Abstract
Chemokines are thought to be important for the recruitment of granulocytes and mononuclear cells and thus for the maintenance of inflammation in ulcerative colitis (UC). We have studied the expression of interferon-γ inducible protein-10 (IP-10), interleukin-8 (IL-8), monocyte chemoattractant protein (MCP)-1, MCP-3, and macrophage inflammatory protein (MIP)-1α in UC patients and control individuals to assess the role of these chemokines in disease progression. Colonic biopsies were taken endoscopically from patients and controls, frozen immediately and subsequently stained for IP-10, IL-8, MCP-1, MCP-3, and MIP-1α in serial sections. Cells infiltrating the lamina propria but not epithelial cells express the analyzed chemokines. They were differentiated and counted, and chemokine-expressing cells were quantified by image analysis. The percentage of cells expressing IP-10, IL-8, MCP-1, and MCP-3 was significantly enhanced in all UC samples as compared to controls. Expression in the controls was borderline, except for IP-10. No expression of MIP-1α was found in controls and UC. IP-10 was also markedly expressed in the mucosa of control biopsies and therefore could have a role in activated T lymphocytes’ recruitment into the healthy mucosa.
Ulcerative colitis (UC) is a chronic relapsing disease of unknown etiology with a prominent leukocyte infiltrate, which is confined to the mucosa and superficial submucosa of the bowel wall and contributes largely to the tissue damage. 1,2 The recruitment and activation of leukocytes in inflamed tissues is a complex process driven by chemokines and possibly other attractants that induces cell adhesion and locomotion. 3 Numerous studies have described the expression of CXC and CC chemokines in diseased tissues, underlining their role as the main attractants for different types of inflammatory cells. 4,5 Receptor expression and local generation of different chemokines are the bases for the selectivity of leukocyte recruitment in different diseases. 3 Chemokines are produced by resident cells such as tissue macrophages, mast cells, fibroblasts, and endothelial and epithelial cells, but also by infiltrating leukocytes. In situ analysis shows that CXC and CC chemokines are often expressed concomitantly. 4
The relationship between clinical activity, endoscopic severity, and chemokine production in inflammatory bowel diseases has been repeatedly investigated. 1 Several studies have provided evidence for the role of interleukin-8 (IL-8) in mediating infiltration of neutrophils into the gut wall. 6-11 Monocyte chemoattractant protein (MCP)-1, on the other hand, was reported to elicit the infiltration of monocytes into the colonic mucosa of patients affected by UC. 12-14
In the present study we analyzed the expression of five chemokines with distinct, but overlapping target selectivity to gain information on their role in UC. In addition to the chemokines that were analyzed previously we have included interferon-γ inducible protein-10 (IP-10), a selective attractant of activated T lymphocytes, and MCP-3, a powerful CC chemokine. 5 Serial sections from colonic biopsies, which were taken endoscopically from assessing UC disease activity, were examined by immunohistochemistry and evaluated by image analysis. All biopsies stained positively for IP-10, IL-8, MCP-1, and MCP-3 but not for macrophage inflammatory protein-1α (MIP-1α), and the number of chemokine-expressing cells increased significantly in dependence on disease activity.
Materials and Methods
Patients
Colonic biopsies were obtained from 30 consenting patients with UC (9 female and 21 male; median age 40 years; range, 22–63 years) undergoing colonoscopy for diagnostic purposes as approved by the institutional review board of the Bologna University Hospital. The diagnoses were based on clinical, radiological, endoscopic, and histological criteria.
Disease duration, histological disease activity, and drug treatment are listed for each patient in Table 1 ▶ . Five additional colonic biopsies were obtained as controls from consenting patients undergoing endoscopy to rule out a neoplastic disease. Endoscopic appearance of the colonic mucosa was assessed according to the criteria of Baron et al. 15 Histological disease activity was assessed according to the criteria of Truelove and Richard. 16 A single pathologist blinded to patient identification, clinical status, and treatment assigned histological disease activity index scores as follows: 0, normal; 1, chronic inflammatory cell infiltrate in lamina propria; 2, mild crypt injury with acute cell infiltrate, some crypt abscesses; 3, marked crypt destruction with crypt abscesses and ulceration. Patients with UC were divided into two groups according to histological disease activity scores. Grade 1 corresponds to patients with inactive disease (n = 17) and grade 2/3 corresponds to patients with mild to moderate or severe disease activity (n = 13).
Table 1.
Characteristics of Ulcerative Colitis Patients
| Patients | Age | Sex | Disease duration (years) | Disease extent | Histological grade | Treatment |
|---|---|---|---|---|---|---|
| FF | 44 | F | 11.2 | Sub-total colitis | Severe | 5-ASA* |
| CA | 47 | M | 6.4 | Left-sided colitis | Severe | 5-ASA |
| CL | 63 | F | 18.4 | Total colitis | Severe | SASP§ |
| FA | 49 | F | 7.4 | Proctosigmoiditis | Severe | 5-ASA |
| LC | 42 | M | 5.8 | Total colitis | Severe | 5-ASA |
| MR | 30 | M | 9.8 | Total colitis | Severe | 5-ASA |
| SA | 38 | F | 10.4 | Left-sided colitis | Severe | SASP |
| TR | 23 | F | 4.2 | Proctosigmoiditis | Severe | 5-ASA |
| VB | 49 | M | 5.8 | Sub-total colitis | Severe | Steroids+ 5-ASA |
| VR | 33 | M | 8.6 | Total colitis | Severe | 5-ASA |
| CF | 46 | M | 3.7 | Left-sided colitis | Moderate | 5-ASA |
| PR | 44 | M | 7.2 | Left-sided colitis | Moderate | Steroids |
| MR | 30 | M | 9.6 | Total colitis | Moderate | 5-ASA |
| CL | 63 | F | 18.3 | Total colitis | Inactive | SASP |
| GG | 22 | M | 3.5 | Proctosigmoiditis | Inactive | 5-ASA |
| NM | 37 | M | 6.2 | Proctitis | Inactive | none |
| PF | 42 | M | 12.6 | Total colitis | Inactive | SASP |
| PM | 47 | M | 5.4 | Proctosigmoiditis | Inactive | 5-ASA |
| SG | 39 | F | 8.6 | Left-sided colitis | Inactive | 5-ASA |
| ZB | 27 | F | 3.3 | Proctosigmoiditis | Inactive | 5-ASA |
| VG | 32 | M | 7.5 | Sub-total colitis | Inactive | 5-ASA |
| II | 37 | M | 5.7 | Total colitis | Inactive | 5-ASA |
| SN | 42 | M | 3.2 | Left-sided colitis | Inactive | 5-ASA |
| BF | 46 | M | 13.6 | Proctosigmoiditis | Inactive | SASP |
| CA | 31 | M | 4.8 | Total colitis | Inactive | 5-ASA |
| MC | 41 | M | 11.6 | Sub-total colitis | Inactive | 5-ASA |
| RI | 33 | F | 10.2 | Total colitis | Inactive | 5-ASA |
| TR | 31 | M | 5.6 | Proctosigmoiditis | Inactive | none |
| LA | 37 | M | 3.4 | Left-sided colitis | Inactive | 5-ASA |
| ZA | 45 | M | 8.7 | Proctitis | Inactive | none |
*5-Aminosalicylic acid; §Sulfalazine.
Histological disease activity of “severe” corresponds to grade 3, “moderate” to grade 2, and “inactive” to grade 1. See Materials and Methods.
Immunohistochemistry
Tissue samples were immediately embedded in OCT compound (Sakura Inc., Torrance, CA), frozen in liquid nitrogen, and stored at −70°C. Cryostatic sections 4 μm thick were fixed and stained as described. 17 Endogenous peroxidase activity in colon mucosa was blocked at room temperature for 1 hour with 1% hydrogen peroxide in phosphate buffered saline with 0.1% saponin. Serial sections were incubated overnight with 5 μg/ml of a monoclonal antibody (mAb) against IP-10, IL-8, MCP-1, MCP-3, or MIP-1α. 18 Lymphocytes, macrophages, and neutrophils were identified using anti-CD3 at 1:50, anti-CD68 at 1:100, and anti-neutrophil elastase at 1:200 (all from Dako, Glostrup, Denmark). Species and isotype-matched immunoglobulins (Sigma, St. Louis, MO) were used as controls. After washing, the slides were incubated for 30 minutes with biotin-labeled sheep anti-mouse antibody (Amersham, Arlington Heights, IL) at 1:800. After additional washes, the slides were incubated for 30 minutes with streptavidin-biotin-horseradish peroxidase (Dako). All incubations were performed at room temperature. The color reaction was developed with 3-amino-9-ethyl-carbazole (Sigma). The slides were counterstained with Mayer’s hematoxylin. All chemokine mAbs were kindly provided by LeukoSite, Inc. (Cambridge, MA). Their specificity was assessed by absorption. The mAbs were incubated overnight at 4°C with the respective chemokine, and the supernatants of this reaction were then used for the immunohistochemistry procedure.
Analysis of Tissue Sections
The cells expressing chemokines in the lamina propria were evaluated using the NIH Image analyzer. All epithelial or intraepithelial cells were excluded. Several magnification fields (20× to 40×) of each chemokine-stained section were analyzed. Two acquisitions per field were made, one in bright field for counting the total number of cells and the second in bright field with an interpose blue filter (Kodak Wratten 47B) for enhancing the brown peroxidase product. The second image was used for counting chemokine-positive cells. The same method was used to analyze cells positive for CD68, CD3, and elastase. The results are expressed as percentage of positive cells with respect to the total number of cells counted.
Statistical Analysis
The parameters were submitted to the Kruskal-Wallis test, a nonparametric one-way analysis of variance. After the global analysis, multiple comparisons between pairs of groups were carried out according to Dunn’s test. 19
Results
Chemokine expression was analyzed in serial sections of biopsies from UC patients and controls. The intestinal mucosa of control biopsies was free of pathological changes and contained on average in the lamina propria 41 × 10−4 cells/μm2. Much higher numbers of cells were counted in samples from UC patients. The infiltrate consisted mainly of lymphocytes (CD3-positive cells) and macrophages (CD68-positive cells) (Table 2) ▶ . In the lamina propria an average of 52 × 10−4cells/μm 2 and 57 × 10−4cells/μm 2 was observed in grade 1 and grade 2/3 biopsies, respectively. Cell-associated positivity for IP-10, IL-8, MCP-1, and MCP-3 was found in all UC samples in the lamina propria, but not in the epithelium (Figure 1) ▶ . Staining for MIP-1α, by contrast, was not observed. The percentage of chemokine-positive cells in the three groups, as obtained by image analysis, is shown in Figure 2 ▶ .
Table 2.
Image Analysis of CD68-, CD3-, and Neutrophil Elastase-Positive Cells in the Mucosa of Ulcerative Colitis and Control Biopsies
| Positivity | Area analyzed (μm2)* | Number of cells analyzed* | Cells/μm2 × 10−4 | Percentage of positive cells |
|---|---|---|---|---|
| CD68 | ||||
| Control | 110310 (61822–250834) | 456 (237–1031) | 42 | 61.8 |
| Grade 1 | 75994 (45512–178684) | 397 (243–750) | 53 | 56.6 |
| Grade 2/3 | 126678 (59637–202788) | 737 (247–1315) | 57 | 48.2 |
| CD3 | ||||
| Control | 75906 (63865–85853) | 312 (270–351) | 41 | 27.6 |
| Grade 1 | 88346 (42521–176411) | 378 (233–743) | 51 | 36.3 |
| Grade 2/3 | 98813 (55233–130430) | 565 (234–796) | 57 | 28.5 |
| Elastase | ||||
| Control | 108363 (56663–264906) | 435 (238–1067) | 40 | 0.2 |
| Grade 1 | 69967 (43462–97240) | 357 (240–513) | 52 | 0.3 |
| Grade 2/3 | 93211 (60494–124122) | 542 (258–930) | 57 | 16.4 |
*Mean value (range).
Figure 1.
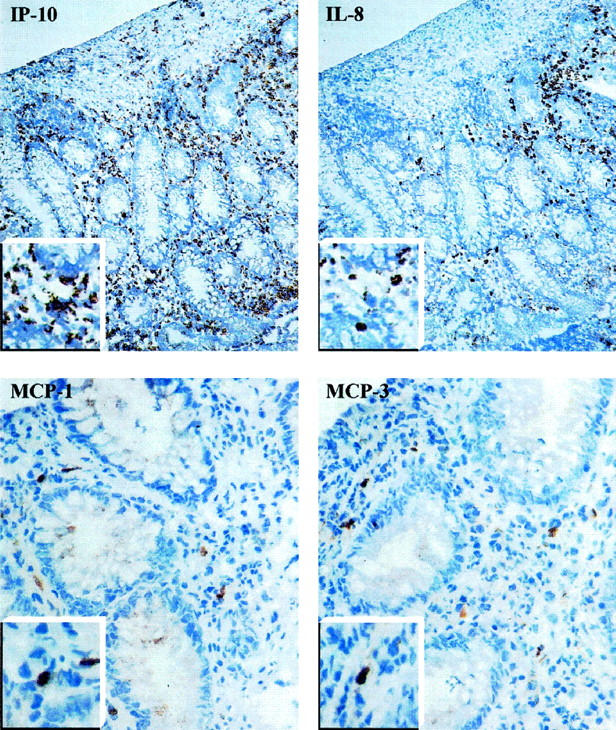
Micrograph of serial sections from a representative UC colonic mucosa stained for IP-10, IL-8, MCP-1, and MCP-3. Higher magnification of chemokine-positive cells are shown in the insets. Original magnifications, ×20 (IP-10 and IL-8) and ×40 (MCP-1 and MCP-3).
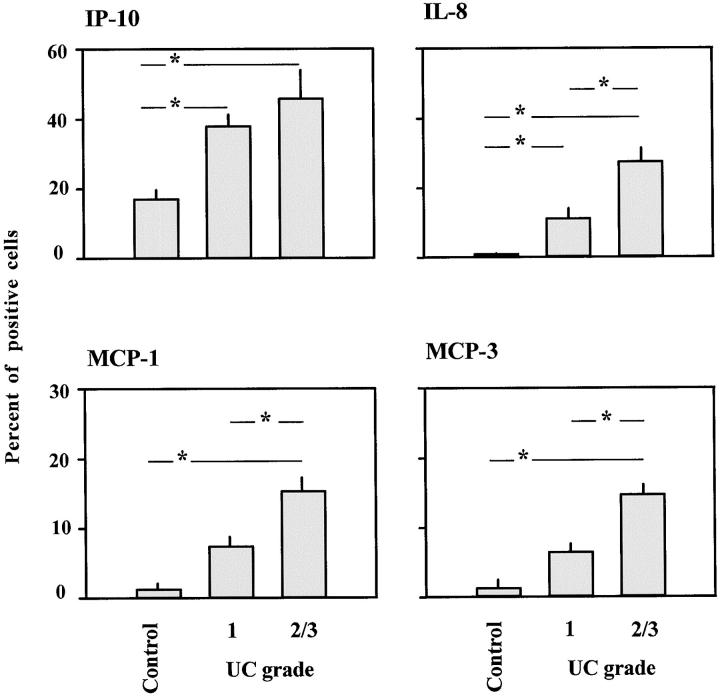
Figure 2.
Percentage of IP-10, IL-8, MCP-1, and MCP-3 positive cells in the lamina propria of UC colonic mucosa and controls. Mean ± SE of each group studied is shown. The asterisk represents a significant difference between groups (P < 0.01).
IP-10-positive cells were found in all samples analyzed. Their incidence in the lamina propria was 17% in control, 38% in UC grade 1, and 46% in UC grade 2/3 samples (global value from Kruskall-Wallis test 7.303, P < 0.05). The difference between controls and UC samples was highly significant (P < 0.01). No significant difference was observed, however, between UC grade 1 and UC grade 2/3 (Figure 2) ▶ .
IL-8-positive cells were found in all of the UC samples and only rarely in the controls. In the lamina propria, IL-8-positive cells amounted to 0.25% in control, 11% in UC grade 1, and 27% in UC grade 2/3 samples (global value from Kruskall-Wallis test 17.12, P < 0.01). Highly significant differences (P < 0.01) were observed between controls and UC, and between UC grade 1 and UC grade 2/3 (Figure 2) ▶ .
MCP-1- and MCP-3-positive cells were found in all controls and UC samples, but the percentages were lower than for cells expressing IP-10 or IL-8. The incidence of MCP-1- and MCP-3-positive cells in the lamina propria was 0.7 and 1.2% in controls, 6.9 and 6.8% in UC grade 1, and 14.6 and 14.8% in UC grade 2/3. For MCP-1, the global value from the Kruskall-Wallis test was 14.38 (P < 0.01); for MCP-3, the value was 15.80 (P < 0.01). For both chemokines the values were significantly higher in UC grade 2/3 than in UC grade 1 and controls (P < 0.01), but the differences between UC grade 1 and controls were not statistically significant (Figure 2) ▶ .
Immunohistological staining of serial sections for chemokines, macrophages, neutrophils, and T lymphocytes indicated that the mononuclear cells were the major source of the chemokine. In particular, IP-10 and, to a lesser extent, IL-8 were also associated with CD3-positive cell areas. IL-8 expression was occasionally observed in endothelial cells of UC grade 2/3, but no chemokine was detected in epithelial cells.
Discussion
We have analyzed the expression of five chemokines in the lamina propria of colonic biopsies taken from patients with UC and have found that their expression increases with disease activity. The chemokines were chosen because they are frequently present in inflamed tissues and are known to attract different types of leukocytes that are found in the lesions. IL-8 is the major attractant and activator of neutrophils. 20-22 IP-10 selectively attracts activated T lymphocytes, which are the only inflammatory cells expressing the chemokine receptor CXCR3. 23 MCP-1, MCP-3, and MIP-1α attract monocytes and activated lymphocytes via different receptors, CCR1, CCR2, and CCR5. 24-28
Several papers have reported chemokine expression in chronic inflammatory diseases of the colon. They showed that chemokines acting on neutrophils and mononuclear cells are found most frequently in UC and Crohn’s disease, but value of the data was limited by the small number of patients and the lack of quantitative evaluation of the chemokines expressed and the inflammatory cells present. 29
In addition, most studies were performed with tissue samples obtained by resection from patients with end-stage disease. We have studied a large population of UC patients who were treated only with 5-aminosalicylic acid or Sulfasalazine. All samples were obtained by endoscopy in the first days of disease relapse, thus ensuring a careful staging and homogeneity of the groups. In addition to the chemokines analyzed previously, we have included IP-10, a selective attractant of activated T lymphocytes, 23 and MCP-3, a powerful CC chemokine that acts on monocytes, activated T lymphocytes, eosinophils, and basophils. 30-34
By analyzing the cells infiltrating the lamina propria we found that IL-8, MCP-1, and MCP-3, which are barely detectable in normal tissue, are significantly increased not only in active but also in clinically inactive disease. A correlation of IL-8 expression and number of positive cells with the histological stage of the disease was formerly reported for samples obtained by surgical resection. 6,8 In addition, IL-8 was detected in homogenates of surgery samples from UC patients with different degrees of inflammation. 9 Higher expression of MCP-1 was also reported and suggested to derive mainly from cells of the lamina propria and the epithelia. 12,14 Data on the expression of MCP-1 by epithelial cells are, however, contradictory. 13 In the present study epithelial cells were always negative, although it was shown previously that freshly isolated epithelial cells can produce IL-8 and MCP-1. 35-37 The expression of MCP-1 and MCP-3 was virtually identical in terms of the number of positive cells and their distribution. Although always considerably lower than that of IP-10 and IL-8, the expression of both MCPs was always significantly enhanced in mucosal samples from patients with severe disease.
In control biopsies few leukocytes were observed and positivity for IL-8, MCP-1, or MCP-3 was extremely rare. The high percentage of IP-10-positive cells observed in controls suggests that in contrast to IL-8, MCP-1, and MCP-3, IP-10 is normally expressed by some resident cells. IP-10 may be involved in the recruitment of activated lymphocytes into the normal mucosa. It is interesting, however, that in active UC, IP-10 expression was always up-regulated to a greater extent than that of other chemokines.
Acknowledgments
We thank Dr. Beatrice Dewald (Theodor Kocher Institute, University of Bern) for critical reading of the manuscript and Dr. Antoinette Wetterwald (Institute for Clinical Research, University of Bern) for advice on histology.
Footnotes
Address reprint requests to Prof. Marco Baggiolini, Theodor Kocher Institute, Freiestrasse 13012, Bern, Switzerland. E-mail: marco.baggiolini@tki.unibe.ch.
Supported in part by the Swiss National Science Foundation.
References
- 1.Fiocchi C: Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998, 115:182-205 [DOI] [PubMed] [Google Scholar]
- 2.MacDermott RP, Sanderson IR, Reinecker HC: The central role of chemokines (chemotactic cytokines) in the immunopathogenesis of ulcerative colitis and Crohn’s disease. Inflam Bowel Dis 1998, 4:54-67 [DOI] [PubMed] [Google Scholar]
- 3.Luster AD: Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini M: Chemokines and leukocyte traffic. Nature 1998, 392:565-568 [DOI] [PubMed] [Google Scholar]
- 6.Grimm MC, Elsbury SKO, Pavli P, Doe WF: Interleukin 8: cells of origin in inflammatory bowel disease. Gut 1996, 38:90-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsuyama K, Toyonaga A, Sasaki E, Watanabe K, Tateishi H, Nishiyama T, Saiki T, Ikeda H, Tsuruta O, Tanikawa K: IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn’s disease. Clin Exp Immunol 1994, 96:432-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA, Mueller C: Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol 1994, 144:997-1007 [PMC free article] [PubMed] [Google Scholar]
- 9.Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V: Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 1996, 38:216-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen OH, Rüdiger N, Gaustadnes M, Horn T: Intestinal interleukin-8 concentration and gene expression in inflammatory bowel disease. Scand J Gastroenterol 1997, 32:1028-1034 [DOI] [PubMed] [Google Scholar]
- 11.Ina K, Kusugami K, Yamaguchi T, Imada A, Hosokawa T, Ohsuga M, Shinoda M, Ando T, Ito K, Yokoyama Y: Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol 1997, 92:1342-1346 [PubMed] [Google Scholar]
- 12.Reinecker H-C, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP: Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 1995, 108:40-50 [DOI] [PubMed] [Google Scholar]
- 13.Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C: Differential in situ expression of the genes encoding the chemokines MCP-1, and RANTES in human inflammatory bowel disease. J Pathol 1996, 178:201-206 [DOI] [PubMed] [Google Scholar]
- 14.Grimm MC, Elsbury SKO, Pavli P, Doe WF: Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol 1996, 59:804-812 [DOI] [PubMed] [Google Scholar]
- 15.Baron JH, Connel AM, Lennard Jones JE: Variation betwwen observers in describing mucosal appearances in proctocolitis. Br Med J 1964, 1:89-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truelove SC, Richard WCD: Biopsy studies in ulcerative colitis. Br Med J 1956, 1:1315-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulfgren A-K, Lindblad S, Klareskog L, Andersson J, Andersson U: Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis 1995, 54:654-661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA: Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol 1996, 149:1459-1467 [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn OJ: Multiple comparisons using rank sums. Tecnometrics 1964, 6:241-252 [Google Scholar]
- 20.Mahida YR, Ceska M, Effenberger F, Kurlak L, Lindley I, Hawkey CJ: Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin Sci 1992, 82:273-275 [DOI] [PubMed] [Google Scholar]
- 21.Raab Y, Gerdin B, Ahlstedt S, Hällgren R: Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut 1993, 34:1203-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, MacDermott RP, Sanderson IR: Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol 1999, 94:704-712 [DOI] [PubMed] [Google Scholar]
- 23.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B: Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med 1996, 184:963-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uguccioni M, D’Apuzzo M, Loetscher M, Dewald B, Baggiolini M: Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1α and MIP-1β on human monocytes. Eur J Immunol 1995, 25:64-68 [DOI] [PubMed] [Google Scholar]
- 25.Loetscher P, Seitz M, Baggiolini M, Moser B: Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med 1996, 184:569-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M: Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry 1996, 35:3362-3367 [DOI] [PubMed] [Google Scholar]
- 27.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF: Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem 1996, 271:17161-17166 [DOI] [PubMed] [Google Scholar]
- 28.Combadiere C, Ahuja SK, Tiffany HL, Murphy PM: Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1α, MIP-1β, and RANTES. J Leukoc Biol 1996, 60:147-152 [DOI] [PubMed] [Google Scholar]
- 29.Grimm MC, Doe WF: Chemokine in inflammatory bowel disease mucosa: expression of RANTES, macrophage inflammatory protein (MIP)-1α, MIP-1β, and γ-interferon-inducible protein-10 by macrophages, lymphocytes, endothelial cells, and granulomas. Inflamm Bowel Dis 1996, 2:88-96 [PubMed] [Google Scholar]
- 30.Van Damme J, Proost P, Lenaerts J-P, Opdenakker G: Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med 1992, 176:59-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minty A, Chalon P, Guillemot JC, Kaghad M, Liauzun P, Magazin M, Miloux B, Minty C, Ramond P, Vita N, Lupker J, Shire D, Ferrara P, Caput D: Molecular cloning of the MCP-3 chemokine gene and regulation of its expression. Eur Cytokine Netw 1993, 4:99-110 [PubMed] [Google Scholar]
- 32.Noso N, Proost P, Van Damme J, Schröder J-M: Human monocyte chemotactic proteins-2 and 3 (MCP-2 and MCP-3) attract human eosinophils and desensitize the chemotactic responses towards RANTES. Biochem Biophys Res Commun 1994, 200:1470-1476 [DOI] [PubMed] [Google Scholar]
- 33.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B: The monocyte chemotactic proteins, MCP-1, MCP-2 and MCP-3, are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J 1994, 8:1055-1060 [DOI] [PubMed] [Google Scholar]
- 34.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, Minty A, Baggiolini M: Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med 1994, 179:751-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang SK, Eckmann L, Panja A, Kagnoff M: Differential and regulated expression of CXC, CC, and C chemokines by human colon epithelial cells. Gastroenterology 1997, 113:1214-1223 [DOI] [PubMed] [Google Scholar]
- 36.Jung HC, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF: A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995, 95:55-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson P, Rosella O: Interleukin 8 secretion by colonic crypt cells in vitro: response to injury suppressed by butyrate and enhanced in inflammatory bowel disease. Gut 1995, 37:536-543 [DOI] [PMC free article] [PubMed] [Google Scholar]