Abstract
The immunohistochemical expression pattern of β-catenin has been correlated with β-catenin gene mutations, clinicopathological features, and disease outcome in 69 stage I and II ovarian carcinomas. β-Catenin expression was localized in the nuclei, in addition to the cytoplasm and membrane, in 11 tumors (16%): nine endometrioid carcinomas with widespread nuclear expression and two serous carcinomas with focal nuclear expression. The remaining 58 carcinomas (84%) only had membranous β-catenin expression. All but one of the endometrioid carcinomas with nuclear β-catenin expression had considerable squamous metaplasia, and five of these cases had large areas of endometrioid tumor of low malignant potential. In addition, β-catenin nuclear expression was observed in atypical epithelial cells in endometriotic glands adjacent to an endometrioid carcinoma. Sequencing was performed on 25 tumors and corresponding normal tissue: all 13 endometrioid tumors as well as 12 carcinomas of other histological types (four serous, two clear cell, two mucinous, and two mixed). There were oncogenic mutations in the phosphorylation sequence for GSK-3β in exon 3 of the β-catenin gene in seven endometrioid carcinomas with β-catenin nuclear expression. Three mutations affected codon 32 (D32G, D32Y, and D32Y), one affected codon 33 (S33C), two affected codon 37 (S37C and S37F), and one affected codon 41 (T41A). No mutations were observed in the other 18 carcinomas analyzed, comprising two endometrioid and two serous carcinomas with β-catenin nuclear expression, and 14 carcinomas of different histological types with only membranous expression. In the univariate and multivariate survival analyses, β-catenin nuclear expression was selected as an indicator of good prognosis, because no patient whose tumor expressed β-catenin in the nuclei showed relapses or died, in contrast to the 19 relapses and deaths among patients with tumors that only had β-catenin membranous expression, including three of the four patients with endometrioid carcinomas. Oncogenic β-catenin mutation is characteristic of a group of endometrioid carcinomas with a good prognosis, most of which originate from previous benign or borderline lesions. Endometrioid carcinomas with exclusively membranous expression of β-catenin seem to represent a different subgroup of carcinomas that probably have a worse prognosis. In early-stage ovarian cancer, determination of the β-catenin expression pattern could prove to be a useful marker for selecting low-risk patients.
Ovarian cancer is a highly agressive gynecological malignancy affecting approximately 27,000 women per year in the United Sates and producing almost 15,000 deaths yearly. 1 Although the prognosis of early-stage ovarian cancer is considerably better than in advanced disease, 30–40% of early-stage patients will eventually die as a result of their tumors. 2 High-grade histology, tumor cells in ascites or peritoneal washings, a ruptured capsule, or the presence of a tumor on the external surface have been described as unfavorable prognostic factors in early-stage ovarian cancer, 3,4 and adjuvant chemotherapy is strongly recommended. 2
Relatively little is known about the molecular events that lead to the development of ovarian cancer, and no molecular markers are generally accepted as prognostic indicators. Although most cases of epithelial tumors of the ovary seem to have a common origin in the surface epithelium covering the ovary, different pathways and genetic alterations have been implicated in the development of cystadenomas, borderline tumors, and carcinomas of different histological subtypes and stages of progression. For example, loss of heterozygocity and a complete loss of chromosome 17 is characteristic of the advanced stages of serous carcinomas, 5 whereas ras mutation participates in the development of tumors with mucinous differentiation, but not in other histological types. 6 Recently, we have reported data that strongly suggest that β-catenin may function as an oncogen in the initiation of some ovarian endometrioid carcinomas in which β-catenin is mainly expressed in the nucleus and cytoplasm. 7 However, the clinical implications of this finding remain to be established.
β-Catenin is a multifunctional protein involved in at least two important biological processes: cell-cell adhesion and signal transduction (transcriptional activation). 8 The role of β-catenin in adhesion is well established. In the adherens junctions of epithelial cells, the cytoplasmic domain of E-cadherin organizes a peripheral protein complex, including α-catenin, β-catenin, and γ-catenin (plakoglobin), that is necessary for adhesion to occur. 9 The role of β-catenin in the linkage between α-catenin and E-cadherin in this complex is probably regulated by epidermal growth factor-mediated tyrosine phosphorylation of the β-catenin. 8,9 Molecules of the cadherin/catenin complex have been implicated in differentiation and tumor progression, because their reduced expression is not only frequent in poorly differentiated tumors, but also might produce loss of adhesiveness and increased invasive and metastatic potential. 10-13
In addition, β-catenin participates in the transduction of signals and activates transcription by forming complexes with DNA binding proteins in the T-cell factor-lymphoid enhancer factor (TcF-lef) family. 14 The free (cytoplasmic) β-catenin level is low in normal cells because the protein is targeted for destruction in the ubiquitin-proteasome system 15 by adenomatous polyposis coli (APC) protein together with glycogen synthetase kinase-3β (GSK-3β) 16 and other molecules such as axin or conductin 8 ; thus wild-type APC plays an essential role in the clearance of unnecessary β-catenin from the cytoplasm. Disruption of the APC-mediated regulation of the β-catenin-Tcf pathway was first implicated in the development of colon carcinomas and melanoma. 17,18 Mutations that inactivate APC increase cytoplasmic levels of free β-catenin, 19 which may then act as an oncoprotein through constitutive β-catenin-Tcf-regulated transcription. 14 In the same manner, activating β-catenin mutations render this pathway insensitive to the effect of WT APC. 17,18 To date, oncogenic β-catenin mutations have been described in several malignancies, such as carcinomas of the colon, 17,20-23 ovary, 7 prostate, 24 liver, 25 and endometrium 26 and medulloblastomas. 27
This study analyzes the expression pattern of β-catenin in a series of 69 stage I and II ovarian carcinomas and their relationships with β-catenin gene mutation, clinicopathological features, and survival to better understand the biological and clinical relevance of β-catenin pathway activation in ovarian cancer. Some of the clinicopathological and genetic features of 30 cases have previously been reported. 7
Materials and Methods
Patients
This study was conducted on 69 primary ovarian carcinomas diagnosed at the Department of Pathology of La Paz Hospital, Madrid. All cases were in stage I or II and had a minimum follow-up period of 5 years. Staging was done following the International Federation of Gynecology and Obstetrics (FIGO) system. Retrospective reviews of patient files were performed to obtain all pertinent data on the primary tumor, type of surgery, adjuvant treatment, recurrence, and survival. If necessary, patients were retrospectively assigned a FIGO stage on the basis of their clinical history. The tumors were routinely fixed in 10% formalin and embedded in paraffin. Histological typing was performed according to the World Health Organization classification. Histological, immunohistochemical, and DNA studies were performed on formalin-fixed, paraffin-embedded tissue samples.
Immunohistochemistry
Immunohistochemistry for β-catenin was performed by the avidin-biotin-alkaline phosphatase method, as previously reported. 7 A heat-induced antigen retrieval step (deparaffinized sections were immersed in 0.01 mol/L sodium citrate buffer (pH 6.0) and incubated in a pressure cooker for 3 minutes) was performed. The mouse anti-human β-catenin monoclonal antibody (Transduction Laboratories, Lexington, KY) was applied at a dilution of 1:200. In negative controls, the primary antibody was omitted or replaced with an irrelevant antibody.
Evaluation of Immunohistochemical Staining
Two patterns of β-catenin expression were considered: membranous, if β-catenin was localized in the cell membrane only; and nuclear, when β-catenin was expressed in the nucleus, irrespective of the percentage of stained nuclei or simultaneous expression of β-catenin in membrane and cytoplasm.
A semiquantitative estimation of membranous β-catenin expression was made, using a composite score obtained by adding the values of the immunoreaction intensity and relative abundance of the β-catenin immunoreactive cells, as previously reported for cadherins. 28,29 Briefly, the intensity was graded from 0 (equivalent to background staining of the acellular stroma) to +3 (intense stain equivalent to normal surface epithelium). The abundance of β-catenin-positive cells was graded from 0 to 4 (0 = less than 5% of positive cells; 1 = 5–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%). β-Catenin expression was considered preserved when the composite score was 6 or 7. Cases with scores between 0 and 5 were considered to be tumors with reduced β-catenin expression.
DNA Analysis
Polymerase chain reaction (PCR) was performed as previously reported 7 on 25 tumors and corresponding normal tissue: all 13 endometrioid tumors as well as 12 carcinomas of other histological types (four serous, two clear cell, two mucinous, and two mixed). DNA was extracted from paraffin blocks containing a large proportion of epithelial tumor cells (>75%), but no microdissection of selected areas was performed. Normal tissue in each case consisted of samples from fallopian tube or myometrium.
The primers used were 240F (ATG GAA CCA GAC AGA AAA GC) and 439F (GCT ACT TGT TCT GAG TGA AG), which amplified a 200-bp fragment of exon 3 of the β-catenin gene, encompassing the sequence for GSK-3β phosphorylation. After purification, PCR products were sequenced in a 373 Automated Sequencer or a 310 Automater Sequencer (Applied Biosystems, Foster City, CA), using the Abi Prism dRodhamine Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). PCR primers were used as the primers for sequencing the two strands of the amplified products. Cases with abnomal DNA sequences were subjected to DNA extraction from additional tissue sections, PCR, and sequencing, to confirm the presence of true mutations.
Statistical Methods
The χ 2 test was used to analyze the statistical significance of the relationship between β-catenin expression and the clinicopathological and immunohistochemical variables. Relapse-free survival and overall survival, defined as the time from diagnosis to relapse or death, respectively, were used as a measure of prognosis. Univariate survival curves were estimated using the Kaplan and Meier method and compared using the log rank test. Multivariate analysis was done using Cox’s proportional hazards regression model. Analyses were carried out using JMP (Version 3.0.1; SAS Institute, Cary, NC).
Results
Clinicopathological Features
Some clinicopathological, inmunohistochemical, and genetic features of 30 cases have previously been reported. 7 The mean age of the 69 patients was 55 years (range 19–77). There were 19 (27.5%) stage Ia, 4 (6%) stage Ib, 28 (40.5%) stage Ic, 5 (7%) stage IIa, 9 (13%) stage IIb, and 4 (6%) stage IIc tumors. There were 27 (39%) grade I carcinomas, 28 (41%) grade II, and 14 (20%) grade III. No patient showed macroscopic residual disease after surgery. All patients received postoperative therapy: 17 (24.6%) radiotherapy and 52 (75.4%) chemotherapy: 33 melphalan and adryamicine, 15 melphalan alone, and 4 cisplatin-based chemotherapy.
β-Catenin Expression and Correlations with Clinicopathological Features
All analyzed tumors showed membrane β-catenin expression with variable extension and intensity, but simultaneous nuclear β-catenin expression (nuclear pattern) was observed in only 11 (16%) ovarian carcinomas (Figure 1A) ▶ ; the remaining 58 tumors (84%) only had membrane expression (membranous pattern) (Figure 1B) ▶ . The expression pattern (nuclear versus membranous) correlated significantly with the histological type (Table 1) ▶ , inasmuch as 9 cases with nuclear expression were endometrioid carcinomas. Nuclear expression was also observed in two serous carcinomas. In endometrioid lesions, the percentage of stained nuclei ranged between 25% and 90% of the neoplastic cells. In contrast, in serous carcinomas the percentage of immunoreactive nuclei did not exceed 5%. The different β-catenin expression patterns did not correlate with tumor grade and stage. In addition, postoperative therapy was similar in tumors with a nuclear or membranous β-catenin expression pattern (Table 1) ▶ .
Figure 1.
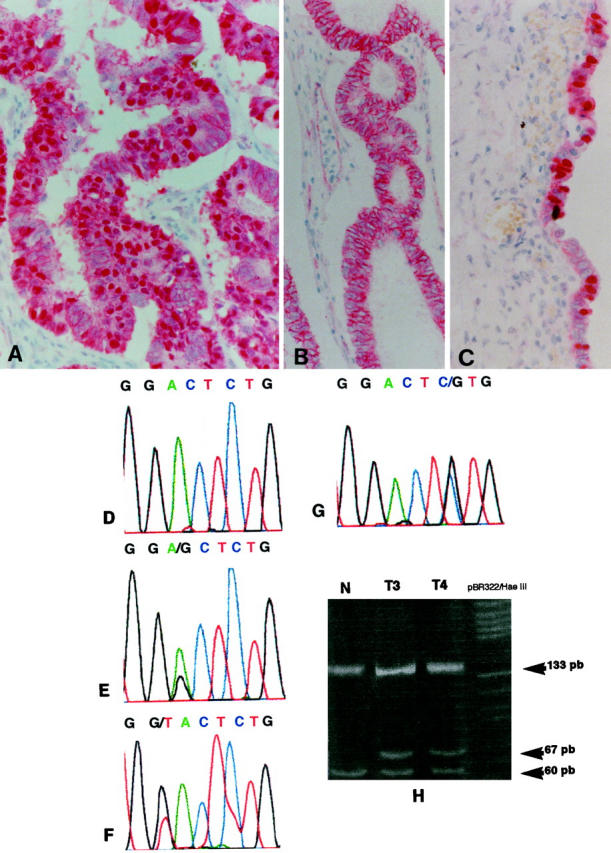
A: Nuclear β-catenin expression pattern in an endometrioid carcinoma. B: Membranous β-catenin expression pattern in an endometrioid carcinoma. C: Nuclear β-catenin expression in some nuclei of epithelial cells of an endometriotic cyst. D: Normal sequence of codons 32 and 33 in exon 3 of β-catenin gene. E: GAC to GGC change in codon 32 (D32G) in tumor 1. F: GAC to TAC change in codon 32 (D32Y) in tumor 3. G: TCT to TGT change in codon 33 (S33C) in tumor 4. H: Analysis with HinfI restriction endonuclease of PCR products from cases 3 and 4. In normal tissue (N) digestion produces three fragments of 133, 60, and 7 bp. In tumor tissues, heterozygous mutations in codons 32 and 33 eliminate a restriction site in the mutated allele and generate an additional fragment of 67 bp.
Table 1.
Relationships between β-Catenin Expression and Clinicopathological Features in Stage I and II Ovarian Carcinomas*
| Nuclear β-catenin† expression pattern | Membrane β-catenin expression pattern | P value | |
|---|---|---|---|
| Histological type | |||
| Endometrioid (n = 13) | 9 (70%) | 4 (30%) | |
| Serous (n = 16) | 2 (12.5%) | 14 (87.5) | |
| Mucinous (n = 13) | 0 | 13 (100%) | |
| Clear cell (n = 18) | 0 | 18 (100%) | |
| Mixed (n = 9) | 0 | 9 (100%) | 0.000 |
| Grade | |||
| I (n = 27) | 5 (18.5%) | 22 (81.5%) | |
| II (n = 28) | 5 (18%) | 23 (82%) | |
| III (n = 14) | 1 (7.2%) | 13 (96.8%) | 0.552 |
| FIGO stage | |||
| Ia-Ib (n = 23) | 5 (21.7%) | 18 (78.3%) | |
| Ic-II (n = 46) | 6 (15.2%) | 40 (84.8%) | 0.36 |
| Postoperative therapy | |||
| Adriamycine and melphalan (n = 33) | 5 (15.2%) | 28 (84.8%) | |
| Melphalan (n = 15) | 2 (13.4%) | 13 (86.6%) | |
| Cisplatinun (n = 4) | 0 | 4 (100%) | |
| Radiotherapy (n = 17) | 4 (23.5%) | 13 (76.5%) | 0.663 |
*χ2 test.
†Nuclear β-catenin expression pattern: When the protein was expressed in the nucleus, irrespective of the percentage of stained nuclei or simultaneous expression of β-catenin in membrane and cytoplasm; membranous β-catenin expression pattern: if β-catenin was localized in the cell membrane only.
All but one endometrioid carcinoma with nuclear β-catenin expression also had evident squamous metaplasia, and five of these cases had large areas of endometrioid tumor of low malignant potential with an adenofibromatous, papillary, or mixed growth pattern (Table 2) ▶ . Endometrioid carcinomas with β-catenin membranous expression only did not have squamous metaplasia, and two cases had a growth pattern resembling that of sex-cord stromal tumors. Four mixed carcinomas with an endometrioid component showed only β-catenin membranous expression. Nuclear β-catenin expression was observed in epithelial cells of endometriotic cyst (Figure 1C) ▶ adjacent to an endometrioid carcinoma with β-catenin nuclear expression.
Table 2.
Clinicopathological, Immunohistochemical, and Genetic Characteristics of 13 Endometrioid Carcinomas
| Case | Age | Stage | Grade | Endometriosis | ETLMP | Squamous metaplasia | β-Catenin pattern | Expression membrane | Mutated codon | Base change | Amino acid change | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | 1c | 1 | Absent | Present | Present | Nuclear | Reduced | 32 | GAC to GGC | Asp to Gly | NED 6 |
| 2 | 49 | 1b | 1 | Absent | Present | Present | Nuclear | Preserved | 32 | GAC to TAC | Asp to Tyr | NED 6 |
| 3 | 47 | 1b | 2 | Absent | Absent | Present | Nuclear | Reduced | 32 | GAC to TAC | Asp to Tyr | NED 16 |
| 4 | 50 | 2b | 2 | Absent | Absent | Present | Nuclear | Reduced | 33 | TCT to TGT | Ser to Cys | NED 11 |
| 5 | 48 | 1a | 1 | Absent | Present | Present | Nuclear | Preserved | 37 | TCT to TGT | Ser to Cys | NED 8 |
| 6 | 64 | 2a | 1 | Present | Present | Present | Nuclear | Preserved | 37 | TCT to TTT | Ser to Phe | NED 11 |
| 7 | 50 | 2a | 2 | Present | Absent | Present | Nuclear | Preserved | 41 | ACC to GCC | Thr to Ala | NED 12 |
| 8 | 38 | 1c | 2 | Present | Present | Present | Nuclear | Reduced | NS | NED 22 | ||
| 9 | 58 | 1c | 3 | Absent | Absent | Absent | Nuclear | Reduced | NS | NED 14 | ||
| 10 | 66 | 2c | 2 | Present | Absent | Absent | Membranous | Reduced | NS | NED 6 | ||
| 11 | 49 | 1c | 2 | Absent | Absent | Absent | Membranous | Reduced | NS | DD 2 | ||
| 12 | 61 | 2c | 2 | Absent | Absent | Absent | Membranous | Reduced | NS | DD 2 | ||
| 13 | 27 | 1a | 3 | Absent | Absent | Absent | Membranous | Reduced | NS | DD 3 |
Endometriosis in case 10 affected the contralateral ovary, whereas the tumorous ovary was affected in cases 6, 7, and 8. This last case arose directly from a endometriotic cyst. ETLMP: Endometrioid tumor of low malignant potential. NS: normal sequence. NED: No evidence of disease. DD: Died of disease.
As previously noted, β-catenin expression was observed in the membrane in all of the 69 carcinomas, but only 15 cases (22%) had preserved membrane expression (score 6–7), and the remaining 54 carcinomas had reduced membrane expression (score 3–5). The status of membrane β-catenin expression (preserved versus reduced) did not correlate to the histological type, grade, or stage of the patient’s tumor (Table 3) ▶ .
Table 3.
Relationships between β-Catenin Membrane Expression and Clinicopathological Features in Stage I and II Ovarian Carcinomas*
| Preserved β-catenin expression pattern (score 6–7) | Reduced β-catenin membrane expression (score 0–5) | P value | |
|---|---|---|---|
| Histological type | |||
| Endometrioid (n = 13) | 5 (38%) | 8 (62%) | |
| Serous (n = 16) | 1 (6%) | 15 (94%) | |
| Mucinous (n = 23) | 3 (23%) | 20 (77%) | |
| Clear cell (n = 18) | 5 (28%) | 13 (72%) | |
| Mixed (n = 9) | 1 (11%) | 8 (89%) | 0.210 |
| Grade | |||
| I (n = 27) | 8 (30%) | 19 (70%) | |
| II (n = 28) | 5 (17%) | 23 (83%) | |
| III (n = 14) | 2 (14%) | 12 (86%) | 0.431 |
| FIGO stage | |||
| Ia-Ib (n = 23) | 5 (22%) | 18 (78%) | |
| Ic-II (n = 46) | 10 (22%) | 36 (78%) | 1 |
*χ2 test.
β-Catenin Gene Mutations
Direct sequence analysis of the PCR amplification products showed heterozygous substitution mutations of β-catenin gene exon 3 in seven endometrioid carcinomas with nuclear β-catenin expression: three at codon 32 (Figure 1E and 1F) ▶ , one at codon 33 (Figure 1F) ▶ , two at codon 37, and one at codon 41. At codon 32, two GAC to TAC (D32Y) changes and a GAC to GGC (D32G) change were observed; at codon 33, a TCT to TGT change (S33C) was observed; at codon 37, a TCT to TTT change (S37F) and a TCT to TGT change (S37C) were observed; and finally, an ACC to GCC change at codon 41 (T41A) was observed. To further confirm sequencing data in the cases with mutation in S37, PCR products were digested with XmnI restriction endonuclease. Mutations affecting codons 32 and 33 were confirmed by HinfI restriction analysis (Figure 1H) ▶ . All detected mutations were also confirmed by resequencing.
No β-catenin gene mutations were observed in two endometrioid carcinomas with nuclear β-catenin expression pattern, four endometrioid carcinomas with a membranous β-catenin pattern (Table 3) ▶ , or 12 non-endomterioid carcinomas, two serous carcinomas with focal nuclear β-catenin expression, and 10 carcinomas with membranous β-catenin pattern.
Outcome Analysis
The mean follow-up time of the study was 8.9 ± 6.8 years (range 1–24 years; median follow-up period 7 years). Among the 69 patients, 19 (33.3%) showed a relapse, which occurred between 4 and 46 months after surgery. All patients who relapsed subsequently died (13–48 months after surgery) of the disease.
The variables analyzed were the type of postoperative therapy (radiotherapy, melphalan, melphalan and adryamicine, and cisplatinun- based chemotherapy), histological type, histological grade, stage (Ia-b versus Ic-II), β-catenin expression pattern (nuclear versus membranous), and membrane β-catenin expression (preserved versus reduced).
Relapse-free survival was significantly related to histological grade (χ2 = 26.61; P = 0.0000), stage (χ2 = 9.61; P = 0.0019), and β-catenin expression pattern (χ2 = 5.82; P = 0.016), but was unrelated to the type of postoperative therapy (χ2 = 5.11; P = 0.163), membrane β-catenin expression (χ2 = 1.93; P = 0.164), and histological type (χ2 = 6.88; P = 0.153) in the univariate analysis. Multivariate analysis also selected histological grade (χ2 = 16.82; P = 0.0022; risk ratio = 4.63; 95% confidence interval = 1.40–20.82), stage (χ2 = 7.96; P = 0.0048; risk ratio = 5.81; 95% confidence interval = 1.61–37.18), and β-catenin pattern (χ2 = 1028; P = 0.003; risk ratio = 0.000005) as independent predictors of relapse-free survival.
Overall survival was also significantly related to histological grade (χ2 = 21.12; P = 0.0000), stage (χ2 = 9.92; P = 0.0016), and β-catenin expression pattern (χ2 = 6.74; P = 0.0094), but was unrelated to the type of postoperative therapy (χ2 = 3.39; P = 0.334), membrane β-catenin expression (χ2 = 1.38; P = 0.51), and histological type (χ2 = 6.37; P = 0.17) in the univariate analysis. Multivariate analysis also selected histological grade (χ2 = 12.25; P = 0.0022; risk ratio = 4.13; 95% confidence interval = 1.25–18.54), stage (χ2 = 7.54; P = 0.0060; risk ratio = 5.54; 95% confidence interval = 1.54–35.41), and β-catenin pattern (χ2 = 8.91; P = 0.0028; risk ratio = 0.000005), as independent predictors of OS. The nuclear pattern of β-catenin expression was an indicator of good prognosis, inasmuch as not one recurrence or death occurred among these patients.
Discussion
This study confirms our previous observation 7 in a reduced number of cases that β-catenin expression in ovarian cancer has two patterns: nucleocytoplasmic and membranous, which reflect the two main known functions of β-catenin: signal transduction and cell adhesion. Widespread β-catenin nuclear expression is mainly observed in endometrioid carcinoma, suggesting that activation of the β-catenin-Tcf signaling pathway mediates development in a group of cases of this subtype of ovarian cancer. Although the activation of this signaling pathway could hypothetically be achieved by different molecular events, this study demonstrated that most ovarian endometrioid carcinomas with nucleocytoplasmic expression of β-catenin have β-catenin-gene mutations.
Ovarian carcinomas may arise de novo from surface ovarian epithelium (or epithelial inclusion cysts) or from benign or borderline lesions. 30 It has been suggested that most serous carcinomas arise de novo, whereas most mucinous carcinomas originate from benign or borderline tumors. 31 A proportion of endometrioid carcinomas may arise from previous benign lesions such as endometriosis and benign tumors of the same histological type. 32,33 Endometrioid tumors of the ovary are either benign, usually taking the form of an adenofibroma or cystoadenofibroma, or overtly malignant. Between these two well-established categories, there is a spectrum of lesions that have received different names in the literature (proliferating endometrioid adenofibromas, 32 atypical and borderline endometriod adenofibromas, 33 proliferative endometrioid adenofibromas, and endometrioid tumors of low malignant potential 34,35 ). Proliferating, borderline, or low-malignant-potential endometrioid tumors are characterized by adenocystadenofibromatous or papillary growth, epithelial proliferation, and atypia without stromal invasion. 32-35 In addition, they frequently show squamous metaplasia and have an excellent prognosis, although carcinomas may arise in them. 32-35 We previously reported β-catenin mutation in one borderline or low-malignant-potential endometrioid tumor. In this study we observed β-catenin nuclear expression in five carcinomas with large areas of endometrioid tumor of low malignant potential, four of which had β-catenin mutations. Although we have not studied benign endometrioid adenofibromas, these data indicate that β-catenin mutation would be implicated in the transformation process leading to the development of a subgroup of endometrioid carcinomas that have borderline or low-malignant-potential endometrioid tumors as their precursor. In addition, the observation of β-catenin nuclear expression in endometriotic glands adjacent to one carcinoma with β-catenin gene mutation suggests that β-catenin could be implicated in the malignant transformation of some cases of endometriosis.
We also observed β-catenin mutations in three endometrioid carcinomas without associated preinvasive areas but with evident squamous metaplasia. In contrast, none of the four endometrioid tumors with only membranous expression of β-catenin and without β-catenin mutations had squamous metaplasia. This finding may indicate that β-catenin mutations could be implicated in the genesis of so-called adenoacanthomas, a suggestion that must be confirmed in a larger series of cases.
Since the original description of oncogenic β-catenin mutation, several tumor types have been analyzed. β-Catenin point mutations have been reported in 2%, 5%, 13%, and 19% of medulloblastomas, 27 prostate carcinomas, 24 endometrial carcinomas, 26 and hepatocellular carcinomas, 25 respectively. Candidus et al 36 have not found evidence for mutation in this gene in gastric (diffuse and intestinal subtypes) and breast (ductal and lobular subtypes) carcinomas, although their sample was small. In ovarian carcinomas, we have observed β-catenin mutations in only 10% of all stage I and II tumors analyzed, but in 50% of the endometrioid carcinomas. This is the highest frequency of β-catenin mutation so far reported in any histological type of human carcinoma and is probably due to the characteristics of the sample analyzed (low stages and a high proportion of cases originated in preinvasive lesions).
Most studies on β-catenin mutations have only studied the consensus sequence for GSK-3β phosphorylation in exon 3. Mutations affecting this sequence, mainly in the amino acids implicated in the down-regulation of β-catenin through phosphorylation by this serine/threonine kinase (Ser, 33 Ser,37, Thr41, and Ser45), 17 probably render a fraction of cellular β-catenin insensitive to APC-mediated down-regulation and are responsible for the up-regulation of cytoplasmic β-catenin and its distribution in the nuclei of tumor cells, as was immunohistochemically detected in the present series. Mutation at codon 32 changing a highly conserved aspartic acid was observed in three ovarian carcinomas. Mutations have been reported at this codon in hepatocellular 25 and prostate 25 carcinomas, and it has been suggested that the alteration may change the protein structure and inhibit phosphorylation.
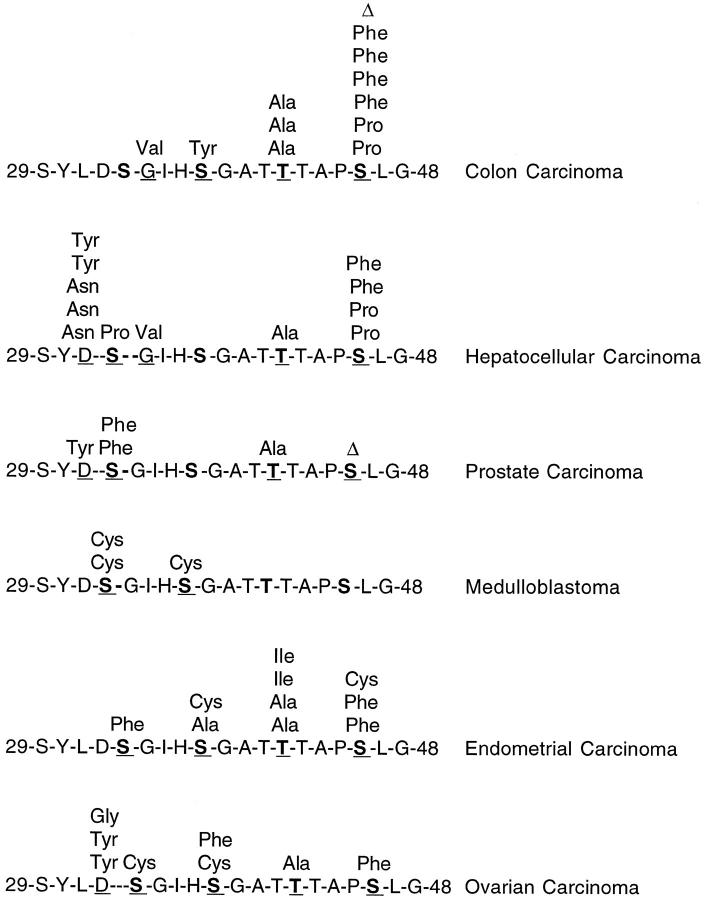
Although all reported β-catenin point mutations in human carcinoma samples affect the sequence for GSK-3β phosphorylation, the frequency of mutations at each codon and the type of mutation differ by tumor type, probably indicating different etiologies (Figure 2) ▶ . For example, in hepatocellular carcinoma, but not in other tumor types, the change from G to A or T predominates. 25 These types of β-catenin mutations have been reported in colon carcinomas induced by azoxymethane 37 and heterocyclic amines, 38 which have a preference for forming DNA adducts at guanines. In endometrial carcinoma, 50% of the mutations are C to T changes, suggesting the action of carcinogens that induce deamination of DNA bases. 26
Figure 2.
Reported point mutation in exon 3 of β-catenin gene in human carcinoma samples (excluding preinvasive lesions and cell lines). Bold letters indicate phosphorylated amino acids by GSK-3β. Underlined letters indicate mutated amino acids (D Asp; S Ser; G Gly; T Thr) ( refs. 17, 21, 23-27, and this series).
We did not find β-catenin gene mutations in two endometrioid carcinomas with nuclear and cytoplasmic β-catenin expression; however, our DNA analysis was very limited, and genetic alterations other than mutations in the sequence for GSK-3β phosphorylation have been described by others. 18,20,22,23,25 The entire coding sequence of β-catenin has been analyzed in colon 22 and hepatocellular carcinomas 25 and cell lines derived from colon cancer 20 and melanoma. 18 These studies indicate that interstitial deletions affecting exon 3 may occur, whereas mutations outside exon 3 of the β-catenin gene are infrequent. 20 Thus insertional deletion affecting exon 3 has been reported in 10% of colon carcinomas without APC mutations, 22 and in 3% of hepatocellular carcinomas. 25
It is also possible that the nucleocytoplasmic pattern of β-catenin immunoexpression in the two cases without β-catenin gene mutations was due to some alteration in other molecules of the β-catenin-Tcf signaling pathway. At present, it appears that the intracellular pool of β-catenin is regulated by an active GSK-3β that recognizes an APC-β-catenin complex. 8 The role of APC in this complex is still poorly understood, but it has been suggested that APC may be involved in directing β-catenin to proteasomes. 15 APC contains β-catenin binding and degradation sites, as well as binding sites for GSK-3β. Thus the cytoplasmic β-catenin pool can be increased in response not only to mutations in the β-catenin gene, but also to extracellular Wg/Wnt signaling, 39 which would inactivate GSK-3β, or to genetic defects affecting GSK-3β or APC. Expression of different Wnt factors has been described in human carcinomas, 40,41 but to date their effect in β-catenin levels has not been reported. In addition, the expression pattern for these factors in ovarian cancer remains to be established. The role of GSK-3β in human oncogenesis is not well known. Recent studies do not report any alterations of GSK-3β in medulloblastomas and colon carcinomas, some of which have mutations of the β-catenin gene. 23,27 Increased β-catenin cytoplasmic and nuclear levels can also be observed in cases of APC inactivation (ie, mutations of APC in colorectal polyps and carcinomas 19,42 and other conditions, such as sporadic aggressive fibromatosis 43 ). Loss of heterozygosity has been reported at the APC locus in sporadic ovary carcinomas with a frequency of 50% in a series that included only four endometrioid carcinomas 44 ; nevertheless, this seems to be a late event in tumor progression and does not correlate with mutation in the APC gene. 44
We did not observe β-catenin mutations in serous, mucinous clear cell, or mixed carcinomas, including four mixed tumors with an endometrioid component and two serous carcinomas with focal nuclear β-catenin immunoexpression. In these two cases we did not perform microdissection of the nuclear immunostained areas, and we could not exclude mutations in these cases. β-Catenin mutations have been reported as occurring focally in some prostate cancers, suggesting that these mutations may occur during tumor progression. 24 Interestingly, two of the colon cancers reported by Kitaeva et al 21 and two colon cancer cell lines (HCT 116 and SW48), which have β-catenin gene mutations, also show a replication error-positive phenotype, suggesting that the activating mutations in β-catenin that occur in some tumors may be associated with an underlying defect in DNA mismatch repair.
In the present series, the significant prognostic value of the traditional clinicopathological variables (grade and stage) as predictors of relapse-free and overall survival at 5 years in early-stage ovarian cancer 2-4 was confirmed. In addition, the β-catenin expression pattern was selected as an independent prognostic marker, indicating that cases with nuclear expression have an excellent prognosis, with no relapses or deaths among them. Obviously, these results must be taken as preliminary because of the small number of cases, but they do suggest β-catenin as a potential prognostic marker that could be analyzed in larger series from different institutions. Although we did not observe any deaths among endometrioid carcinomas with a nuclear β-catenin expression pattern, three of the four patients with endometrioid carcinomas with only membranous expression died. These findings suggest two oncogenic pathways in endometrioid carcinomas with different clinical implications. Identification of molecular defects in the subgroup of patients without nuclear β-catenin expression and with apparently poor prognosis would be helpful in selecting a specific therapy for each patient.
In addition to the role of β-catenin in signal transduction, this molecule has a pivotal role in cell adhesion through its participation in the cadherin/catenin complex of adherens junctions. Changes in the expression and structure of β-catenin lead to loss of adhesiveness and may promote invasiveness and metastasis in tumor cells. 9,12,13 Mutations of β-catenin disrupting the interaction between E-cadherin and α-catenin have been described in tumor cell lines that grow as loose aggregates or single cells. 45,46 In human tumors, the expression of membranous β-catenin has been analyzed in head and neck, 47 esophageal, 48,49 gastric, 50 colon, 51 breast, 52 and ovarian 53,54 carcinomas. It has been observed that 88% of head and neck squamous cell carcinomas, 70% of esophageal carcinomas, 50% of gastric carcinomas, and 30% of infiltrating ductal carcinomas of the breast show reduced membranous β-catenin expression. In some reports, reduced expression of β-catenin has been correlated with poorly differentiated tumors, advanced stage, and poor survival. 48,50
Davis et al 54 observed reduced immunohistochemical β-catenin expression in 21% of a series of 34 ovarian carcinomas, but Fujimoto et al 53 did not find differences in mRNA β-catenin expression between normal ovary tissue and ovarian carcinomas. We observed reduced membranous β-catenin expression in 78% of our ovarian carcinomas. β-Catenin expression did not correlate with histological type, tumor differentiation, progression (stage), or survival. However, it should be remembered that other molecules of the cadherin/catenin complex may be more important than β-catenin in modulating adhesion in ovarian carcinomas. For example, α-catenin was reduced in approximately 60% of cases in a large series of stage I and II ovarian carcinomas, and severe reduction of α-catenin expression (<30%) in stage I ovarian carcinomas was a predictor of poor prognosis in the univariate and multivariate analysis. 55
In summary, the pattern of β-catenin expression differs between ovarian carcinomas of differing histological types. APC-β-catenin-Tcf signaling pathway activation secondary to oncogenic β-catenin mutation is characteristic of a group of endometrioid carcinomas with nuclear β-catenin expression and a good prognosis, most of which originate in previous benign or borderline lesions. Endometrioid carcinomas with only membrane expression of β-catenin seem to represent a different subgroup of carcinomas that is not associated with the β-catenin signaling pathway and which, probably, has a worse prognosis. Determination of the β-catenin expression pattern in early-stage ovarian cancer might prove to be a useful marker for selecting low-risk patients.
Acknowledgments
The authors thank Vicente Sánchez, Petra Rubio, and Inmaculada Briones for technical assistance with the immunohistochemical study.
Footnotes
Address reprint requests to Dr. Carlos Gamallo, Departamento de Anatomía Patológica, Hospital La Paz, Paseo de la Castellana 261, 28046 Madrid, Spain. E-mail: cgamallo@hulp.es.
Supported by research grants 98/0151 from the Fondo de Investigaciones Sanitarias de la Seguridad Social, CAM 08.1/0020/1997 from the Comunidad Autónoma de Madrid, and SAF 98-0085-C03-03 from the Ministerio de Educación y Cultura, Spain.
References
- 1.Parker SL, Tong T, Bolden S, Wingo PA: Cancer statistics, 1997. CA Cancer J Clin 1997, 47:5-27 [DOI] [PubMed] [Google Scholar]
- 2.Canistra SA: Cancer of the ovary. N Engl J Med 1993, 329:1550-1559 [DOI] [PubMed] [Google Scholar]
- 3.Dembo AJ, Davy M, Stenwig AE, Berle EJ, Bush RS, Kjorstad K: Prognostic factors in patients with stage I epithelial ovarian cancer. Obstet Gynecol 1990, 75:263-273 [PubMed] [Google Scholar]
- 4.Vergote IB, Kaern J, Abeler VM, Pettersen EO, De Vos LN, Tropé CG: Analysis of prognostic factors in stage I epithelial ovarian carcinoma: importance of degree of differentiation and deoxyribonucleic acid ploidy in predicting relapse. Am J Obstet Gynecol 1993, 169:40-52 [DOI] [PubMed] [Google Scholar]
- 5.Pieretti M, Powell DE, Gallion HH, Case EA, Conway PS, Turker MS: Genetic alterations on chromosome 17 distinguish different types of epithelial ovarian tumors. Hum Pathol 1995, 26:393-397 [DOI] [PubMed] [Google Scholar]
- 6.Cuatrecasas M, Villanueva A, Matías-Guiu X, Prat J: K-ras mutations in mucinous ovarian tumors: a clinicopathological and molecular study of 95 cases. Cancer 1997, 79:1581-1586 [DOI] [PubMed] [Google Scholar]
- 7.Palacios, Gamallo C: Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 1998, 58:1344–1347 [PubMed]
- 8.Bath AIM, Nätke IS, Nelson WJ: Cadherins, catenins, and APC protein: interplay between cytoeskeletal complexes and signalling patways. Curr Opin Cell Biol 1997, 9:683-690 [DOI] [PubMed] [Google Scholar]
- 9.Ilyas M, Tomlison IPM: The interaction of APC, E-cadherin and β-catenin in tumor development and progression. J Pathol 1997, 182:128-137 [DOI] [PubMed] [Google Scholar]
- 10.Pignatelli M: E-cadherin: a biological marker of tumour differentiation. J Pathol 1993, 171:81-82 [DOI] [PubMed] [Google Scholar]
- 11.Takeichi M: Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol 1993, 5:806-811 [DOI] [PubMed] [Google Scholar]
- 12.Shiozaki H, Oka H, Inoue M, Tamura S, Monden M: E-cadherin mediated adhesion system in cancer cells. Cancer 1996, 77:1605-1613 [DOI] [PubMed] [Google Scholar]
- 13.Hirohashi S: Inactivation of E-cadherin mediated cell adhesion system in human cancers. Am J Pathol 1998, 153:333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korinek V, Barker N, Morin PJ, Van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 15.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R: β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997, 16:3797-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P: Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 1996, 272:1023-1026 [DOI] [PubMed] [Google Scholar]
- 17.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signalling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 18.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 19.Inomata M, Ochiai A, Akimoto S, Kitano S, Hirohashi S: Alteration of β-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res 1996, 56:2213-2217 [PubMed] [Google Scholar]
- 20.Ilyas M, Tomlison IPM, Rowan A, Pignatelli M, Bodmer WF: β-catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci USA 1997, 94:10330-10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitaeva MN, Grogan L, Williams JP, Dimond E, Nakahara K, Hausner P, DeNobile JW, Soballe PW, Kirsch IR: Mutations in β-catenin are uncommon in colorectal cancer occurring in occasional replication error-positive tumors. Cancer Res 1997, 4478–4481 [PubMed]
- 22.Iwao K, Nakamori S, Kameyama M, Imaoka S, Kinoshita M, Fukui T, Ishiguro S, Nakamura Y, Miyoshi Y: Activation of the β-catenin gene by interstitial deletion involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res 1998, 58:1021-1026 [PubMed] [Google Scholar]
- 23.Spark AB, Morin PJ, Volgestein B, Kinzler KW: Mutational analysis of APC/β-catenin/Tcf patway in colorectal cancer. Cancer Res 1998, 58:1130-1134 [PubMed] [Google Scholar]
- 24.Voeller HJ, Truica CI, Gelman EP: β-catenin mutations in prostate cancer. Cancer Res 1998, 2520–2523 [PubMed]
- 25.Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y: Activation of β-catenin gene in primary hepatocellular carcinomas by somatic alteration involving exon 3. Cancer Res 1998, 58:2524-2527 [PubMed] [Google Scholar]
- 26.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hiroashi S: β-catenin mutations in carcinoma of the uterine endometrium. Cancer Res 1998, 58:3526-3528 [PubMed] [Google Scholar]
- 27.Zurawel RH, Chiappa SA, Allen C, Raffel C: Sporadic medulloblastomas contain oncogenic β-catenin mutations. Cancer Res 1998, 58:896-899 [PubMed] [Google Scholar]
- 28.Gamallo C, Palacios J, Suárez A, Pizarro A, Navarro P, Quintanilla M, Cano A: Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol 1993, 142:987-993 [PMC free article] [PubMed] [Google Scholar]
- 29.Palacios J, Benito N, Pizarro A, Suárez A, Espada J, Cano A, Gamallo C: Anomalous expression of P-cadherin in breast carcinoma. Correlation with E-cadherin expression, and pathological features. Am J Pathol 1995, 146:605–612 [PMC free article] [PubMed]
- 30.Bell DA, Scully RE: Early de novo ovarian cancer: a study of fourteen cases. Cancer 1994, 73:1859-1864 [DOI] [PubMed] [Google Scholar]
- 31.Puls LE, Powel DE, DePriest PD, Gallion HH, Hunter JE, Kryscio RJ, van Nagell JR, Jr: Transition from benign to malignant epithelium in mucinous and serous ovarian cystadenocarcinoma. Gynecol Oncol 1992, 47:53-57 [DOI] [PubMed] [Google Scholar]
- 32.Roth LM, Czernobilsky B, Langley FA: Ovarian endometrioid adenofibromatous and cystadenofibromatous tumors: benign, proliferating and malignant. Cancer 1981, 48:1838-1845 [DOI] [PubMed] [Google Scholar]
- 33.Bell DA, Scully RE: Atypical and borderline endometrioid adenofibromas of the ovary. A report of 27 cases. Am J Surg Pathol 1985, 9:205–214 [DOI] [PubMed]
- 34.Snyder RR, Norris HJ, Tavassoli F: Proliferative endometrioid tumors and endometrioid tumors of low malignant potential of the ovary. A clinicopathological study of 46 cases. Am J Surg Pathol 1989, 12:661–671 [DOI] [PubMed]
- 35.Norris HJ: Proliferative endometrioid tumors and endometrioid tumors of low malignant potential of the ovary. Int J Gynecol Pathol 1993, 12:134-140 [PubMed] [Google Scholar]
- 36.Candidus S, Bischoff P, Becker KF, Höfler H: No evidence for mutations in the α- and β-catenin genes in human gastric and breast cancer. Cancer Res 1996, 56:49-36 [PubMed] [Google Scholar]
- 37.Takahashi M, Fukuda K, Sugimura T, Wakabayashi K: β-Catenin is frequently mutated and demonstrates altered cellular location in azoxymethane-induced rat colon tumors. Cancer Res 1998, 58:42-46 [PubMed] [Google Scholar]
- 38.Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M: High frequency of β-catenin (Ctnnb1) mutation in colon tumors induced by two heterocyclic amines in the F334 rat. Cancer Res 1998, 58:1127-1128 [PubMed] [Google Scholar]
- 39.Peifer M, Pai LM, Casey M: Phosphorylation of the Drosophila adherens junction protein Armadillo: roles of Wingless signal and Zeste-whit 3 kinase. Dev Biol 1994, 543–546 [DOI] [PubMed]
- 40.Huget EL, McMahon JA, McMahon AP, Bicknell R, Harris AL: Differential expression of human Wnt genes 2, 3, 4, and 7b in human breast cell lines and normal and disease state of human breast tissue. Cancer Res 1994, 54:2615-2621 [PubMed] [Google Scholar]
- 41.Iozzo RV, Eichteiter I, Danielson KG: Aberrant expression of the growth factor Wnt 5A in human malignancy. Cancer Res 1995, 55:1495-3499 [PubMed] [Google Scholar]
- 42.Valizadeh A, Karayiannakis AJ, El-Hariry I, Kmiot W, Pignatelli M: Expression of E-cadherin associated molecules (α, β, and γ-catenins and p120) in colorectal polyps. Am J Pathol 1997, 150:1977-1984 [PMC free article] [PubMed] [Google Scholar]
- 43.Alman BA, Li C, Pajerski ME, Díaz-Cano S, Wolfe HJ: Increased β-catenin protein, and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol 1997, 151:324-329 [PMC free article] [PubMed] [Google Scholar]
- 44.Allan GJ, Cottrell S, Trowsdale J, Foulkes WD: Loss of heterozygosity on chromosome 5 in sporadic ovarian carcinoma is a late event and is not associated with mutations in APC at 5q21–22. Hum Mutat 1994, 3:283-291 [DOI] [PubMed] [Google Scholar]
- 45.Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, Nagafuchi A, Tsukit S, Shibamoto S, Ito F, Takeichi M, Matsuda H, Hiroashi S: A truncated β-catenin disrupts the interaction between E-cadherin and β-catenin. A cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res 1994, 54:6282–6287 [PubMed]
- 46.Kawanishi J, Kato J, Sasaki K, Fujii S, Watanabe N, Niitsu Y: Loss of E-cadherin-dependent cell-adhesion due to mutation of β-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol 1995, 15:1175-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrews NA, Jones AS, Heliwell TR, Kinsella AR: Expression of E-cadherin-catenin cell adhesion complex in primary squamous cell carcinomas of the head and neck and their nodal metastases. Br J Cancer 1997, 75:1474-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnadath K, Tilanus HW, Van Blankestein M, Hop WCJ, Kremer ED, Dinjens WND, Bosman FT: Reduced expression of the cadherin/catenin complex in oesophageal adenocarcinomas correlates with poor prognosis. J Pathol 1997, 182:331-338 [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S: Expression of E-cadherin, α-catenin, β-catenin and plakoglobin in esophageal carcinoma and its prognostic significance. Oncology 1997, 54:158-165 [DOI] [PubMed] [Google Scholar]
- 50.Jawhari A, Jordan S, Poole S, Browne P, Pignatelli M, Farthing M: Abnormal immunoreactivity of E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology 1997, 112:46-54 [DOI] [PubMed] [Google Scholar]
- 51.Hao X, Tomlinson I, Ilyas M, Palazzo JP, Talbot IC: Reciprocity between membranous and nuclear expression of b-catenin in colorectal tumors. Virchows Arch 1997, 431:167-172 [DOI] [PubMed] [Google Scholar]
- 52.Hashizume R, Koizumi H, Ihara A, Ohta T, Uchikoshi T: Expression of β-catenin in normal breast carcinoma: a comparative study with epithelial cadherin and α-catenin. Histopathology 1996, 29:139-146 [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto J, Ichigo S, Hirose R, Sakaguchi H, Tamaya T: Expression of E-cadherin and α- and β-catenin mRNAs in ovarian cancers. Cancer Lett 1997, 115:207-212 [DOI] [PubMed] [Google Scholar]
- 54.Davies BR, Worsley SD, Ponder BAJ: Expression of E-cadherin, α-catenin and β-catenin in normal ovarian surface epithelium and epithelial ovarian cancer. Histopathology 1998, 32:69-80 [DOI] [PubMed] [Google Scholar]
- 55.Anttila M, Kosma V-M, Ji H, Wei-Ling X, Puolakka J, Juhola M, Saarikoski S, Syrjänen K: Clinical significance of α-catenin, collagen IV, and Ki-67 expression in epithelial ovarian cancer. J Clin Oncol 1998, 16:2591-2600 [DOI] [PubMed] [Google Scholar]