Abstract
The transmembrane tyrosine kinase receptor c-met with its ligand, hepatocyte growth factor/scatter factor (HGF/SF), acts as a mitogen, motogen, and morphogen in many normal epithelia. HGF/SF-met signaling has also been implicated in neoplastic progression and metastasis. In this study, immunofluorescence staining and quantitative laser scanning confocal microscopy were used to measure c-met expression in ovarian surface epithelial tumors from 17 oophorectomy specimens. These specimens were from patients aged 25 to 81 (mean age, 52) and included 10 malignant tumors, 4 borderline tumors, and five benign tumors including a Brenner tumor. For comparison, c-met expression was measured in normal tissues from the same patients, including 4 ovarian surface epithelia, 4 fallopian tube epithelia, 2 endometria, and 3 endocervical epithelia, as well as 3 cases of endometriosis. Relative pixel intensity values of c-met expression ranged from 0.4 in a normal ovarian surface epithelium to 22.3 in a borderline serous tumor. Malignant tumors (mean, 9.6) and borderline tumors (mean, 12.9) had higher average c-met expression levels than normal tissues (mean, 3.6) and endometriosis (mean, 1.8). The expression levels of benign tumors were intermediate (mean, 7.9). Among the normal tissues, c-met expression in fallopian tubes (mean, 8.2; range, 3.4–12.9) was higher than that of the other normal epithelia (mean, 1.6; range, 0.4–4.3). In eight cases where both normal and malignant tissues were sampled, c-met expression was significantly greater in malignant than in normal epithelia (P = 0.01). These findings indicate that c-met plays a role in the biology of the normal tissues examined. They confirm that its expression increases in the malignant progression of ovarian surface epithelial tumors, and suggest that increases comparable to those in frankly malignant carcinomas have already been reached in borderline lesions, ie, early in the neoplastic process.
The transmembrane tyrosine kinase c-met is the receptor for hepatocyte growth factor/scatter factor (HGF/SF). c-met activation by its ligand leads to a variety of cellular effects, including the induction of proliferation, morphogenesis, and motogenesis, under both normal and pathological conditions. c-met is expressed predominantly by epithelial tissues, whereas HGF/SF is characteristically produced by stromal cells and serves as an important mediator of epithelio/mesenchymal interactions. 1 In addition to such paracrine mechanisms, autocrine and endocrine regulation by HGF/SF-met has also been described. 2 HGF/SF-met play a role in the development of tumors, their progression to malignancy, and the onset of invasion by stimulating proliferation, scattering, and invasiveness. 2-6 Because c-met expression has been reported to be elevated in several types of malignancies, including ovarian carcinomas, 7-8 the present study was undertaken as a quantitative comparison between c-met expression in epithelial ovarian neoplasms and that in normal epithelia of the female reproductive system to which this group of tumors is related either by origin or by their characteristic ectopic differentiation along the lines of Mullerian duct-derived epithelia.
The epithelial ovarian carcinomas are the prime cause of death from gynecological cancers among North American women. The pathogenesis of these tumors is not well understood, and the patients’ overall prognosis is very poor. In addition to their clinical importance, the epithelial ovarian carcinomas exhibit two characteristics that make them particularly interesting for investigations of HGF/SF-met action.
First, differentiated ovarian adenocarcinomas frequently form papillae and gland-like structures, whereas the ovarian surface epithelium (OSE), which gives rise to these carcinomas, is a simple mesothelium that lacks such complex features. The OSE is derived from the coelomic epithelium (mesothelium) of the embryonic gonad. This epithelium is contiguous with that which penetrates the adjacent mesenchyme to form the Mullerian ducts. This histogenetic proximity is reflected in the lines of ectopic differentiation seen in ovarian surface epithelial tumors: characteristics of fallopian tube epithelium are seen in serous neoplasms, those of endometrial epithelium in endometrioid tumors, and endocervix-like epithelium in mucinous tumors. The capacity of adult human OSE to undergo these varied forms of Mullerian differentiation reflects its uncommitted pluripotent developmental state and accounts for the great phenotypic diversity and complexity found among ovarian carcinomas. The distribution of c-met in the human ovary follows the characteristic pattern where the ligand is presumably produced by the stromal components while c-met is expressed by the surface epithelium and the surface epithelial inclusion cysts, ie, the sites where ovarian carcinomas are thought to arise. 7-9 Because HGF/SF-met can induce mesenchymal-epithelial conversion under normal 1 and experimental 10 conditions and contributes to gland morphogenesis and branching, 11-12 this ligand/receptor combination might also influence the glandular differentiation that accompanies ovarian epithelial neoplastic progression.
Secondly, among the serous papillary adenocarcinomas, which are the most common form of epithelial ovarian cancers, the predominant means of spread beyond the ovary is not by direct invasion of adjacent tissues but by the detachment of clusters of cancer cells from the primary tumor on the ovarian surface, and their seeding of the peritoneum. Therefore, the stimulation by HGF/SF-met of basement membrane degradation and cell migration in the invasive process, which appears to be highly significant in other types of malignancies, 5 may be more important in the less common endometrioid and mucinous ovarian carcinomas, whereas mechanisms that interfere with adhesion between carcinoma cells might be more significant in serous ovarian carcinomas. Ovarian cancers utilize N- and E-cadherin for intercellular adhesion, 13-15 and HGF/SF has the capacity to interfere with this function. 16 c-met signaling may contribute to the detachment process and dissemination of serous ovarian carcinoma cells by similar means.
In spite of these interesting features, information about c-met expression in ovarian cancers is limited. Overexpression of c-met was demonstrated by Western blot analysis in 28% of 67 ovarian carcinomas but not in benign tumors. 7 In this study, the overexpression of c-met correlated positively with premenopausal status but not with clinical stages, was highest in differentiated carcinomas, but was not associated with met-gene amplification or with specific histological subtypes. In contrast, Moghul et al 8 did report a correlation with histological subtypes as immunohistochemistry revealed more intense c-met staining in 5 out of 5 cases of ovarian clear cell carcinoma than in serous carcinomas. In the present study, we placed particular emphasis on the precise selection of defined areas of normal ovarian and Mullerian epithelia and of ovarian tumors, wherever possible from the same specimens, to better define the relationships of c-met expression to neoplastic progression and differentiation in ovarian carcinogenesis.
Materials and Methods
Formalin-fixed, paraffin-embedded tissue from 17 oophorectomy cases was obtained from the archives of the Vancouver General Hospital. The cases include 4 benign tumors, 4 borderline tumors, and 10 primary ovarian carcinomas. Normal ovarian surface epithelium was present in 4 cases. In the other cases, tissue blocks containing fallopian tube epithelium were analyzed in 5 cases, endometrium in 2 cases, and endocervix in 3 cases as representative of normal epithelia of Mullerian origin. In addition, endometriosis occurred in 3 of the above cases. Six 4-μm sections were cut from each tissue block. The first and last were stained with haematoxylin and eosin and the remaining four were processed for quantitative immunofluorescence microscopy. C28 anti-Met antibody from Santa Cruz Biotechnology (Santa Cruz, CA) was used to label the Met. 10,17 Labeled cells were examined using a Zeiss 310 laser scanning confocal microscope (CLSM) having the following configuration: LSM 310:25 mW Argon external and internal HeNe lasers generating excitation lines for 488-, 514-, and 543-nm wave lengths. Photographs were made using a Codonics (Middleburg Heights, OH) NP 1600 dye sublimation printer. Tumors and adjacent tissues were evaluated visually using Nomarski optics to identify the histopathology. Areas were then imaged using the appropriate laser line for met excitation (488). Images were stored on an optical disk and color coded for image (pixel) intensity values. Overlay images were created by superimposing the fluorescent (488) image onto the Nomarski image. Analysis of each sample was made with the same laser intensity, brightness, power and scan rate. Parameter files of the microscope settings were stored in parameter files and used in the analysis. Laser fluctuations were eliminated by evaluating for day to day variation with fluorescent beads that standardize the laser beam (Molecular Probes, Eugene, OR). The relative amounts of fluorescent intensity were then measured qualitatively using the look-up table glow scale and quantitatively using the Optimas 5.0 image analysis program (Bothell, WA). Measurements were done as described in Klineberg et al. 17 In brief, the histology of the samples was evaluated and a cursor drawing delineating the region of interest was made and called a mask. That region/mask was then applied to the corresponding fluorescent image and the fluorescence intensity of that area was measured. Wherever possible, more than one area per tissue was sampled. Cases were then ranked and scored using only viable, well-preserved, and appropriate epithelia, either normal or neoplastic.
Statistical analysis of the results from cases in which both normal and malignant epithelia were analyzed was performed using the Student’s t-test and the signed rank test.
Results
The ages, tissues examined, and relative levels of c-met expression of the 17 patients in this study are listed in Table 1 ▶ . The age of the patients ranged from 25 to 81 years, and the mean age and standard deviation (SD) of the patients was 52 ± 15.1 years. Measurements of multiple areas within the same specimens showed that heterogeneity in c-met expression was particularly great within malignant lesions. The 13 normal epithelia had a mean ± SD c-met level of 3.6 ± 3.9 (range, 0.38–12.9). Fallopian tube epithelium exhibited markedly more intense c-met staining (8.2 ± 3.9, range, 3.35–12.9) than the other normal epithelia (1.6 ± 1.2, range, 0.38–4.25) suggesting that, perhaps, the physiological role of HGF differs between the fallopian tube epithelium and other epithelia of the female genital tract. In 3 cases, foci of endometriosis had c-met levels resembling those of normal epithelia (mean 1.8, range, 1.08–2.6).
Table 1.
Relative Levels of c-met Expression in Normal Epithelia of the Female Reproductive Tract, Benign and Borderline Ovarian Epithelial Tumors, and Ovarian Epithelial Carcinomas
| Case No. | Age | Epithelium | c-met intensity2 | |
|---|---|---|---|---|
| Values/area | Mean* | |||
| 188 | 25 | Serous adenoma | 5.4, 7.2, 27.8 | 13.47 |
| Mucinous adenoma | 4.4, 4.6 | 4.5 | ||
| 528 | 27 | Serous adenoma | 6.3, 9.7 | 8.0 |
| 980 | 34 | Normal OSE | 1.9, 2.0 | 1.95 |
| Mucinous borderline tumor | 2.8, 26.7 | 14.75 | ||
| 816 | 39 | Normal fallopian tube | 5.8, 10.0, 11.0 | 8.9 |
| Serous papillary borderline tumor | 8.8, 13.2 | 11.0 | ||
| 787 | 44 | Normal endocervix | 1.8, 6.7 | 4.25 |
| Mucinous adenoma | 1.0, 2.2 | 1.60 | ||
| 558 | 46 | Normal fallopian tube | 12.9 | 12.90 |
| Serous papillary borderline tumor | 16.6, 28.0 | 22.30 | ||
| 670 | 49 | Normal endocervix | 0.58, 1.0 | 0.79 |
| Clear cell carcinoma | 1.4, 1.5, 3.6 | 2.17 | ||
| 209 | 50 | Normal endocervix | 1.7, 3.2 | 2.45 |
| Mucinous adenocarcinoma | 7.6, 34.0 | 20.8 | ||
| 160 | 52 | Normal OSE | 0.56 | 0.56 |
| Endometriosis | 2.6 | 2.60 | ||
| Mixed borderline tumor | 2.8, 4.2 | 3.50 | ||
| Clear cell carcinoma | 8.4 | 8.4 | ||
| 961 | 59 | Mucinous adenocarcinoma | 2.0, 18.0, 24.8 | 14.93 |
| 534 | 59 | Normal fallopian tube | 3.0, 3.7 | 3.35 |
| Clear cell carcinoma | 9.5, 4.5 | 7.0 | ||
| 426 | 60 | Endometrium, early prolif. | 0.88, 1.3 | 1.09 |
| Endometriosis | 1.8 | 1.80 | ||
| Endometrioid carcinoma | 5.8, 2.4, 2.8, 3.2, 7.0 | 4.24 | ||
| 188 | 61 | Normal OSE | 1.85 | 1.85 |
| 060 | 64 | Serous papillary carcinoma | 6.1, 7.5, 15.2 | 9.60 |
| 325 | 65 | Normal fallopian tube | 7.7 | 7.70 |
| Serous papillary carcinoma | 29.4, 6.1, 18.4 | 17.97 | ||
| 991 | 69 | Normal OSE | 0.38 | 0.38 |
| Brenner tumor | 3.9, 19.9 | 11.90 | ||
| Serous papillary carcinoma | 4.7 | 4.7 | ||
| 827 | 81 | Atrophic endometrium | 0.56, 1.1 | 0.83 |
| Endometriosis | 0.73, 1.0, 1.5 | 1.08 | ||
| Endometrioid carcinoma | 2.7, 5.1, 6.1, 12.3 | 6.55 |
*Values listed as means represent the average in cases for which more than one area within the same specimen was analyzed.
Square value of c-met staining intensity.
Five benign tumors were analyzed. Among these, the Brenner tumor had a mean level of c-met protein of 11.90. Mean c-met levels in the two serous adenomas were 8.0 and 13.47, whereas the two mucinous adenomas had lower levels of 1.6 and 4.5. Among four borderline ovarian tumors, two serous papillary borderline tumors had mean c-met levels of 11.0 and 22.3. The third was of the mucinous subtype with a mean level of 14.75, while the fourth was a mixed tumor with a mean level of 3.5. The ten malignant tumors had a mean c-met level of 9.6 ± 6.2 (range, 2.17–20.80), which was in the same range as the borderline tumors. As shown in Table 1 ▶ , c-met expression increased with neoplastic progression in all cases where more than one stage of progression was examined within one biopsy specimen. In the 8 cases in which both normal and malignant epithelium were studied, c-met levels in the malignant specimens were significantly higher by both parametric analysis with the t-test (P < 0.009) and nonparametric analysis with the signed rank test (P < 0.008). Representative illustrations of sections used for measurements are shown in Figure 1 ▶ . They demonstrate the characteristic differences between normal epithelia such as the relatively weak staining of endometrium compared to fallopian tube epithelium, as well as the increases in c-met expression in neoplastic lesions. As expected, the staining was most intense along the plasma membranes, except for Figure 1D ▶ where the whole cytoplasm of the cells was strongly fluorescent. This apparent expansion of c-met staining into cytoplasmic regions likely reflects amplification of the stained regions due to the tissue processing: we used a polyclonal rabbit antibody to the c-terminal (cytoplasmic) region of c-met and amplified this antibody by the second antibody. Furthermore, the use of paraffin-cut sections enhances penetration of the antibodies into the cells and results in staining of tangentially cut membranes. Previous electron microscopic examination of whole cells has shown that the antibody to c-met, used in the present study, localizes clearly to the plasma membrane (data not shown). In Figure 2 ▶ , the relative fluorescence values of all cases are shown in a scatter histogram. Where multiple measurements were taken of the same specimen (see Table 1 ▶ ). Figure 2 ▶ shows the mean value. Even though this series is small, a definite trend for c-met expression to increase with neoplastic progression is apparent. Among the malignant tumors, the serous and mucinous carcinomas had higher c-met values than the clear cell and endometrioid carcinomas.
Figure 1.
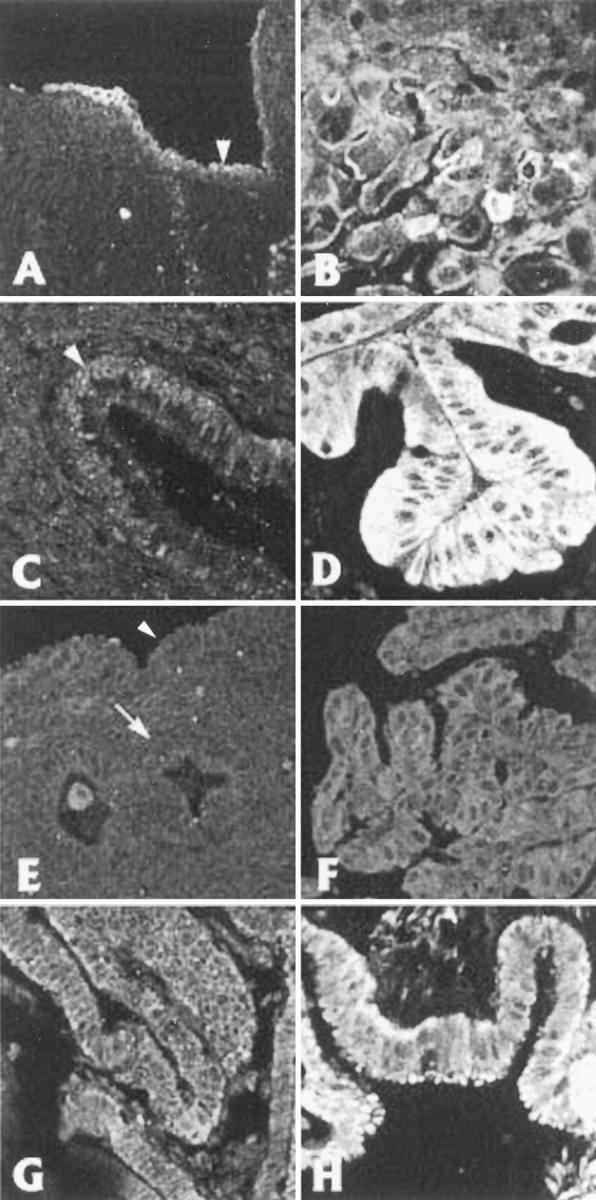
Representative areas used for measurements. Each pair of figures (A and B, C and D, E and F, and G and H) is taken from normal and abnormal areas within the same specimen, respectively. The numbers in parentheses refer to the case numbers in Table 1 ▶ . Figures D, F, and H were chosen to compare neoplastic lesions to normal epithelia with similar types of differentiation. A, normal ovarian surface epithelium (#980) (note intercellular variation in c-met expression). B, mucinous borderline tumor (#980). C, arrowhead: normal endocervical epithelium (#209). D, mucinous adenocarcinoma (#209). E, normal endometrium (#827) on the endometrial surface (arrowhead) and lining a gland (arrow). F, endometrioid carcinoma (#827). G, normal fallopian tube (#816). H, serous papillary borderline tumor (#816). Magnification, ×100.
Figure 2.
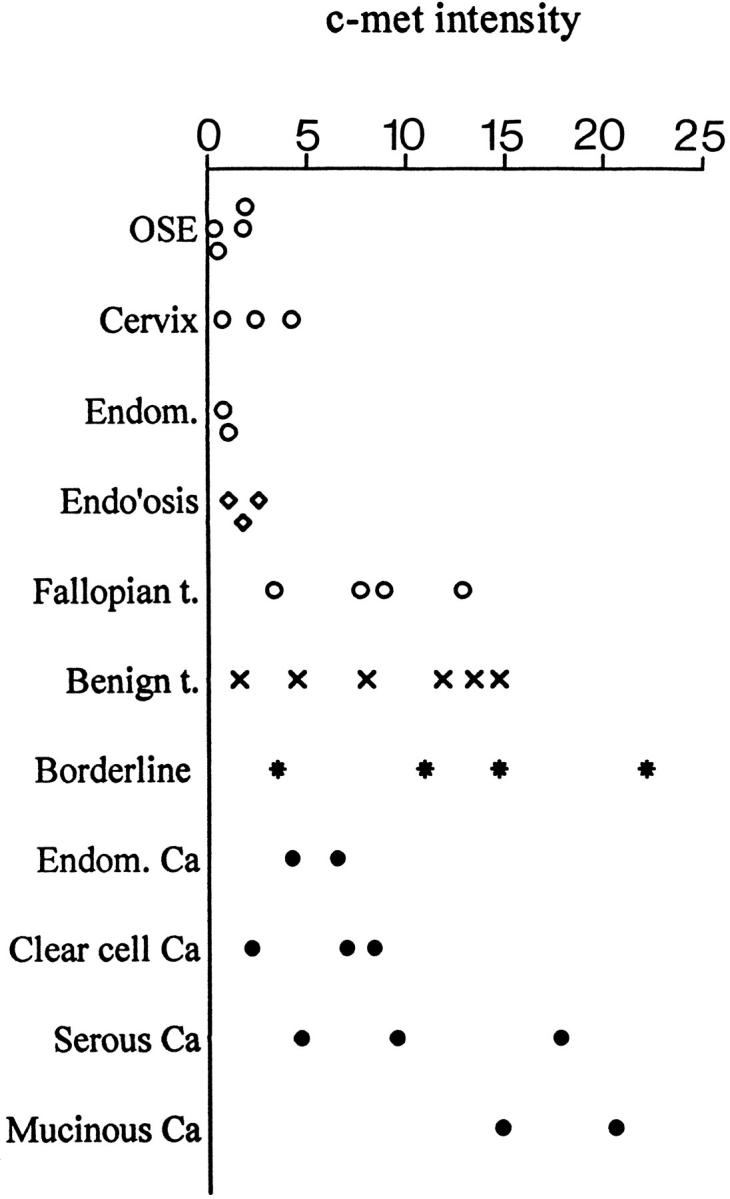
Scatter histogram of pixel intensity values for c-met fluorescence of areas analyzed versus histopathological diagnoses. Benign t., benign tumors; Ca, carcinoma; Endom, endometrium; Endo’osis, endometriosis; Fallopian t., fallopian tube; OSE, ovarian surface epithelium.
Discussion
The results of the present study confirm by quantitative laser scanning confocal microscopy other indications based on histochemistry 8 and Western blot densitometry 7 that c-met expression is increased in ovarian epithelial carcinomas compared to their tissue of origin, the ovarian surface epithelium (OSE). The c-met values of all benign, borderline, and malignant tumors exceeded the highest value among normal OSE specimens. The results also demonstrate considerable heterogeneity in c-met expression within individual tumors, similar to the heterogeneity in breast carcinomas, 4 and suggests that c-met expression may be influenced by genetic instability and microenvironmental factors. Finally, our paper presents the first quantitative comparison of c-met expression between epithelial ovarian carcinomas and the normal epithelia of the oviduct, endometrium, and endocervix, which epithelial ovarian cancers resemble as a result of the ectopic differentiation that takes place during neoplastic progression in these tumors.
The number of cases in our study is small, so that few statistical conclusions can be drawn. Within these limits, some of the trends that were observed in previous studies were not confirmed. Thus, in contrast to the study by Moghul, 8 our clear cell carcinomas expressed less c-met than serous carcinomas and, in contrast to a large series of tumors in Di Renzo’s study 7 but in keeping with Moghul et al, the high levels of c-met expression in serous and mucinous carcinomas compared to clear cell and endometrioid carcinomas in our series did suggest a correlation with histological variants. Furthermore, in our study, all borderline and frank carcinomas expressed increased c-met levels compared to the OSE specimens, whereas in the study by DiRenzo et al, c-met expression was increased in only 28% of carcinomas. This difference may be due to the difference in measurement techniques between the two studies.
The variation in c-met levels that we observed among the normal epithelia suggests that HGF/SF-met may influence their differentiation and functions and perhaps also those of the OSE-derived tumors. c-met was detected in normal OSE, supporting previous observations 7-9 that found this to be the only c-met-expressing cell type in the ovary. There was intercellular variation in c-met expression between and within OSE samples in our study, reminiscent of similar fluctuations in the expression of other proteins, such as E-cadherin and CA125. 13,18 The role of c-met in OSE physiology remains to be determined. It is interesting in this regard that OSE secretes interleukin-1, 19 a cytokine that regulates c-met levels in ovarian cancer lines 8 as well as HGF production in stromal tissues. 1 This relationship suggests a possible route for autocrine/paracrine regulation of HGF/SF-met functions in normal and neoplastic OSE. Among the other normal epithelia, the oviductal epithelium expressed the highest values of c-met, suggesting that HGF/SF-met may play a different role in fallopian tube physiology than in the other epithelia studied here.
Ovarian epithelial tumors tend to differentiate along the lines of Mullerian duct-derived epithelia and, therefore, changes in c-met expression could be related to such differentiation. In the present study, the c-met expression of mucinous borderline tumors and mucinous carcinomas exceeded those of normal endocervix, and c-met expression of the endometrioid carcinomas was higher than that of normal endometrium. The c-met levels in serous tumors overlapped with those of normal fallopian tube, but this epithelium was found to express particularly high levels of c-met. Thus, in none of the histological subtypes could differentiation account for the increases in c-met that accompanied the ectopic differentiation of neoplastic OSE. However, although the number of specimens is to small for conclusions, the high c-met expression in serous adenomas compared to mucinous adenomas suggests a possible relationship of c-met levels to differentiation pathways. Compared to normal epithelia, benign OSE-derived tumors expressed c-met levels that were intermediate between normal and malignant specimens. The consistent increase in c-met among all histological subtypes of borderline and frankly malignant tumors in our study confirms that c-met overexpression is an integral component in the development of all these forms of ovarian epithelial neoplasms.
In a number of tissues, c-met becomes increasingly overexpressed as tumors become poorly differentiated, invasive, and metastatic. 2,6 However, the relationship of c-met expression to tumor differentiation seems to vary among organ sites. In breast cancer cell lines, an inverse relationship between differentiation and c-met expression was reported. However, in the same study, no such correlation was observed among primary breast cancer biopsies, though metastases from the same tumors all showed greatly increased c-met expression. 4 In renal cell carcinomas, a close correlation was observed between the overexpression of c-met and the chromophilic subtype with a papillary growth pattern. 20 Di Renzo et al 17 found c-met to be most overexpressed in ovarian carcinomas that were differentiated. In the present study, c-met expression was enhanced in most benign tumors and appeared to be maximally overexpressed in differentiated borderline tumors and differentiated ovarian carcinomas.
In the course of neoplastic progression, the simple phenotype of normal OSE characteristically converts to tumor cells with complex glandular epithelial phenotypes. It is interesting in this regard, that HGF has the capacity to induce epithelial tubular/glandular morphogenesis. 21 The high levels of c-met in benign tumors and in differentiated borderline and malignant epithelial neoplasms therefore raise the possibility that HGF/SF action may be implicated not only in the invasion and metastatic spread of advanced ovarian carcinomas, but also in the morphogenesis that is part of the Mullerian differentiation of many types of ovarian tumors. 6 Such a dual function would be in keeping with the pleiotropic nature of HGF/SF.
Acknowledgments
We are very grateful to Alice Labed for her help with the statistical analysis and to Dr. P. B. Clement for his valuable advice and help in deriving histopathological diagnoses.
Footnotes
Address reprint requests to Dr. Nelly Auersperg, Department of Obstetrics & Gynecology, University of B.C., 2H30 B.C. Women’s Hospital, 4490 Oak Street, Vancouver, B.C., V6H 3V5, Canada. E-mail: auersper@interchange.ubc.ca.
Supported by a grant from the National Cancer Institute of Canada with funds from the Terry Fox Run and by the U.S. National Cancer Institute, under contract with ABL.
References
- 1.Matsumoto K, Nakamura T: Hepatocyte growth (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Comm 1997, 239:639-644 [DOI] [PubMed] [Google Scholar]
- 2.Koochekpour S, Jeffers M, Rulong S, Taylor G, Klineberg E, Hudson EA, Resau JH, Vande Woude GF: Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res 1997, 57:5391–5398 [PubMed]
- 3.Maggiora P, Gambarotta G, Olivero M, Giordano S, Di Renzo MF, Comoglio PM: Control of invasive growth by the HGF receptor family. J Cell Physiol 1997, 173:183-186 [DOI] [PubMed] [Google Scholar]
- 4.Beviglia L, Matsumoto K, Lin C-H-S, Ziober BI, Kramer RH: Expression of the c-met/HGF receptor in human breast carcinoma: correlation with tumor progression. Int J Cancer (Pred Oncol) 1997, 74:301–309 [DOI] [PubMed]
- 5.Jeffers M, Rong S, Vande Woude G: Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J Mol Med 1996, 74:505–513 [DOI] [PubMed]
- 6.To CTT, Tsao MS: The roles of hepatocyte growth factor/scatter factor and Met receptor in human cancers (review). Oncol Rep 1998, 5:1013-1024 [DOI] [PubMed] [Google Scholar]
- 7.Di Renzo MF, Olivero M, Katsaros D, Crepaldi T, Gaglia P, Zola P, Sismondi P, Comoglio PM: Overexpression of the MET/HGF receptor in ovarian cancer. Int J Cancer 1994, 58:658-662 [DOI] [PubMed] [Google Scholar]
- 8.Moghul A, Lin L, Beedle A, Kanbour-Shakir A, DeFrences MC, Liu Y, Zarnegar R: Modulation of c-MET proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: evidence for rapid decay of the 8 kb c-MET transcript. Oncogene 1994, 9:2045-2052 [PubMed] [Google Scholar]
- 9.Prat M, Narsimhan RP, Crepaldi T, Nicotra MR, Natali PG, Comoglio PM: The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer 1991, 49:323-328 [DOI] [PubMed] [Google Scholar]
- 10.Tsarfaty I, Resau JH, Rulong S, Keydar I, Faletto DL, Vande Woude GF: The met proto-oncogene receptor and lumen formation. Science 1992, 257:1258–1261 [DOI] [PubMed]
- 11.Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R: Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci 1995, 108:413-430 [DOI] [PubMed] [Google Scholar]
- 12.Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A: Hepatocyte growth factor stimulates proliferation, migration, and lumen formation of human endometrial epithelial cells in vitro. Biol Reprod 1997, 57:936-942 [DOI] [PubMed] [Google Scholar]
- 13.Maines-Bandiera SL, Auersperg N: Increased E-Cadherin expression in ovarian surface epithelium: an early step in metaplasia and dysplasia? Int J Gynecol Pathol 1997, 16:250-255 [DOI] [PubMed] [Google Scholar]
- 14.Sundfeldt K, Piontkewitz, Ivarsson K, Nilsson O, Hellberg P, Brannstrom M, Janson PO, Enerback S, Hedin L: E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int J Cancer 1997, 74:275–280 [DOI] [PubMed]
- 15.Peralta SA, Knudsen KA, Tecson-Miguel A, McBrearty FX, Han AC, Salazar H: Expression of E-cadherin and N-cadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum Pathol 1997, 28:734-739 [DOI] [PubMed] [Google Scholar]
- 16.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F: Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun 1994, 1:295–305. [DOI] [PubMed]
- 17.Klineberg E, Tsarfaty I, Alvord WG, Sathyanarayana BK, Resau J: Correction and quantification of normal differentiation in human epithelium: application for Optimas 4.0 image analysis program. Cell Vision 1996, 3:402–406
- 18.Auersperg N, Maines-Bandiera S, Booth JH, Lynch HT, Godwin AK, Hamilton TC: Expression of two mucin antigens in cultured human ovarian surface epithelium: influence of a family history of ovarian cancer. Am J Obstet Gynecol 1995, 173:558-565 [DOI] [PubMed] [Google Scholar]
- 19.Ziltener HJ, Maines-Bandiera S, Schrader JW, Auersperg N: Secretion of bioactive interleukin-1, interleukin-6, and colony-stimulating factors by human ovarian surface epithelium. Biol Reprod 1993, 49:635-641 [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Karashima T, Chikazawa M, Iliyama T, Yoshikawa C, Furihata M, Ohtsuki Y, Shuin T: Overexpression of c-met proto-oncogene associated with chromophilic renal cell carcinoma with papillary growth. Virchows Arch 1998, 433:511-515 [DOI] [PubMed] [Google Scholar]
- 21.Tsafarty I, Rong S, Resau J, Rulong S, da Silva P, Vande Woude G: The met proto-oncogene in mesenchymal to epithelial conversion. Science 1994, 263:98–101 [DOI] [PubMed]


