Abstract
The retinal pigment epithelium (RPE) maintains the choriocapillaris (CC) in the normal eye and is involved in the pathogenesis of choroidal neovascularization in age-related macular degeneration. Vascular endothelial growth factor-A (VEGF) is produced by differentiated human RPE cells in vitro and in vivo and may be involved in paracrine signaling between the RPE and the CC. We investigated whether there is a polarized secretion of VEGF by RPE cells in vitro. Also, the localization of VEGF receptors in the human retina was investigated. We observed that highly differentiated human RPE cells, cultured on transwell filters in normoxic conditions, produced two- to sevenfold more VEGF toward their basolateral side as compared to the apical side. In hypoxic conditions, VEGF-A secretion increased to the basal side only, resulting in a three- to 10-fold higher basolateral secretion. By immunohistochemistry in 30 human eyes and in two cynomolgus monkey eyes, KDR (VEGFR-2) and flt-4 (VEGFR-3) were preferentially localized at the side of the CC endothelium facing the RPE cell layer, whereas flt-1 (VEGFR-1) was found on the inner CC and on other choroidal vessels. Our results indicate that RPE secretes VEGF toward its basal side where its receptor KDR is located on the adjacent CC endothelium, suggesting a role of VEGF in a paracrine relation, possibly in cooperation with flt-4 and its ligand. This can explain the known trophic function of the RPE in the maintenance of the CC and its fenestrated permeable phenotype and points to a role for VEGF in normal eye functioning. Up-regulated basolateral VEGF secretion by RPE in hypoxia or loss of polarity of VEGF production may play a role in the pathogenesis of choroidal neovascularization.
The retinal pigment epithelium (RPE) has important functions in both the normal eye and under pathological conditions. Experimental evidence indicates that the RPE is involved in the pathogenesis of choroidal neovascularization (CNV), which occurs in the course of various eye conditions like age-related macular degeneration (AMD). 1-4 In normal healthy conditions, the RPE appears to have a positive survival effect in the maintenance of the highly vascularized, highly permeable fenestrated choriocapillaris (CC) on its outer basal aspect, whereas the photoreceptor layer internal to it is completely avascular. The anatomy of the choroidal vasculature and the preferential localization of the CC fenestrations on the side of the RPE also suggest a modulating role of the RPE on the CC. In addition, clinical observations in AMD and experimental animal studies show that absence of the RPE can cause secondary atrophy of the choriocapillaris. 1-3
On the other hand, in the course of CNV, RPE cells are observed to encapsulate the new vessels, with their basal side toward the endothelial cells, which coincides with the arrest of further vascular growth. 4 These morphological observations in vivo suggest that the RPE can have a function in controlling CNV.
Based on these observations, it seems that the RPE has a dual role in its interactions with the CC, but how the integrity of the RPE/CC microunit is regulated in healthy conditions, how the photoreceptor layer remains avascular, and why pathological changes in the RPE layer lead to choroidal atrophy or to an angiogenic response from the choroid are essentially unknown. It is likely, however, that mediator molecules play an essential role in these intercellular interactions.
Such interactions have previously been investigated in cell cultures of the constituent cells, but from these studies conflicting results have emerged. Whereas some authors were able to demonstrate that RPE cells in vitro produce an inhibitor of endothelial proliferation, 5 others found mitogenic activity for endothelium in RPE conditioned culture medium. 6
We hypothesize that a polarized secretion of growth/inhibitory factors may explain these apparently conflicting in vivo and in vitro observations. Growth factors produced by RPE in vitro include platelet-derived growth factor-BB, transforming growth factor-β, and vascular endothelial growth factor-A (VEGF). 7 VEGF, a vascular permeability and angiogenesis factor, is a good candidate for a role in maintaining the CC. VEGF has been shown to increase vascular permeability in skin and muscle partly by inducing a fenestrated endothelial phenotype. 8 Furthermore, it can act as a vascular survival factor in vivo. 9 In a coculture experiment of RPE and CC endothelium in vitro, RPE-derived VEGF was shown to stimulate tube formation of the underlying endothelial cells. 10
The aim of the present study was to evaluate the polarity of VEGF secretion by cultured human RPE cells and to investigate the localization of VEGF receptors in situ in human and monkey eye tissue sections.
Materials and Methods
RPE Cell Cultures
Four human donor eyes (age of the donors: 9, 15, 17, and 24 years), obtained from the Corneabank, Amsterdam, were used as a source of primary RPE cells. The RPE cells (further designated as RPE cell lines) were isolated within 24 hours post mortem. 11 RPE cells were isolated as described by Holtkamp et al 11 In short, the cornea, the anterior segment, the optic nerve, vitreous, and neural retina were removed from the eye, and the RPE cells were dissociated from the eye with trypsin by two subsequent incubations at 37°C. Cells obtained from the second incubation were plated in 24-well plates (Costar, Cambridge, MA) at 10 5 cells/well in Iscove’s modified Dulbecco’s medium (IMDM) (Gibco BRL) supplemented with 20% fetal calf serum (FCS) (Gibco BRL), penicillin (100 U/ml; Gibco BRL), and streptomycin (100 mg/ml; Gibco BRL). Nonadherent cells were removed after 2 days by washing and refreshing the culture medium. At confluence, cells were detached by trypsin treatment and passed to culture flasks at approximately 4 × 10 4 cells/cm2. For the present experiments, RPE cells were used between the sixth and 11th passages. To investigate whether the RPE cell lines were not contaminated during the isolation procedure, the cells were analyzed morphologically and by immunohistochemistry.
For immunohistochemistry, cells were cultured on tissue chamber slides (Lab-tek; Nunc, Naperville, IL) and stained with antibodies specific to cytokeratin 8/18 (CAM 5:2; Beckton-Dickinson, San Jose, CA) or specific to glucose transporter-1 protein (a kind gift from L. Andersson, University of Uppsala, Uppsala, Sweden). As a negative control an antibody against a nonhuman bacterial protein was used (mouse negative control immunoglobulins; DAKO).
All cells were cultured at 37°C and at 5% CO2. Medium was changed twice a week.
RPE Cell Monolayers on Transwell Filters
RPE cells were cultured on transwell filters (12-mm diameter, 0.4-mm pore size; Costar) according to the method of Holtkamp et al. 11 Briefly, filters were coated with 160 ml of a 1:40 dilution of Matrigel (Collaborative Biomedical Products, Bedford, MA) in medium and air dried overnight. The RPE cell lines were seeded as 1.6 × 10 5 cells/cm 2 in a volume of 200 ml/filter, in IMDM supplemented with 1% normal human serum (NHS) (CLB, Amsterdam, the Netherlands). In the lower compartment, 1000 ml medium was added, thereby leveling the height of the liquid levels to prevent hydrostatic pressure. After 2 days IMDM/1%NHS was added to a final volume of 750 ml in the upper compartment and 1500 ml in the lower compartment.
Transepithelial resistance (TER) was measured once a week with an Endohm chamber and an ohmmeter (World Precision Instruments, Sarasota, FL). TER measurements were corrected for background by subtracting the TER value of a matrigel-coated filter without cells. Filters with RPE monolayers were used for experiments approximately 3 weeks after plating.
Microscopy of RPE Cell Monolayers on Transwell Filters
The possible formation of multilayers of RPE cells was checked by light microscopy on sections of the filters. The filters were washed with phosphate-buffered saline (PBS), frozen in liquid N2, embedded in Tissue-Tek (Miles, Elkhart, IN), cut into 10-μm-thick sections, fixed in acetone for 5 minutes, and air dried. The sections were stained with hematoxylin for 10 minutes, followed by staining with eosin for 1 minute.
The presence of tight junctions was determined by immunofluorescence microscopy. Filters were washed with PBS (pH 7.4), fixed with 4% paraformaldehyde, washed again with PBS, permeabilized by incubation with 0.2% Triton X-100 for 15 minutes, blocked with 5% normal goat serum (Jackson Lab), and incubated with polyclonal antibodies against the tight junction-associated protein ZO-1 (Zymed Laboratories, San Francisco, CA; 1:200) for 1 hour. After washing with PBS, filters were incubated for 1 hour with fluorescein isothiocyanate-conjugated swine anti-rabbit immunoglobulins (Nordic, El Toro, CA; 1:100), washed again in PBS, and sealed with vectashield before being observed in a fluorescence microscope (Leica).
As described previously, 11 the integrity of the RPE cell monolayers was analyzed by electron microscopy (EM). For scanning EM, filters were washed with Hanks’ buffered salt solution, after which the cells were fixed in a 80 mmol/L cacodylate-buffered solution containing 1% glutaraldehyde and 1.25% paraformaldehyde (pH 7.3). After fixation for several days, the filters were dehydrated with ethanol, critical-point dried with CO2 as the intermediate, and coated with a few nanometers of platinum. The filters were inspected and micrographed with a Philips SEM 505 scanning electron microscope (Philips Industries, Eindhoven, the Netherlands) with a secondary emission detector.
For transmission EM, filters were postfixed in OsO4, dehydrated in a graded series of ethanol, and embedded in Epon. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and inspected and photographed in a Philips EM 400 electron microscope (Philips Industries) equipped with an EDAX PV9800 system and an ultrathin window detector (EDAX PV9760/53).
VEGF Secretion of RPE Cell Monolayers on Transwell Filters
Experiments were performed with 12 transwell filters with confluent RPE cells from each donor. Medium was removed from the 48 filters and replaced with fresh medium containing 1% NHS just before the experiments. The filters were incubated in normal growth conditions (24 filters, 37°C, 20% O2, 5% CO2, moist environment) or in hypoxic conditions (24 filters, 37°C, 1% O2, 5% CO2, moist environment) for 24 hours (24 filters) or 48 hours (24 filters). Thus each of the four conditions investigated was tested in triplicate. After 24 hours or 48 hours medium from the upper and lower compartments was collected, snap-frozen, and stored at −20°C until further analysis.
Levels of VEGF production of the RPE cells toward the basal or the apical side were assayed by enzyme-linked immunoabsorbent assay (R&D Systems, Abingdon, England), according to the manufacturer’s description. Each sample of medium was tested in triplicate.
Statistical Analysis
For statistical data analysis, the Independent Samples t-test was used after investigation of equality of variances by Levene’s test for equality of variances (SPSS Software, SPSS Gorinchem, The Netherlands).
Human and Primate Tissue Samples
Eyes from 30 persons with no known eye disease were obtained from the Corneabank, Amsterdam, within 5–15 hours after death. Furthermore, fresh normal eyes from two Cynomolgus monkeys (Macaca fascicularis) were kindly provided by the University of Nijmegen, the Netherlands. All animal experiments in this study were performed in accordance with the ARVO Resolution on the Use of Animals in Ophthalmic and Vision Research. Samples of the posterior segment were dissected. All tissues were snap-frozen and stored at −70°C.
Immunohistochemistry
Air-dried cryosections (10 μm) were fixed in cold acetone for 10 minutes and postfixed for 2 minutes with Zamboni fixative (2% paraformaldehyde in a saturated picric acid solution) and stained by an indirect immunoperoxidase procedure. To reduce nonspecific staining, sections were preincubated in 10% normal goat serum (Jacksons Immuno Research Laboratories, West Grove, PA; catalog no. 005-000-121) in PBS for 15 minutes and then incubated overnight at 4°C with the following antibodies: anti-endothelial monoclonal antibody (mAb) PAL-E (1:1000), anti-VEGF receptor KDR, anti-VEGF receptor flt-1 (both a kind gift of Dr. H. A. Weich, Department of Gene Expression, GBF, 38124 Braunschweig, Germany; 1:200), and anti-VEGF receptor flt-4 (raised at the Haartman Institute, University of Helsinki, Helsinki, Finland; 1:400). Sections were washed and incubated with subsequent compounds of a sensitive avidin-biotin-based Histostain plus kit (Zymed 85–9043) according to the manufacturer’s instructions. Sections were washed again, and staining was performed using 3-amino-9-ethyl carbazole (AEC) containing 0.01% H2O2. 12 The reaction was terminated by rinsing the sections with H2O. Counterstaining was performed with hematoxylin. Sections were washed and mounted in glycerol/glycerine (Sigma; GG-1). In control sections the first antibodies were omitted.
All sections were examined by two independent observers in a masked fashion. Microvascular staining was graded as follows: no staining (−), weak staining (±), or evident staining (+).
Results
Characterization of RPE Cultures on Transwell Filters
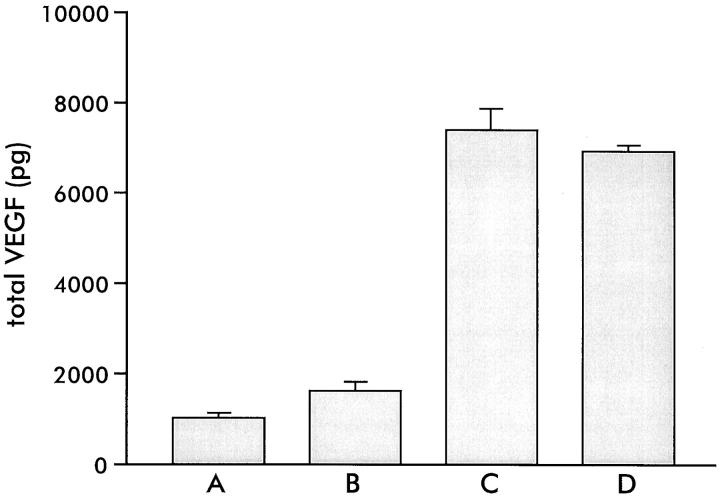
After seeding on transwell filters (12 filters for each donor), the human retinal pigment epithelial (RPE) cell monolayers (passage between 6 and 11) showed a gradual increase in TER. The TER of the matrigel-coated filters without cells was 15 Ohm·cm2. Maximum net TER was reached after approximately 3 weeks. The TER of the four RPE cell lines ranged from 30 ± 1.2 to 100 ± 4.3 Ohm·cm 2 but showed little variation within each cell line (Figure 1) ▶ .
Figure 1.
Transepithelial resistance (TER) (Ohm·cm2 ± SD) of RPE cells of the four donors A, B, C, and D, cultured on transwell filters for 3 weeks (each bar represents 12 filters).
The integrity of the RPE monolayers was verified by light microscopical examination of cross sections of the filters (Figure 2A) ▶ . Transmission electron microscopic studies revealed a monolayer of cells with microvilli and tight junctions at the side of the upper compartment of the transwell system (Figure 2B) ▶ , and deposition of basement membrane material between the lower side of the cells and the filter, indicating that the upper compartment represents the apical (“retinal”) side of the RPE monolayer and the lower compartment the basal (“choroidal”) side. The presence of tight junctional complexes was suggested by immunofluorescence staining of the cell layers for the tight junction-associated protein ZO-1. ZO-1 was present at one level around every cell, in both the cell lines with low (35 Ohm·cm2, Figure 2C ▶ ) and high (100 Ohm·cm2, Figure 2D ▶ ) TER. Staining for ZO-1 also showed that the density of cells on the filters was similar for all four RPE cell lines (approximately 10 6 cells/well). Taken together, these results indicated that the cultured RPE cells are very similar to differentiated resting RPE in vivo.
Figure 2.
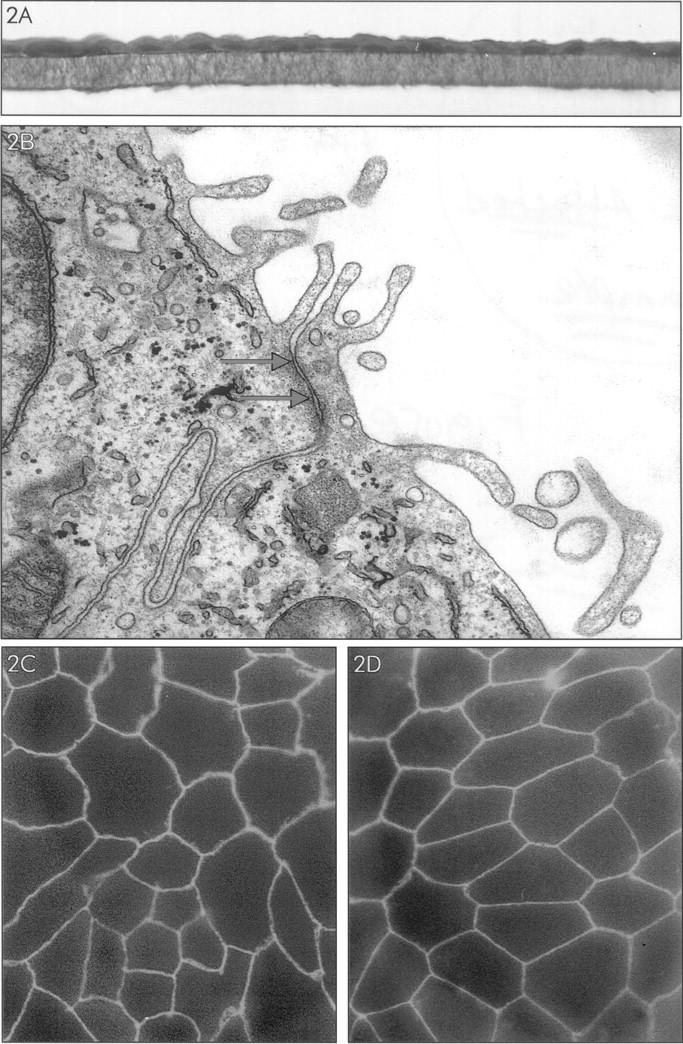
Characterization of RPE monolayers cultured on transwell filter for 3 weeks. A: Light microscopy of RPE cell monolayer (donor B), stained with hematoxylin-eosin. B: Electron microscopy of the apical side of the RPE cell monolayer. Note the presence of apical microvilli and between the cells a structure (indicated by arrows) suggesting the presence of a tight junction. C and D: Immunofluorescence microscopy of RPE cell monolayers of donor B (low TER: 35 Ohm·cm2) (C) and donor D (high TER: 100 Ohm·cm2) (D) stained for tight junction-associated protein ZO-1. Note staining for ZO-1 at the intercellular junctions.
VEGF Production by RPE Cell Lines in Normoxic and Hypoxic Culture Conditions
The RPE cell lines produced vascular endothelial growth factor-A (VEGF) under normal culture circumstances (20% O2, 5% CO2). The total production of VEGF varied between the different RPE cell lines, ranging from 1056 ± 64 to 7410 ± 456 pg/filter (10 6 cells) (24-hour incubation) (Figure 3) ▶ . Interestingly, the TER values of the four RPE cell lines correlated with the total VEGF production (compare Figures 1 and 3 ▶ ▶ ).
Figure 3.
Total VEGF secreted (basal and apical side together, ± SE of mean) in 24 hours by RPE cells of the four donors A, B, C, and D cultured on transwell filters for 3 weeks (each bar represents three filters).
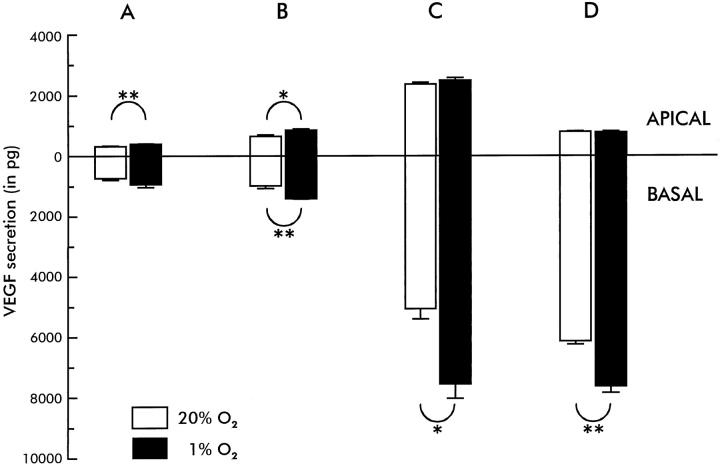
VEGF secretion in all filters examined was highly polarized. This was statistically significant for each donor, both in hypoxic and in normoxic conditions and at both time points (P < 0.05). In normoxic conditions, a two- to sevenfold higher accumulation of VEGF was found at the basal side of the cells compared to the apical side at both time points (Figure 4) ▶ . When RPE monolayers were kept in hypoxic conditions (1% O2, 5% CO2), VEGF production increased between 1.3- and 1.5-fold to the basal side but much less to the apical side. At 24 hours, this basal increase (and consequently higher polarization) was statistically significant for donors B, C, and D (P < 0.05, Figure 4 ▶ ). As a consequence, in hypoxic conditions the amount of VEGF secreted toward the basal side was 3–10 times higher than the amount secreted to the apical side.
Figure 4.
Differential VEGF secretion (± SE of mean) toward the apical and basal sides of transwell filters by RPE cell monolayers of the four donors A, B, C, and D in normal (37°C, 20% O2, 5% CO2, moist environment) and in hypoxic (37°C, 1% O2, 5% CO2, moist environment) conditions in 24 hours (each bar represents three filters). Note that VEGF secretion is higher to the basal side than to the apical side (statistically significant for all conditions, P < 0.05). In hypoxic conditions, VEGF secretion is higher, mainly to the basal side (statistical significance indicated by * = P < 0.05 and ** = P < 0.01).
Localization of VEGF Receptors KDR, flt-1, and flt-4 in the Human and Monkey Eye
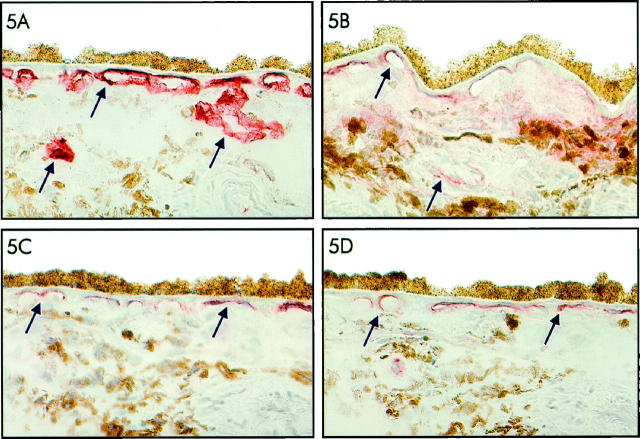
Retina and choroid of 30 human donor eyes and two monkey eyes were stained with antibodies against VEGF receptors flt-1 (VEGFR-1), KDR (VEGFR-2), and flt-4 (VEGFR-3) and with a marker for fenestrated endothelium, PAL-E. Staining with the antibody against PAL-E strongly outlined the endothelial cells of the choriocapillaris as well as the endothelial cells of the other choroidal vessels in both the human donor eyes and the monkey eyes (Figure 5A) ▶ . Staining of the sections with antibodies against the VEGF receptors KDR and flt-4 showed a particular staining pattern of the choriocapillaris in both human and monkey eyes (Figure 5, C and D) ▶ . Only on the side of the choriocapillaris endothelium that faces the RPE was a granular staining for KDR and flt-4 observed. On the scleral side of the choriocapillaris and in the larger choroidal vessels, only sporadic staining for KDR and flt-4 was seen (Figure 5, C and D) ▶ . The microvasculature of adjacent retina was negative. The staining for flt-4 in the choriocapillaris was even more pronounced than that for KDR. In contrast, staining with anti-flt-1 antibodies showed, in addition to marked staining of the inner CC, weak staining in the other choroidal vessels (Figure 5B) ▶ and staining of the entire retinal microvasculature in a pattern suggestive of expression of this receptor by both endothelial cells and pericytes (results not shown).
Figure 5.
Immunoperoxidase staining of frozen tissue sections of human choroid with monoclonal antibody PAL-E, recognizing fenestrated endothelium (A), and antibodies recognizing VEGFR-1 (flt-1) (B), VEGFR-2 (KDR) (C), and VEGFR-3 (flt-4) (D). Note the intense staining of the entire choriocapillaris and other choroidal vessels for PAL-E (A), in contrast to the localized staining of the choriocapillaris at the side of the RPE cell layer for VEGFR-2 (KDR) (C) and VEGFR-3 (flt-4) (D). VEGFR-1 (flt-1) staining can be observed in the inner CC and in a large vessel in the choroid (B). Staining of vascular structures is indicated by arrows.
Discussion
This study indicates that tight monolayers of cultured human RPE cells preferentially secrete VEGF-A to their basal (choroidal) side. Furthermore, in human and monkey tissue sections of the eye the VEGF receptors KDR and flt-4 were found to be specifically located at the retinal side of the choriocapillaris endothelium facing the RPE, whereas flt-1 had a more widespread distribution. These findings suggest that VEGF is involved in a paracrine relation between the choriocapillaris and RPE, and this may help to explain the well-known role of the RPE in maintaining the survival and fenestrated morphology of the choriocapillaris. Furthermore, these findings may have important implications for understanding the pathogenesis of CNV.
Several authors have previously demonstrated that cultured human RPE cells produce VEGF, among other growth factors. 7,13-18 In fact, VEGF was found to be responsible for most, if not all, mitogenic activity for endothelial cells in RPE conditioned medium. 6 In vivo, RPE cells can dedifferentiate and act as multipotent cells in ocular wound healing and proliferative vitreoretinopathy. Therefore, one may argue that human RPE cells in vitro produce VEGF as a result of the specific culture conditions, which may induce an “injury-type” phenotype of cells, comparable to those observed in vivo in wound healing and other pathological conditions. However, several in vivo studies indicate that also in the resting normal eye, 1,6,17,18 VEGF is produced constitutively by RPE cells, suggesting a physiological role of the growth factor in the eye. Furthermore, of our four different RPE cell lines, those with a higher TER produced higher amounts of VEGF. This may indicate that a higher degree of differentiation of RPE cells in vitro, as reflected by a higher TER, 19 leads to a higher production of VEGF. In these tight RPE monolayers with the highest TER, VEGF secretion was also most polarized, with up to 10 times more VEGF produced to the basal side as compared to the apical side. Previous in vitro studies have found that RPE cells secrete between 0.6 and 70 ng VEGF/10 6 cells. 6,20 This correlates well with our results, as the concentrations of VEGF found in the RPE conditioned medium ranged from 0.2 ng/ml on the apical side to 11 ng/ml on the basal side in hypoxic conditions. These concentrations of VEGF are in the biologically active range, as observed in experiments with cultured endothelium. 21-23 From such experiments, VEGF has emerged as an important permeability and angiogenesis factor. 21-23 In vitro, VEGF is mitogenic for endothelial cells and stimulates their migration. When injected in vivo into muscle or skin, it rapidly induces endothelial fenestrations in the continuous endothelium of these tissues. 8 In other conditions, as in the developing retina, VEGF has been shown to act as a survival factor for endothelium. 9
The relation between the choriocapillaris and the RPE has long been a focus of attention from both clinicians and basal scientists. Anatomically, the choroidal vascular plexus is modeled toward the RPE, as if drawn to it by some mysterious force. Furthermore, the choriocapillaris endothelial cells have fenestrations only on their side facing the RPE. It is also clear from clinical observations that the choriocapillaris will disappear after loss of the RPE in geographic age-related macular degeneration (AMD) and in other conditions. 24,25 Animal models also demonstrate that RPE loss leads to choriocapillaris atrophy, preceded by loss of the endothelial fenestrations. 2,26 All of these data suggest a strong modulating influence of the RPE on the choriocapillaris.
It has previously been suggested that VEGF may be involved in this respect, based on the observed production of VEGF by cultured RPE. Furthermore, in a coculture experiment, VEGF derived from RPE monolayers induced capillary tube formation of underlying endothelial cells in a type I collagen gel. 3 These studies did not specify the degree of differentiation of the RPE cells or the direction of secretion of VEGF, however. In fact, these results were confusing, because, when VEGF is secreted by RPE toward the retina in vivo, this would be expected to induce retinal neovascularization. Such neovascularization was demonstrated recently in a transgenic mouse model with constitutive VEGF production by photoreceptor cells. 27 However, in the normal eye, the outer retina is, of course, completely avascular. The data presented in this study suggest that the constitutive secretion of VEGF by RPE in vivo may well be highly polarized and be restricted to the basal choroidal side.
Our observations suggest that VEGF is involved in a physiological paracrine relation between the RPE and the choriocapillaris. The observed specific localization of the VEGFR-2, KDR, at the inner choriocapillaris, which is facing the basal side of the RPE cells, further supports this notion, as expression of VEGFR-2 by these cells would allow paracrine signaling by VEGF-A. In contrast, immunohistochemical staining for the VEGFR-1, flt-1, was also found in other vessels of the choroid and retina, in a pattern suggestive of both endothelial and pericyte staining. It has been proposed that these two VEGF receptors (KDR and flt-1) mediate different functions of VEGF-A. 28 Therefore, differential expression of the receptors in tissues may allow the regulation of VEGF-A activity. A third member of the VEGF receptor family, flt-4, uses other gene products, VEGF-C and VEGF-D, as ligands, whereas its affinity for VEGF-A is negligible. 29,30 Our finding that flt-4 is present in the vessel segments of the choriocapillaris that contain KDR suggests that cooperativity may occur between different types of VEGF molecules. It is unknown, however, whether RPE cells indeed produce VEGF-C or VEGF-D.
In light of the known effects of VEGF on endothelial cells in vivo and in vitro, VEGF may well be very important in both the trophic influence of RPE on the choriocapillaris, as well as in inducing its highly permeable phenotype, reflected by the abundant fenestrations located on the RPE side of the choriocapillaris. 31 Recently, Roberts and Palade 8 and Esser et al 32 found that VEGF is able to induce endothelial fenestrations, both in vivo and in vitro. Likewise, secretion of VEGF by RPE cells to the basal side could induce the fenestrations of the choriocapillaris. It has to be resolved whether the secretion of VEGF-A is sufficient or VEGF-A and VEGF-C or other factors cooperate in this effect. A recent study indicates that such a cooperation exists in endothelial proliferation and tube formation in vitro. 33 Moreover, the VEGF receptors KDR and flt-4 are mainly localized on the RPE side of the choriocapillaris, providing a selectively responsive area of the existing blood vessels. By such a paracrine mechanism VEGF secreted by the RPE cells may also act as a trophic factor on the choriocapillaris.
These findings may also have important implications for the understanding of the pathogenesis of choroidal neovascularization as occurs in AMD. Choriocapillaris atrophy and an increased thickness of Bruch’s membrane and relative impermeability to water-soluble compounds, like VEGF, have been reported in aging and AMD. 34,35 One may speculate that the latter changes in Bruch’s membrane may not only lead to CC atrophy resulting from insufficient amounts of VEGF reaching the choroid, but may lead, on the other hand, to accumulation of VEGF on the basal side of the RPE, as has indeed been observed in AMD in immunohistochemical studies. 36 Here, VEGF may reach a critical concentration and induce CNV under the RPE. This mechanism may be further enhanced by local hypoxia, as we observed up-regulated basolateral production of VEGF by RPE cells in hypoxic conditions. In fact, a relative outer retinal hypoxia has been suggested to occur in AMD as a result of CC atrophy and thickening of Bruch’s membrane. 34,36 In other situations with CNV, defects in the RPE monolayer could lead to misdirection of secreted VEGF and subsequent classical subretinal neovascularization.
Acknowledgments
The authors thank Dr. P. J. Luthert and Dr. J. Greenwood of the Department of Pathology, Institute of Ophthalmology, University College, London, for their helpful advice in initiating these studies; N. Bakker, E. M. Danzmann, and A. A. Put for preparing the photographs; Dr. W. Kamphuis for his helpful advice; Dr. E. Pels and her co-workers of the Corneabank Amsterdam for making available the human eye tissue; the donors and their relatives for their generosity; and BIS/Eurotransplant for its assistance in obtaining the tissues.
Footnotes
Address reprint requests to Dr. R.O. Schlingemann, Department of Ophthalmology, Academic Medical Center, P.O. Box 22660, 1100 DD Amsterdam, The Netherlands. E-mail: r.schlingemann@amc.uva.nl.
Supported by the Stichting Blindenhulp, the Diabetes Fonds Nederland (grant 95.103), the Praeventiefonds (grant 26-1822-3), the Edward en Marianne Blaauwfonds, and the Stichting “De Drie Lichten.”
References
- 1.Yi X, Ogata N, Komada M, Yamamoto C, Takahashi K, Omori K, Uyama M: Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefe’s Arch Clin Exp Ophthalmol 1997, 235:313-319 [DOI] [PubMed] [Google Scholar]
- 2.Korte GE, Gerszberg T, Pua F, Henkind P: Choriocapillaris atrophy after experimental destruction of the retinal pigment epithelium in the rat. A study in thin sections and vascular casts. Acta Anat 1986, 127:171-175 [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto T, Sakamoto H, Murphy TL, Spee C, Soriano D, Ishibashi T, Hinton DR: Vessel formation by choroidal endothelial cells in vitro is modulated by pigment epithelial cells. Arch Ophthalmol 1995, 113:512-520 [DOI] [PubMed] [Google Scholar]
- 4.Miller H, Miller B, Ryan SJ: The role of retinal pigment epithelium in the involution of subretinal neovascularization. Invest Ophthalmol Vis Sci 1986, 27:1644-1652 [PubMed] [Google Scholar]
- 5.Glaser BM: Extracellular modulating factors and the control of intraocular neovascularization. An overview. Arch Ophthalmol 1988, 106:603-607 [DOI] [PubMed] [Google Scholar]
- 6.Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Becse B, D’Amore PA, Folkman J: Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun 1993, 193:631-638 [DOI] [PubMed] [Google Scholar]
- 7.Campochiaro PA, Hackett SF, Vinores SA: Growth factors in the retina and retinal pigmented epithelium. Prog Retinal Eye Res 1996, 15:547-567 [Google Scholar]
- 8.Roberts WG, Palade GE: Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor in vivo. J Cell Sci 1995, 108:2369-2379 [DOI] [PubMed] [Google Scholar]
- 9.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med 1995, 1:1024-1028 [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto T, Sakamoto H, Murphy TL, Spee C, Soriano D, Ishibashi T, Hinton DR, Ryan SJ: Vessel formation by choroidal endothelial cells in vitro is modulated by retinal pigment epithelial cells. Arch Ophthalmol 1995, 113:512-520 [DOI] [PubMed] [Google Scholar]
- 11.Holtkamp GM, Van Rossum M, De Vos AF, Willekens B, Peek R, Kijlstra A: Polarized secretion of IL-6 and IL-8 by human pigment epithelial cells. Clin Exp Immunol 1998, 112:34-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham RC, Lundholm U, Darnofsky MJ: Cytochemical demonstration of peroxidase activity with 3-amino-9-ethylcarbazole. J Histochem Cytochem 1965, 13:150-154 [DOI] [PubMed] [Google Scholar]
- 13.Tanihara H, Yoshida M, Matsumoto M, Yoshimura N: Identification of transforming growth factor-β expressed in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1993, 34:413-419 [PubMed] [Google Scholar]
- 14.Ocrant I, Fay CT, Parmelee JT: Expression of insulin and insulin-like growth factor receptors and binding proteins by retinal pigment epithelium. Exp Eye Res 1991, 52:581-589 [DOI] [PubMed] [Google Scholar]
- 15.Ishigooka H, Aotaki-Keen AE, Hjelmeland LM: Subcellular localization of bFGF in human retinal pigment epithelium in vitro. Exp Eye Res 1992, 55:203-214 [DOI] [PubMed] [Google Scholar]
- 16.Campochiaro PA, Sugg R, Grotendorst G, Hjelmeland LM: Retinal pigment epithelial cells produce PDGF-like proteins and secrete them into their media. Exp Eye Res 1989, 49:217-227 [DOI] [PubMed] [Google Scholar]
- 17.Shima DT, Gougos A, Miller JW, Tolentino M, Robinson G, Adamis AP, D’Amore PA: Cloning and mRNA expression of vascular endothelial growth factor in ischemic retinas of Macaca fascicularis. Invest Ophthalmol Vis Sci 1996, 37:1334-1340 [PubMed] [Google Scholar]
- 18.Stone J, Itin A, Alon T, Peéz J, Gnessin H, Chan-Ling T, Keshet E: Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995, 15:4738-4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konari K, Sawada N, Zhong Y, Isomura H, Nakagawa I, Mori M: Development of the blood-retinal barrier in vitro: formation of tight junctions as revealed by occludin and ZO-1 correlates with the barrier function of chick retinal pigment epithelial cells. Exp Eye Res 1995, 61:99-108 [DOI] [PubMed] [Google Scholar]
- 20.Guerrin M, Scotet E, Malecaze F, Houssaint E, Plouet J: Overexpression of vascular endothelial growth factor induces cell transformation in cooperation with fibroblast growth factor 2. Oncogene 1997, 14:463-471 [DOI] [PubMed] [Google Scholar]
- 21.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1986, 219:983-985 [DOI] [PubMed] [Google Scholar]
- 22.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Conolly DT: Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989, 246:1309-1312 [DOI] [PubMed] [Google Scholar]
- 23.Connolly DR, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J: Tumor vascular permeability facor stimulates endothelial cell growth and angiogenesis. J Clin Invest 1989, 84:1470-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarks SH: Changes in the region of the choriocapillaris in ageing and degeneration. Int. Congr. Ophthalmol. XXIII 1979, 1:228-238 [Google Scholar]
- 25.Young RW: Pathophysiology of age-related macular degeneration. Surv Ophthalmol 1989, 31:291-306 [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura N, Matsumoto M, Shimizu H, Mandai M, Hata Y, Ishibashi T: Photocoagulated human retinal pigment epithelial cells produce an inhibitor of vascular endothelial cell proliferation. Invest Ophthalmol Vis Sci 1995, 36:1686-1691 [PubMed] [Google Scholar]
- 27.Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA: Transgenic mice with increased expression of vascular endothelial growth factor in the retina. Am J Pathol 1997, 151:281-291 [PMC free article] [PubMed] [Google Scholar]
- 28.Schlingemann RO, van Hinsbergh VWM: Role of vascular permeability factor/vascular endothelial growth factor in eye disease. Br J Ophthalmol 1997, 81:501-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lymboussaki A, Partanen TA, Olofsson B, Thomas-Crusells J, Fletcher CDM, de Waal RMW, Kaipainen A, Alitalo K: Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol 1998, 153:395-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA: Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt-4). Proc Natl Acad Sci USA 1998, 95:548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugita A, Hamasaki M, Higashi R: Regional difference in fenestration of choriodal capillaries in Japanese monkey eye. Jpn J Ophthalmol 1982, 26:47-52 [PubMed] [Google Scholar]
- 32.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W: Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 1998, 140:947-959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepper MS, Mandriota SJ, Jeltsch M, Kumar V, Alitalo K: Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial extracellular proteolytic activity. J Cell Physiol 1998, 177:439-452 [DOI] [PubMed] [Google Scholar]
- 34.Bird AC: Bruch’s membrane change with age. Br J Ophthalmol 1992, 76:166-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird AC: Treatment of pigment epithelial detachments in the elderly patient. Curr Opin Ophthalmol 1993, 4:25-32 [Google Scholar]
- 36.Kliffen M, Sharma HS, Mooy CM, et al: Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 1997, 81:154-162 [DOI] [PMC free article] [PubMed] [Google Scholar]