSEMF, and other lamina propria cells (Figures 2A and 3) ▶ ▶ . In contrast, CC specimens displayed a significantly enhanced signal intensity (P < 0.001) in sSEMF cells in an almost linear, uninterrupted distribution (Figures 2B, 2C, and 3) ▶ ▶ . The relative number of cells expressing procollagen α1(I), however, was similar in normal mucosa and CC specimens. Pericryptal subepithelial cells and some of the superficial subepithelial cells stained for smooth-muscle α-actin, identifying these cells as myofibroblasts. By combining immunohistology and in situ hybridization, we found that at least a proportion of approximately 30% of the procollagen α1(I)-expressing cells of the sSEMF sheet were α-actin-positive myofibroblastic cells (Figure 1, G and H) ▶ .
Figure 2.
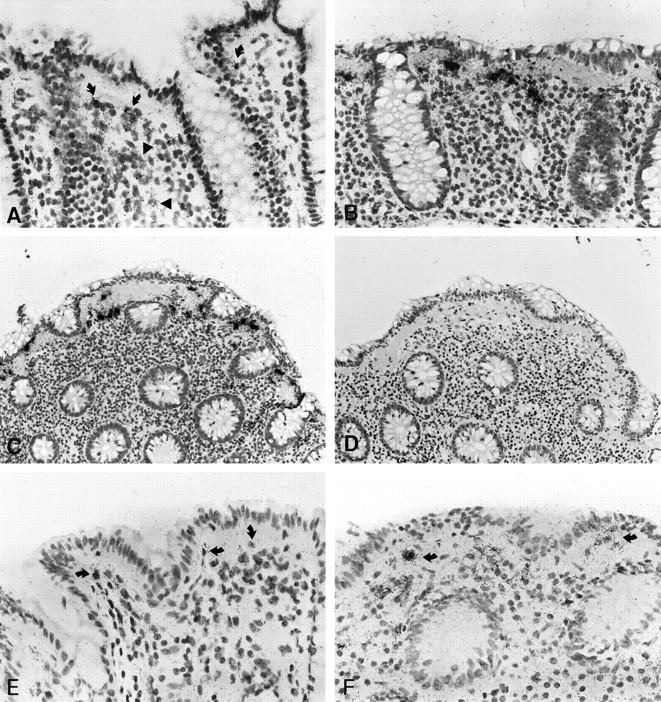
Patterns of procollagen α1(I) and α1(IV) expression in normal mucosa and CC as revealed by in situ hybridization with [35S]-labeled RNA probes. B, C, and D represent adjacent serial sections of the same biopsy (case no. 8). Procollagen α1(I)-specific labeling is displayed by cells of the subepithelial myofibroblast sheet (curved arrows) and the lamina propria (arrowheads) of normal colonic mucosa (A). In CC, the same probe reveals cells with high transcripts levels in an almost linear distribution underneath the surface epithelium (B and C). A weak background signal is seen after hybridization with the sense (control) probe (D). As compared to normal mucosa (E), elevated levels of procollagen α1(IV) transcripts are found in myofibroblasts in a linear distribution underneath the surface epithelium in CC (arrows, case no. 6, F). Autoradiographic exposure time 14 days (A–D) and 36 days (E, F). Original magnification, ×100 (A, B, E, F) and ×50 (C, D).
Figure 3.
Semiquantitative evaluation of autoradiographic signals (silver grains) obtained with the procollagen α1(I) probe in CC cases (closed bars) and in normal mucosa (hatched bars). The histograms represent the average number of all specifically labeled cells (A) and of the strongly labeled cells (>30 grains/cells; B) per microscopic field (mf) in the subepithelial myofibroblast (sSEMF) and pericryptal myofibroblast (pcSEMF) layer and the remainder of the lamina propria (LP). A third panel (C) displays the evaluation of the average number of silver grains per labeled cell. In comparison to normal control mucosa, the number of strongly labeled cells (B) and the average number of grains per labeled cell (C) were significantly increased in the sSEMF sheet of CC biopsies, whereas the relative number of labeled cells did not differ significantly. The evaluation is based on sections of all cases of collagenous colitis (n = 12) and normal controls (n = 7) which had been carried out in parallel through the entire in situ hybridization and autoradiographic procedures.
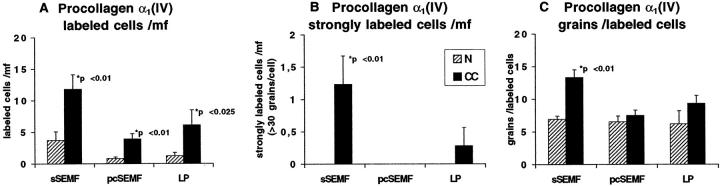
In normal mucosa, the cellular α1(IV) procollagen transcript levels were near the threshold of detection and required extended autoradiographic exposure of up to 36 days to be clearly labeled. These cells were found underneath the surface and crypt epithelium and along small blood vessels (Figure 2E) ▶ . In CC, procollagen α1(IV) RNA expression showed a significantly elevated number of labeled cells in all compartments of the LP and an increased signal intensity in the sSEMF (P < 0.01) (Figures 2F and 4) ▶ ▶ . The procollagen α1(IV)-expressing cells displayed a procollagen α1(I)-like linear distribution pattern with increased transcript levels in sSEMF cells.
Figure 4.
Semiquantitative evaluation of autoradiographic signals (silver grains) obtained with the procollagen α1(IV) probe in CC cases (closed bars) and in normal mucosa (hatched bars) as outlined in Figure 3 ▶ . In CC, the number of cells expressing procollagen α1(IV) transcripts was significantly increased in all compartments of the lamina propria (A). In the sSEMF sheet, the number of strongly labeled cells (B) and the average signal intensity (C) were also significantly higher in CC as compared to normal mucosa.
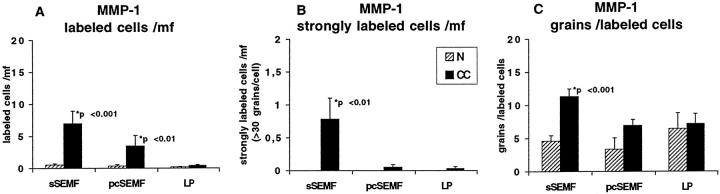
MMP-1 RNA transcript levels were close to the threshold of detection in normal mucosa. In CC, MMP-1 transcript levels were elevated in 8 of 12 cases. The number of MMP-1 expressing cells was increased in both the sSEMF and pcSEMF cells, but cells with highly elevated signal intensity were exclusive to the sSEMF sheet and showed a discontinuous and patchy distribution (Figures 5, A–C, and 6) ▶ ▶ . sSEMF cell clusters with elevated MMP-1 transcript levels alternated with extended regions devoid of any specific signal. The peculiarity of this labeling pattern was evident when compared to procollagen α1(I)-specific hybridizations on neighboring serial sections (Figure 5D) ▶ .
Figure 5.
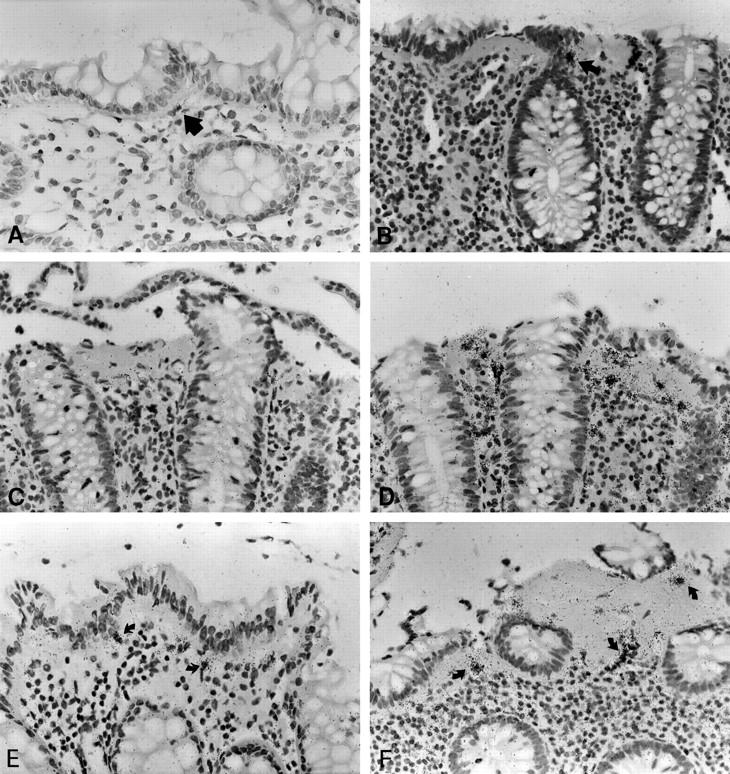
In situ hybridization with MMP-1, procollagen α1(I) and TIMP-1 specific [35S]-labeled RNA probes in normal mucosa and CC. In normal mucosa, MMP-1 expression is restricted to few weakly labeled cells (arrow, A). In CC, MMP-1 labeling is found in few cells clustered within the subepithelial myofibroblast sheet (arrow, B). These foci of expression are interrupted by long stretches of myofibroblasts without detectable MMP-1 RNA transcript levels (B, C). The paucity of these cells is particularly evident when compared to the procollagen α1(I) specific signal on an adjacent serial section (D). TIMP-1 expression is found in a few cells of normal colon (arrows, E), but upregulated in sSEMF, pcSEMF and LP cells of CC specimens (arrows, F). CC cases no. 8 (B, F) and no. 1 (C, D). Autoradiographic exposure time 28 days (A–C), 14 days (D), and 25 days (E, F). Original magnification, ×100 (A–F).
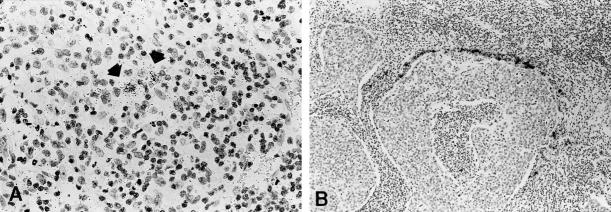
Figure 6.
In situ hybridization with the MMP-13 specific [35S]-labeled RNA probe in Crohn’s disease and a tonsillar squamous cell carcinoma. A clear autoradiographic signal is seen in few submucosal mesenchymal cells in Crohn’s disease (A), whereas a very intense signal is found in the peritumoral mesenchymal cells in the squamous cell carcinoma (B). Autoradiographic exposure time 36 days. Original magnifications, ×100 (A) and ×40 (B).
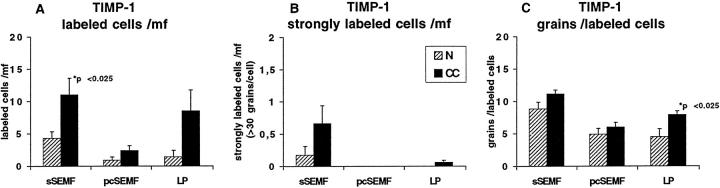
TIMP-1 RNA expression was found in moderate levels underneath the surface epithelium of normal mucosa (Figure 5E) ▶ . In the sSEMF sheet of CC biopsies, TIMP-1 expression was increased as well (P < 0.025), both with regard to the number of cells and the signal intensity per cell (Figures 5F and 7) ▶ ▶ . TIMP-1-positive cells showed an almost linear distribution in the sSEMF sheet including clusters of strongly labeled sSEMF cells. In serial sections, these clusters of strongly TIMP-1 RNA-expressing cells colocalized with clusters of cells displaying excess MMP-1 transcripts. Thus, the cellular distribution of TIMP-1 RNA appeared as a superimposition of both the procollagen α1(I) and the MMP-1 characteristic patterns.
Figure 7.
Semiquantitative evaluation of autoradiographic signals (silver grains) obtained with the MMP-1 probe in CC cases (closed bars) and in normal mucosa (hatched bars) as outlined in Figure 3 ▶ . In comparison to normal control mucosa, the number of MMP-1-expressing cells in general (A) and of cells with high specific transcript levels (B), and the average number of grains per labeled cell is (C) increased in CC. Note that these cells display a clustered distribution (Figure 5) ▶ .
Labeling patterns for the above listed probes in ulcerative colitis and Crohn’s disease specimens were as described previously 27,31 and differed from those in CC by strong labeling of cells throughout the entire LP or even other layers of the bowel wall.
MMP-13 RNA transcripts were not detectable in CC specimens and in three cases of ulcerative colitis. The MMP-13 probe produced, however, a clear signal in few submucosal mesenchymal cells of 1 of 3 cases of Crohn’s disease (Figure 6A) ▶ , and a very intense autoradiographic signal in peritumoral mesenchymal cells in 4 of 9 squamous cell carcinomas of the head and neck region (Figure 6B) ▶ . Hybridizations with sense (control) probes revealed a weak, nonspecific background labeling (Figure 2D) ▶ .
Discussion
Collagenous colitis is a rare, only recently recognized intestinal disease entity of unknown etiology. At variance with other forms of inflammatory bowel diseases, the macroscopic and radiological findings are inconspicuous. Unlike Crohn’s disease and ulcerative colitis, which may involve the entire bowel wall or the full thickness of the mucosa, respectively, CC displays deposits of interstitial ECM confined to a layer beneath the surface epithelium. More recent evidence suggests that, upon fecal stream diversion, these deposits can be removed within periods as short as a few months, 12 pointing to a peculiar composition of the subepithelial ECM layer favoring a high turnover.
The enhanced immunostaining for tenascin in the absence of any undulin/collagen XIV-specific labeling within the ECM layer may provide an explanation for these findings. Tenascin is considered a marker of mesenchymal cell proliferation and migration, and undulin is usually found in dense and differentiated collagen matrices. The tenascin/undulin dichotomy has previously been described in the context of matrix deposits that are subject to rapid remodeling, such as the ECM of tumors or in hepatic fibrosis. 22,32 Thus, an ECM that is subject to rapid turnover, regardless of the collagen composition, is made up of loosely connected interstitial collagen fibers with a high tenascin content. In contrast, matrices of long-standing scar tissue such as those present in Crohn’s disease or cirrhotic liver display a high undulin but a low tenascin content. 22,33-36 Apparently, undulin condenses interstitial ECM by binding and interconnecting collagen type I, III, V, and VI fibers and decorin. 37,38 These staining patterns can explain some aspects of the above-mentioned clinical observations.
In situ hybridization demonstrated synthesis of procollagen α1(I) and α1(IV) at significantly increased transcript levels by resident sSEMF in all of our CC specimens. In case of procollagen α1(I), an almost linear distribution of sSEMF cells with high transcript levels became evident. For procollagen α1(IV), an essentially identical distribution of labeled cells was found, albeit with lower autoradiographic signal intensity requiring extended autoradiographic exposure. These observations indicate a continuously up-regulated synthesis of both prototypic ECM molecules during the course of CC. Our findings are well in keeping with the recently published immunostaining patterns for collagens type I, III, IV, and VI. 39 The nonradioactive in situ hybridization patterns for the corresponding procollagens reported in the latter study are largely congruent with the distribution of (myo-) fibroblasts displaying >30 silver grains in our hybridizations.
In CC, up-regulated MMP-1 expression was restricted to few sSEMF cells in clustered, patchy distribution, contrasting with ECM molecules, whereas its physiological inhibitor, TIMP-1, displayed a partially superimposable expression pattern. Degradation of the various ECM components is a function of an array of enzymes with diverse, partially overlapping substrate specificities. 14-16 The observation of MMP-1 RNA suggests local enzyme synthesis, but does not prove the presence of active enzyme. Similarly, the immunostaining pattern for MMP-1 was inconclusive as it does not distinguish among zymogen, active, and inhibited forms of the enzyme. TIMP-1 expression seems to occur independently of MMP-1, as demonstrated by the weak, linear distribution of TIMP-1 RNA along the subepithelial collagenous layer. In areas with expression of MMP-1, MMP-1 protein, if activated, may be inhibited by TIMP-1 and other inhibitors unless, at balance, MMP-1 molecules prevail. Local and temporary changes of this balance in favor of active MMP-1 may then result in a rapid increase of the fibrolytic potential.
The expression of MMP-13 has previously been documented in ulcerative colitis and Crohn’s disease at the base of ulcerations and proved to be absent within intact mucosa. 40 MMP-13 was also found in squamous cell carcinomas of the head and neck region. 41 Our in situ hybridization results confirm these patterns. In our CC biopsies, however, MMP-13 transcript levels were below the threshold of detection, suggesting that this collagenase may not play a major role in nonulcerating intestinal lesions such as CC. This finding and the observation of only few and scattered granulocytes as potential sources of MMP-8 (neutrophil collagenase) in the LP underline the suggested central role of MMP-1 expression for interstitial collagen type I degradation in CC. A future approach to the study of collagen degradation in CC should analyze the MMP-1 activation cascade including expression patterns of plasmin-generating molecules and stromelysins (MMP-3 and -10), which serve as co-activators of MMP-1. 15,16
MMP expression in inflammatory bowel diseases has recently been studied by several groups reporting enhanced expression of MMP-1, -3, -7, -9, -10, -12, and -13 in ulcerative lesions of Crohn’s disease, ulcerative and ischaemic colitis, and in wound healing. 40,42-44 However, analysis of such lesions does not warrant conclusions about CC, because CC differs from the former lesions and entities by its peculiar clinical presentation and histology as a non-ulcerating, potentially reversible intestinal disorder.
In summary, the patterns of procollagen α1(I)-specific labeling in CC, which is accompanied by an enhanced deposition of tenascin in the absence of undulin, indicate synthesis of an immature, loosely connected interstitial collagenous matrix that may be rapidly degraded. The restricted occurrence of MMP-1 transcripts in the presence of increased TIMP-1 and interstitial procollagen expression, however, suggests a local imbalance of fibrogenesis and fibrolysis in CC, resulting in the characteristic, linear ECM deposits. Thus, future therapeutic strategies may be directed at influencing the levels of TIMP-1 produced by sSEMF cells.
Figure 8.
Semiquantitative evaluation of autoradiographic signals (silver grains) obtained with the TIMP-1 probe in CC cases (closed bars) and in normal mucosa (hatched bars) as outlined in Figure 3 ▶ . In comparison to normal control mucosa, the number of TIMP-1-expressing cells in general (A) and of cells with high specific transcript levels (B), and the average number of grains per labeled cell (C) is increased in CC. The relative frequency of sSEMF cells with strong MMP-1- and TIMP-1-specific labeling is similar. Note that these cells display a clustered distribution (Figure 5) ▶ .
Acknowledgments
We thank Drs. D. Rowe and D. Prockop for the gift of cDNA probes, Mr. H. J. Koppelmeyer for phototechnical work, and Drs. H. Schöpper and M. Rühl for critical reading of the manuscript.
Footnotes
Address reprint requests to Hermann Herbst, M.D., Institut für Pathologie, Universitätskrankenhaus Eppendorf, Martinistr. 52, 20246 Hamburg, Germany. E-mail: herbst@uke.uni-hamburg.de.
Supported by the Deutsche Forschungsgemeinschaft (grants He 1330/2-1 and SFB366 C5) and the Werner-Otto-Stiftung. D. S. was a recipient of a Hermann-und-Lilly-Schilling professorship.
References
- 1.Lindström CG: Collagenous colitis with watery diarrhoea: a new entity? Pathol Eur 1976, 11:87-89 [PubMed] [Google Scholar]
- 2.Bogomoletz WV: Collagenous, microscopic and lymphocytic colitis: an evolving concept. Virchows Arch 1994, 424:573-579 [DOI] [PubMed] [Google Scholar]
- 3.Bogomoletz WV, Flejou JF: Newly recognized forms of colitis: collagenous colitis, microscopic (lymphocytic) colitis and lymphoid idiopathic proctitis. Semin Diagn Pathol 1991, 8:178-189 [PubMed] [Google Scholar]
- 4.Lee E, Schiller LR, Vendrell D, Santa Ana CA, Fordtran JS: Subepithelial collagen table thickness in colon specimens from patients with microscopic colitis and collagenous colitis. Gastroenterology 1992, 103:1780-1796 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Mazzoleni G, Riddell RH: Distribution of collagenous colitis: utility of flexible sigmoidoscopy. Gut 1992, 33:65-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flejou JF, Grimaud JA, Molas G, Baviera E, Potet F: Collagenous colitis: ultrastructural study and collagen immunotyping of four cases. Arch Pathol Lab Med 1984, 108:977-982 [PubMed] [Google Scholar]
- 7.Jessurun J, Yardley J, Giardiello FM, Hamilton SR, Bayless TM: Chronic colitis with thickening of the subepithelial collagen layer (collagenous colitis): histopathologic findings in 15 patients. Hum Pathol 1987, 18:839-848 [DOI] [PubMed] [Google Scholar]
- 8.Jessurun J, Yardley J, Lee E, Vendrell D, Schiller L, Rordtran J: Microscopic and collagenous colitis: different names for the same condition? Gastroenterology 1986, 91:1583-1584 [DOI] [PubMed] [Google Scholar]
- 9.Giardiello FM, Lazenby AJ, Bayless TM, Levine EJ, Bias WB, Ladenson PW, Hutcheon DF, Derevjanik NL, Yardley JH: Lymphocytic (microscopic) colitis: clinicopathologic study of 18 patients and comparison to collagenous colitis. Dig Dis Sci 1989, 34:1730-1738 [DOI] [PubMed] [Google Scholar]
- 10.Riddell R, Tanaka M, Mazzoleni G: Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: a case control study. Gut 1992, 33:683-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen T, Rikardt Andersen J, Tvede M, Franzmann M: Collagenous colitis: are bacterial cytotoxins responsible? Am J Gastroenterol 1993, 88:375-377 [PubMed] [Google Scholar]
- 12.Järnerot G, Tysk C, Bohr J, Eriksson S: Collagenous colitis and fecal stream diversion. Gastroenterology 1995, 109:449-455 [DOI] [PubMed] [Google Scholar]
- 13.Bowling TE, Price AB, al-Adnani M, Fairclough PD, Menzies-Gow N, Silk DB: Interchange between collagenous and lymphocytic colitis in severe disease with autoimmune associations requiring colectomy: a case report. Gut 1996, 38:788–791 [DOI] [PMC free article] [PubMed]
- 14.Woessner JF: Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991, 5:2145-2154 [PubMed] [Google Scholar]
- 15.Birkedahl-Hansen H: Proteolytic remodeling of extracellular matrix. Curr Op Cell Biol 1995, 7:728-735 [DOI] [PubMed] [Google Scholar]
- 16.Nagase H: Activation mechanisms of matrix metalloproteinases. Biol Chem 1997, 378:151–160. [PubMed]
- 17.Windsor LJ, Grenett H, Birkedal-Hansen B, Bodden MK, Engler JA, Birkedal-Hansen H: Cell type-specific regulation of SL-1 and SL-2 genes: induction of the SL-2 gene but not the SL-1 gene by human keratinocytes in response to cytokines and phorbolesters. J Biol Chem 1993, 268:17341-17347 [PubMed] [Google Scholar]
- 18.Knäuper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G: Cellular mechanisms for human procollagenase-3 (MMP-13) activation. J Biol Chem 1996, 271:17124-17131 [DOI] [PubMed] [Google Scholar]
- 19.Grinnell F, Zhu M, Parks WC: Collagenase-1 complexes with alpha2-macroglobulin in the acute and chronic wound environments. J Invest Dermatol 1998, 110:771-776 [DOI] [PubMed] [Google Scholar]
- 20.Becker J, Schuppan D, Benzian H, Bals T, Hahn EG, Cantaluppi C, Reichart P: Immunohistochemical distribution of collagens types IV, V, and VI and of procollagens types I and III in human alveolar bone and dentine. J Histochem Cytochem 1986, 34:1417-1429 [DOI] [PubMed] [Google Scholar]
- 21.Loreal O, Clement B, Schuppan D, Rescan PY, Pissel M, Guillouzo A: Distribution and cellular origin of collagen VI during development and cirrhosis. Gastroenterology 1992, 102:980-987 [DOI] [PubMed] [Google Scholar]
- 22.Schuppan D, Cantaluppi MC, Becker J, Veit A, Bunte T, Troyer D, Schuppan F, Schmid M, Ackermann R, Hahn EG: Undulin, an extracellular matrix glycoprotein associated with collagen fibrils. J Biol Chem 1990, 265:8823-8832 [PubMed] [Google Scholar]
- 23.Genovese C, Rowe D, Kream B: Construction of DNA sequences complementary to rat α1 and α2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry 1984, 23:6210-6216 [DOI] [PubMed] [Google Scholar]
- 24.Pihlajaniemi T, Tryggvason, Myers JC, Kurkinen M, Lebo R, Cheung MC, Prockop DJ, Boyd CD: cDNA clones coding for the pro-α1(IV) chain of human type IV procollagen reveal an unusual homology of amino acid sequences in two halves of the carboxyl-terminal domain. J Biol Chem 1985, 260:7681-7687 [PubMed] [Google Scholar]
- 25.Whitham SE, Murphy G, Angel P, Rahmsdorf HJ, Smith BJ, Lyons A, Harris TJ, Reynolds JJ, Herrlich P, Docherty AJ: Comparison of the human stromelysin and collagenase by cloning and sequence comparison. Biochem J 1986, 240:913-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbst H, Wege T, Milani S, Grappone G, Orzechowski HD, Bauer M, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D: Tissue inhibitor of metalloproteinase-1 and -2 expression in rat and human liver fibrosis. Am J Pathol 1997, 150:1647-1659 [PMC free article] [PubMed] [Google Scholar]
- 27.Milani S, Herbst H, Schuppan D, Surrenti C, Riecken EO, Stein H: Cellular localization of type I, III, and IV procollagen gene transcripts in normal and fibrotic human liver. Am J Pathol 1990, 137:59-70 [PMC free article] [PubMed] [Google Scholar]
- 28.Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabro’ A, Ciancio G, Stefanini F, Burroughs AK, Surrenti C: Differential expression of matrix-metalloproteinases-1 and -2 genes in normal and fibrotic human liver. Am J Pathol 1994, 144:528-537 [PMC free article] [PubMed] [Google Scholar]
- 29.Matthes H, Herbst H, Schuppan D, Stallmach A, Milani S, Stein H, Riecken EO: Cellular localization of procollagen gene transcripts in inflammatory bowel disease. Gastroenterology 1992, 102:431-442 [DOI] [PubMed] [Google Scholar]
- 30.Freije JMP, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, Lopez-Otin C: Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem 1994, 269:16766-16773 [PubMed] [Google Scholar]
- 31.Matthes H, Ulrich K, Stallmach A, Herbst H, Schuppan D, Riecken EO: Collagenase type I mRNA expression as a predictive parameter to discriminate between Crohn’s disease and ulcerative colitis. Gastroenterology 1994, 106:A730(abstract) [Google Scholar]
- 32.Knittel T, Odenthal M, Schuppan D, Schwoegler S, Just M, Meyer zum Büschenfelde KH, Ramadori G: Synthesis of undulin by rat liver fat-storing cells: comparison with fibronectin and tenascin. Exp Cell Res 1992, 203:312-320 [DOI] [PubMed] [Google Scholar]
- 33.Klingel R, Ramadori G, Schuppan D, Knittel T, Meyer zum Buschenfelde KH, Köhler H: Coexpression of extracellular matrix glycoproteins undulin and tenascin in human autosomal dominant polycystic kidney disease. Nephron 1993, 65:111-118 [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Schuppan D, Becker J, Reichart P, Geldeblom HR: Distribution of undulin, tenascin, and fibronectin in the human periodontal ligament and cementum: comparative immunoelectron microscopy with ultra-thin cryosections. J Histochem Cytochem 1993, 41:245-251 [DOI] [PubMed] [Google Scholar]
- 35.Becker J, Schuppan D, Muller S: Immunohistochemical distribution of collagens type I, III, IV and VI, of undulin and of tenascin in oral fibrous hyperplasia. J Oral Pathol Med 1993, 22:463-467 [DOI] [PubMed] [Google Scholar]
- 36.Milani S, Grappone C, Schuppan D, Calabro’ A, Pellegrini G, Herbst H, Casini A, Pinzani M, Surrenti C: Undulin RNA and protein expression in normal and fibrotic human liver. Hepatology 1994, 20:908-916 [DOI] [PubMed] [Google Scholar]
- 37.Ehnis T, Dieterich W, Bauer M, Lampe B, Schuppan D: A chondroitin/dermatan sulfate form of CD44 is a receptor for collagen XIV (undulin). Exp Cell Res 1996, 229:388-397 [DOI] [PubMed] [Google Scholar]
- 38.Ehnis T, Dieterich W, Bauer M, Kresse H, Schuppan D: Localization of a binding site for the proteoglycan decorin on collagen XIV (undulin). J Biol Chem 1997, 272:20414-20419 [DOI] [PubMed] [Google Scholar]
- 39.Aigner T, Neureiter D, Müller S, Küspert G, Belke J, Kirchner T: Extracellular matrix composition and gene expression in collagenous colitis. Gastroenterology 1997, 113:136-143 [DOI] [PubMed] [Google Scholar]
- 40.Vaalamo M, Karjalainen-Linsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U: Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinase-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 1998, 152:1005-1014 [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson N, Airola K, Grenman R, Kariniemi AL, Saarialho-Kere U, Kähäri VM: Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol 1997, 151:499-508 [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA: Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. J Clin Pathol 1994, 47:113-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saarialho-Kere U, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML: Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol 1996, 148:519-526 [PMC free article] [PubMed] [Google Scholar]
- 44.Madlener M, Parks WC, Werner S: Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res 1998, 242:201-210 [DOI] [PubMed] [Google Scholar]