Abstract
E-Cadherin alterations have been reported frequently in sporadic diffuse type gastric and lobular breast carcinomas. Germline mutations of this gene have been identified recently in several gastric cancer families. We analyzed seven patients with a family history of the disease who had diffuse type gastric cancer diagnosed before the age of 45 for germline mutations in CDH1, the gene encoding the E-cadherin protein. We identified a frameshift mutation in exon 3 in one patient with a strong family history of gastric cancer. The same germline mutation was found in the patient’s mother, who had metachronous development of lobular breast and diffuse type gastric carcinomas. Immunohistochemistry for E-cadherin protein expression revealed an abnormal staining pattern in both of these tumors, suggesting complete inactivation of the cell adhesion molecule. Thus, our finding suggests that besides diffuse type gastric cancer, lobular breast carcinomas may be associated with germline CDH1 mutations.
E-cadherin is a calcium-dependent cell adhesion molecule whose intact function is crucial for the establishment and maintenance of epithelial tissue polarity and structural integrity. 1 The extracellular portion of the molecule mediates homophilic cellular interactions, 1 whereas its intracellular part provides a link to the actin cytoskeleton through an association with the catenins. 2 One of the catenins is β-catenin, an important multifunctional protein involved not only in cell adhesion but also in signal transduction. 3
Somatic mutations of CDH1, the E-cadherin-encoding gene, have been implicated in the carcinogenesis of some gynecological cancers 4 and in a substantial proportion of diffuse type gastric and invasive lobular breast carcinomas. 5-9 The latter two tumor types have in common a diffuse growth pattern, which is considered to be the result of loss of cell adhesion mediated by E-cadherin. Due to the frequent finding of E-cadherin alterations in advanced tumor stages and in the process of metastasis, it was initially categorized as a tumor invasion suppressor gene. 10 However, the finding of somatic mutations in lobular carcinomas in situ of the breast 11 and in intramucosal gastric carcinomas 7 as well as the involvement of the protein in the transition from adenomas to carcinomas in a transgenic mouse model of pancreatic b-cell carcinogenesis, 12 also suggests a role for CDH1 in early steps of tumor development. Furthermore, germline mutations in CDH1 have been demonstrated recently to be the underlying genetic cause of a familial gastric cancer syndrome characterized by the occurrence of diffuse type gastric carcinoma and a predominant early onset of the disease. So far, germline mutations have been reported in three kindreds of Maori descent 13 and for five diffuse type gastric cancer families of European origin. 14,15 Interestingly, no germline mutations have been identified yet in families with intestinal type gastric carcinoma. 14
In this study we analyzed a group of diffuse type gastric cancer patients suspected to have a genetic predisposition for the disease, based on a young age at diagnosis and a positive family history of the disease, for germline mutations in CDH1. We found a new germline mutation that was also present in another family member who had metachronous development of lobular breast and diffuse type gastric carcinoma. Because somatic E-cadherin mutations have frequently been reported in both these tumor types, this finding suggests that lobular breast carcinoma may be a component of the tumor spectrum associated with germline E-cadherin mutations.
Materials and Methods
Patients and Tumors
We studied seven gastric cancer patients with Laurén’s diffuse type gastric carcinoma diagnosed before the age of 45 (range, 22–43). In the families of four of these patients there were at least two first-degree relatives with gastric cancer. In one of these families the mother of the index patient had metachronous development of carcinoma of the breast and stomach. Histological examination confirmed diffuse type gastric cancer according to Laurén’s classification and invasive lobular breast carcinoma. The gastric carcinoma of this patient was limited to the stomach and perigastric lymph nodes without evidence of distant metastasis. In the families of the three remaining patients there were two second-degree relatives with gastric carcinoma.
DNA Isolation
Genomic DNA from frozen or paraffin-embedded normal gastric mucosa or lymph nodes was isolated according to standard procedures which have been described previously. 16 DNA from the gastric carcinoma of the patient with the E-cadherin germline mutation was isolated by manual microdissection from a tumor area, which permitted an isolation of at least 50% tumor cells, to perform somatic mutational analysis of CDH1. In addition, DNA of normal and gastric carcinoma cells of this patient and her mother was isolated by a laser-based microdissection technique using a high resolution UV laser microbeam as previously described. 17 DNA from the lobular breast carcinoma was isolated using both microdissection techniques. Poor amplification rates of the DNA preparations of the breast carcinoma prevented further analysis, however.
RNA Isolation and cDNA Synthesis
Total RNA was isolated from normal gastric mucosa with the RNeasy isolation kit (Quiagen, Hilden, Germany) according to the protocol provided by the manufacturer. Reverse transcription was performed using SuperScript reverse transcriptase (Gibco BRL, Karlsruhe, Germany) and random hexamers according to standard procedures.
Haplotype Analysis
Haplotype analysis was performed on two affected family members with and one unaffected member without an E-cadherin germline mutation. Six microsatellite markers in the vicinity of CDH1 on chromosome 16q22.1 were amplified using genomic DNA isolated from the normal gastric mucosa or from a tumor-free lymph node as a template. The markers analyzed were D16S503, D16S400, D16S3057, D16S301, D16S512, and D16S522. Detailed information about the markers is available in the Genome Database. Polymerase chain reaction (PCR) using a fluorescent labeled primer and analysis of the PCR products on an automated sequencing system (ABI 377, Applied Biosystems, Foster City, CA) was performed essentially as described. 16
Mutation Analysis
Mutation analysis was carried out by direct sequencing of PCR products. For four patients, germline mutation analysis was performed from genomic DNA and for three patients, from cDNA isolated from the normal gastric mucosa. Somatic mutation analysis was performed from genomic DNA isolated from manually microdissected gastric carcinoma cells of the patient with an E-cadherin germline mutation for which sufficient DNA was available for this analysis. Genomic DNA isolated from laser-based microdissected gastric carcinoma cells of the proband and her mother was used to examine loss of heterozygosity (LOH). LOH was analyzed by direct sequencing of the exon with the germline mutation. The primers and PCR conditions for the amplification of exons 1 to 16 from genomic DNA were essentially according to published procedures. 8 For cDNA PCR amplification, the E-cadherin cDNA was divided into five overlapping fragments. The primers and PCR conditions used are available from the authors on request. The PCR products were purified from agarose gels using a gel extraction kit (Quiagen). Cycle sequencing was performed using the Ready Reaction Big Dye Terminator Cycle Sequencing kit (Applied Biosystems) and an automated sequencing system (ABI 377, Applied Biosystems). Sequencing variants suspected to represent mutations were analyzed twice, beginning with a new PCR reaction with sequencing of the forward and reverse strands.
Immunohistochemical Analysis
Immunohistochemical analysis of the two gastric carcinomas and the one lobular breast carcinoma from the family members with an E-cadherin germline mutation was performed on 4-μm sections of paraffin-embedded, formalin-fixed tissues. The sections were pretreated in a pressure cooker for 4 minutes. Two monoclonal E-cadherin antibodies, AEC (Transduction Laboratories, Hamburg, Germany) and HECD-1 (Takara Biomedicals, Shiga, Japan), were used in dilutions of 1:1000 and 1:500, respectively. The AEC antibody has been raised by immunization with a C-terminal protein fragment encompassing the intracellular domain of the E-cadherin molecule, which corresponds to exons 14–16 of the gene. The antibody HECD-1 has been demonstrated to recognize an epitope of the extracellular domain, 18 which could be assigned to the protein region corresponding to exon 8 (Becker and Handschuh, unpublished observation). For immunohistochemical analysis of β-catenin a monoclonal antibody (Dianova, Hamburg, Germany) in a dilution of 1:300 was used. Staining was carried out using the streptavidin-biotin-peroxidase system (Vectastain, Vector Laboratories, Burlingame, CA). Appropriate positive and negative controls were included with each reaction. E-cadherin and β-catenin expression was normal when a minimum of 90% of the tumor cells showed a clear, membrane-associated stain. Irregular membranous, cytoplasmic, or nuclear staining was considered to be abnormal expression.
Results
Germline Mutation Analysis
CDH1 germline mutation analysis of the seven unrelated patients with diffuse type gastric cancer revealed a heterozygous, 1-bp deletion in a run of six cytosines at cDNA position 372–377 in exon 3 in the normal DNA of one patient, indicating a germline mutation (Figure 1A) ▶ . The patient’s family had a strong clustering of gastric cancer with four affected individuals, three of whom were very young (36, 37, and 15 years) at the time of diagnosis (Figure 1B) ▶ . The same mutation was also present in the DNA isolated from the normal gastric mucosa and from a tumor-free lymph node from the proband’s mother, who had developed metachronous lobular breast and diffuse type gastric carcinoma at the ages of 49 and 58, respectively. Because diffuse type gastric carcinomas frequently show somatic mutations of CDH1, 5-7 we repeated the analysis of exon 3 in both family members using laser-microdissected normal gastric mucosa cells to exclude any possible contamination of the normal cells with tumor cells. Sequence analysis revealed the same results in both analyses, unequivocally demonstrating a germline mutation. The mutation was absent in a 69-year-old unaffected family member. Consistent with this was the finding in the two affected family members of a common haplotype with 6 microsatellite markers in the vicinity of CDH1 on chromosome 16q22.1 that was not present in the analyzed unaffected individual, who did not carry the mutation.
Figure 1.
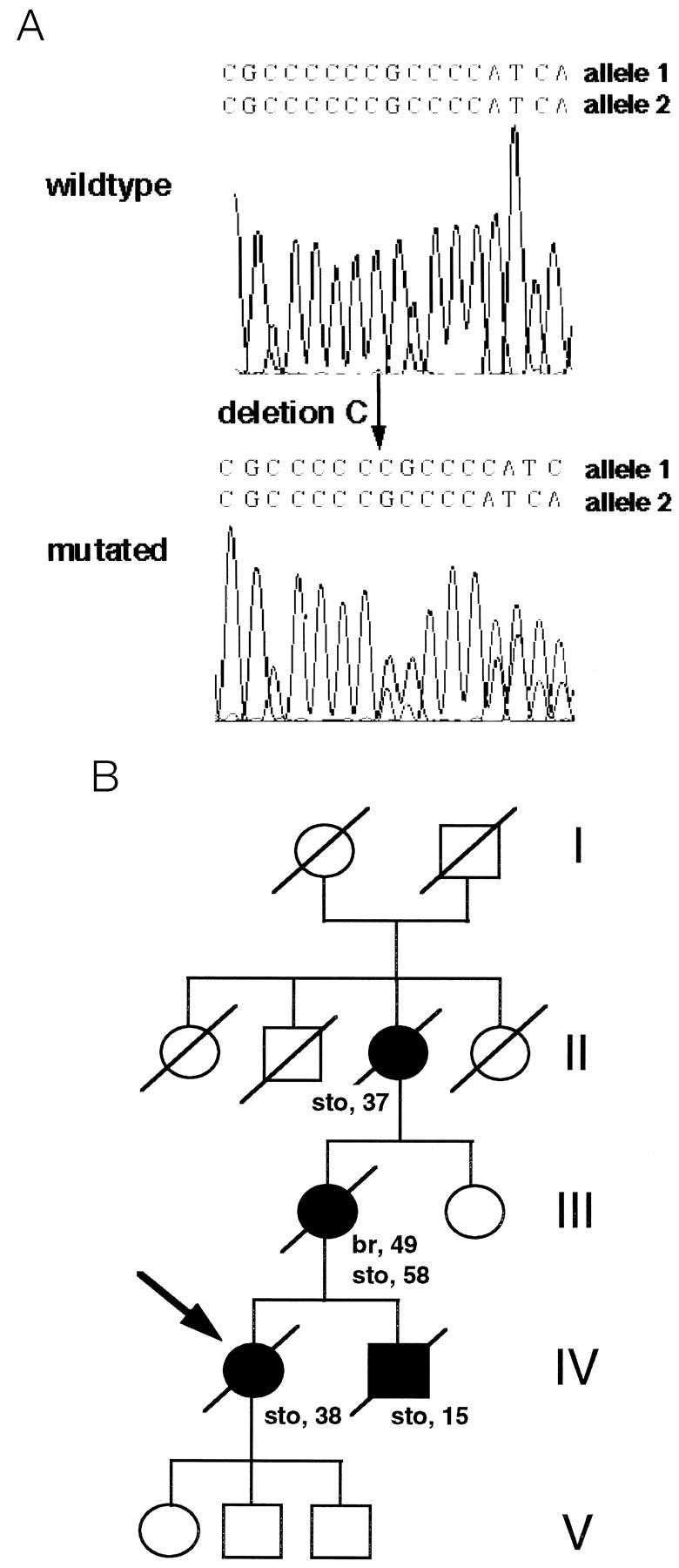
Sequence analysis of exon 3 of the E-cadherin gene and pedigree of the gastric cancer family with the E-cadherin germline mutation. A: The wild-type sequence in comparison with the heterozygote mutation, found in one of the seven unrelated analyzed gastric cancer patients, is shown. An overlapping pattern is observed (arrow) starting at the deletion point of the “C” corresponding to nucleotide position 372–377. B: Pedigree of the family. Squares represent males; circles, females; symbols marked with a diagonal represent deceased individuals. Open symbols indicate no neoplasm and filled symbols represent persons with carcinomas of the stomach (sto) and breast (br). Numbers beside the symbols indicate age at diagnosis; arrow, analyzed proband. The germline mutation in exon 3 in the E-cadherin gene was found in the normal DNA of the proband and in the normal DNA of her mother. The mutation was not found in the normal DNA of the 69-year-old sister of the mother of the proband. The children of the proband were not analyzed.
Somatic Mutation Analysis and LOH
Somatic mutation analysis of the whole E-cadherin gene of the tumor of the proband with the E-cadherin germline mutation revealed no additional mutation. Sequencing of exon 3 starting from DNA isolated from laser-microdissected tumor cells of the gastric carcinoma of the proband and her mother revealed that the mutation in exon 3 was also present in the heterozygote state in the tumor, indicating that there was no LOH. Poor amplification rates of the DNA extracted from the lobular breast carcinoma precluded our attempts to analyze the E-cadherin gene for LOH or somatic mutations in this tumor. The results are summarized in Table 1 ▶ .
Table 1.
Summary of E-Cadherin Immunohistochemistry, LOH, and Somatic Mutation Analysis of the Carcinomas of the Family Members with an E-Cadherin Germline Mutation in Exon 3
| Carcinomas | Immuno- histochemistry with antibody | LOH* | Somatic mutation† | |
|---|---|---|---|---|
| HECD-1 | AEC | |||
| Proband | ||||
| Gastric carcinoma | ++‡ | ++‡ | − | − |
| Mother of the proband | ||||
| Gastric carcinoma | +§ | − | − | n.d. |
| Breast carcinoma | − | +¶ | n.d. | n.d. |
*For LOH, sequencing analysis of exon 3 from DNA isolated from laser microdissected tumor cells was performed.
†Somatic mutation analysis was performed by sequencing all exons of the E-cadherin gene from DNA of manually microdissected tumor cells.
‡++: positive immunostaining of the cell membrane and diffuse and granular cytoplasmatic staining of the tumor cells.
§+: reduced granular cytoplasmic staining pattern
¶+: reduced diffuse cytoplasmatic and nuclear staining
n.d., not done
Immunohistochemistry
Immunohistochemistry for E-cadherin in the gastric carcinoma of the proband revealed positive staining with both antibodies, HECD-1 and AEC, which was both membranous and cytoplasmic with a diffuse or granular distribution (Figure 2, a and b) ▶ . The gastric carcinoma of the proband’s mother revealed reduced, diffuse cytoplasmic staining with the HECD-1 antibody, but no protein expression with the antibody AEC (Figure 2, c and d) ▶ .
Figure 2.
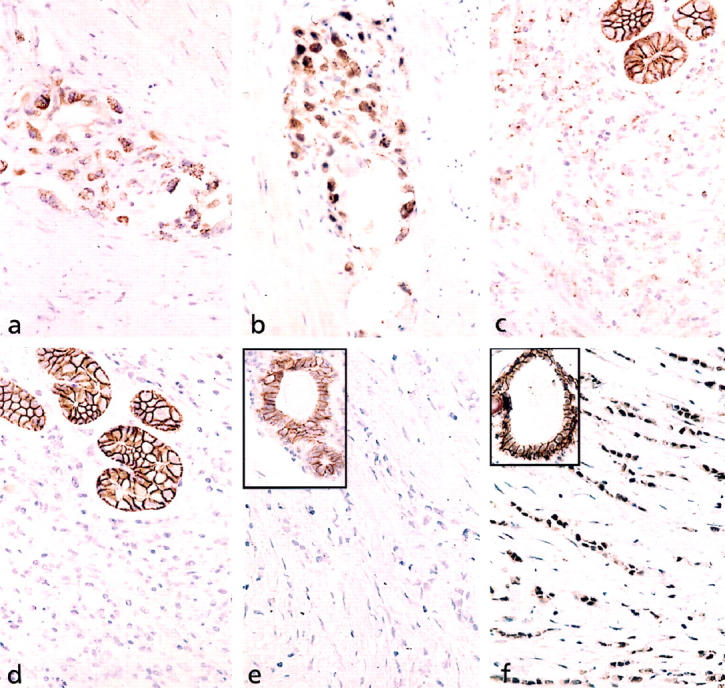
Immunohistochemical analysis of E-cadherin expression. a and b: Invasive gastric carcinoma in the muscularis propria of the stomach of the proband (autopsy material). Positive immunostaining of the cell membrane and diffuse and granular cytoplasmic staining of the tumor cells with the antibodies HECD-1 (a) and AEC (b) are shown. Magnification, ×880. c and d: Invasive carcinoma cells of the gastric diffuse type carcinoma of the proband’s mother (resection specimen). A reduced granular cytoplasmic staining pattern with antibody HECD-1 (c) is shown. No protein expression was detected with antibody AEC (d). The normal gastric glands used as internal controls exhibited a strong cell membrane-associated immunostaining with both antibodies. Magnification, ×880. e and f: Lobular breast carcinoma of the proband’s mother (resection specimen). No immunoreactivity in the carcinoma was detected with antibody HECD-1 (e). Antibody AEC (f) revealed nuclear and reduced diffuse cytoplasmic staining of the tumor cells. Magnification, ×880. Insert shows normal cell membrane-associated staining of normal ductal cells for each antibody. Magnification, ×880.
The invasive lobular breast carcinoma of the proband’s mother had an abnormal E-cadherin staining pattern with both antibodies. Immunohistochemistry with HECD-1 showed no staining, whereas the AEC antibody demonstrated abnormal, diffuse cytoplasmic and nuclear staining (Figure 2, e and f) ▶ . Immunohistochemistry for β-catenin, which was performed in this tumor to clarify whether the abnormal nuclear staining of E-cadherin was associated with nuclear staining of β-catenin, revealed no immunoreactivity for this molecule.
The results of the immunohistochemical analyses are summarized in Table 1 ▶ .
Discussion
In this study we found a germline E-cadherin mutation in one of seven analyzed patients. This patient had a strong clustering of gastric cancer in her family and the mutation was also identified in another family member with metachronous diffuse type gastric and lobular breast carcinomas. So far, E-cadherin germline mutations have been reported in three gastric cancer families of Maori origin, 13 and in three of ten 14 and two of seven 15 European diffuse type gastric cancer families. Thus, our result represents additional evidence that CDH1 is important for genetic predisposition in a subset of diffuse type gastric carcinomas. The identified 1-bp deletion results in a frameshift mutation leading to a truncated E-cadherin protein, which is predicted to be completely functionally inactive inasmuch as the affected portions are responsible for its correct processing and integration into the cell membrane. In addition, none of the calcium binding sites essential for normal E-cadherin function remain intact.
Based on the limited data to date, diffuse type gastric carcinomas are the most common tumors in families with E-cadherin germline mutations, although individuals with colorectal and prostate cancer have also been reported. 13-15 Of interest is that the two tumors found in the proband’s mother with an E-cadherin germline mutation, diffuse type gastric and invasive lobular breast carcinoma, are by far the most frequent tumor types reported to have somatic E-cadherin mutations. 19 E-cadherin protein expression abnormalities detected by immunohistochemistry and complete gene inactivation due to LOH affecting the remaining wild-type allele have also been demonstrated for these tumors, 19,20 indicating that loss of function of this molecule follows the classical two-hit inactivation mechanism for tumor suppressor genes. E-cadherin germline mutations therefore would predispose not only to diffuse type gastric carcinoma, but also to lobular breast carcinoma, a relationship which could only be proven by observing segregation of both types of carcinomas with the germline mutation in families. However, an analysis of the E-cadherin for a second tumor-specific alteration in our study could also provide further evidence in support of this idea.
The immunohistochemical staining pattern of both antibodies showed normal membranous and abnormal diffuse or granular cytoplasmic E-cadherin expression in the gastric carcinoma of the proband. The significance of the latter finding is unclear because this analysis was performed on autopsy material, which is prone to artifacts. The lack of LOH fits with the positive protein expression pattern. Mutational analysis of the whole gene failed to reveal an additional mutation. Thus, there was no evidence for a second, inactivating hit in this case. Possible explanations include the following: i) a larger deletion including exons not corresponding to the recognition sites of the antibody may have been present, which would not have been detectable by sequencing each exon; ii) contamination by nontumorous tissue cells leading to failure to detect a mutation; iii) aberrant posttranslational modifications; or iv) transdominant negative defects in other cadherins or genes coding for E-cadherin binding partners. Silencing of gene expression by methylation of the promotor region of the E-cadherin gene has been reported in various human carcinomas, 21 but this is unlikely to be the second, inactivating hit in this case, since both antibodies indicated that there was E-cadherin protein expression.
In contrast, the proband’s mother’s gastric carcinoma showed reduced, diffuse cytoplasmic HECD-1 antibody staining, but no staining with the AEC antibody. Reduced E-cadherin staining with weak cytoplasmic staining has been observed frequently in tumors including diffuse type gastric carcinoma. 20 The difference between the two antibodies indicates an inactivation of the second E-cadherin allele at the AEC-specific epitope in the E-cadherin protein cytoplasmic domain corresponding to exons 14 to 16. The positive staining with HECD-1, which recognizes an E-cadherin protein epitope coded for by exon 8 (Becker and Handschuh, unpublished observation) argues for an intact extracellular domain, and is consistent with our failure to identify LOH at exon 3 in this tumor.
The proband’s mother’s invasive lobular breast carcinoma stained abnormally with both E-cadherin antibodies; HECD-1 was negative, and AEC demonstrated abnormal, diffuse cytoplasmic and nuclear staining. This difference most likely reflects the different epitopes they recognize, though nuclear E-cadherin staining has never been reported in tumors before. Because cadherin-associated cytoplasmic β-catenin can translocate to the nucleus, 3 the possibility of abnormal transfer of E-cadherin by β-catenin to the nucleus was analyzed by immunohistochemistry for β-catenin, which was negative. Thus, the E-cadherin nuclear staining pattern remains unclear.
Although it is theoretically possible that the gastric carcinoma of the proband’s mother was a metastasis from the lobular breast carcinoma, the different homogeneous staining pattern with both antibodies in these tumors clearly argues against this.
In conclusion, our identification of an E-cadherin germline mutation in a gastric cancer family emphasizes the importance of this gene for a genetic predisposition for diffuse type gastric cancer. Furthermore, the metachronous development of lobular breast and diffuse type gastric carcinoma in association with an E-cadherin germline mutation, indicates that lobular breast carcinoma might be part of the tumor spectrum associated with E-cadherin germline mutations. The abnormal E-cadherin expression in both tumors of this patient indicates that there was complete functional loss of the E-cadherin molecule due to a second somatic inactivation hit. These findings represent an important insight with respect to the clinical management and surveillance of patients with germline E-cadherin mutations.
Acknowledgments
We thank Andrea Montag de Palma and Anja Müller for their excellent technical assistance.
Footnotes
Address reprint requests to Gisela Keller, Institute of Pathology, Laboratory for Molecular Pathology, Technische Universität München, Trogerstrasse 18, 81675 München, Germany. E-mail: gisela.keller@lrz.tum.de.
Supported by the Deutsche Krebshilfe Grant 10–1345-Hö 2.
References
- 1.Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991, 251:1451-1455 [DOI] [PubMed] [Google Scholar]
- 2.Aberle H, Schwartz H, Kemler R: Cadherin-catenin complex: Protein interactions and their implications for cadherin function. J Cell Biochem 1996, 61:514-523 [DOI] [PubMed] [Google Scholar]
- 3.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 4.Risinger JI, Berchuck A, Kohler MF, Boyd J: Mutations of the E-cadherin gene in human gynecologic cancers. Nat Genet 1994, 7:98-102 [DOI] [PubMed] [Google Scholar]
- 5.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H: E-cadherin mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994, 54:3845-3852 [PubMed] [Google Scholar]
- 6.Tamura G, Sakata K, Nishizuka S, Maesawa C, Suzuji Y, Takeshi I, Terashima M, Saito K, Satodate R: Inactivation of the E-cadherin gene in primary gastric carcinomas and gastric carcinomas cell lines. Jpn J Cancer 1996, 87:1153-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muta H, Noguchi M, Kanai Y, Ochiai A, Nawata H, Hirohashi S: E-cadherin gene mutations in signet ring cell carcinoma of the stomach. Jpn J Cancer Res 1996, 87:843-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJF, van de Vijver MJ, Cornelisse C, van Roy F: E-cadherin is a tumor/invasion suppressor gene mutated in human lobular breast cancers. EMBO J 1995, 14:6107-6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJF, Nollet F, van Roy F, Cornelisse CE: E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout ist extracellular domain. Oncogene 1996, 13:1919-1925 [PubMed] [Google Scholar]
- 10.Frixen UH, Behrens J, Sachs M, Eberle G, Voss G, Voss B, Warda A, Lochner D, Birchmeier W: E-cadherin-mediated cell-cell adhesion prevents invasivness of human carcinoma cells. J Cell Biol 1991, 113:173-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos CBJ, Cleton-Jansen AM, Berx G, de Leeuw WJF, ter Haag NT, van Roy F, Cornelisse CJ, Peterse JL, van de Vijver MJ: E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer 76:1131–1133 [DOI] [PMC free article] [PubMed]
- 12.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G: A causal role of the E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392:190-193 [DOI] [PubMed] [Google Scholar]
- 13.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE: E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392:402-405 [DOI] [PubMed] [Google Scholar]
- 14.Gayther SA, Gorringe KL, Ramus SJ, Huntsman D, Roviello F, Grehan N, Machado JC, Pinto E, Seruca R, Halling K, MacLeod P, Powell SM, Jackson CE, Ponder BAJ, Caldas C: Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res 1998, 58:4086-4089 [PubMed] [Google Scholar]
- 15.Richards FM, McKee SA, Rajpar MH, Cole TRP, Evans DGR, Jankowski JA, McKeown C, Sanders DSA, Maher ER: Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer, and colorectal cancer. Hum Mol Genet 1999, 8:607-610 [DOI] [PubMed] [Google Scholar]
- 16.Keller G, Rudelius M, Vogelsang H, Grimm V, Wilhelm MG, Mueller J, Siewert JR, Höfler H: Microsatellite instability and loss of heterozygosity in gastric carcinoma in comparison to family history. Am J Pathol 1998, 152:1281-1289 [PMC free article] [PubMed] [Google Scholar]
- 17.Becker I, Becker KF, Röhrl MH, Minkus G, Schütze K, Höfler H: Single-cell mutation analysis of tumors from stained histologic slides. Lab Invest 1996, 75:801-807 [PubMed] [Google Scholar]
- 18.Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O: Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res 1989, 49:2128-2133 [PubMed] [Google Scholar]
- 19.Berx G, Becker KF, Höfler H, van Roy F: Mutations of the human E-cadherin (CDH1) gene. Hum Mutat 1998, 12:226-237 [DOI] [PubMed] [Google Scholar]
- 20.Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 21.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S: Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci 1995, 92:7416-7419 [DOI] [PMC free article] [PubMed] [Google Scholar]


