Abstract
Postinflammatory scarring is characterized by changes in extracellular matrix (ECM) composition and progressive loss of normal resident cells. In glomerular inflammation there is now evidence that unscheduled apoptosis (programmed cell death) of mesangial and other resident cells may mediate progression to irreversible glomerulosclerosis. In the current study we examined the hypothesis that ECM components may differ in their capacity to support mesangial cell survival by suppression of apoptosis. Using a well-established in vitro model of mesangial cell apoptosis, we found that collagen IV and laminin, components of normal mesangial ECM, protected rat mesangial cells from apoptosis induced by serum starvation and DNA damage, by a β1 integrin-mediated, but arg-gly-asp (RGD)-independent mechanism. In contrast, collagen I, fibronectin, and osteonectin/SPARC, which are overexpressed in diseased glomeruli, failed to promote rat mesangial cell survival. However, the survival-promoting effect of collagen IV and laminin was not associated with changes in cellular levels of apoptosis regulatory proteins of the Bcl-2 family. These experiments demonstrate that glomerular mesangial cell survival is dependent on interactions with ECM and provide insights into potential mechanisms by which resident cell loss may occur during acute inflammation and postinflammatory scarring of the kidney and other organs.
Irreversible scarring is an important, unpredictable, and untreatable complication of tissue inflammation. Changes in the amount and composition of extracellular matrix (ECM) coincide with undesirable loss of normal resident cells from the perturbed tissue. 1 In glomerular inflammation progressing to glomerulosclerosis this undesirable loss of resident cells occurs by unscheduled apoptosis (programmed cell death). 2 This is associated with marked changes in the ECM of the glomerular mesangium, including expression of type I and other collagens, which are normally limited to the interstitium, and accumulation of plasma-type fibronectin. 3 In non-inflamed systems there is now growing evidence to suggest that ECM can confer survival on epithelial and endothelial cells. 4-12 This raises the possibility that unscheduled loss of mesangial and other cells during development of glomerular scarring may reflect disruption of survival signaling consequent to perturbations of the ECM.
However, despite a central role in regulation of the glomerular inflammatory response, 13-16 remarkably little is known of the mechanisms by which mesangial cell survival is signaled. Our own work has shown that soluble survival factors such as insulin-like growth factor-1 (IGF-1) can suppress apoptosis induced in mesangial cells by serum deprivation as a model of diminished survival factor supply, or by etoposide used as a model of cell death triggered by DNA damage. 17 Preliminary data are also available on possible roles for mesangial cell survival signals derived from adherence to extracellular matrix. Singhal and colleagues, building on earlier work, 18 showed that despite the presence of serum, murine mesangial cells deprived of adherence exhibited greater than 10-fold increases in apoptosis compared with cells grown on plastic. 19 Surprisingly, type IV collagen (a normal constituent of mesangial ECM) and Matrigel (a heterogeneous preparation of ECM from the murine Engelbreth-Holm-Schwarm tumor) appeared to induce mesangial cell apoptosis in this system. 19 This finding contrasted with preliminary data on rat mesangial cells from Sugiyama and colleagues 20 in which qualitative assays of apoptosis induced by serum deprivation suggested that Matrigel provided survival signals, partial blockade by antisense oligonucleotides to the integrin β1 chain implicating such receptors in Matrigel-mediated survival.
The current study used a well-established quantitative assay of apoptosis to assess the effects of defined ECM components on apoptosis induced in mesangial cells by serum deprivation or etoposide. Because of difficulty in obtaining human tissue, we used rat mesangial cells, which in previous studies have displayed survival properties similar to those observed in human mesangial cells. 17,21 We report that mesangial cell apoptosis was suppressed by normal constituents of the mesangial ECM (collagen IV and laminin), but not by ECM proteins which are expressed in the diseased glomerulus (collagen I, osteonectin/secreted protein acidic and rich in cysteine (SPARC), and plasma-type fibronectin) by a β1 integrin-dependent but arg-gly-asp (RGD)-independent mechanism. These findings imply that alterations in mesangial ECM seen in progressive glomerular inflammation are likely to dysregulate the signaling of survival to glomerular cells.
Materials and Methods
Tissue Culture Materials
All reagents were from Sigma Chemical Co. (St Louis, MO) unless otherwise stated. Culture media (Dulbecco’s modified Eagle medium nutrient mix f-12) and supplements (100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal calf serum) were from Gibco Laboratories (Grand Island, NY). Sterile tissue culture plastic ware was from Falcon Plastics (Cockeysville, MD).
Culture of Mesangial Cells
A well characterized rat mesangial cell clone was used in all experiments. 17,21-23 Cells were cultured in Dulbecco’s modified Eagle medium nutrient mix f-12 with 10% fetal calf serum and supplements as above, and underwent serial trypsinization/subculture on achieving confluency. The cells were used up to passage 15 after subculture into 24- or 96-well plates. Considerable care was taken to verify the phenotype of these mesangial cells, as in our previous studies. 17,24 Mesangial cells exhibited typical stellate morphology when subconfluent, while on becoming confluent they adopted the well recognized elongated conformation; if cultured beyond confluence, typical hillocks were seen. 25 On electron microscopy, mesangial cells exhibited characteristic features such as abundant microfilaments and, on immunofluorescence, contained organized α-smooth muscle actin, emphasizing the myofibroblast-like nature of these cells. These cells did not exhibit macrophage markers such as acetylated low density lipoprotein uptake, nor did they exhibit macrophage functional properties such as phagocytosis of opsonized zymosan particles. Furthermore, in addition to lack of acetylated low density lipoprotein uptake, these cells showed a lack of immunostaining for factor VIII-related antigen and cytokeratin, as expressed by glomerular endothelial and epithelial cells respectively.
Morphology of Apoptotic Cultured Mesangial Cells
Cells that had undergone apoptosis were recognized and counted by adding acridine orange (final concentration 10 μmol/L) to culture wells, using inverted fluorescent microscopy to recognize and count total cells and apoptotic cells in randomly selected fields at 200× magnification on a Nikon Optiphot fluorescent microscope. Apoptotic cells were recognized by cell shrinkage, nuclear chromatin condensation, and, occasionally, budding into apoptotic bodies. This fluorescent technique enabled quantitation of changes in apoptotic rates and has previously been carefully verified against mesangial cell apoptosis demonstrated by classical electron and light microscopic features or DNA “laddering” and quantified by assays of DNA fragmentation and flow cytometric demonstration of apoptotic cells. 17,21 Furthermore, in contrast to many other methods for quantitation of apoptosis, the method we used has the distinct advantage that the cell culture need not be disturbed before counting, avoiding the potential hazard of losing delicate cells in the later stages of apoptosis and, importantly, preventing the need to disrupt cell-matrix interactions, which were the primary subject of study in this series of experiments.
Induction and Assay of Apoptosis in Cultured Mesangial Cells
To trigger apoptosis, soluble growth factor deprivation was achieved by gentle washing (×3), then incubation of adherent mesangial cell cultures in RPMI 1640 medium with no added supplements. In some experiments, etoposide 50 μmol/L was added to inhibit topoisomerase II and induce DNA strand breaks as an alternative pro-apoptotic stimulus. In each experiment some wells were returned after washing to full medium (ie, RPMI 1640 plus 10% fetal calf serum and insulin/selenium/transferrin growth supplement) as a control. Cells were then incubated at 37°C/5%CO2 for 8 hours routinely, but in some experiments for 24 hours. Apoptosis was quantified by inverted fluorescent microscopy after addition of acridine orange to otherwise undisturbed wells. Total cell number and apoptotic cells were counted first in the plane of the monolayer and then, by altering the plane of focus, in the supernatant above. However, almost invariably apoptotic cells were resting immediately on top of, or next to, their healthy neighbors. Three randomly selected fields in each well were counted, and three wells were examined for each culture condition at every time point (a total of 800-1000 cells per time point).
Assessment of Survival Effects of Matrix Proteins
To assess the effects of matrix proteins, tissue culture-treated wells were treated with solutions of up to 1 mg/ml of the following proteins dissolved in phosphate-buffered saline; collagen I (rat tail purified), collagen IV (purified from human placenta), laminin (from basement membrane of Engelbreth-Holm-Schwarm mouse sarcoma), fibronectin (from bovine plasma), and osteonectin/SPARC (recombinant human protein, prepared as previously described 26, 27 ). The protein solution was added to the well in an amount sufficient to cover the bottom of the well, after which the culture dish was incubated at 37°C in 5% CO2 for 16 hours. The excess protein solution was then aspirated, the well washed, and 10 5 mesangial cells seeded on top in full medium, plus rabbit polyclonal anti-murine β1 integrin blocking antibody 100 μg/ml, rabbit polyclonal anti rat α1 integrin blocking antibody 100 μg/ml (Cambridge Bioscience), rabbit polyclonal anti rat α2 integrin blocking antibody 100 μg/ml (Cambridge Bioscience), murine monoclonal anti rat α3 integrin blocking antibody (Chemison, GA), or control antibody or preimmune serum, or arginine-glycerine-aspartate serine tetrapeptide (RGDS) at varying concentrations in some experiments. Sixteen hours after seeding, the cells were subjected to a pro-apoptotic stimulus as above.
Quantification of Protein Adherent to Wells
Wells of a 96-well tissue culture dish were treated with each matrix protein (100 μg/ml) and incubated at 37°C for 16 hours. Excess fluid was aspirated and discarded and wells treated with 40 μl acetonitrile to strip protein from the plastic. Proteins were treated with acetonitrile to hydrolyze peptide bonds then amino acid composition was determined on a ABI gas phase amino acid analyzer, and quantities compared to known amino acid compositions. In each case, a well was coated with phosphate-buffered saline containing no matrix protein and amino acid content of this well was subtracted from the amount of protein measured in the positive wells to give the final amount, which was comparable in all cases (fibronectin, 0.12 μg/well; laminin, 0.12 μg/well; collagen IV, 0.10 μg/well; collagen I, 0.08 μg/well).
Flow Cytometry
Flow cytometry was used to assess Bcl-2, Bax, Bcl-x, and Bak protein expression by the mesangial cells using antibodies of well-established specificity, as previously described. 21 Briefly, cells were incubated in full medium in wells coated with matrix proteins as above for 16 hours. They were dissociated from adherent culture wells by trypsin/EDTA treatment and fixed and permeabilized with Permeafix (Ortho Diagnostics, NJ) for 30 minutes at room temperature. Primary staining was with P-20 (rabbit polyclonal anti-human Bax IgG) 1 μg/ml, S-18 (rabbit polyclonal anti-human Bcl-x IgG) 1 μg/ml, Clone 124 5 μg/ml (monoclonal mouse anti-human Bcl-2 IgG) (DAKO Ltd., Denmark) and rabbit polyclonal anti-human Bak (kind gift of G. I. Evan, Imperial Cancer Research Fund, London) and secondary staining with fluoroscein isothiocyanate-labeled sheep anti-mouse IgG and fluoroscein isothiocyanate-labeled sheep anti-rabbit IgG (Sigma) as appropriate. Negative controls omitted primary antibody and positive controls used rabbit polyclonal or mouse monoclonal anti-human actin (Sigma) as appropriate. Cell staining was assessed on a Becton Dickinson FACScan flow cytometer.
Statistical Analysis
Results were analyzed by analysis of variance, comparing the effect of matrix proteins and etoposide to controls. Significant results are recorded in the figure legends
Results
Collagen IV and Laminin Inhibit Apoptosis Induced in Rat Mesangial Cells by Serum Starvation
To determine whether mesangial matrix proteins regulate mesangial cell apoptosis we studied two ECM proteins, collagen type IV and laminin, which are expressed in normal glomeruli. 25 We found that cultured rat mesangial cells plated 16h before onto tissue culture-treated wells pretreated with collagen IV and laminin were resistant to apoptosis subsequently induced by 8 hours serum starvation, compared to cells plated directly onto tissue culture plastic (Figure 1) ▶ . Serum starvation represents a well-established model of reduction of survival factor supply as a trigger of apoptosis. 17,21 Although 8 hours was the preferred time point for assay of mesangial cell apoptosis because no increase in cell necrosis nor any difference in the number of cells attached could be detected for any substrate (data not shown), the pro-survival effect of type IV collagen and laminin versus tissue culture plastic alone was clearly demonstrable at later time points. For example, in a series of experiments in which 20.8 ± 5.9% (mean ± SE) of mesangial cells had undergone apoptosis after 24h serum starvation, cells plated on type IV collagen (9.9 ± 3.0%, P < 0.05) and laminin (10.5 ± 2.5%, P < 0.05) were protected against apoptosis. Furthermore, in the case of both type IV collagen and laminin the protective effect at 8h serum starvation was dependent on the concentration of matrix protein used to treat the plate, being maximal at the highest tested dose of 100 μg/ml. However, significant protection was seen with protein treatment concentrations as low as 0.1 μg/ml (collagen IV) and 1 μg/ml (laminin). Nevertheless, the protective effect of laminin was generally less than that observed for collagen IV (Figure 1, A and B) ▶ .
Figure 1.
Effect of matrix proteins on rat mesangial cell apoptosis in vitro. Rat mesangial cells were subcultured onto different ECM proteins for 16 hours in full medium then washed 3 times and incubated for 8 hours in RPMI with no additives. Results are expressed as percentage of cells undergoing apoptosis compared to control. A: Collagen IV, mean ± SE, n = 9,*P < 0.05, in this series of experiments, control apoptosis signal = 23.2 ± 3.3%. B: Laminin, mean ± SE, n = 6,*P < 0.05, control apoptosis signal = 22.3 ± 2.5%. C: osteonectin SPARC, mean ± SE, n = 4, control apoptosis signal = 23.8 ± 6.9%. D: Collagen I, mean ± SE, n = 6, control apoptosis signal = 31.6 ± 1.8%. E: Fibronectin, mean ± SE, n = 8, control apoptosis signal = 25.2 ± 2.2%.
Other Matrix Proteins Do Not Promote Mesangial Cell Survival
Having established that normal ECM proteins could supply survival signals, we went on to examine ECM proteins which in glomerulonephritis are characteristically (over)expressed, using an identical protocol; again, we observed no differences in numbers of cells attaching to different substrates (data not shown). Osteonectin/SPARC, which is expressed in experimental self-limited mesangial proliferative nephritis at a time when proliferation ceases and cell number falls, 28 failed to have any protective effect at the same treatment concentrations as collagen IV and laminin (Figure 1C) ▶ . Similarly, no protective effect was observed when plates were treated with plasma-type fibronectin or collagen I, ECM proteins which are usually only expressed in glomerulosclerosis (Figure 1, D and E) ▶ . 3 To exclude the unlikely possibility that this effect was due to different quantities of protein adhering to the wells, these were quantified and found to be comparable, as detailed in Methods.
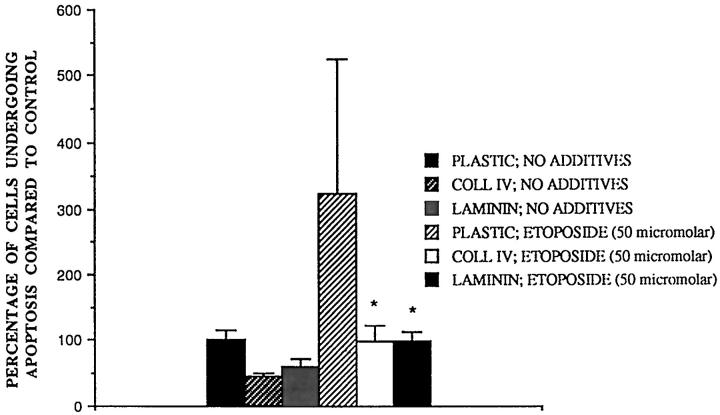
Collagen-IV and Laminin Protect Rat Mesangial Cells from Apoptosis Induced by Etoposide
Inhibition of topoisomerase II with etoposide is a standard tool in cell death studies for inducing DNA damage, which in inflamed glomeruli might result, for example, from generation of reactive oxygen species by activated leukocytes. 29 Fifty micromolar etoposide increased the apoptotic signal approximately threefold compared to serum starvation alone, though sometimes by as much as sevenfold, resulting in a large spread in the data (Figure 2) ▶ . However, both collagen IV and laminin clearly protected rat mesangial cells from apoptosis induced by etoposide.
Figure 2.
Effect of collagen IV and laminin on etoposide-induced apoptosis in rat mesangial cells in vitro. Rat mesangial cells were subcultured onto collagen IV (100 μg/ml) and laminin (100 μg/ml) in full medium for 16 hours then washed 3 times and incubated with RPMI with no additives or RPMI plus etoposide 50 μM. After 8 hours the number of apoptotic cells was counted. In this series of experiments, control apoptotic signal was 27.6 ± 4.1%. Mean ± SE, n = 7, *P < 0.05 compared to etoposide treated cells.
Collagen-IV- and Laminin-Mediated Inhibition of Apoptosis Induced by 8 Hours’ Serum Deprivation Does Not Correlate with Changes in Cellular Levels of Bcl-2 Family Members
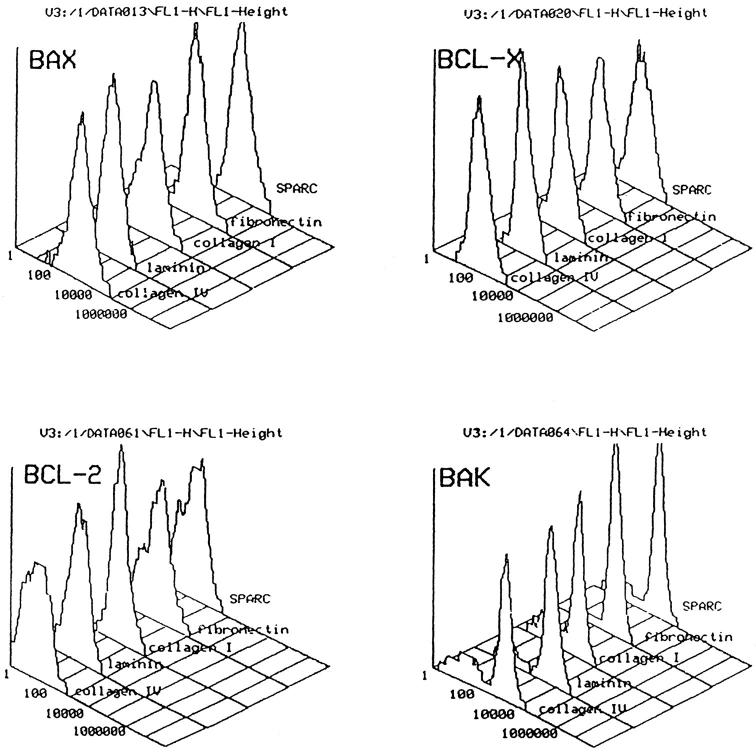
Bcl-2 is the index member of a growing family of intracellular counterregulatory proteins which, via a series of complex heterodimerizations, regulate cell susceptibility to apoptosis. 30,31 Because in previous studies of integrin-mediated, ECM protein-dependent survival, β1 integrin ligation was associated with increased Bcl-2 expression, 9 we studied the expression of Bcl-2 family members in rat mesangial cells subcultured onto protective or nonprotective ECM proteins. Flow cytometry of permeabilized cells is well established as a functionally relevant, quantitative assay of the expression of Bcl-2 family members, 32 which we have previously adapted to study of the mesangial cell. 21 However, flow cytometric analysis of mesangial cells stained for Bcl-2, Bax, Bcl-x, and Bak showed no difference in the expression of these regulatory proteins when subcultured for 16 hours on any of the ECM proteins (Figure 3) ▶ . Although in each case there was a subpopulation of cells which expressed low levels of the pro-apoptotic protein, Bak, the size of this subpopulation did not vary between different ECM proteins. Because up-regulation of Bcl-2 has been reported in survival mediated by the RGD (arg-gly-asp)-dependent integrins αvβ3 7 and α5β1, 9 we went on to seek confirming evidence that such adhesion receptors were unlikely to be mediating the pro-survival effects of type IV collagen and laminin.
Figure 3.
ECM proteins do not affect mesangial cell expression of Bcl-2, Bax, Bcl-x, or Bak. Typical flow cytometric histogram analysis of rat mesangial cells stained for Bcl-2, Bax, Bcl-x, and Bak by indirect immunofluorescence. Cells had been preincubated on the ECM protein shown for 16 hours, and positive staining was confirmed by microscopy (data not shown), but no difference in levels of staining was seen with any of the matrix proteins tested.
Collagen IV and Laminin Survival Signaling Is Independent of RGD Peptide Ligation
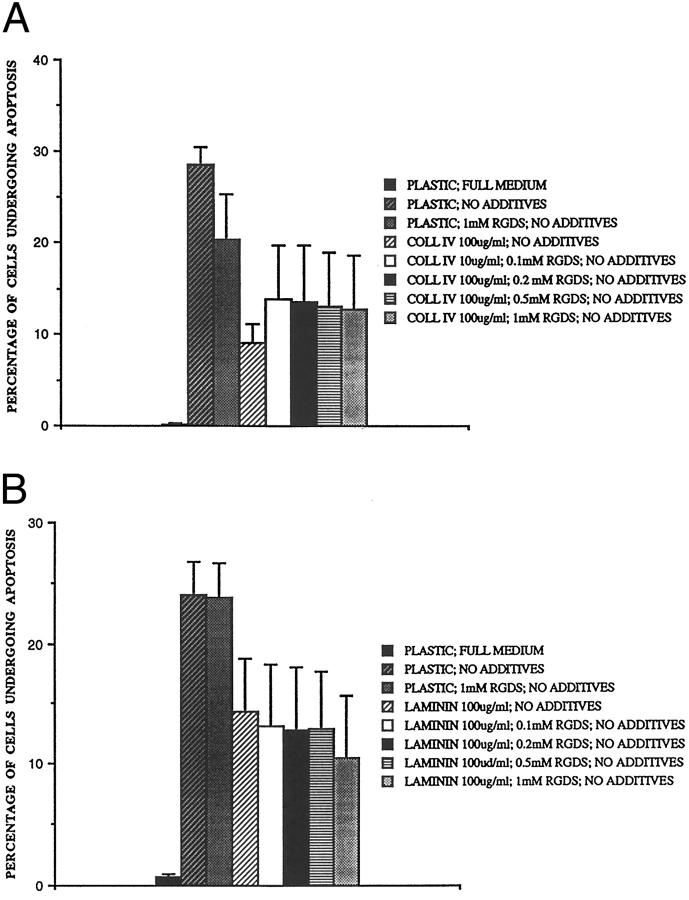
To date, those integrins that mediate survival (α5β1 and αvβ3) by up-regulation of Bcl-2 7,9 share the property of recognizing the arg-gly-asp (RGD) sequence in their ligands; their function can be inhibited with RGD-bearing peptides such as RGDS at around 1 mmol/L. 33 Various concentrations of RGDS were added to the rat mesangial cells at the time of subculture on ECM protein-coated plastic in an attempt to inhibit such interactions, and the results are shown in Figure 4 ▶ . The addition of 1 mmol/L RGDS (but not 1 mmol/L arg-gly-glu-ser, RGES) to the cells inhibited their adhesion to tissue culture-treated plastic. However, despite the presence of 1 mmol/L RGDS cells adhered normally to collagen IV- and laminin-treated wells (data not shown), indicating that binding to the protective proteins did not depend on RGD-mediated integrin binding. Furthermore, after 8 hours’ serum deprivation, RGDS failed significantly to reduce the protective effect of collagen IV or laminin compared to untreated wells. Therefore, in view of this strong evidence against involvement of RGD-dependent integrins, we went on to investigate roles for other adhesion receptors expressed by mesangial cells. 34
Figure 4.
Effect of RGD peptides on the protective effect of collagen IV and laminin. Rat mesangial cells were subcultured onto (A) collagen IV (100 μg/ml) and (B) laminin (100 μg/ml) coated wells in full medium plus increasing concentrations of RGD peptides as shown. After 16 hours, they were washed 3 times, and incubated in RPMI plus no additives for 8 hours and the number of cells undergoing apoptosis was counted. Mean ± SE, n = 3.
Blocking Antibody to β1 Integrins Abrogates the Protective Effect of Collagen IV and Laminin
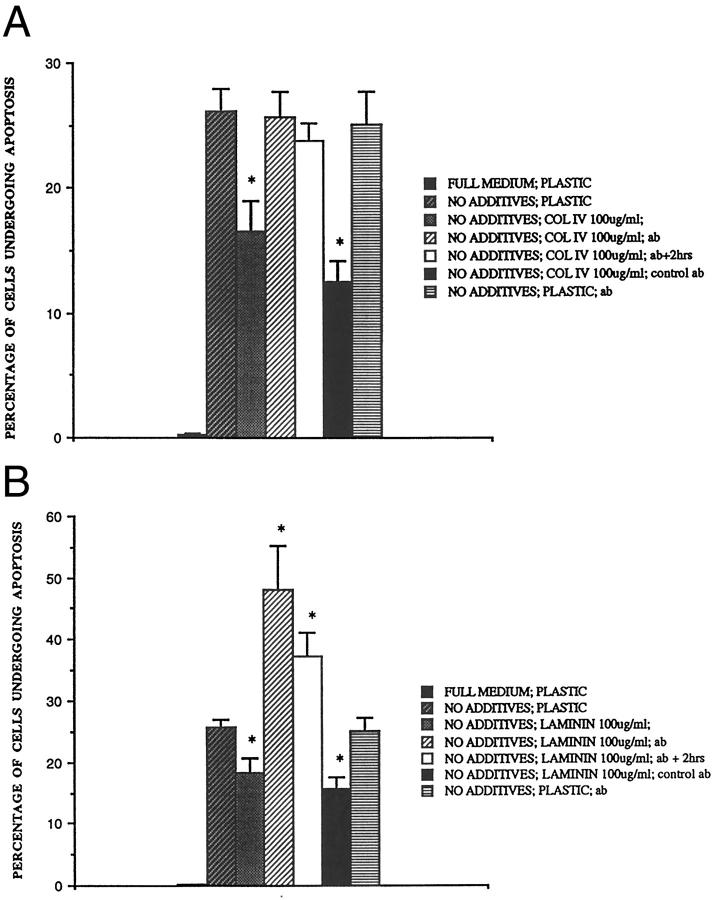
Two RGD-independent integrins of the β1 family have been implicated in signaling survival; α2β1 in kidney cells 10 and α3β1, 35 which has been implicated in glomerular cell survival by impaired glomerular development in mice with targeted mutation of the α-3 integrin gene. 36 To seek a role for RGD-independent integrin ECM receptors of the β1 family, a polyclonal rabbit anti-rodent β1 integrin antibody of well established specificity 37,38 was added to the rat mesangial cells at the time of subculture and 2 hours later. Addition of this antibody increased the apoptotic signal of cells plated onto collagen IV-treated wells to the same level as that seen in cells plated directly onto tissue culture plastic, completely abrogating its protective effect (Figure 5) ▶ . Indeed, when added to cells subcultured onto laminin-treated wells, the apoptotic signal was increased above control levels by a factor of approximately two. Therefore blocking β1 integrin interactions rendered rat mesangial cells growing on laminin more susceptible to serum starvation induced apoptosis. The antibody was equally effective whether added at the time of subculture or 2 hours later, when cell adhesion and spreading had begun. Control preimmune serum had no detectable effect when added at the same concentration. Furthermore, it is particularly important to note that (i) anti-β1 antibody did not increase mesangial cell apoptosis when cells were attached to plastic alone (Figure 5, A and B) ▶ and (ii) under no conditions was anti-β1 antibody observed to cause a reduction in cell attachment to the plate, as would have been the case had anti-β1 antibody merely induced cell detachment and so-called secondary apoptosis. 19 Thus, when the number of cells attached to plastic alone was counted in each well after 8 hours’ serum starvation and standardized as 100% of control, anti-β1 antibody did not diminish attachment to tissue culture-treated plastic (106 ± 11%, mean ± SE, n = 3), or to type IV collagen- (109 ± 16% of control) or laminin- (95.1 ± 8.0% of control) treated tissue culture-treated plastic. The data strongly implied that laminin- and collagen IV-mediated survival is dependent on β1 integrin ligation of a non-RGD peptide sequence.
Figure 5.
Effect of anti-β1 integrin blocking antibody on the protective effect of collagen IV and laminin. Rat mesangial cells were subcultured onto (A) collagen IV (100 μg/ml) and (B) laminin (100 μg/ml) in full medium plus antibody or control preimmune serum (100 μg/ml). In some cases, the antibody was added two hours after subculture at which time cell adhesion and spreading had begun (data not shown). After 16 hours, the cells were washed 3 times and incubated for 8 hours in RPMI plus no additives and the number of apoptotic cells were counted. Mean ± SE, *P < 0.05 compared to cells plated onto matrix protein.
Furthermore, it should be apparent that the use of tissue culture-treated plastic treated in turn with type IV collagen or laminin provided a system in which survival-promoting properties of β1 integrins could be isolated from their role in cell adhesion, which was essential for cell anchorage in previous studies of mesangial cell adhesion to substrates different from those used here, such as untreated plastic coated with ECM proteins. 39 We did not characterize this non-survival adhesion further, but it presumably depended on non-integrin receptors and/or non-β1 integrins such as α6β4 40
However, attempts to use our antibody blockade approach to define which β1 integrin mediated the pro-survival effects of type IV collagen- and laminin-treated substrates were inconclusive. Because α5 and αv appeared unlikely to partner β1 given the RGD-independence of survival signaling on type IV collagen and laminin (see above), we examined the effects of function-blocking antibodies to rat α1, α2, and α3 chains. No antibody affected cell attachment after 8 hours’ serum starvation and neither α1 nor α2 antibodies abrogated type IV collagen- or laminin-mediated mesangial cell survival (data not shown). However, although α3 antibody appeared to inhibit these effects (in a series of experiments in which 8 hours’ serum starvation induced 10.3 ± 5.3% apoptosis, α3 antibody increased apoptosis on type IV collagen-treated substrate from 3.7 ± 3.3% to 8.6 ± 4.2% and on laminin-treated substrates from 4.7 ± 6.6% to 8.7 ± 5.1%, mean ± SE, n = 3) no firm conclusions could be drawn because α3 antibody also increased apoptosis in cells cultured on plastic alone (in the same series of experiments, from 10.3 ± 5.3% to 16.5 ± 0.7%).
Discussion
Inflammation evolved as a mechanism to protect tissue from injury and preserve organ function, but postinflammatory scarring represents a failure of this process, as it is associated with loss of functional resident cells and their replacement with a hypocellular scar. In glomerulosclerosis, there is evidence that apoptosis underlies this undesirable loss of mesangial and other cells, 2 which is associated with replacement of the glomerular tuft by abnormal ECM proteins. 3 We now report that in studies of rat mesangial cells subcultured on tissue culture-treated wells treated in turn with different ECM proteins, collagen IV and laminin, but not collagen I, fibronectin, or osteonectin/SPARC, protected cells from apoptosis induced by serum starvation and DNA damage. The protective effect of collagen IV and laminin could be abrogated by blocking antibody to β1 integrins but not by the common integrin recognition peptide, RGDS, which inhibits the function of the αvβ3 vitronectin receptor and α5β1 fibronectin receptor. In contrast to other cell systems in which these RGD-dependent integrins are implicated, ECM protein-mediated suppression of apoptosis did not correlate with changes in intracellular levels of apoptosis regulatory proteins of the Bcl-2 family. However, it was not possible to characterize which α integrin chain(s) partner(s) β1 in survival signaling under the conditions used.
From these data we draw a number of conclusions which will help to elucidate mechanisms controlling mesangial cell numbers in inflamed glomeruli. First, although cell adhesion is recognized to have generally suppressive effects on apoptosis, particular ECM proteins can supply additional survival signals to mesangial cells. It is important to note that mesangial cell apoptosis in our culture system is not merely an example of apoptosis that follows cell detachment; as previously established, 17,21 the vast majority of apoptotic cells were found in the plane of the cultured monolayer, apparently still attached to substrate rather than floating in the medium. Our second conclusion is that ECM-derived survival signals may be particularly important in regulating susceptibility of mesangial cells to pro-apoptotic stimuli, because blocking antibody to β1 integrins induced massive apoptosis (approximately 50% at 8 hours) in serum-deprived mesangial cells on laminin-bearing substrate. Third, this suggests in turn that a search for interruption of ECM-derived survival signals might be productive in instances of unscheduled mesangial cell apoptosis in glomerular disease. Although not addressed in the current study, our data suggest the interesting possibility that in progressive glomerular scarring the accumulation of nonprotective abnormal ECM constituents might disrupt survival signaling from normal ECM proteins, perhaps promoting undesirable glomerular apoptosis as glomerular inflammation progresses to hypocellular scar.
However, some caution in interpretation of the data are required. First, although cultured mesangial cells adopt the smooth muscle-like phenotype observed in diseased glomeruli in vivo, our data were obtained with a simple two-dimensional culture model. Nevertheless, data obtained in two-dimensional culture have proved predictive of mesangial cell behavior in vivo, for example in defining the roles of platelet-derived growth factor and transforming growth factor-β in mesangial cell proliferation and excess matrix accumulation respectively. 41-43 Secondly, the experiments were performed on rat mesangial cells, which necessitated the use of some ECM proteins derived from human or bovine rather than rodent sources. However, the primary, secondary, and tertiary structures of the various ECM proteins are well conserved between species, as is their relative expression in disease states. 3 For example, the amino acid sequence of human osteonectin/SPARC is 96% identical with the rat protein, and there is strong evidence of functional equivalence. 28 Nevertheless, differences in reagents and experimental conditions appear likely to account for differences between the current data and those reported in studies of SV40-transformed murine mesangial cells. 19 However, such discrepancies emphasize that it will be necessary to devise approaches by which to dissect ECM-mediated signaling of glomerular cells in vivo.
The current data add importantly to previous work in which we showed that mesangial cell survival is also under cytokine control, especially by the autocrine/paracrine actions of secreted IGF-I. 21 Further studies will be required to dissect how these different survival signals are integrated, but it is worth noting that inhibition by β1 integrin-blocking antibody is very strong evidence of a direct effect of ECM proteins and mitigates against the unlikely possibility that survival effects of ECM represent an artifact of bound passenger cytokines. Furthermore, our blocking antibody data extend and confirm Sugiyama and colleagues’ antisense oligonucleotide studies implicating β1 integrins in Matrigel-mediated survival of serum-stained rat mesangial cells. 20 However, neither the current study nor previous work 20 has succeeded in identifying the α integrin chain(s) which partner(s) the β1 chain in signaling mesangial cell survival on ECM-treated substrates. Nevertheless, taken together with mesangial cell defects in mice deleted for the α3 integrin chain, 36 our data suggest that future studies should develop approaches beyond antibody blockade to learn whether a dominant role in this process exists for the α3β1 integrin, because α3 antibody increased apoptosis (without affecting cell attachment) on all substrates, including plastic.
Although we did not set out to dissect completely the integrin-initiated intracellular pathways by which matrix proteins signal survival of mesangial cells, we obtained evidence indicating that survival signaling could be independent of changes of intracellular expression of Bcl-2 family members (unlike reported increases in Bcl-2 expression in β1 integrin-mediated survival signaling in Chinese hamster ovary cells 9 ). However, the data do not exclude more subtle effects such as changes in intracellular location of these regulatory proteins or small but functionally important alterations in expression of Bcl-2 family members which might not be detected by the techniques used. Clearly, future studies will need to delineate more specifically intracellular signaling of ECM-mediated survival of mesangial cells, but such work is beyond the scope of this report.
To conclude, we have shown that the normal mesangial matrix proteins collagen IV and laminin promote mesangial cell survival in vitro by inhibition of apoptosis, via a β1 integrin-dependent but RGD ligation-independent mechanism. We propose that evidence of interruption of such survival signaling should be sought in postinflammatory glomerular scarring in which unscheduled mesangial cell apoptosis appears to lead to undesirable glomerular hypocellularity. Furthermore, this should be considered as a generally applicable candidate mechanism for unscheduled and undesirable loss of resident cells from tissues undergoing postinflammatory scarring.
Acknowledgments
The gift of the rat mesangial cells from Dr. Masanori Kitamura (University College London, UK) is gratefully acknowledged; Judith Hayes typed the manuscript.
Footnotes
Address reprint requests to Professor John Savill, Department of Clinical and Surgical Sciences (Internal Medicine), Royal Infirmary, Lauriston Place, Edinburgh, UK EH3 9YW.
A. M. received a Medical Research Council Clinical Training Fellowship; R. B. was supported by the National Kidney Research Fund. Additional support came from The Wellcome Trust (047273).
References
- 1.Border WA, Ruoslahti E: Transforming growth factor-β in disease: the dark side of tissue repair. J Clin Invest 1992, 90:1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugiyama H, Kashihara N, Makino H, Yamasaki Y, Ota Z: Apoptosis in glomerular sclerosis. Kidney Int 1996, 49:103-111 [DOI] [PubMed] [Google Scholar]
- 3.Couchman JR, Bevan LA, McCarthy KJ: Glomerular matrix: synthesis turnover and role in mesangial expansion. Kidney Int 1994, 45:328-335 [DOI] [PubMed] [Google Scholar]
- 4.Meredith J, Fazeli B, Schwartz M: The extracellular matrix as a survival factor. Mol Biol Cell 1993, 4:953-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates RC, Buret A, Van Helden DF, Horton MA, Burns GF: Apoptosis induced by inhibition of intercellular contact. J Cell Biol 1994, 125:403-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisch SM, Harris H: Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994, 124:619-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks P, Montgomery AMP, Rosenfield M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 8.Boudreau N, Sympson CJ, Werb Z, Bissell MJ: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995, 276:891-892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Vuori K, Reed JC, Ruoslahti E: The α5β1 integrin supports survival of cells on fibronectin, and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA 1995, 92:6161-6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saelman EUM, Keely PJ, Santoro SA: Loss of MDCK cell α2β1 integrin expression results in reduced cyst formation, failure of hepatocyte growth-factor scatter factor-induced branching morphogenesis, and increased apoptosis. J Cell Sci 1995, 108:3531-3540 [DOI] [PubMed] [Google Scholar]
- 11.O’Brien V, Frisch SM, Juliano RL: Expression of the integrin α5 subunit in HT29 colon-carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res 1996, 224:208-213 [DOI] [PubMed] [Google Scholar]
- 12.Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH: Requirement of basement-membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci 1996, 109:631-642 [DOI] [PubMed] [Google Scholar]
- 13.Johnson R: The glomerular response to injury: progression or resolution? Kidney Int 1994, 45:1769-1782 [DOI] [PubMed] [Google Scholar]
- 14.Striker LJ, Peten EP, Elliot SJ, Doi T, Striker GE: Mesangial cell turnover: effect of heparin and peptide growth factors. Lab Invest 1991, 64:446-456 [PubMed] [Google Scholar]
- 15.Mene P, Simonson MS, Dunn MJ: Physiology of the mesangial cell. Physiol Rev 1989, 69:1374-1424 [DOI] [PubMed] [Google Scholar]
- 16.Abboud HE: Nephrology forum: growth factors in glomerulonephritis. Kidney Int 1993, 43:252-267 [DOI] [PubMed] [Google Scholar]
- 17.Baker AJ, Mooney A, Hughes J, Lombardi D, Johnson RJ, Savill J: Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest 1994, 94:2105-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal PC, Hays RM: Actin filament morphology in living and nonliving cultured mesangial cells: formation and dissolution. Nephron 1988, 50:28-33 [DOI] [PubMed] [Google Scholar]
- 19.Singhal PC, Franki N, Kumari S, Sanwal V, Wagner JD, Mattana J: Extracellular matrix modulates mesangial cell apoptosis and mRNA expression of cathepsin-B and tissue transglutaminase. J Cell Biochem 1998, 68:22-30 [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama H, Kashihara N, Maeshima Y, Okamoto K, Kanao K, Sekikawa T, Makino H: Regulation of survival and death of mesangial cells by extracellular matrix. Kidney Int 1998, 54:1188-1196 [DOI] [PubMed] [Google Scholar]
- 21.Mooney A, Jobson T, Bacon R, Kitamura M, Savill J: Cytokines regulate glomerular mesangial cell survival by stimulus-dependent inhibition of apoptosis. J Immunol 1997, 159:3949-3960 [PubMed] [Google Scholar]
- 22.Yokoo T, Kitamura M: IL-1β depresses expression of the 70-kilodalton heat shock protein and sensitises glomerular cells to oxidant-initiated apoptosis. J Immunol 1997, 159:2886-2892 [PubMed] [Google Scholar]
- 23.Kitamura M, Kawachi H: Creation of an in vivo cytosensor using engineered mesangial cells. J Clin Invest 1997, 100:13941399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savill JS, Smith J, Ren Y, Sarraf C, Abbott F, Rees AJ: Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int 1992, 42:924-936 [DOI] [PubMed] [Google Scholar]
- 25.Lovett DH, Sterzel RB: Cell culture approaches to the analysis of glomerular inflammation. Kidney Int 1986, 30:246-254 [DOI] [PubMed] [Google Scholar]
- 26.Bassuk JA, Braun LP, Motamed K, Baneyx F, Sage EH: Renaturation of SPARC expressed in Escherichia coli requires isomerization of disulfide bonds for recovery of biological activity. Int J Biochem Cell Biol 1996, 28:1031-1043 [DOI] [PubMed] [Google Scholar]
- 27.Bassuk JA, Baneyx F, Vernon RB, Funk SE, Sage EH: Expression of biologically active human SPARC in Escherichia coli. Arch Biochem Biophys 1996, 325:8-19 [DOI] [PubMed] [Google Scholar]
- 28.Pichler R, Bassuk JA, Hugo C, Reed MJ, Eng E, Gordon KL, Pippin J, Alpers CE, Couser WG, Sage EH, Johnson RJ: SPARC is expressed by mesangial cells in experimental mesangial proliferative nephritis and inhibits platelet-derived-growth-factor-mediated cell proliferation in vitro. Am J Pathol 1996, 148:1153-1167 [PMC free article] [PubMed] [Google Scholar]
- 29.Henson PM, Johnston RB: Tissue injury in inflammation: oxidants, proteinases and cationic proteins. J Clin Invest 1987, 79:669-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994, 124:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ: Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA 1995, 92:7834-7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akbar AN, Borthwick N, Salmon M, Gombert W, Bofill M, Shamsadeen N, Pett S, Grundy JE, Janossy G: The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infection: the role of apoptosis in T cell memory. J Exp Med 1993, 178:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruoslahti E, Pierschbacher MD: Arg-Gly-Asp: a versatile cell recognition signal. Cell 1986, 44:517-518 [DOI] [PubMed] [Google Scholar]
- 34.Hafdi Z, Lesavre P, Tharaux PL, Bessou G, Baruch D, Halbwachs-Mecarelli L: Role of α (V) integrins in mesangial cell adhesion to vitronectin and von Willebrand factor. Kidney Int 1997, 51:1900-1907 [DOI] [PubMed] [Google Scholar]
- 35.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH: Complementation of dominant suppression implicates CD98 in integrin activation. Nature 1997, 390:81-85 [DOI] [PubMed] [Google Scholar]
- 36.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R: α3β1 integrin has a crucial role in kidney and lung organogenesis. Development 1996, 122:3537-3547 [DOI] [PubMed] [Google Scholar]
- 37.Milner R, Wilby M, Nishimura S, Boylen K, Edwards G, Fawcett J, Streuli C, Pytela R, Constant C: Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Dev Biol 1997, 185:215-228 [DOI] [PubMed] [Google Scholar]
- 38.Edwards GM, Streuli CH: Preparing a polyclonal antibody to mouse ss1 integrin with function-blocking activity. Integrin Protocols. Methods in Molecular Biology, vol. 129. Edited by A Howlett. Totowa, NJ, Humana Press, 1999, pp 135–152 [DOI] [PubMed]
- 39.Mendrick DL, Kelly DM, duMont SS, Sandstrom DJ: Glomerular epithelial and mesangial cells differentially modulate the binding specificities of VLA-1 and VLA-2. Lab Invest 1995, 72:367-375 [PubMed] [Google Scholar]
- 40.Falk-Marzillier J, Domanico SZ, Pelletier A, Mullen L, Quaranta V: Characterization of a tight molecular complex between integrin α6β4 and laminin-5 extracellular matrix. Biochem Biophys Res COmmun 1998, 251:49-55 [DOI] [PubMed] [Google Scholar]
- 41.Johnson RJ, Raines EW, Floege J, Yoshimura A, Pritzl P, Alpers CE, Ross R: Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med 1992, 175:1413-1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E: Suppression of experimental glomerulonephritis by antiserum against growth factor-β. Nature 1990, 346:371-374 [DOI] [PubMed] [Google Scholar]
- 43.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E: Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature 1992, 360:361-364 [DOI] [PubMed] [Google Scholar]