Abstract
Germline mutations of c-met oncogene at 7q31 have been detected in patients with hereditary papillary renal cell carcinoma. In addition, c-met mutations were shown to play a role in 13% of patients with papillary renal cell carcinoma and no family history of renal tumors. The histopathology of papillary renal cell carcinoma with c-met mutations has not been previously described. We analyzed the histopathology of 103 bilateral archival papillary renal cell carcinomas and 4 metastases in 29 patients from 6 hereditary papillary renal cell carcinoma families with germline c-met mutations and 6 papillary renal cell carcinomas with c-met mutations from 5 patients with no family history of renal tumors. Twenty-five sporadic renal tumors with prominent papillary architecture and without somatic c-met mutations were evaluated for comparison. All papillary renal cell carcinomas with c-met mutations were 75 to 100% papillary/tubulopapillary in architecture and showed chromophil basophilic, papillary renal cell carcinoma type 1 histology. Fuhrman nuclear grade 1–2 was seen in tumors from 23 patients, and nuclear grade 3 was observed focally in 8 patients. Seventeen patients had multiple papillary adenomas and microscopic papillary lesions in the surrounding renal parenchyma. Clear cells with intracytoplasmic lipid and glycogen were focally present in tumors of 94% papillary renal cell carcinoma patients. Clear cells of papillary renal cell carcinoma had small basophilic nuclei, and clear cell areas lacked a fine vascular network characteristic of conventional (clear) cell renal cell carcinoma. We conclude that papillary renal cell carcinoma patients with c-met mutations develop multiple, bilateral, papillary macroscopic and microscopic renal lesions. Renal tumors with c-met genotype show a distinctive papillary renal cell carcinoma type 1 phenotype and are genetically and histologically different from renal tumors seen in other hereditary renal syndromes and most sporadic renal tumors with papillary architecture. Although all hereditary and sporadic papillary renal cell carcinomas with c-met mutations share papillary renal cell carcinoma type 1 histology, not all type 1 sporadic papillary renal cell carcinomas harbor c-met mutations.
Papillary renal carcinomas (PRC) occur in sporadic and hereditary forms. Sporadic PRC is the second most common carcinoma of the kidney, comprising 10–15% of renal neoplasms 1-3 and is a predominant tumor type in acquired renal cystic disease. 4 Sporadic PRC are usually solitary tumors but multiple papillary adenomas were described in the surrounding renal parenchyma in some cases. 5 Kovacs et al 6 established criteria for papillary renal neoplasm stipulating that the tumor should be 75% papillary and/or tubulopapillary in architecture to be classified as a PRC. In 1986 Thoenes et al 7 included PRC in a morphological classification of kidney tumors as a chromophil carcinoma with basophilic, eosinophilic and mixed (basophilic/eosinophilic) histological subtypes. In the two current classifications of renal tumors PRC is included as a separate entity. 1, 8 Delahunt and Eble 9 recently suggested subdividing all PRC into type 1 and type 2 tumors which basically correspond to the chromophil basophilic and chromophil eosinophilic carcinoma subtypes, respectively, in Thoenes’ classification. PRC lack chromosome 3p genetic alterations characteristic of clear cell carcinomas in von Hippel-Lindau disease and sporadic conventional (clear) renal carcinoma. 10 Jiang et al 11 recently suggested the presence of cytogenetic differences between PRC type 1 and type 2.
Hereditary PRC is a recently described form of familial kidney cancer characterized by a predisposition to develop multiple, bilateral papillary renal tumors 12, 13 with trisomy of chromosomes 7 and 17. 14 Hereditary PRC is characterized by autosomal dominant transmission with reduced penetrance. 12, 13, 15 Recently the hereditary PRC gene was localized to chromosome 7q31.1-34 by genetic linkage analysis. 15 Germline missense mutations in the tyrosine kinase domain of the c-met proto-oncogene were detected in affected members of 6 of 7 hereditary PRC families. 15, 16 In addition, c-met mutations have been identified in tumors of 13% of PRC patients with no family history of renal tumors. 17 To date 15 different missense mutations have been identified in hereditary and sporadic PRC, located in the tyrosine kinase domain of c-met. These mutations have been shown to be transforming when transfected into NIH3T3 cells and cause ligand-independent constitutive phosphorylation of the c-met protein. 16-18 Trisomy 7 harboring non-random duplication of the mutant c-met proto-oncogene is thought to play a causative role in the development of multiple renal tumors in hereditary PRC patients. 19, 20
The c-met proto-oncogene, located at chromosome 7q31, is a member of the receptor tyrosine kinase family of proteins. 21, 22 Binding of its ligand, hepatocyte growth factor (HGF), to the extracellular portion of c-met, triggers autophosphorylation of critical tyrosines in the intracellular tyrosine kinase domain of c-met, activating a downstream signaling cascade via an SH2-domain docking site in the COOH-terminus of the protein. 23 Activation of the c-met/HGF signaling pathways has been shown to be involved in a number of biological activities including cell motility, 24 morphogenic differentiation, 25 cell proliferation, 26 and invasion. 27 In addition, c-met has been shown to be overexpressed in a number of human cancers. 28-30
The histopathology of PRC harboring c-met mutations has not been previously described. We examined the histopathology of multiple, bilateral archival renal tumors and adjacent renal parenchyma in patients from 6 hereditary PRC families with germline c-met mutations and in patients with PRC with c-met mutations and no family history of renal tumors. Sporadic renal tumors with prominent papillary/tubulopapillary architecture and without somatic c-met mutations were evaluated for comparison.
Materials and Methods
Patient Characteristics
Twenty-nine patients from 6 hereditary PRC families with germline c-met mutations and 5 patients with PRC who had no family history of renal tumors and had a documented mutation in the c-met oncogene were included in the study (Table 1) ▶ . All patients gave informed consent in a protocol approved by the NCI Institutional Review Board or Institutional Review Boards in their country. Hereditary PRC families were from Sweden (family 152), the Netherlands (family 162), Canada (family 160), and the United States (families 158, 150 and 5946). 13, 15, 16 Hereditary PRC patients included 18 women and 11 men with a mean age 48 years (age range 29–72 years) at the time of diagnosis (Table 2) ▶ . Most patients with hereditary PRC were asymptomatic at presentation and their tumors were discovered only during screening by computed tomography or magnetic resonance (CT/MRI) imaging. Radiologically, all tumors were relatively hypovascular and enhanced on contrast CT/MRI scans 31 ; additional smaller tumors were seen on intraoperative ultrasound or upon sectioning of renal specimens. Five patients underwent partial nephrectomy or tumor enucleation, 8 patients underwent unilateral nephrectomy, 6 patients were treated by bilateral nephrectomy, and kidneys in 2 patients were examined at autopsy. No information about procedure type was available in 6 of 7 patients from family 152. In 2 patients (3740 and 5153) primary kidney tumors were not available for evaluation and metastases obtained at autopsy were examined (Table 2) ▶ . No information about patients’ age, sex, or type of surgery was available on 5 PRC patients with c-met mutations who had no family history of renal tumors.
Table 1.
Genetic and Tumor Characteristics of PRC Patients with c-met Mutations
| Family no. or patient/DNA no. | Family history PRC (yes/no) | c-met mutation | No. patients examined | No. PRC examined | Tumor size range (cm) | Histology |
|---|---|---|---|---|---|---|
| 152 | Yes | M1149T germline | 7 | 10 | 0.6–1.5 | PRC Type 1 |
| 162 | Yes | M1149T germline | 3 | 3 | 0.6–2.0 | PRC Type 1 |
| 158 | Yes | V1206L germline | 1 | 5 | 1.0–5.0 | PRC Type 1 |
| 150 | Yes | H1112R germline | 5 | 49 | 0.6–6.0 | PRC Type 1 |
| 160 | Yes | H1112R germline | 11 | 22 | 1.0–11.0 | PRC Type 1 |
| 5946 | Yes | V1110I germline | 2 | 14 | 0.6–9.0 | PRC Type 1 |
| 6088 | No | V1110I germline | 1 | 1 | 2.0 | PRC Type 1 |
| 6059 | No | Y1248D germline | 1 | 2 | 0.6–0.7 | PRC Type 1 |
| 6082 | No | M1268T germline | 1 | 1 | 2.2 | PRC Type 1 |
| 6052 | No | H1124D somatic | 1 | 1 | 2.5 | PRC Type 1 |
| 6046 | No | Y1248C somatic | 1 | 1 | 2.0 | PRC Type 1 |
PRC, papillary renal carcinoma.
Table 2.
Histopathology of PRC with c-met Mutations in 34 Patients
| Family no. or DNA no. | Patient no. | Patient Age/Sex | No. PRC examined | Architecture* | Histology* | Fuhrman nuclear grade* (1–4) | Clear cells* (%) | Foamy macrophage/psammoma bodies* | Hemorrh/necrosis* | No. PA; no. micro- scopic lesions | Metastases |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 152 | T41-76† | 42F | 2 | pap/tubpap/solid | bas/eos‡ | 2–3 | 10 | yes /no | yes /yes | NA | Skeletal muscle |
| N1124-76 | 51F | 1 | pap/tubpap/solid | bas | 2–3 | 10 | yes /yes | yes /yes | NA | ||
| 4371 | 53F | 1 | tubpap/pap | bas | 2 | 40 | yes /yes | no /no | NA | ||
| 4374 | 34F | 1 | pap | bas | 2–3 | 50 | yes /no | yes /yes | NA | ||
| 0260-69 | 37F | 2 | pap/tubpap | bas | 1–2 | 30 | yes /yes | yes /no | 1; 5 | ||
| 0690-68† | 43M | 2 | pap/tubpap | bas | 1–2 | 30 | no /no | no /no | 1; 4 | Lung | |
| 4375 | 29F | 1 | pap | bas/eos | 2 | 20 | yes /no | yes /no | NA | ||
| 162 | 4813 | 42F | 1 | pap/solid | bas/eos | 2–3 | 0 | yes /no | no /no | NA | |
| 5227 | 64F | 1 | pap | bas | 2 | 70 | yes /no | yes /no | NA | ||
| 5228 | 54F | 1 | pap | bas | 2 | 50 | yes /yes | yes /no | NA | ||
| 158 | 5935 | 60F | 5 | pap/tubpap | bas | 1–2 | 30 | yes /yes | yes /yes | 5; 3 | |
| 150 | 4599 | 45M | 40 | pap/tubpap/solid/MA-like | bas/eos | 1–2 | 70 | yes /yes | no /no | 13; 36 | |
| 3740† | 72M | 2 mets | Kidney NA for evaluation | Lymph nodes, lung | |||||||
| 5226 | 55M | 2 | pap/tubpap | bas/eos | 2–3 | 50 | yes /no | yes /yes | 0; 3 | ||
| 6092-92 | 72M | 6 | pap | bas | 2 | 40 | yes /yes | yes /no | none | ||
| 3766 | 40M | 1 | pap | bas | 2 | 40 | yes /no | yes /no | 0; 2 | ||
| 160§ | 4967 | 21M | |||||||||
| 5161 | 50F | 2 | tubpap | bas/eos | 1–2 | 70 | yes /yes | yes /no | mult; mult | ||
| 5100 | 42M | 3 | pap/tubpap | bas | 2 | 20 | yes /yes | no /no | mult; mult | ||
| 5126 | 48M | 3 | pap/tubpap | bas | 1–2 | 20 | yes /yes | yes /yes | mult; mult | ||
| 5153† | 57M | 2 mets | Kidney NA for evaluation | Lung, skeletal muscle, lymph node | |||||||
| 160 | 5011 | 47F | 4 | pap/tubpap/solid/MA-like | bas | 1–2 | 20 | yes /no | yes /yes | 0; 1 | |
| 5154 | 56F | 1 | pap/tubpap | bas | 1–2 | 10 | yes /yes | yes /yes | 0; 6 | ||
| 5052 | 36F | 1 | pap/tubpap/solid | bas | 1–2 | 50 | no /no | no /no | 0; 2 | ||
| 5151 | 33F | 2 | pap/tubpap/MA-like | bas/eos | 2–3 | 1 | yes /no | no /no | 15; 21 | ||
| 5020 | 45M | 4 | pap/tubpap | bas/eos | 2–3 | 10 | yes /yes | yes /yes | 3; 9 | ||
| 5150 | 69F | 2 | pap/tubpap | bas | 2 | 20 | yes /no | yes /no | mult; mult | ||
| 5946 | 5946 | 41F | 13 | pap/tubpap | bas/eos | 2–3 | 30 | yes /yes | yes /yes | 15; 29 | |
| 2400-74 | 45F | 1 | pap/tubpap | bas | 1–2 | 1 | yes /no | yes /yes | NA | ||
| 6088 | 6088 | NA | 1 | pap/tubpap | bas | 1–2 | 10 | yes /no | yes /no | none | |
| 6059 | 6059 | NA | 2 | pap/tubpap | bas | 1–2 | 1 | yes /no | yes /no | 2; 0 | |
| 6082 | 6082 | NA | 1 | pap/tubpap | bas | 1–2 | 1 | yes /no | yes /no | none | |
| 6052 | 6052 | NA | 1 | tubpap | bas | 1–2 | 15 | yes /no | no /no | none | |
| 6046 | 6046 | NA | 1 | tubpap/solid | bas | 2 | 0 | yes /yes | yes /no | none |
Age, age at the time of diagnosis; PRC, papillary renal carcinoma; PA, papillary adenoma; hemorrh, hemorrhage; pap, papillary; tubpap, tubulopapillary; MA-like, metanephric adenoma-like; bas, chromophil basophilic (7); NA, not available; mult, multiple.
*Combined histopathological characteristics present in tumors from each patient.
†Patients with metastases.
‡Eos-eosinophilic cytoplasm when seen was present focally only and all tumors met the criteria of PRC, type 1 (9).
§No tumors present in bilateral kidneys on autopsy at age 21.
Morphological Examination of Tumors
One hundred nine PRC from 34 patients with c-met mutations were included in the study. Twenty-five renal tumors with prominent papillary/tubulopapillary architecture from 25 sporadic patients with no c-met mutations were evaluated for comparison. Formalin-fixed, paraffin-embedded tumors and gross reports were obtained from the files of the Laboratory of Pathology at the National Cancer Institute, NIH, and Laboratory of Immunobiology, National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, MD. In 4 hereditary PRC patients metastases were available for review (Table 2) ▶ . Tumors were evaluated on hematoxylin and eosin (H&E) stain.
Papillary renal tumors with c-met mutations were classified as papillary adenoma (≤0.5 cm) or papillary carcinoma based on established histopathological criteria. 1, 8 Microscopic renal lesions in surrounding renal parenchyma were also evaluated. Tumor histology was evaluated and recorded according to Delahunt and Eble criteria for papillary renal carcinoma as PRC type 1 or PRC type 2 9 and according to Thoenes criteria for chromophil carcinoma as basophilic, eosinophilic, or basophilic/eosinophilic (mixed) 7 (Tables 1 and 2) ▶ ▶ .
Mucicarmine stain and immunohistochemistry stains for vimentin (1:40; DAKO, Carpintaria, CA) and Ulex europaeus lectin (1:50; Vector, Burlingame, CA) were performed in eight representative PRC. The stains were performed for differential diagnosis between PRC type 1 and other renal tumors with prominent papillary architecture. Mucicarmine stain and U. europaeus lectin are thought to be specific for collecting duct carcinoma and are negative in PRC. PRC type 1 have been shown to have significantly greater vimentin staining than PRC type 2. 9 Three tumors with prominent clear cell component were examined by electron microscopy. For electron microscopic examination, 2.5% glutaraldehyde-fixed, osmium-postfixed tumor tissue was embedded in Maraglas 655 (Ladd Research Industries, Burlington, VT). Thin sections were stained with uranyl acetate and lead citrate and were reviewed in a Philips CM10 transmission electron microscope.
c-met Germline and Somatic Mutations and Trisomy 7
Mutation analysis of patient tumor and germline DNAs was performed by polymerase chain reaction (PCR)-based single strand conformation polymorphism (SSCP) as described previously 32 followed by direct sequencing of PCR products in which aberrant SSCP bands were observed (Figure 1D) ▶ . Primers and PCR protocols were reported previously. 33 Trisomy 7 in multiple tumors from hereditary PRC patients 4599 and 5161 was documented by FISH (Figure 1C) ▶ . The trisomy 7 FISH data, trisomy 7 harboring non-random duplication of the mutant MET allele and X-chromosome inactivation analysis of multiple renal tumors from these two patients was published in detail previously. 19
Figure 1.
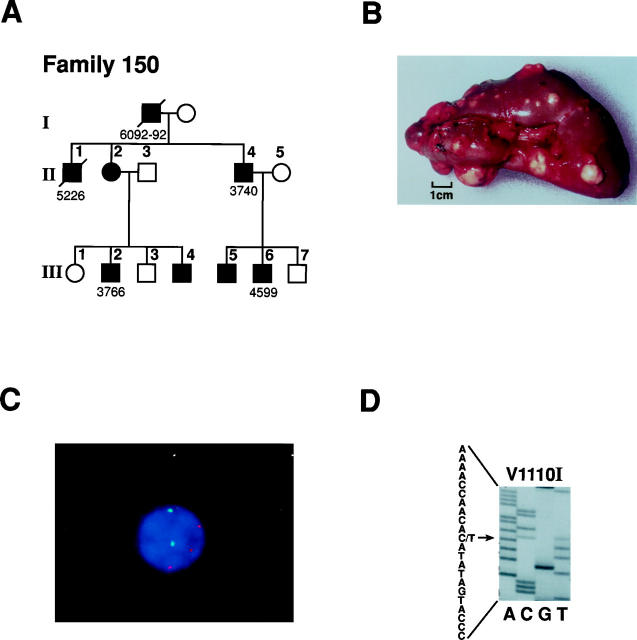
A: Pedigree of hereditary PRC family 150. Solid symbols indicate individuals with renal tumors. Diagonal slash indicates patient is deceased. Patient numbers correspond to individuals listed in Table 2 ▶ . B: Gross photograph of multiple renal tumors in a nephrectomy specimen from hereditary PRC patient 4599 (family 150) with H1112R mutation (from Zbar et al12). The tumors display prominent yellow color. C: Interphase FISH on a tumor cell touch preparation from hereditary PRC patient 4599 with H1112R mutation (family 150). Three copies of MET (trisomy 7; rhodamine, red signal) are seen in the tumor cell, using c182b3 combined with a centromeric α-satellite probe (FITC, green signal) specific for chromosome 1719; D: DNA sequence of hereditary PRC patient 5946 (family 5946) showing germline mutation C to T transition (antisense, arrow) at nucleotide 3522, resulting in a valine to isoleucine change at codon 1110.
Results
Genetic and tumor characteristics of patients from 6 hereditary PRC families with germline c-met mutations and of 5 patients with PRC and c-met mutations but no family history of renal tumors are shown in Table 1 ▶ . Family 152 from Sweden and family 162 from the Netherlands had an identical c-met mutation, M1149T. Family 150 from the United States (Figure 1A) ▶ and family 160 from Canada shared the same mutation, H1112R, with a documented Founder effect. 16 Family 5946 and a PRC patient 6088 with no family history of renal tumors had the same germline mutation, V1110I (Figure 1D) ▶ .
The number of gross renal tumors examined varied from 1 to 40 per patient (Table 2) ▶ , and all hereditary PRC patients had multiple bilateral renal lesions clinically and on gross examination. The size of gross tumors ranged from 0.6 to 11 cm (Table 1) ▶ . Fibrous pseudocapsule was a common feature of PRC more than 0.7 cm in size (see Figure 3A ▶ ) and was usually absent in smaller renal lesions. The pseudocapsule appeared to be overgrown by the tumor papillae rather than invaded by them, and no desmoplastic reaction was noted in the pseudocapsule around the tumor. A retraction artifact between the pseudocapsule and tumor was common in larger tumors and created the impression of a “cyst wall” lined by a layer of tumor cells (Figure 3A) ▶ .
Figure 3.
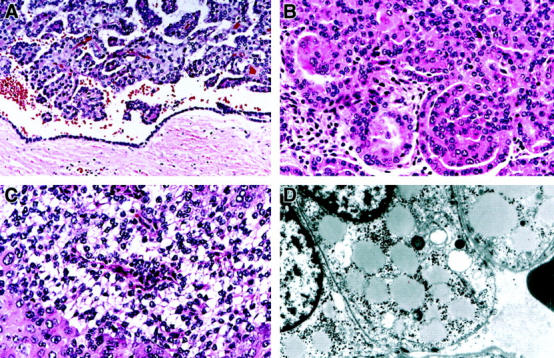
Histopathology of representative PRC and an ultrastructure of clear cells in hereditary PRC patients 5946 with V1110I mutation and 4599 with H1112R mutation. A: Fibrous pseudocapsule surrounds a 4 cm tumor from patient 5946; a retraction artifact gives an impression of a “cyst wall lined by a layer of tumor cells” (H&E, ×200). B: A tumor from patient 4599 shows a “metanephric adenoma-like” architecture and contains cells with low grade basophilic nuclei and amphophilic and eosinophilic cytoplasm (H&E, ×400). C: A tumor from patient 5946 demonstrates an area composed of sheets of cells with basophilic Fuhrman nuclear grade 1 nuclei and clear cytoplasm and adjacent cells with Fuhrman nuclear grade 3 nuclei and eosinophilic cytoplasm. Thin fibrovascular papillary cores are seen in cross-section in the area of clear cells (H&E, ×400). D: Electron micrograph of clear cells from a hereditary PRC patient 5946 shows prominent intracytoplasmic lipid droplets and glycogen (magnification, ×6600).
All tumors with c-met mutations demonstrated a predominance of PRC type 1 histology by Delahunt and Eble criteria and chromophil basophilic histological subtype by Thoenes criteria (Table 2 ▶ , Figure 2 ▶ ). All tumors showed papillary/tubulopapillary architecture with slender, short papillae and delicate fibrovascular cores lined by neoplastic cells (Table 2 ▶ , Figure 2, A and B ▶ ). Six patients had areas of solid architecture present in their tumors. Areas of “metanephric adenoma-like” architecture with complex papillae resembling glomeruloid bodies (Figure 3B) ▶ were present in one member of family 150 (patient 4599) and two members of family 160 (patients 5011 and 5151); both families shared the H1112R mutation. No sarcomatous differentiation was seen in any tumor.
Figure 2.
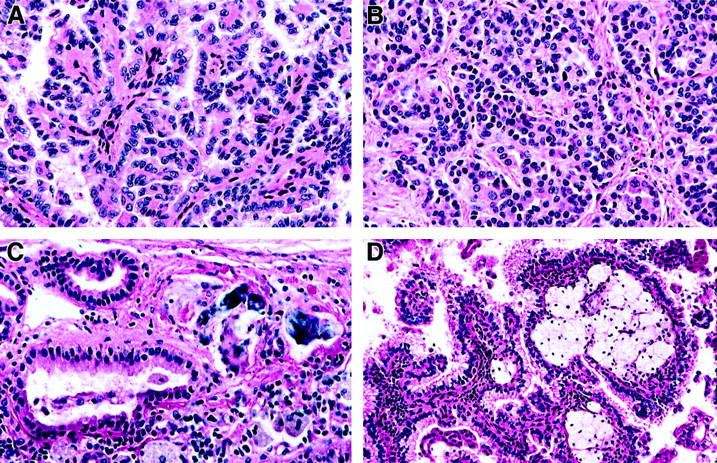
Representative PRC from patient 4599 (family 150) with H1112R mutation. The tumors show type 1 PRC (chromophil basophilic) histology and Fuhrman nuclear grade 1–2. A: Papillary architecture characterized by delicate fibrovascular cores lined by small cells with basophilic nuclei and scant amphophilic cytoplasm (H&E, ×400). B: Tubulopapillary architecture and small cells with basophilic nuclei and amphophilic cytoplasm (H&E, ×400). C: Psammoma bodies and foamy hemosiderin-laden macrophages are prominent microscopic features (H&E, ×400). D: Papillary architecture, foamy macrophages in fibrovascular cores and cells with small basophilic nuclei and scant amphophilic and focal clear cytoplasm (H&E, ×200).
The papillae were lined by a single layer of small cells with small basophilic nuclei and inconspicuous amphophilic cytoplasm (Figures 2 and 3A) ▶ ▶ ; however, focal areas of cells with eosinophilic cytoplasm were present in tumors from 9 hereditary PRC patients (Figure 3, B and C) ▶ . Fuhrman nuclear grade 1-2 predominated in all PRC with c-met mutations but focal areas of Fuhrman nuclear grade 3 were seen in tumors from 9 HPRC patients (Table 2 ▶ , Figure 3C ▶ ). Nuclear crowding and “grooves” were prominent in some PRC (family 152). Tumors from all but two patients (0690-68 and 5052) demonstrated foamy macrophages in fibrovascular cores, and psammoma bodies were present in tumors from 14 patients (Table 2 ▶ , Figure 2C ▶ ). Areas of hemorrhage and necrosis were seen in tumors of 23 and 11 PRC patients, respectively.
Areas of clear cells were common in PRC with c-met mutations regardless of size and were seen in renal tumors from 29 patients. The number of clear cells ranged from 1 to 70% (Table 2 ▶ , Figure 3C ▶ ). Like clear cells of the conventional (clear) cell RCC, clear cells of PRC contained intracytoplasmic lipid and glycogen demonstrated by electron microscopy (Figure 3D) ▶ . However, unlike in conventional (clear) cell carcinoma, clear cells of PRC had small basophilic nuclei and the tumor areas composed of clear cells lacked a fine vascular network. Delicate papillae cut in cross-section were seen in clear cell areas of PRC (Figure 3C) ▶ .
In 16 of 21 PRC patients in whom normal renal parenchyma was available for histological evaluation, papillary renal adenomas (<0.5 cm in size) and/or multiple microscopic papillary renal lesions (<0.5 mm in size) were detected (Table 2 ▶ , Figure 4, A–C ▶ ). The number of papillary adenomas ranged from one to 15 on slides available for evaluation, and four patients (5161, 5100, 5126, and 5150) had multiple adenomas recorded at the time of gross examination of kidney specimens. Microscopic renal lesions were common in kidneys of hereditary PRC patients, and patients 4599 and 5946 had 36 and 29 bilateral microscopic lesions, respectively. The microscopic lesions showed the architecture and histology identical to those of papillary adenomas and PRC type 1 (Figure 4B, C) ▶ . A detailed pathological examination of both kidneys of a 21-year-old man (patient 4967, family 160) who was a H1112R mutation carrier and died of a gunshot wound revealed no renal lesions. Four hereditary PRC patients (T42-76 and 0690-68 from family 152, 3740 from family 150, and patient 5153 from family 160) had metastases to lymph nodes, skeletal muscle and/or lungs (Figure 4 ▶ , Table 2 ▶ ).
Figure 4.
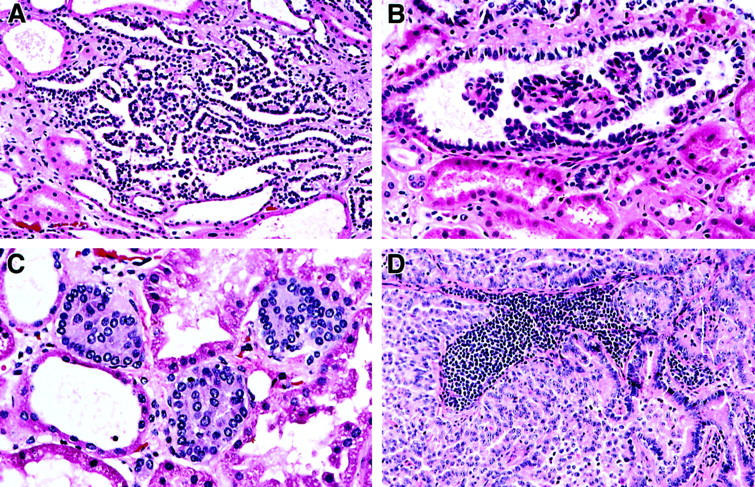
Multiple adenomas and microscopic papillary lesions in the surrounding kidney parenchyma and PRC metastases in hereditary PRC patients with c-met mutations have the architecture and histology which is similar to the architecture and histology of PRC. A: Papillary adenoma in patient 5946 with V1110I mutation (H&E, ×200). B: A microscopic papillary lesion in patient 4599 with H1112R mutation (H&E, ×400). C: Microscopic papillary lesions in patient 5946 with V1110I mutation (H&E, ×400). D: A lymph node metastasis in patient 3740 with H1112R mutation (family 150) (H&E, ×200).
Mucicarmine stain was negative for intracytoplasmic mucin in all 8 PRC with c-met mutations examined. Vimentin immunohistochemistry stain was positive and U. europaeus lectin was uniformly negative in all 8 tumors stained. Electron microscopy revealed intracytoplasmic lipid and glycogen in clear cells of 3 tumors evaluated.
Histological examination of sporadic PRC from 5 patients without c-met mutations (patients/DNA 6056, 6058, 6080, 6083 and 609117) revealed PRC type 1 histology identical to the histology seen in PRC with c-met mutations. Tumors with papillary/tubulopapillary architecture and no detectable c-met mutations were histologically evaluated and classified as PRC type 2 (5 cases), collecting duct carcinoma (4 cases), renal cell carcinoma with proven 1;X translocation (3 cases), metanephric adenoma (5 cases) and conventional (clear) cell carcinoma (3 cases).
Discussion
The Union Internationale Contre le Cancer (UICC) and American Joint Committee on Cancer (AJCC) and Heidelberg classifications of renal neoplasms recognize PRC as a distinct histopathological entity. 1, 8 Recent cytogenetic and molecular genetic studies demonstrate a genetic heterogeneity of PRC, 10 and therefore, the presence of the “papillary” architecture alone is no longer sufficient for the diagnosis of PRC. Papillary/tubulopapillary architecture may be seen and predominate in several types of renal tumors such as PRC type 1 and type 2, collecting duct carcinoma, conventional (clear) cell carcinoma, renal cell carcinoma with 1;X translocation and metanephric adenoma. 9, 34-36
Hereditary PRC is a recently described entity and is characterized by multiple, bilateral renal neoplasms which are hypovascular and enhance poorly after contrast on CT/MRI. 12, 13, 31 The disease is indolent; most patients are asymptomatic and are only found to have renal tumors during radiological screening. The genetic defect in hereditary PRC was mapped to the long arm of chromosome 7, and germline mutations of c-met oncogene at 7q31 were detected in affected patients. 15, 16 Trisomies 7 and 17 are common in hereditary PRC, 14 and trisomy 7 harboring non-random duplication of the mutant c-met proto-oncogene is thought to play a causative role in the development of multiple renal tumors. 19, 20 Multiple bilateral renal tumors of hereditary PRC were shown to arise as different clones in the renal parenchyma. 19
We report here the genotype-phenotype correlation in PRC with c-met mutations. We show that papillary renal neoplasms from the members of different PRC families and from patients with PRC who have no family history of renal tumors but have a mutation in the c-met oncogene (c-met genotype) share the same histological features of chromophil basophilic, type 1 PRC (PRC Type 1 phenotype). The neoplasms were multiple in kidney parenchyma and ranged in size from microscopic (less than a size of a single kidney tubule) to papillary adenomas (less than 0.5 cm) to PRC (more than 0.5 cm). Tumors of different sizes were histologically similar to one another, suggesting that the histopathological classification based on size into microscopic renal lesion, adenoma and carcinoma is most likely arbitrary.
Papillary and/or tubulopapillary architecture was seen in all tumors but areas with solid and “metanephric adenoma-like” architectural pattern were also seen. Delicate fibrovascular cores lined by small cells with low grade (Fuhrman nuclear grade 1-2) basophilic nuclei and scant amphophilic cytoplasm predominated in all lesions regardless of size. However, tumors in 8 patients showed focal areas with Fuhrman nuclear grade 3 cells and prominent eosinophilic cytoplasm intermixed with Fuhrman nuclear grade 1–2 basophilic cells. Foamy macrophages and psammoma bodies were common in PRC with c-met mutations.
Clear cells were common in PRC tumors with c-met mutations regardless of size and constituted as much as 70% of some tumors. The cytoplasmic clearing was most prominent in tumors with necrosis and hemorrhage. In such areas clear cells showed foamy cytoplasm identical to the cytoplasm of foamy macrophages and could be secondary to phagocytosis of cellular debri by neoplastic epithelial cells. Clear cells contained intracytoplasmic lipid and glycogen and their predominance in some PRC raised the differential diagnosis of conventional (clear) cell carcinoma. Clear cells of PRC had small basophilic nuclei and the tumor areas composed of clear cells lacked a fine vascular network characteristic of clear cell carcinoma. Delicate papillae cut in cross-section were consistently seen in clear cell areas of PRC. Predominance of other architectural patterns in clear cell carcinoma also helped to distinguish clear cell carcinoma from c-met positive PRC with clear cell areas.
The differential diagnosis of type 1 PRC with c-met mutations includes other renal tumors with papillary architecture such as PRC type 2, collecting duct carcinoma, renal cell carcinoma with 1;X translocation, conventional (clear) cell carcinoma, and metanephric adenoma. 9, 34-36 Most of these neoplasms present as a solitary primary renal tumor, and all tumors histologically evaluated in this study for comparison lacked c-met mutations by molecular genetic analysis. PRC type 1 with c-met mutations are composed of small cells with basophilic, Fuhrman grade 1–2 nuclei, while both PRC type 2 and collecting duct carcinoma of the kidney are composed of larger cells with prominent eosinophilic cytoplasm and high grade nuclei (Fuhrman nuclear grade 3–4). Collecting duct carcinoma cells demonstrate focal intracytoplasmic mucin on mucicarmine stain which is negative in both type 1 and type 2 PRC. U. europaeus lectin, the stain commonly positive in collecting duct carcinoma of the duct of Bellini, 9 was uniformly negative in PRC type 1 with c-met mutations.
Renal cell carcinomas of children and young adults with chromosome 1;X translocations may have prominent papillary architecture and often contain a mixture of large eosinophilic and clear cells with high grade nuclei and, in our experience, can be widely metastatic and have poor prognosis. The TFE3 gene was identified at the translocation breakpoint PRCC with 1;X translocation supporting a separate genetic mechanism for this type of renal cell carcinoma. 37, 38
Metanephric adenoma, in our experience, is the most common tumor type confused with PRC type 1 on histological examination. “Metanephric adenoma-like” architecture was focally observed in tumors from three hereditary PRC patients, however, nuclear and cytoplasmic features of cells in such areas were similar to the classic features of cells of type 1 PRC seen elsewhere in the tumor. True metanephric adenoma has predominately tubulopapillary architecture with solid areas and “glomeruloid-like” areas and lacks fibrovascular cores. The cells of metanephric adenoma show small densely basophilic nuclei which lack nucleoli, have a very scant cytoplasm 35 and have distinct ultrastructural and immunohistochemical characteristics. 36 Although one study suggested that metanephric adenoma may be related to PRC, 39 no c-met mutations were found in several metanephric adenomas studied by Schmidt et al. 17
PRC type 1 with c-met mutations have metastatic potential. Documented metastases to the lymph nodes, lungs, and skeletal muscle were present in 4 patients with hereditary PRC. The metastases retained the architecture and histology of the primary tumors. The presence of papillae, psammoma bodies, basophilic nuclei with nuclear overlap and “grooves” should raise a differential diagnosis of papillary thyroid carcinoma if the tumor presents as a lymph node or lung metastasis. Negative thyroglobulin immunohistochemistry stain in metastatic cells will exclude the diagnosis of thyroid carcinoma.
c-met mutations were recently shown to play a role in 13% of patients with PRC and no family history of renal tumors. 17 Interestingly, only half of these patients were found to harbor somatic c-met mutations and whereas another half harbored germline c-met mutations. Therefore, the rate of c-met mutations in truly sporadic PRC patients may be as low as 7%. We evaluated and report here histopathology of 6 PRC in five of the patients with c-met mutations and no family history of renal tumors: three with germline c-met mutations and two with somatic c-met mutations (Tables 1 and 2) ▶ ▶ . All 6 PRC showed type 1 PRC histology identical to the histology of hereditary PRC patients.
A detailed pathological examination of the both kidneys of a 21-year-old man (patient 4967) from a PRC family 160 who was a H1112R mutation carrier and died of a gunshot wound revealed no renal lesions, which indicates that in hereditary PRC patients multiple PRC may not develop until later in life. Furthermore, affected individuals in PRC families are commonly asymptomatic. The tumors may not be detected until the intensive radiological screening of the family members is done and, therefore, the patient may report a negative family history for renal tumors. These reasons could contribute to the detection of the “germline” c-met mutations in reportedly “sporadic” PRC cases. 17
In summary, patients with PRC with c-met mutations develop a spectrum of multiple bilateral renal lesions with papillary histology: PRC, papillary adenomas, and microscopic papillary lesions. Hereditary and sporadic PRC with a c-met genotype show a distinctive PRC type 1 phenotype. c-met positive PRC are genetically and histologically different from renal tumors seen in other hereditary renal syndromes (von Hippel-Lindau and familial renal oncocytoma) 40, 41 and from most sporadic renal tumors with prominent papillary architecture (PRC type 2, RCC with 1;X translocation, collecting duct carcinoma, conventional (clear) RCC and metanephric adenoma). Although all hereditary and sporadic PRC with c-met mutations show a distinctive PRC type 1 histology, not all type 1 sporadic PRC harbor c-met mutations. The study supports the notion of the genetic heterogeneity of renal cell carcinomas in general and of PRC in particular and stresses the importance of the classification of renal neoplasms based on combined pathological and molecular genetic criteria.
Acknowledgments
We thank Dr. C. J. M. Lips, University Hospital, Utrecht, The Netherlands for providing material on the Dutch family 162 and Dr. Ulf Bergerheim, Department of Surgery, Karolinska Hospital, Stockholm, Sweden for providing material on the Swedish family 152. We also thank Dr. Maria Tsokos, Laboratory of Pathology, National Cancer Institute, NIH, for assistance with electron microscopy, and Cynthia A. Harris and Sarah A. Delay, Laboratory of Pathology, National Cancer Institute, NIH, for assistance with immunohistochemistry staining.
Footnotes
Address reprint requests to Irina A. Lubensky, Laboratory of Pathology, National Cancer Institute, NIH, Building 10, Room 2A33, 10 Center Drive, Bethesda, MD 20892. E-mail: lubensky@box-l.nih.gov.
Supported in part by the National Cancer Institute, National Institutes of Health, contract NO1-CO-56000.
References
- 1.Störkel S, Eble JN, Adlakha K, Amin M, Blute ML, Bostwick DG, Darson M, Delahunt B, Iczkowski K: Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and American Joint Committee on Cancer (AJCC). Cancer 1997, 80:987-989 [DOI] [PubMed] [Google Scholar]
- 2.Mancilla-Jimenez R, Stanley RJ, Blath RA: Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer 1976, 38:2469-2480 [DOI] [PubMed] [Google Scholar]
- 3.Amin MB, Corless CL, Renshaw MD, Tickoo SK: Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol 1997, 21:621-635 [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa I, Kovacs G: High incidence of papillary renal cell tumours in patients on chronic haemodialysis. Histopathology 1993, 22:135-139 [DOI] [PubMed] [Google Scholar]
- 5.Kovacs G: Molecular cytogenetics of renal tumours. Adv Cancer Res 1993, 62:89-124 [DOI] [PubMed] [Google Scholar]
- 6.Kovacs G: Papillary renal cell carcinoma: a morphologic and cytogenetic study of 11 cases. Am J Pathol 1989, 134:27-34 [PMC free article] [PubMed] [Google Scholar]
- 7.Thoenes W, Störkel S, Rumpelt HJ: Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas): the basic cytological and histopathological elements and their use for diagnostics. Pathol Res Paract 1986, 181:125-141 [DOI] [PubMed] [Google Scholar]
- 8.Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros LJ, Moch H, Reuter VE, Ritz E, Roos G, Schmidt D, Srigley JR, Störkel S, van den Berg E, Zbar B: The Heidelberg classification of renal cell tumours. J Pathol 1997, 183:131-133 [DOI] [PubMed] [Google Scholar]
- 9.Delahunt B, Eble JN: Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 1997, 107:537-544 [PubMed] [Google Scholar]
- 10.Zbar B, Lerman M: Inherited carcinomas of the kidney. Adv Cancer Res 1998, 75:164-201 [DOI] [PubMed] [Google Scholar]
- 11.Jiang F, Richter J, Schraml P, Bubendorf L, Gasser T, Sauter G, Mihatsch MJ, Moch H: Chromosomal imbalances in papillary renal cell carcinoma: genetic differences between histologic subtypes. Am J Pathol 1998, 153:1467-1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zbar B, Tory K, Merino M, Schmidt L, Glenn G, Choyke P, Walther MM, Lerman M, Linehan WM: Hereditary papillary renal cell carcinoma. J Urol 1994, 151:561-566 [DOI] [PubMed] [Google Scholar]
- 13.Zbar B, Glenn G, Lubensky I, Choyke P, Walther MM, Magnusson G, Bergerheim US, Pettersson S, Amin M, Hurley K, Linehan WM: Hereditary papillary renal cell carcinoma: clinical studies in 10 families. J Urol 1995, 153:907-912 [PubMed] [Google Scholar]
- 14.Bentz M, Bergerheim USR, Li C, Joos S, Werner CA, Baudis M, Gnarra J, Merino M, Zbar B, Linehan WM, Lichter P: Chromosome imbalances in papillary renal cell carcinoma and first cytogenetic data of familial cases analyzed by comparative genomic hybridization. Cytogenet Cell Genet 1996, 75:17-21 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt L, Duh F-M, Chen F, Kishida T, Glenn G, Choyke P, Scherer S, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim U, Feltis T, Casadevall C, Zamarron A, Bernues M, Richard S, Lips CJM, Walther M, Tsui L-C, Geil L, Orcutt ML, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson M, Moch H, Störkel S, Lerman MI, Linehan WM, Zbar B: Germline and somatic mutations in the tyrosine kinase domain of the MET prot-oncogene in papillary renal carcinomas. Nature Genet 1997, 16:68-73 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt L, Junker K, Weirich G, Glenn G, Choyke P, Lubensky I, Zhuang Z, Jeffers M, Vande Woude G, Neumann H, Walther M, Linehan WM, Zbar B: Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res 1998, 58:1719-1722 [PubMed] [Google Scholar]
- 17.Schmidt L, Junker K, Nakaigawa N, Kinjerski T, Weirich G, Miller M, Lubensky I, Neumann HPH, Brauch H, Decker J, Vocke C, Brown JA, Jenkins R, Richard S, Bergerheim U, Gerrard B, Dean M, Linehan WM, Zbar B: Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 1999, 18:2343-2350 [DOI] [PubMed] [Google Scholar]
- 18.Jeffers M, Schmidt L, Nakaigawa N, Webb C, Weirich G, Kishida T, Zbar B, Vande Woude J: Activating mutations for the Met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA 1997, 94:11445-11450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang Z, Park WS, Pack S, Schmidt L, Vortmeyer AO, Pak E, Pham T, Weil R, Candidus S, Lubensky IA, Linehan WM, Zbar B, Weirich G: Trisomy 7-harboring nonrandom duplication of the mutant c-met proto-oncogene allele in hereditary papillary renal carcinomas. Nature Genet 1998, 20:66-69 [DOI] [PubMed] [Google Scholar]
- 20.Fischer J, Palmedo G, von Knobloch R, Bugert P, Prayer-Galetti T, Pagano F, Kovacs G: Duplication and overexpression of the mutant allele of the MET proto-oncogene in multiple hereditary papillary renal cell tumors. Oncogene 1998, 17:733-740 [DOI] [PubMed] [Google Scholar]
- 21.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF: Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984, 311:29-34 [DOI] [PubMed] [Google Scholar]
- 22.Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande Woude GF: Sequence of MET proto-oncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA 1987, 84:6379-6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comoglio PM, Vigna E: Structure and function of the HGF receptor (c-Met). Liver Regeneration and Carcinogenesis. Academic Press Inc, 1995, pp 51–70
- 24.Gherardi E, Stoker M: Hepatocyte growth factor-scatter factor: mitogen, motogen and MET. Cancer Cells 1991, 3:227-232 [PubMed] [Google Scholar]
- 25.Brinkman V, Foroutan H, Sachs M, Weidner KM, Birchmeier W: Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol 1995, 131:1573-1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin JS, Bottaro DP, Aaronson SA: Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta 1993, 1155:357-371 [DOI] [PubMed] [Google Scholar]
- 27.Jeffers M, Rong S, Vande Woude GF: Hepatocyte growth factor/scatter factor-MET signalling in tumorigenesis and invasion/metastasis. J Mol Med 1996, 74:505-513 [DOI] [PubMed] [Google Scholar]
- 28.DiRenzo MF, Poulson R, Olivero M, Comoglio PM, Lemoine NR: Expression of the MET/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 1995, 55:1129-1138 [PubMed] [Google Scholar]
- 29.DiRenzo MF, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R, Pierotti MA, Comoglio PM: Overexpression of the c-met/HGF receptor gene in human thyroid carcinomas. Oncogene 1992, 7:2549-2553 [PubMed] [Google Scholar]
- 30.Rong S, Jeffers M, Resau JH, Tsarfaty I, Oskarsson M, Vande Woude GF: Met expression and sarcoma tumorigenicity. Cancer Res 1993, 53:5355-5360 [PubMed] [Google Scholar]
- 31.Choyke P, Walther M, Glenn G, Wagner J, Venzon D, Lubensky I, Zbar B, Lenehan WM: Imaging features of hereditary papillary renal cancers. J Comput Assist Tomogr 1997, 21:737-741 [DOI] [PubMed] [Google Scholar]
- 32.Latif F, Tory K, Gnarra J, Yao M, Duh F-M, Orcutt M, Stackhouse T, Kuzmin I, Modi W, Geil L, Schmidt L, Zhou F, Li H, Wei M, Chen F, Glenn G, Choyke P, Walther M, Weng Y, Duan D, Dean M, Glavac D, Richards F, Crossey P, Ferguson-Smith M, Paslier D, Chumakov I, Cohen D, Chinault A, Maher E, Linehan W, Zbar B, Lerman M: Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993, 260:1317-1320 [DOI] [PubMed] [Google Scholar]
- 33.Duh F-M, Scherer S, Tsui L-C, Lerman M, Zbar B, Schmidt L: Gene structure of the human MET proto-oncogene. Oncogene 1997, 15:1583-1586 [DOI] [PubMed] [Google Scholar]
- 34.Srigley JR, Eble JN: Collecting duct carcinoma of kidney. Semin Diagn Pathol 1998, 15:54-67 [PubMed] [Google Scholar]
- 35.Grignon DJ, Eble JN: Papillary and metanephric adenomas of the kidney. Semin Diagn Pathol 1998, 15:41-53 [PubMed] [Google Scholar]
- 36.Martin L, Justrabo E, Michel F, Billerey C, Yaziji N: L’adenome metanephrique du rein. Une etude anatomoclinique, immunohistochimique et ultrastructurale de deux cas. Ann Pathol 1998, 18:120-124 [PubMed] [Google Scholar]
- 37.Weterman MA, Wilbrink M, Dijkhuizen T, van den Berg E, Geurts van Kessel: A Fine mapping of the 1q21 breakpoint of the papillary renal cell carcinoma-associated (X;1) translocation. Hum Genet 1996, 98:16–21 [DOI] [PubMed]
- 38.Sidhar SK, Clark J, Gill S, Hamoudi R, Crew AJ, Gwilliam R, Ross M, Linehan WM, Birdsall S, Shipley J, Cooper CS: The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses a novel gene PRCC to the TFE3 transcription factor gene. Human Mol Genet 1996, 5:1333-1338 [DOI] [PubMed] [Google Scholar]
- 39.Brown JA, Anderl KL, Borell TJ, Qian J, Bostwick DG, Jenkins RB: Simultaneous chromosome 7 and 17 gain and sex chromosome loss provide evidence that renal metanephric adenoma is related to papillary renal cell carcinoma. J Urol 1997, 158:370-374 [PubMed] [Google Scholar]
- 40.Lubensky IA, Gnarra J, Bertheau P, Walther M, Linehan WM, Zhuang Z: Allelic deletions of the VHL gene detected in multiple microscopic clear cell renal lesions in von Hippel-Lindau disease patients. Am J Pathol 1996, 149:2089-2094 [PMC free article] [PubMed] [Google Scholar]
- 41.Weirich G, Glenn G, Junker K, Merino M, Störkel S, Lubensky I, Choyke P, Pack S, Amin M, Walther MM, Linehan WM, Zbar B: Familial renal oncocytoma: clinicopathological study of 5 families. J Urol 1998, 160:335-340 [DOI] [PubMed] [Google Scholar]