Abstract
Estrogens have been implicated in prostatic cancerogenesis and tumor progression. The mechanisms underlying estrogen signaling in human prostate tissue, however, remain poorly understood. Using immunohistochemical and in situ hybridization (ISH) techniques, the present study demonstrates the classical estrogen receptor (ERα) in premalignant lesions and prostatic adenocarcinoma through the various stages of the disease. Conversely, the novel characterized ERβ subtype was undetectable in human prostate tissue. High-grade prostatic intraepithelial neoplasia revealed ERα mRNA and protein expression in 28% and 11% of cases evaluated. Focal ER immunoreactivity was detected in a minority of low- to intermediate-grade adenocarcinoma. High-grade (primary Gleason grade 4 and 5) tumors revealed ER protein expression in 43% (62% respectively) of cases. The most significant ERα gene expression on mRNA and protein levels was observed in hormone refractory tumors and metastatic lesions, including lymph node and bone metastases. Results of the current study suggest that estrogens can affect prostatic cancerogenesis and neoplastic progression through an ER-mediated process in human prostate tissue.
Since the pioneering work of Huggins and co-workers, estrogens have been widely used in the medical treatment of hormone refractory prostate cancer. The regulatory impact of estrogens on normal and abnormal prostatic growth, however, still remains a matter of great speculation. Both epidemiological and experimental data suggest that estrogens are involved in prostatic carcinogenesis. 1,2 The high incidence of clinical prostate cancer in Western countries has been related to several demographic and dietary factors, including increasing age, weight, and a fat-rich diet. 1-8 These potential risk factors may be associated with an increase in estrogen levels or high estrogen/androgen levels in circulating blood. 1 Some evidence supporting cancerogenic effects of estrogens has been documented in animal models. 1,2 Chronic treatment with testosterone of Noble rats results in a high incidence of prostate cancer when combined with estrogens. 9-11 More recent studies have reported that neonatal estrogenization induces dysplastic changes of the mouse prostatic epithelium, which shares morphological characteristics with high-grade prostatic intraepithelial neoplasia (HGPIN) of the human prostate. 1,2 Although there is a growing amount of literature dealing with the potential implications of estrogens in prostatic carcinogenesis, there has never been a clear demonstration of direct estrogenic effects on transformed epithelial cells through a receptor-mediated process in human prostate tissue.
The role of estrogens in prostate cancer progression is even less well understood. It is generally accepted that estrogens influence prostatic growth indirectly through effects at the hypothalamic and pituitary levels, reducing gonadotrophin secretion and hence the synthesis of testicular testosterone. 12 This concept is supported by immunohistochemical and in situ hybridization (ISH) studies that failed to demonstrate detectable levels of the estrogen receptor (ER) in epithelial compartments of human prostate cancer. 13-19 Nevertheless, ER gene expression has been reported in several human prostate cancer cell lines, including LNCaP, PC-3, and DU-145, 20,21 although these data have not been confirmed by others. 19 A recent study using RT-PCR, ligand binding assays, and immunohistochemistry (IHC) failed to demonstrate detectable ER in prostate cancer cell lines and tissue sections from patients with metastatic disease. 19 Regardless of these divergent findings, the recent discovery of an additional ER subtype has shed new light on the role of estrogens in prostate tissue. 22-24 The novel ERβ cloned from a rat prostate cDNA library is expressed at high levels in epithelial compartments of the rat prostate gland. 22,25 Its presence in human prostate tissue and prostatic adenocarcinoma, however, has not yet been reported in the literature. The ERβ shares high homology with the known ER protein (consequently ERα) in the DNA binding domain but differs in the ligand and transactivation domains. 22-24 In human prostate tissue, the classical ER has been detected exclusively in stromal compartments and in subsets of basal cells. 13,14,16,17 Thus it is generally believed that ERα-mediated effects on the prostatic epithelium are exerted via paracrine mechanisms.
The controversial issue of the potential implications of estrogens in neoplastic prostate growth prompted us to investigate ER gene expression in human prostate tissue by combined immunohistochemical and mRNA ISH techniques. Using improved antigen retrieval methods and the well-characterized antibody NCL-ER-6F11 directed against the whole protein of the classical ER, 26 we were able to demonstrate the nuclear ER in 11% of HGPIN and in a significant number of high-grade, metastatic, and hormone-insensitive prostatic adenocarcinomas. On the other hand, the ERβ was undetectable in prostate tissue by IHC. ERα gene expression was confirmed by ISH showing high steady-state levels of ERα mRNA in premalignant and malignant human tissue, even in the absence of detectable ERα protein.
Materials and Methods
Tissue Selection
Formalin-fixed, paraffin-embedded, and fresh frozen tissue sections were obtained from 28 patients who underwent radical prostatectomy and pelvic lymphadenectomy for prostate cancer during the period from 1991 to 1998. The pathological stages included pT3c (n = 14), pT3a,b (n = 9), pT2 (n = 5), pN1 (n = 9) (Table 1) ▶ . Tissue sections from radical prostatectomy specimens submitted for study contained the Gleason primary grades 2 (n = 8), 3 (n = 25), 4 (n = 28), and 5 (n = 18). High-grade prostatic intraepithelial neoplasia (HGPIN) was identified in all prostatectomy specimens. The extend of HGPIN was arbitrarily defined by one microscopic field at low magnification (×100). One hundred seventy-six foci of HGPIN were submitted for study. The material further contained bone metastasis from two patients without previous hormonal therapy and palliative transurethral resection specimens from 18 patients with recurrent prostatic adenocarcinoma after orchiectomy (Table 1) ▶ . Antibody specificity for the ERα and β subtypes was tested in ovarian tissue obtained from five patients with cervical carcinomas. Paraffin-embedded tissue sections were cut 1 day before pretreatment and staining.
Table 1.
Pathological Stages and Grades
| n* | Stages | Primary Gleason grades |
|---|---|---|
| 6 | pT3c,pN1 | 5 (n = 6), 4 (n = 6), 3 (n = 3) |
| 8 | pT3c,pN0 | 5 (n = 8), 4 (n = 8), 3 (n = 8) |
| 3 | pT3a,b;pN1 | 5 (n = 3), 4 (n = 3), 3 (n = 3) |
| 6 | pT3a,b;pN0 | 5 (n = 1), 4 (n = 6), 3 (n = 6), 2 (n = 3) |
| 5 | pT2, pN0 | 4 (n = 5), 3 (n = 5), 2 (n = 5) |
| 2 | bone metastases | ND |
| 18 | recurrent carcinomas | ND |
*Number of patients.
ND, not determined.
The primary Gleason grades refer to the cancerous lesions obtained from 28 radical prostatectomy specimens. The number of Gleason primary grades submitted for study is indicated in parentheses. Metastatic and recurrent lesions were not graded.
Immunohistochemical Analysis
Tissue sections were deparaffinized, rehydrated through graded alcohol, and subsequently incubated in H2O2 (0.3%) to block endogenous peroxidase. For microwave-based antigen retrieval, sections were microwaved (750 W for 5 minutes and 450 W for 5 minutes) in 10 mmol/L citrate buffer (pH 6.0). After pretreatment, the sections were incubated for 30 minutes in a normal rabbit serum (Dako, Hamburg, Germany). The mouse monoclonal antibody NCL-ER-6F11, directed against the full-length ER molecule (Novocastra Laboratories, Newcastle upon Tyne, UK) was obtained by recombinant protein preparation from MCF-7 cells. 26 Specificity was tested by Western blotting and by IHC on breast and other tissues. 26 In comparative studies, NCL-ER-6F11 was recognized to be more effective than other available ER antibodies. 26 To localize the ERα, we have used the NCL-ER-6F11 antibody in a dilution of 1:200. Sections were incubated overnight in a humid chamber. After incubation with the secondary biotinylated rabbit anti-mouse immunglobulin (Dako) for 30 minutes, the horseradish peroxidase-labeled avidin-biotin complex (ABC-HRP) method (Dako) was performed according to the manufacturer’s instructions. A signal amplification method based on the deposition of biotinylated tyramine was used to enhance immunodetection of the ER. Preparation of the biotinylated tyramine reagent was recently described in greater detail. 27 After precipitation of the biotinylated tyramine (10 minutes at room temperature) through the enzymatic action of HRP and H2O2 (0.1%), the biotin precipitate was detected with an additional application of the HRP-labeled avidin-biotin complex (Dako) for 30 minutes in a humid chamber. The peroxidase reaction was developed by 3,3′-diaminobenzidine (Sigma, Deisenhofen, Germany), leaving a brown end product. Negative controls were performed on consecutive sections by replacing the primary antibody with a nonimmune mouse serum.
To localize the ERβ subtype, we have tested two commercially available antibodies in routinely processed and frozen tissue sections. The 65-kd anti-rat estrogen receptor β (Upstate Biotechnology, Lake Placid, NY) and the 210–180-C050 antibody (Alexis Corporation, Nottingham, UK) were obtained by immunizing rabbits with synthetic peptides representing the N-terminal amino acids 46–63 and the C-terminal amino acid residues 467–485 of human ERβ, respectively. The specificity of these antibodies was tested by Western blot analysis. For microwave-based antigen retrieval, the deparaffinized slides were microwaved in Target Retrieval Solution (pH 6.1) (Dako) for 30 minutes according to the manufacturer’s instructions. Frozen sections did not require microwave pretreatment. After incubation of the slides with a normal swine serum (Dako), the ERβ antibodies were applied in a dilution of 1:100 overnight. Detection was achieved as described above, except that the secondary biotinylated rabbit anti-mouse immunglobulin was replaced by the biotinylated swine anti-rabbit antibody (Dako).
In Situ Hybridization Analysis
Twenty-four base cDNA oligonucleotide antisense and sense probes (antisense: 5′-CTC CAG CTC GTT CCC TTG GAT CTG-3′; sense: 5′-CAG ATC CAA GGG AAC GAG CTG GAG-3′) complementary to human ERα mRNA coding for amino acids 17–24 were synthesized from MWG-Biotech (Ebersberg, Germany). This antisense probe sequence is located within a 72-base region of ERα mRNA that shows no homology with glucocorticoid, mineralocorticoid, or progesterone receptors. 28,29
Using the BLAST sequence similarity search tool provided by the National Center for Biotechnology Information (Bethesda, MD), the published ERβ cDNA sequences 30,31 revealed no homology of the oligonucleotide probe used for the mRNA-ISH assay.
The oligonucleotides (antisense probe and sense probe for negative control) were biotin-16-2′-desoxyuridine-5-triphosphate (bio-16-dUTP) (Boehringer Mannheim, Mannheim, Germany) 3′-tailed with the DNA tailing kit (Boehringer Mannheim). The labeling procedure was performed according to the manufacturer’s instructions (Boehringer Mannheim). The efficiency of labeling reaction was checked on dot blot dilution series.
A standard nonradioactive in situ hybridization method was used as described elsewhere, 32 with slide modifications. In brief, 5-μm sections were deparaffinized in xylene (2 × 15 minutes) (Merck, Darmstadt, Germany), rehydrated through graded alcohol, and pretreated with 10 μg/ml proteinase K (30 minutes, 37°C) (Boehringer Mannheim). After they were washed twice in 2× standard saline citrate (20× SSC stock solution: 3 mol/L NaCl, 0.3 mol/L sodium citrate, pH 7.0) (Merck), the slides were covered with 40 μl of the prehybridization buffer containing 2× SSC, 1× Denhardt’s solution (50× Denhardt’s stock solution: 1% polyvinylchloride, 1% pyrrolidone, 2% bovine serum albumine) (Oncor, Heidelberg, Germany), 10% dextran sulfate (Boehringer Mannheim), 50 mmol/L phosphate buffer (pH 7.0) (Merck), 50 mmol/L 1,4-dithiothreitol (DTT) (Boehringer Mannheim), 250 μg/ml yeast tRNA (Boehringer Mannheim), 100 μg/ml polyadenylic acid (Boehringer Mannheim), 500 μg/ml denatured and sheared DNA from fish sperm (Boehringer Mannheim), and 26.7% deionized formamide (Oncor) and incubated for 2 hours at 37°C in a humid chamber. The sections were hybridized overnight at 37°C with 40 μl hybridization mixture containing the hybridization buffer and 10 pmol/L labeled oligonucleotide probe per slide.
After the slides were washed in graded concentrations of SSC (2×, 1×, 0.25×); slides were washed twice in each concentration for 30 minutes in a shaking water bath at 37°C). Detection was achieved by the ABC method with the tyramine amplification method described above, leaving a brown end product. The slides were counterstained with hematoxylin.
To prove the specificity of the hybridization process, the following negative controls were performed in each case. The slides were hybridized with the corresponding sense probe. The hybridization procedure was then performed by omitting the sense and antisense probes. In addition, slides pretreated with RNase (Boehringer Mannheim) were hybridized as described above.
Quantitative Analysis of Staining
The staining results obtained by IHC and ISH analysis were classified into six categories ranging from 0 to 5: 0: no staining detectable; 1: less than 5% positive tumor cells; 2: 5–25% positive tumor cells; 3: 25–50% positive tumor cells; 4: 50–75% positive tumor cells; 5: more than 75% positive tumor cells.
Differences in staining intensities obtained by IHC or ISH results were not considered in the scoring system.
HGPIN was evaluated by reporting the presence or absence of detectable ERα protein and mRNA expression in each lesion defined by one microscopic field at low magnification (×100).
Statistical Analysis
Contingence table and χ 2 analyses were used to study the relation between the ER-IHC score and the primary Gleason grade of primary adenocarcinomas, recurrent disease, and metastases. Statistical analyses were performed with SPSS software (SPSS ASC GmbH, Erkrath, Germany). P < 0.05 was regarded as statistically significant.
Results
Differential Expression of ERα and ERβ in Benign Ovarian and Prostate Tissue
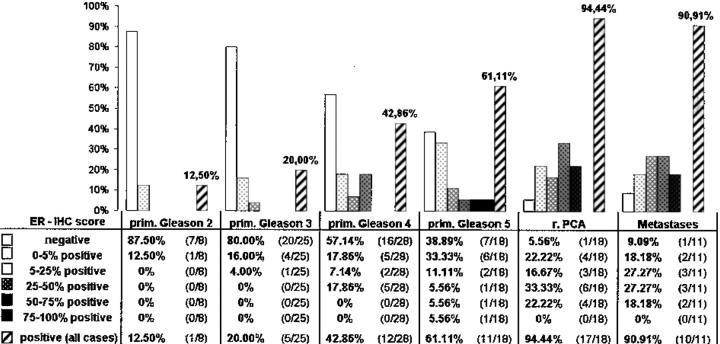
Among the three antibodies tested in the current study, only the NCL-ER-6F11 antibody directed against the classical ER yielded distinct nuclear immunolocalization in benign and malignant prostate tissue. Using the polyclonal 210-180-C050 and the 65-kd anti-rat ERβ antibody, we were unable to demonstrate any reliable nuclear staining in both routinely processed and frozen sections of prostate tissue. On the other hand, distinct nuclear ERβ positivities were identified in granulosa and theca cells, which served as positive controls (Figure 1a) ▶ . To test the specificity of the NCL-ER-6F11 antibody for ERα, immunohistochemical and ISH analyses were performed in ovarian tissue expressing ERβ. We failed to demonstrate immunoreactive granulosa and theca cells, although these cells revealed detectable levels of ERα mRNA. In benign prostate tissue, ERα mRNA and protein expression was restricted to stromal and basal cells (Figure 1b) ▶ but was undetectable in secretory luminal cells reported for the presence of the ERβ at high levels in rat tissue. 22,24,25
Figure 1.
Differential expression of ERα and β subtypes in ovarian and prostate tissue. Ovarian tissue reveals strong ERβ immunoreactivity in granulosa and theca cells, and subsets of endothelial cells (a). The same specimen was unreactive for the classical ER using the NCL-ER-6F11 antibody. In situ hybridization detects high steady-state levels of ERα mRNA in the basal cell layer of benign prostatic acini (b). Secretory luminal cell types lack ERα mRNA, whereas stromal cells exhibit weak hybridization signals (b). Immunohistochemical stainings performed on adjacent sections failed to demonstrate detectable levels of ERβ in epithelial and stromal compartments. Original magnification: a, ×400; b, ×100
ERα Expression in HGPIN and Prostate Cancer
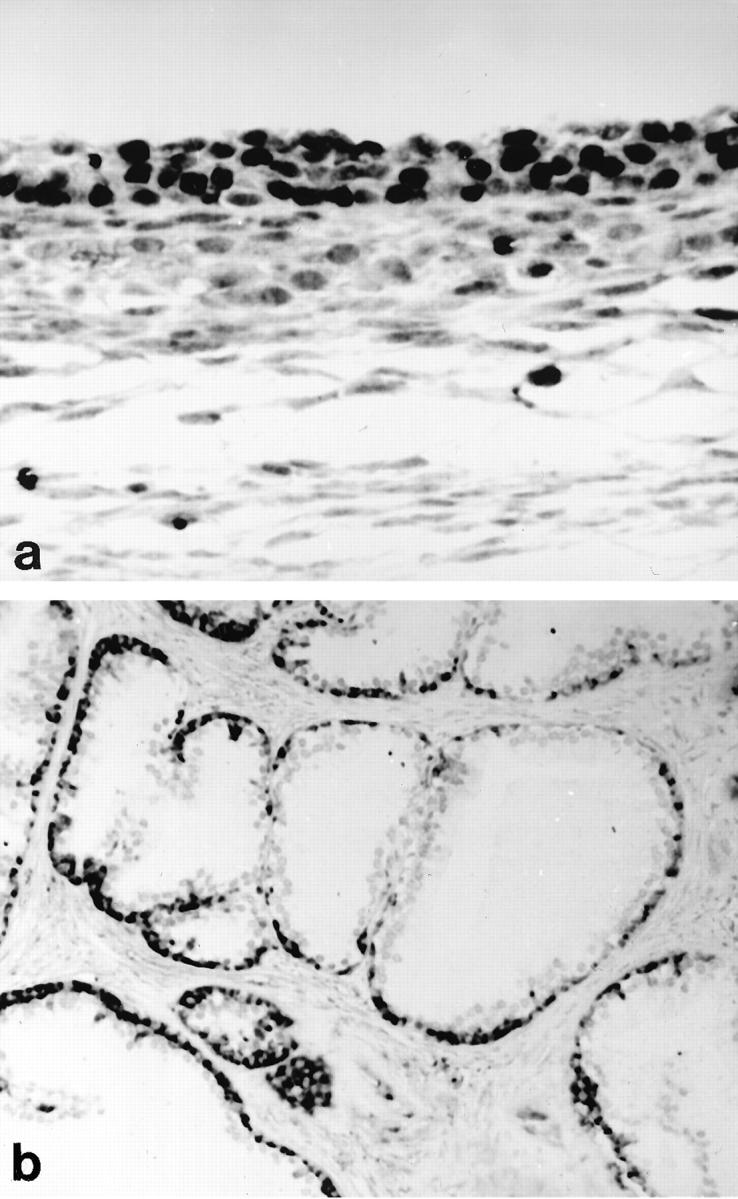
Using the NCL-ER-6F11 antibody and the staining protocol described above, we were able to demonstrate ERα gene expression in both stromal and epithelial compartments of HGPIN and prostatic adenocarcinoma (Figures 2–4) ▶ ▶ ▶ . The IHC and ISH results obtained in epithelial compartments are summarized in Tables 2 and 3 ▶ ▶ . ER protein expression was found in 11% of 176 HGPIN lesions evaluated (Figure 2a) ▶ . Forty-seven of 168 cases of HGPIN submitted for ISH analysis (28%) revealed detectable mRNA transcripts in the dysplastic epithelium (Figure 2b) ▶ . Low- to intermediate-grade prostatic adenocarcinoma (primary Gleason grades 2 and 3) showed focal ER positivities in up to 20% of cases (Table 2) ▶ . Gleason grade 4 and 5 carcinomas variably expressed the ER protein in 43% (respectively 61%) of cases (Figure 4a) ▶ . Five Gleason grade 4 and three Gleason grade 5 tumors were identified with significant staining (>25% immunoreactive tumor cells). Statistical analysis showed a significant positive correlation between the ER-IHC score and the primary Gleason grade (P < 0.001). Recurrent adenocarcinoma after hormonal therapy expressed the nuclear ER in 94% of cases (Table 2) ▶ . Significant staining (>25% immunoreactive tumor cells) was recorded in 10 of 18 cases (55.5%) of recurrent adenocarcinoma. Eight lymph node metastases and two bone metastases were ER positive (Figure 4b) ▶ . Significant staining (>25% positive tumor cells) was noted in five of 11 cases (45.5%) of metastatic lesions. Statistical analyses comparing high-grade (primary Gleason grades 4 and 5) adenocarcinoma with recurrent and metastatic tumors showed a significant positive correlation with the ER status (P < 0.001).
Figure 2.
High-grade prostatic intraepithelial neoplasia (HGPIN). a: Immunohistochemistry using the NCL-ER-6F11 antibody demonstrates moderate to strong nuclear ER expression in the dysplastic epithelium, subsets of stromal cells, and basal cells. b: High steady-state levels of ERα mRNA are found in a focus of HGPIN lacking detectable ER immunoreactivity. Adjacent nondysplastic ducts reveal ERα mRNA expression predominantly in basal cells (arrow). Original magnification: a, ×200; b, ×40
Figure 3.

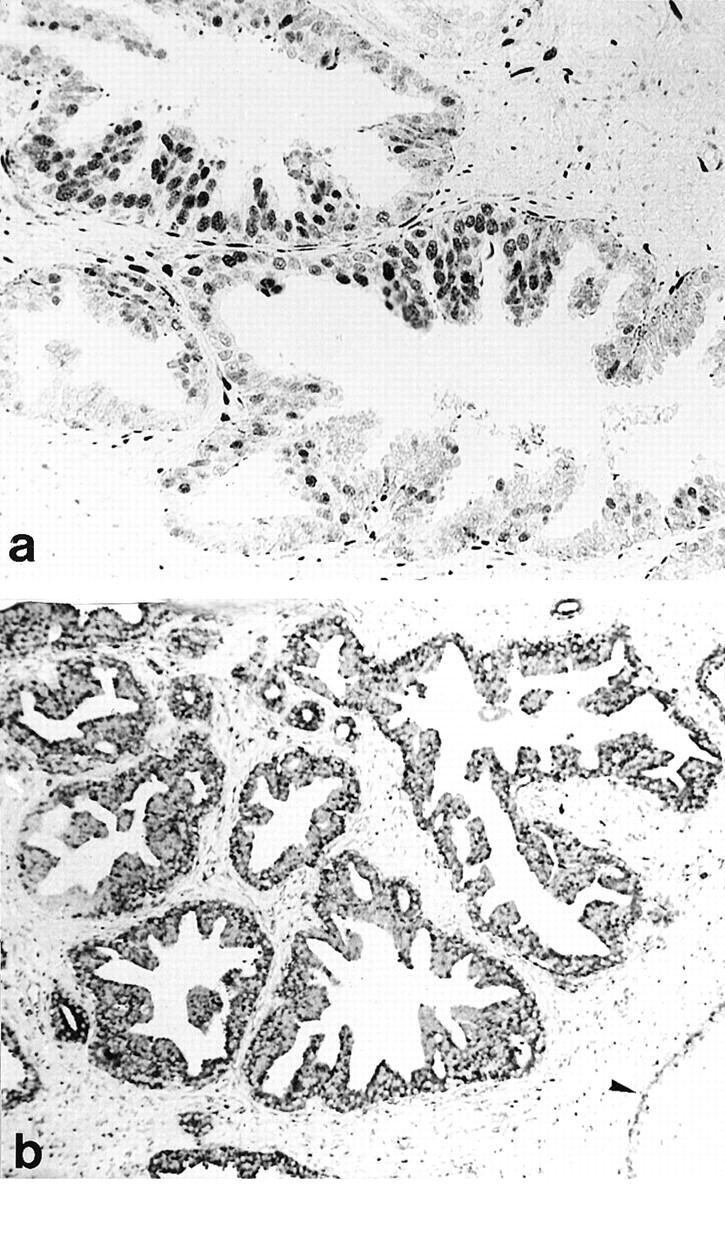
Intermediate-grade prostatic adenocarcinoma (primary Gleason grade 3). a: Intense immunoreactivity for the ER is found in numerous stromal cells, whereas neoplastic acini lack the ER protein. b: On adjacent sections, in situ hybridization detects high steady-state levels of ERα mRNA in the neoplastic lesion. Preexistent glands show hybridization signals predominantly in basal cells (arrow). Some stromal cells reveal low steady-state levels of ERα mRNA. Original magnification: a, ×100; b, ×120.
Figure 4.

High-grade (primary Gleason grade 4) adenocarcinoma (a) and lymph node metastases (b). Using the NCL-ER-6F11 antibody, strong and extensive nuclear ER protein expression is detected in both cancerous lesions. Original magnification: a, ×100; b, ×120.
Table 2.
Comparative Evaluation of the Classical ER in Primary Prostatic Adenocarcinoma (Primary Gleason Grades 2–5), Recurrent Carcinoma after Hormonal Therapy (r, PCA), and Distant Metastases
The ER immunohistochemical (IHC) score was evaluated by a grading system ranging from 0 to 5. ER protein expression progressively increases from primary Gleason grade 2 tumors to recurrent and metastatic lesions. Statistical analyses revealed a significant positive correlation between the ER-IHC score and the primary Gleason grade, and recurrent and metastatic disease (P < 0.001).
Table 3.
Comparative Evaluation of ERα mRNA Expression in Primary Prostatic Adenocarcinoma (Primary Gleason grades 2–5), Recurrent Carcinoma after Hormonal Therapy (r. PCA), and Distant Metastases Lacking ER Expression at the Protein Level
| ER-ISH score | prim. Gl. 2 | prim. Gl. 3 | prim. Gl. 4 | prim. Gl. 5 | r. PCA | Met. |
|---|---|---|---|---|---|---|
| Negative | 2 /4 | 6 /15 | 2 /15 | 2 /7 | 2 /6 | 0 /2 |
| 0–5% positive | 0 /4 | 0 /15 | 0 /15 | 0 /7 | 0 /6 | 0 /2 |
| 5–25% positive | 0 /4 | 0 /15 | 3 /15 | 0 /7 | 0 /6 | 1 /2 |
| 25–50% positive | 0 /4 | 1 /15 | 3 /15 | 1 /7 | 1 /6 | 0 /2 |
| 50–75% positive | 1 /4 | 1 /15 | 1 /15 | 0 /7 | 2 /6 | 1 /2 |
| 75–100% positive | 1 /4 | 7 /15 | 6 /15 | 4 /7 | 1 /6 | 0 /2 |
| Positive (all cases) | 2 /4 | 9 /15 | 13 /15 | 5 /7 | 4 /6 | 2 /2 |
Staining intensities obtained by ISH analysis (ER-ISH score) were evaluated by a grading system ranging from 0 to 5. Thirty-five of 49 cancerous lesions (71.5%) lacking ER positivities revealed detectable steady-state levels of ERα mRNA.
Cancerous lesions expressing the ER protein also revealed high steady-state levels of ERα mRNA. Table 3 ▶ summarizes the ISH results obtained in specimens lacking the ER protein in epithelial compartments. A significant number of ER-negative lesions (71.5% of 49 cases) revealed detectable steady-state levels of ERα mRNA (Figure 3) ▶ .
Discussion
Estrogens probably have a dual function in prostate cancer growth. Their therapeutic efficiency in hormone refractory disease is widely believed to be related to their suppressive effect on the pituitary release of gonadotrophins, reducing the testicular output of androgens. 12 Results of the current study suggest that estrogens can also affect neoplastic prostatic growth through a receptor-mediated process. To our knowledge, this is the first report documenting ER gene expression in human prostatic adenocarcinoma and its precursor lesions. The discrepancy with the results of previous studies 13-19 that failed to demonstrate ER immunoreactivity in prostate cancer cells may be related to antibody specificity, more effective antigen retrieval, and tissue processing. For example, we were unable to demonstrate the nuclear ERβ in human prostate tissue, although the antibodies used in the current study allowed reliable detection of the ERβ subtype in ovarian granulosa cells. These negative results contrast with recent data reporting the presence of the ERβ variant in the rat prostatic epithelium. 22,24,25 Irrespective of possible explanations, the relative distribution of the ERβ protein in human tissues is currently unknown. More effective antibodies are probably required to address this issue. Among the antibodies tested in the present study, only the NCL-ER-6F11 antibody was suitable for detection of the nuclear ER in prostatic adenocarcinoma and its precursor lesions. This commercially available antibody is directed against the whole protein of the classical ER (ERα) and has a wide application in routine practice in the assessment of the ER status in breast and endometrial neoplasias. 26 There is currently no convincing evidence that this antibody cross-reacts with ERβ. For example, granulosa and theca cells expressing ERβ were unreactive with NCL-ER-6F11. Furthermore, the differential expression of ERα mRNA and protein obtained in human prostate tissue differed significantly from the immunolocalization of ERβ reported in the rat prostate gland. 22,24,25
HGPIN is considered to be the most likely precursor of intermediate- and high-grade prostatic adenocarcinoma. 33,34 In our series, approximately 11% of these precursor lesions expressed the classical ER in the dysplastic epithelium. In addition, ISH analysis revealed ERα transcripts in 28% of HGPIN investigated. No correlation was found between the ER status in HGPIN and that of invasive cancer present in the same tissue section. The presence of ERα clearly indicates that subsets of HGPIN are estrogen-responsive through a receptor-mediated process. Interestingly, a recent study has identified the estrogen-inducible PS-2 protein in a significant number of nonneoplastic and dysplastic prostate tissue from patients with locally advanced prostate cancer, but not in prostate tissue from patients without evidence of malignant disease. 35 These data, along with evidence from experimental and epidemiological studies, 1-11 suggest that estrogens can affect early phases of prostatic carcinogenesis through an ER-mediated pathway. This warrants further investigations of surrogate markers of estrogen action and estrogen-regulated genes to define their role in the malignant transformation of the prostatic epithelium.
Results of the current study clearly demonstrate the presence of the nuclear ER in stromal and epithelial compartments of common prostatic adenocarcinoma. In primary tumors, the extent of detectable ER in epithelial compartments correlated significantly with the primary Gleason grade. Low- to intermediate-grade adenocarcinoma expressed the ER protein in a minority of cases. On the other hand, high-grade (primary Gleason grades 4 and 5) tumors revealed at least focal ER positivities in 43% (respectively 61%) of cases. The most consistent and extensive ERα mRNA and protein expression observed in the current study was detected in recurring carcinomas after hormonal therapy and in metastatic lesions, including lymph node and bone metastases. In particular, hormone refractory and metastatic lesions showed significant ER protein expression (>25% immunoreactive tumor cells) in 55.5% and 45.5% of cases evaluated. It is noteworthy that the levels of ERα detected by ISH were significantly higher than that obtained by IHC. These apparent discrepancies in ER protein and mRNA expression are difficult to interpret. It is possible that receptor saturation by ligand binding and subsequent degradation of the ER-ligand complex accounts for decreased detection of the ER protein by IHC. Whether more powerful IHC techniques can increase the detection rate of nuclear ERα in prostatic adenocarcinoma remains to be established.
In summary, the preliminary data presented here suggest that the progressive emergence of the classical ER in common prostatic malignancies runs parallel to the process of dedifferentiation, metastasis, and androgen insensitivity. This observation may have clinical implications, inasmuch as androgen ablation therapy increases the estrogen/androgen ratio in cancerous tissue. Tumor cells expressing the ER protein are potentially estrogen-responsive and may survive in an androgen-deprived milieu by using estrogens for their continuous maintenance and growth. Much more work is needed to define the molecular bases of ER gene expression and its downstream effector pathways during prostatic carcinogenesis and progression to androgen-insensitive disease. Progress in this field may lead to novel therapeutic strategies interfering with the ER-mediated process. The occurrence of the classical ER in a substantial number of androgen-insensitive and metastatic carcinomas raises the question of whether these tumors should be treated with antiestrogens when the ER protein is detectable in cancerous tissue.
Footnotes
Address reprint requests to Prof. H. Bonkhoff, Institute of Pathology, University of the Saarland, 66421 Homburg/Saar, Germany. E-mail: pahbon@med-rz.uni-sb.de.
Supported by Deutsche Forschungsgemeinschaft grant Bo 1018/2-2.
References
- 1.Santti R, Newbold RR, Makela S, Pylkkänen L, McLachlan JA: Developmental estrogenization and prostatic neoplasia. Prostate 1994, 24:67-78 [DOI] [PubMed] [Google Scholar]
- 2.Pylkkänen L, Mäkelä S, Santti R: Animal models for the preneoplastic lesions of the prostate. Eur Urol 1996, 30:243-248 [DOI] [PubMed] [Google Scholar]
- 3.Flanders WD: Review: prostate cancer epidemiology. Prostate 1984, 5:621-629 [DOI] [PubMed] [Google Scholar]
- 4.Carter HB, Coffey DS: The prostate: an increasing medical problem. Prostate 1990, 16:39-48 [DOI] [PubMed] [Google Scholar]
- 5.Nomura AM, Kolonel LN: Prostate cancer: a current perspective. Epidemiol Rev 1991, 13:200-227 [DOI] [PubMed] [Google Scholar]
- 6.Carter BS, Carter HB, Isaacs JT: Epidemiologic evidence regarding predisposing factors to prostate cancer. Prostate 1990, 16:187-197 [DOI] [PubMed] [Google Scholar]
- 7.Graham S, Haughey B, Marshall J, Priore R, Byers T, Rzepka T, Mettlin C, Pontes JE: Diet in the epidemiology of carcinoma of the prostate gland. J Natl Cancer Inst 1983, 70:687-692 [PubMed] [Google Scholar]
- 8.Snowdon DA, Phillips RL, Choi W: Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol 1984, 120:244-250 [DOI] [PubMed] [Google Scholar]
- 9.Drago JR: The induction of NB rat prostatic carcinomas. Anticancer Res 1984, 4:255-256 [PubMed] [Google Scholar]
- 10.Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D: Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst 1988, 80:1045-1053 [DOI] [PubMed] [Google Scholar]
- 11.Leav I, Merk FB, Kwan PW, Ho SM: Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate 1989, 15:23-40 [DOI] [PubMed] [Google Scholar]
- 12.Sandberg AA: Endocrine control and physiology of the prostate. Prostate 1980, 1:169-184 [DOI] [PubMed] [Google Scholar]
- 13.Wernert N, Gerdes J, Loy V, Seitz G, Scherr O, Dhom G: Investigations of the estrogen (ER-ICA-test) and the progesterone receptor in the prostate and prostatic carcinoma on immunohistochemical basis. Virchows Arch A Pathol Anat Histopathol 1988, 412:387-391 [DOI] [PubMed] [Google Scholar]
- 14.Schulze H, Claus S: Histological localization of estrogen receptors in normal and diseased human prostates by immunocytochemistry. Prostate 1990, 16:331-343 [DOI] [PubMed] [Google Scholar]
- 15.Brolin J, Skoog L, Ekman P: Immunohistochemistry and biochemistry in detection of androgen, progesterone, and estrogen receptors in benign and malignant human prostatic tissue. Prostate 1992, 20:281-295 [DOI] [PubMed] [Google Scholar]
- 16.Ehara H, Koji T, Deguchi T, Yoshii A, Nakano M, Nakane PK, Kawada Y: Expression of estrogen receptor in diseased human prostate assessed by non-radioactive in situ hybridization and immunohistochemistry. Prostate 1995, 27:304-313 [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu M, Maehara I, Orikasa S, Sasano H: Immunolocalization of oestrogen and progesterone receptors in prostatic hyperplasia and carcinoma. Histopathology 1996, 28:163-168 [DOI] [PubMed] [Google Scholar]
- 18.Kruithof-Dekker IG, Tetu B, Janssen PJA, van der Kwast TH: Elevated estrogen receptor expression in human prostatic stromal cells by androgen ablation therapy. J Urol 1996, 156:1194-1197 [PubMed] [Google Scholar]
- 19.Hobisch A, Hittmair A, Daxenbichler G, Wille S, Radmayr C, Hobisch-Hagen P, Bartsch G, Klocker H, Culig Z: Metastatic lesions from prostate cancer do not express oestrogen and progesterone receptors. J Pathol 1997, 182:356-361 [DOI] [PubMed] [Google Scholar]
- 20.Castagnetta LA, Miceli MD, Sorci CM, Pfeffer U, Farruggio R, Oliveri G, Calabro M, Carruba G: Growth of LNCaP human prostate cancer cells is stimulated by estradiol via its own receptor. Endocrinology 1995, 163:2309-2319 [DOI] [PubMed] [Google Scholar]
- 21.Carruba G, Pfeffer U, Fecarotta E, Coviello DA, D’Amato E, Lo Castro M, Vidali G, Castagnetta L: Estradiol inhibits growth of hormone non-responsive PC3 human prostate cancer cells. Cancer Res 1994, 54:1190-1193 [PubMed] [Google Scholar]
- 22.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA: Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 1996, 93:5925-5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA: Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 1997, 138:863-870 [DOI] [PubMed] [Google Scholar]
- 24.Kuiper GG, Gustafsson JA: The novel estrogen receptor-β subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett 1997, 23:87-90 [DOI] [PubMed] [Google Scholar]
- 25.Saunders PT, Maguire SM, Gaughan J, Millar MR: Expression of oestrogen receptor β (ER β) in multiple rat tissues visualised by immunohistochemistry. J Endocrinol 1997, 154:R13-R16 [DOI] [PubMed] [Google Scholar]
- 26.Bevitt DJ, Milton ID, Piggot N, Henry L, Carter MJ, Toms GL, Lennard TW, Westley B, Angus B, Horne CH: New monoclonal antibodies to oestrogen and progesterone receptors effective for paraffin section immunohistochemistry. J Pathol 1997, 183:228-232 [DOI] [PubMed] [Google Scholar]
- 27.Kerstens HMJ, Poddighe PJ, Hanselaar AGJM: A novel in situ hybridization signal amplification method based on the deposition on biotinylated tyramine. J Histochem Cytochem 1995, 43:347-352 [DOI] [PubMed] [Google Scholar]
- 28.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P: Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 1986, 13:134-139 [DOI] [PubMed] [Google Scholar]
- 29.Graham DM, Jin L, Lloyd RV: Detection of estrogen receptor in paraffin-embedded sections of breast carcinoma by immunohistochemistry and in situ hybridization. Am J Surg Pathol 1991, 15:475-485 [DOI] [PubMed] [Google Scholar]
- 30.Mosselman S, Polman J, Dijkema R: ER β: identification and characterization of a novel human estrogen receptor. FEBS Lett 1996, 19:49-53 [DOI] [PubMed] [Google Scholar]
- 31.Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M: The complete primary structure of human estrogen receptor β (hER β) and its heterodimerization with ER α in vivo and in vitro. Biochem Biophys Res Commun 1998, 243:122-126 [DOI] [PubMed] [Google Scholar]
- 32.Hankin CR, Lloyd RV: Detection of messenger RNA in routinely processed tissue sections with biotinylated oligonucleotide probes. Am J Clin Pathol 1989, 92:166-171 [DOI] [PubMed] [Google Scholar]
- 33.Bostwick DG: Prospective origins of prostate carcinoma. Prostatic intraepithelial neoplasia and atypical adenomatous hyperplasia. Cancer 1996, 78:330-336 [DOI] [PubMed] [Google Scholar]
- 34.Montironi R, Bostwick D, Bonkhoff H, Cockett A, Helpap B, Troncoso P, Waters D: Origins of prostate cancer. Cancer 1996, 78:362-365 [DOI] [PubMed] [Google Scholar]
- 35.Bonkhoff H, Stein U, Welter C, Remberger K: Differential expression of the pS2 protein in the human prostate and prostate cancer. Association with premalignant changes and neuroendocrine differentiation. Hum Pathol 1995, 26:824-828 [DOI] [PubMed] [Google Scholar]