Abstract
In this study, we report coexpression of transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) in pancreatic carcinoma tissue associated with significantly elevated levels of both cytokines in the sera of pancreatic carcinoma patients. Using conditioned media (CM) of pancreatic carcinoma cells, we further demonstrate that tumor cell-derived TGF-β and IL-10 inhibited in an additive fashion both proliferation and the development of Th1-like responses in peripheral blood mononuclear cell (PBMC) preparations derived from normal donors. The antiproliferative and Th1-suppressive activities contained in CM of pancreatic carcinoma cells were due primarily to IL-10 and/or TGF-β, as shown by the capacity of cytokine-specific neutralizing antibodies to reverse these effects. Finally, as compared to normal controls, PBMC derived from pancreatic carcinoma patients displayed a Th2-like cytokine expression pattern upon activation with either anti-CD3 antibody or Staphylococcus aureus strain Cowan I. Taken together, these results suggest that aberrant production of TGF-β and IL-10 in pancreatic tumor patients skews T-cell cytokine production patterns in favor of a Th2 immunophenotype.
The recent isolation from tumor patients of T lymphocytes reactive with tumor-associated antigens1-3 underscores the notion that tumors can be immunogenic and, thus, are potential targets for immune destruction. Eliciting or restoring an effective antitumor immune response provides an attractive goal for the development of cancer vaccines and cancer immunotherapy. A thorough understanding of the mechanisms by which neoplastic cells evade detection or destruction by the immune system is required to guide these efforts. Tumor cells produce a variety of immunomodulatory cytokines that can stimulate or inhibit the host response to tumor cells (for a review see Ref. 4 ). The present study was performed to explore the immunomodulatory activities of two such cytokines, transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), both of which are aberrantly produced by human pancreatic carcinoma cells (this study).
TGF-β is a 25-kd dimeric cytokine with pleiotrophic effects on a wide spectrum of target cells. Three highly conserved isoforms of human TGF-β (1–3) encoded by separate genes are known; the TGF-β isoforms share considerable structural and sequence homology and exert similar effects when tested in biological systems.5 Aberrant expression of different TGF-β isoforms is widespread among human tumors,6 including pancreatic carcinoma,7,8 breast carcinoma,9 glioma,10-12 and malignant melanoma.13-15 In support of a significant tumor-protective role of TGF-β in vivo, transfection of TGF-β1 into highly immunogenic mouse tumor cells has been shown to enable these cells to survive in immunocompetent syngeneic animals.16 Conversely, increased immunogenicity and decreased tumorigenicity in syngeneic animals was observed when TGF-β production in rat gliobastoma cells was suppressed using antisense TGF-β2.17 In addition, neutralizing antibodies to TGF-β have been shown to inhibit the tumorigenicity of human breast cancer cells in nude mice; this effect was associated with increased NK cell activity.18 That tumor-derived TGF-β may also contribute to immune evasion of human tumor cells is suggested by studies in glioma. An activity that inhibits proliferation of T cells and autologous lymphokine-activated killer (LAK) cells was isolated from conditioned medium (CM) of glioma cells19 and cyst fluid recovered from the tumor bed of subtotally resected glioblastomas20 ; this activity was shown to be TGF-β2.
IL-10, like TGF-β, is a cytokine that exerts multiple effects on the immune system relating to antigen presentation, B- and T-cell proliferation, cytokine production, and monocyte/macrophage function (for a review see Ref. 21 ). IL-10 prevents up-regulation of B-7 expression during macrophage activation22 and down-modulates expression of a broad range of cytokines in peripheral blood mononuclear cells (PBMC) preparations, including interferon-γ (IFN-γ),23 IL-2,24 and tumor necrosis factor-α (TNF-α).25 IL-10 has been found to be commonly expressed in human carcinoma cells26,27 and in melanomas.28,29 In melanoma patients increased serum IL-10 levels have been described.30 Whether IL-10 production by tumor cells is relevant to local and/or systemic antitumor immune responses in cancer patients is currently unclear.
In this study, we have examined expression patterns and functional consequences of TGF-β and IL-10 production by human pancreatic carcinoma cells for systemic T-cell responses in vitro and in vivo. To characterize the relative contributions of tumor-derived TGF-β and IL-10 on systemic immune parameters in pancreatic cancer patients, we determined, in the presence and absence of specific neutralizing antibodies to these cytokines, the effects of CM from pancreatic carcinoma cells on proliferative potential, cytotoxic activity, and the Th1/Th2-like cytokine profiles upon activation of PBMC derived from normal donors. To assess the potential effects of tumor-derived TGF-β and IL-10 in pancreatic cancer patients themselves, we also determined the Th1/Th2-like cytokine profiles of PBMC preparations obtained from these patients.
Materials and Methods
Tissue Samples and Patients
A group of 10 pancreatic carcinoma patients (seven men and three women, aged 46–71) who underwent surgical resections at the Department of Medical-Surgical Disciplines (University of Torino, Torino, Italy) were included in this study. All patients were affected with histopathologically confirmed primary pancreatic duct adenocarcinomas representing stage II (n = 2), stage III (n = 3), and stage IV (n = 5) pancreatic neoplasms according to the classification by Warshaw and Fernandez-del Castillo.31 Pancreatic cancer tissue samples and normal pancreatic tissue were frozen in liquid nitrogen immediately after surgical removal and before RNA extraction. Venous blood from pancreatic carcinoma patients was collected before anesthesia and surgery. PBMCs from patients and age- and sex-matched healthy donors were separated by Ficoll-Hypaque gradient centrifugation and used immediately for analysis. Donor and patient serum samples were frozen at −70°C until analysis.
Cell Lines and CM
Human pancreatic carcinoma cell lines Capan2 (American Type Culture Collection (ATCC), Rockville, MD), PT45, and BxPC3 (kindly provided by Dr. M. F. DiRenzo, Department of Biomedical Sciences and Human Oncology, University of Torino, Torino, Italy) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (GIBCO, Grand Island, NY). All cell lines were routinely screened for Mycoplasma contamination, using the Hoechst dye H33258. To obtain serum-free CM, Capan2, PT45, and BxPC3 cells were trypsinized, extensively washed with phosphate-buffered saline (pH 7.3), and seeded at 3 × 105/ml in 5 ml of serum-free DMEM containing 0.25 vol% fatty acid-free bovine serum albumin fraction V (Boehringer Mannheim). After a 48-hour incubation in a humidified atmosphere containing 5% CO2, cell-free supernatants were collected after centrifugation, concentrated five-fold by filtration with Amicon Diaflo concentrators equipped with YM5 membranes (Danvers, MA), and stored at −70°C until use.
Antibodies and Reagents
The hybridoma-producing monoclonal antibody (mAb) OKT3 (anti-CD3) was obtained from the ATCC. Neutralizing anti-IL-10 goat and panspecific anti-TGF-β rabbit polyclonal antibodies were from R&D Systems Europe (Abingdon, England). For immunohistochemistry, rabbit antisera reacting specifically with TGF-β1, TGF-β2, or TGF-β3 (epitopes corresponding to amino acid sequences mapping at the carboxy terminus of the precursor forms of TGF-β1, TGF-β2, and TGF-β3 of human origin, respectively) from Santa Cruz Biotechnology (Santa Cruz, CA) and mAbs to IL-10 (JES-9D7 and 12G8) from Pharmingen (San Diego, CA) were used. Recombinant human TGF-β1, TGF-β2, and TGF-β3 isoforms were obtained from R&D Systems Europe. Staphylococcus aureus strain Cowan I (SAC) was from Calbiochem (La Jolla, CA) and was used at 1:10,000 final dilution.
Cytokine Mapping by Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA from normal and neoplastic pancreatic tissues and from the three pancreatic carcinoma cell lines included in this study was extracted with a commercially available kit based on the single-step RNAzol method (Cinna/Biotex, Houston, TX). Reverse transcription (RT) was performed at 37°C for 1 hour, using oligo-dT primer in a final reaction volume of 20 μl containing 20 U of MMLV reverse transcriptase, 1× reverse transcriptase buffer, 24 U of RNAse inhibitor, and 0.5 mmol/L dNTP mix. For each polymerase chain reaction (PCR), 10 μl of first-strand cDNA was added to 20 μl of PCR mix containing 100 ng each of 5′ and 3′ cytokine-specific primers and 1 U Taq polymerase. All PCR reagents were purchased from Life Technologies (Paisley, Scotland). Human IL-10-specific primers were 5′-ATGCCCCAAGCTGAGAACCAAGACCCA-3′ (sense) and 5′-AAGTCTCAAGGGGCTGGGTCAGCTA-3′ (antisense). PCR conditions were as follows: 3 minutes at 94°C, 20 seconds at 60°C, and 30 seconds at 72°C (32 cycles). The predicted size of IL-10 amplimers was 325 bp. Human TGF-β1-specific primers were 5′-GCCCTGGACACCAACTATTGC-3′ (sense) and 5′-GCACTTGCAGGAGCGCA-3′ (antisense). PCR conditions were as follows: 4 minutes at 95°C, 1 minute at 58°C, and 35 seconds at 72°C (32 cycles). The predicted size of TGF-β1 amplimers was 333 bp. Human TGF-β2 and TGF-β3-specific primers were 5′-AAATGGATACACGAACCCAA-3′ (sense) and 5′-GCTGCATTTGCAAGACTTTAC-3′ (antisense); 5′-AAGTGGGTCCATGAACCTAA-3′ (sense) and 5′-GCTACATTTACAAGACTTCAC-3′ (antisense), respectively. PCR conditions were as follows: 4 minutes at 94°C, 20 seconds at 51°C, and 25 seconds at 72°C (32 cycles). The predicted size of both TGF-β2 and TGF-β3 amplimers was 247 bp. Diagnostic restriction enzyme digestion of the PCR amplimers13 was used to confirm the specificity of the primers used for the TGF-β isoforms targeted. Human β-actin primers and amplification conditions have been described by us previously.32 PCR products were analyzed by size fractionation, using 2% agarose gels stained with ethidium bromide.
Immunohistochemical Detection of Cytokines
Sections from paraffin-embedded pancreatic carcinoma tissue samples of the patients studied here were stained with antibodies to IL-10 and TGF-β as described previously.32 TGF-β was detected using rabbit antisera reacting specifically with either TGF-β1, TGF-β2, or TGF-β3. In the case of IL-10 a combination of two rat mAbs (JES3-9D7 and 12G8) was used as described.33 Specificity of the antibodies used was tested by preincubation with saturating amounts of the appropriate recombinant TGF-β isoforms or with IL-10 followed by immunostaining of tissue sections.
Generation of LAK Cells and Cytotoxicity Assay
PBMCs isolated by Ficoll-Hypaque gradient separation were cultured for 6 days in RPMI 1640 medium supplemented with 100 U/ml of IL-2 (a gift from Glaxo, Geneva, Switzerland) in the presence and absence of 20 vol% of 5× concentrated CM of pancreatic carcinoma cell lines Capan2, PT45, and BxPC3. LAK activity of these PBMC preparations was evaluated using standard 4-hour 51Cr release assays,34 using the Burkitt lymphoma cell line Daudi as a target.
PBMC Proliferation Assay
The proliferative response of T cells in response to anti-CD3 mAb OKT3 was determined by measuring [3H]thymidine (TdR) uptake. PBMCs were cultured at 2 × 105 cells/well in OKT3-coated flat-bottom 96-well plates in the presence and absence of increasing concentrations of 5× concentrated CM of pancreatic carcinoma cell lines BxPC3, Capan2, and PT45. After 48 hours of incubation, the cells were pulsed for 6 hours with 1 μCi [3H]TdR (6.7 mCi/mmol; NEN-Dupont, Boston, MA) and harvested on glass fiber filters. [3H]TdR uptake was measured using a β-scintillation counter. In selected experiments, cell line supernatants were preincubated for 1 hour at room temperature with neutralizing antisera to IL-10 (10 μl/ml) and TGF-β (5 μl/ml) or control antibodies before addition to PBMC cultures.
Determination of Cytokine Concentrations in Sera and CM
IFN-γ, IL-10, and IL-4 protein concentrations were determined by enzyme-linked immunosorbent assay (ELISA), using kits commercially available from Biosource (Camarillo, CA). TGF-β1 and TGF-β2 concentrations were determined using ELISA kits available from Genzyme (Cambridge, MA) and from R&D Systems (Minneapolis, MN), respectively. The lower threshold of sensitivity of the IFN-γ, IL-10, and IL-4 assays was 5 pg/ml, whereas the TGF-β1 and TGF-β2 assays had a sensitivity threshold of 50 pg/ml. IL-12 levels were determined using a radioimmunoassay (RIA) specific for the p40 chain of IL-12 heterodimer, as previously described.35 The lower threshold of detection of this assay was 30 pg/ml.
Statistical Analysis
To assess statistically significant differences between data sets, Student’s t-tests for independent samples were performed using SigmaPlot software.
Results
Production of TGF-β and IL-10 by Pancreatic Carcinoma Cells in Vivo and in Vitro
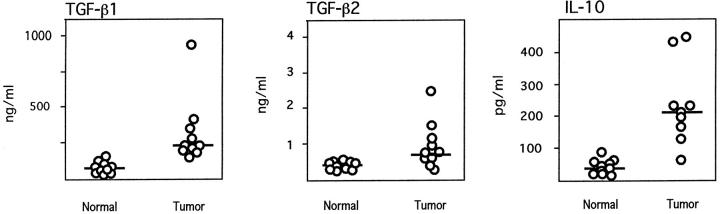
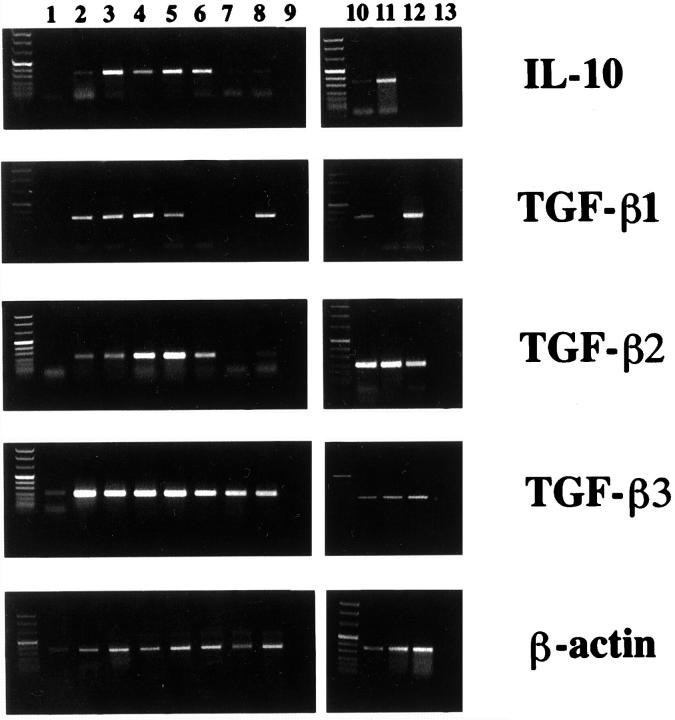
To assess IL-10 and TGF-β production in pancreatic carcinoma patients, we determined IL-10, TGF-β1, and TGF-β2 serum concentrations in 10 pancreatic carcinoma patients. As compared to normal age- and sex-matched control donors not affected by obvious neoplastic or inflammatory diseases, significantly elevated mean serum levels of all three cytokines were observed in the majority of pancreatic cancer patients (Figure 1)▶ . The mean serum level of TGF-β1 in pancreatic carcinoma patients was 320.4 ± 252 ng/ml (mean ± SD) as compared to 70.8 ± 27.4 in normal controls (P < 0.05; Student’s t-test for independent samples). The mean serum level of TGF-β2 in pancreatic carcinoma patients was 947 ± 620 pg/ml, as compared to 380 ± 89 in normal controls (P < 0.05). The mean serum level of IL-10 in cancer patients was 233.7 ± 13.6 pg/ml, as compared to 40.4 ± 17.2 in normal controls (P < 0.05). In seven of the patients tumor tissue was available for RT-PCR analysis of IL-10 and TGF-β1, -β2, and -β3 transcripts. Either IL-10 and/or TGF-β amplimers of the expected size were identified in several tumor tissue samples (Figure 2)▶ . Whereas expression of TGF-β1, TGF-β2, and IL-10 mRNAs was variable between different tumors, TGF-β3 amplimers were identified at comparably high levels in all pancreatic carcinoma tissue samples. In normal pancreatic tissue, RT-PCR products corresponding to TGF-β3, but not TGF-β1, TGF-β2, or IL-10, were identified. Immunohistochemical analysis of TGF-β1, -β2, and -β3 and IL-10 expression confirmed expression of these cytokines by pancreatic carcinoma cells. Representative results are shown in Figure 3▶ , revealing moderate to strong staining for all cytokines in tumor cells.
Figure 1.
Elevated TGF-β1, TGF-β2, and IL-10 concentrations in the sera of pancreatic carcinoma patients (tumor) as compared to sera of donors free of inflammatory or neoplastic diseases (normal). Bars represent median values; as determined by Student’s t-test, TGF-β1, TGF-β2, and IL-10 levels were significantly higher (P < 0.05) in sera from pancreatic carcinoma patients as compared to normal controls.
Figure 2.
Expression of TGF-β1, -β2, -β3, and IL-10 transcripts in pancreatic carcinoma tissues (Lanes 2–8) and cell lines (Lane 10, BxPC3; Lane 11, Capan2; Lane 12, PT45) when compared to normal pancreatic tissue (Lane 1) . Ethidium bromide-stained RT-PCR products generated by using cytokine-specific primers and separated by agarose gel electrophoresis are shown. Lanes 9 and 13 show lack of amplification products in the absence of mRNA template. To control for RNA integrity and quantity, RT-PCR products generated by using β-actin primers are shown in the bottom panel. The specificity of TGF-β primers was assessed by diagnostic enzyme digestion of RT-PCR products.
Figure 3.
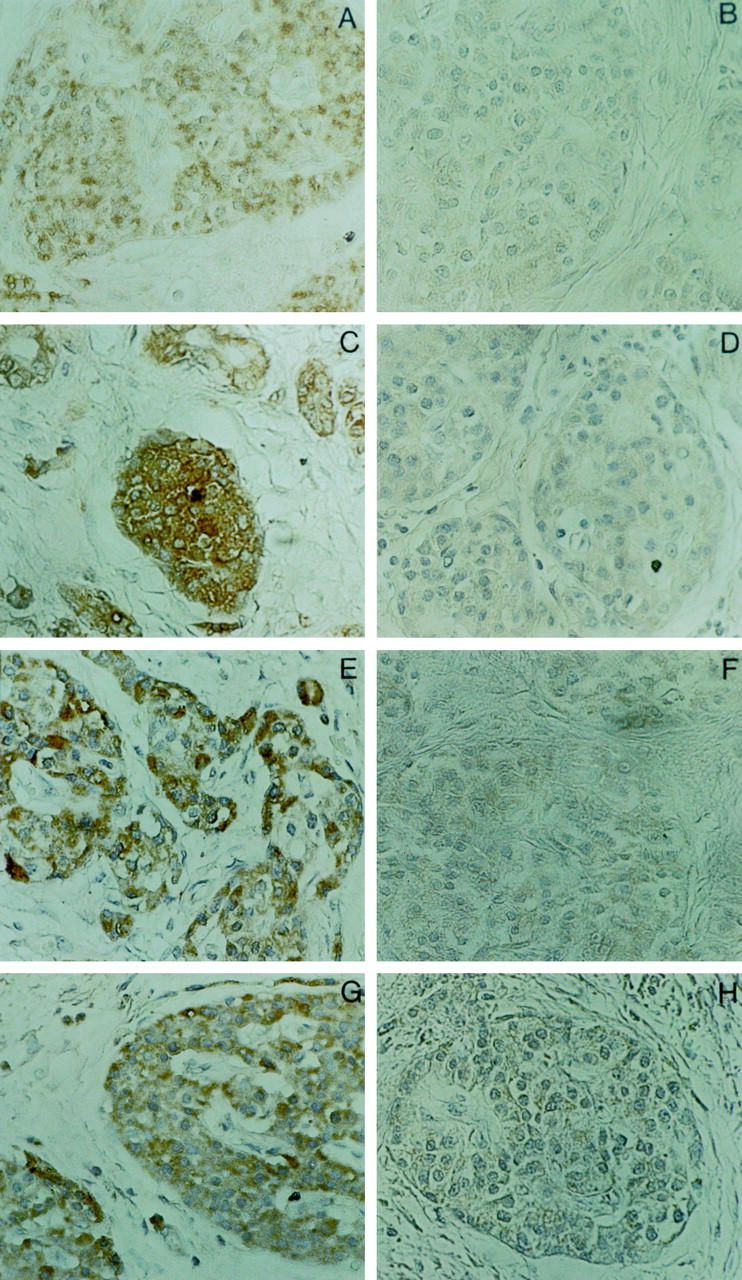
Representative immunohistochemical analysis of TGF-β1, -2, -3, and IL-10 expression in pancreatic carcinoma tissue. Antibodies reacting specifically with TGF-β1 (A), TGF-β2 (C), TGF-β3 (E), or IL-10 (G) were used. B, D, F, and H show negative controls stained with antibodies preincubated with saturating amounts of the appropriate recombinant proteins. Magnification, ×400.
We next examined production of TGF-β and IL-10 by pancreatic carcinoma cell lines BxPC3, Capan2, and PT45. As assessed by RT-PCR analysis, all three cell lines expressed either IL-10 mRNA alone (Capan2), or TGF-β1 mRNA alone (PT45), or both cytokine mRNAs (BxPC3) (Figure 2)▶ . To account for overall TGF-β expression, we also determined by RT-PCR expression of TGF-β2 and TGF-β3 mRNA in these cells. Primers specific for TGF-β2 and -β3 yielded amplification products of the predicted size in all three cell lines tested. Consistent with the RT-PCR results, TGF-β1 protein was secreted by BxPC3 and PT45 but not Capan2 cells, and IL-10 protein was detected by ELISA in CM of Capan2 and BxPC3 cells but not PT45 cells (Table 1)▶ . In summary, whereas IL-10 was secreted by 2/3 pancreatic carcinoma cell lines, at least 2/3 known TGF-β isoforms were expressed by all three cell lines.
Table 1.
Secretion of TGF-β1, TGF-β2, and IL-10 by Pancreatic Carcinoma Cell Lines
| Cell line | TGF-β1 (ng/ml)* | TGF-β2 (pg/ml)† | IL-10 (pg/ml)‡ |
|---|---|---|---|
| Capan2 | <0.2 | 1872 | 47.2 |
| PT45 | 193.2 | 2661 | <5 |
| BxPC3 | 2006.4 | 1276 | 112 |
* As determined by TGF-β1-specific ELISA.
† As determined by TGF-β2-specific ELISA.
‡ As determined by IL-10-specific ELISA.
Effects of Media Conditioned by Pancreatic Carcinoma Cell Lines on LAK Activity and Proliferation of PBMCs
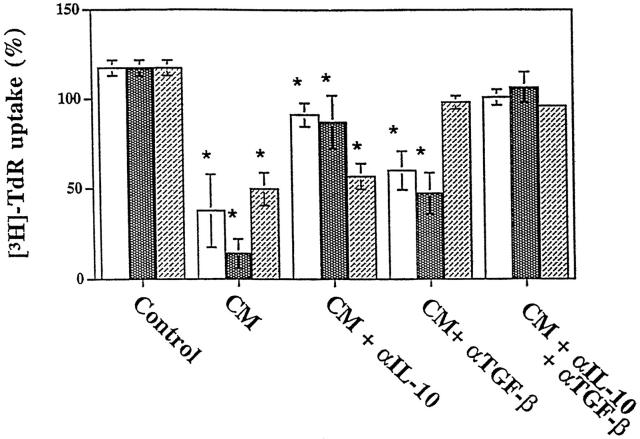
To investigate functional consequences of IL-10 and TGF-β production by pancreatic carcinoma cells on immune parameters potentially relevant to tumor development, we next determined the effects of media conditioned by pancreatic carcinoma cell lines on lymphokine-activated cytotoxicity and proliferative capacity of PBMC. First, the effects of CM on LAK activity of PBMCs derived from two normal donors was tested. To this end, PBMCs were incubated for 6 days with 5× concentrated CM of pancreatic carcinoma cell lines added at 20 vol% to RPMI 1640 medium supplemented with IL-2. When LAK activity was determined by 51Cr release assays using Daudi target cells at effector-to-target ratios from 40:1 to 5:1, no significant change of cytotoxic activity was observed when compared to medium controls (not shown). Second, we determined the effects of pancreatic carcinoma CM on anti-CD3-dependent proliferation of PBMC obtained from three normal donors. This was based on previous reports that both TGF-β and IL-10 affect proliferation of several types of immune cells, including T and B lymphocytes36-40 and NK cells.36,41 The addition of 5× concentrated pancreatic carcinoma CM to culture media at concentrations ranging from 5 to 20 vol% resulted in dose-dependent inhibition of [3H]TdR uptake in all three PBMC preparations tested (Figure 4)▶ . To determine the relative contribution of TGF-β and IL-10 to this phenomenon, we used neutralizing antibodies known to inhibit the biological activity of either all three TGF-β isoforms and/or IL-10. These experiments demonstrated that a combination of the two neutralizing antibodies almost completely (>90%) reversed the PBMC growth-inhibitory activity produced by the three pancreatic carcinoma cell lines (Figure 5)▶ . As predicted by the cytokine production patterns (see Table 1▶ and Figure 2▶ ), the anti-IL-10 antibody had no significant effect in the case of PT45 cells that do not produce IL-10. However, the TGF-β antibody was sufficient to neutralize the growth-inhibitory activity present in PT45 conditioned medium. By contrast, in Capan2 and BxPC3 cells, the TGF-β antibody only partially reversed PBMC growth inhibition, whereas the IL-10 antibody was more effective. In summary, these results show that the PBMC antiproliferative activity present in pancreatic carcinoma CM was primarily accounted for by the combined action of IL-10 and TGF-β.
Figure 4.
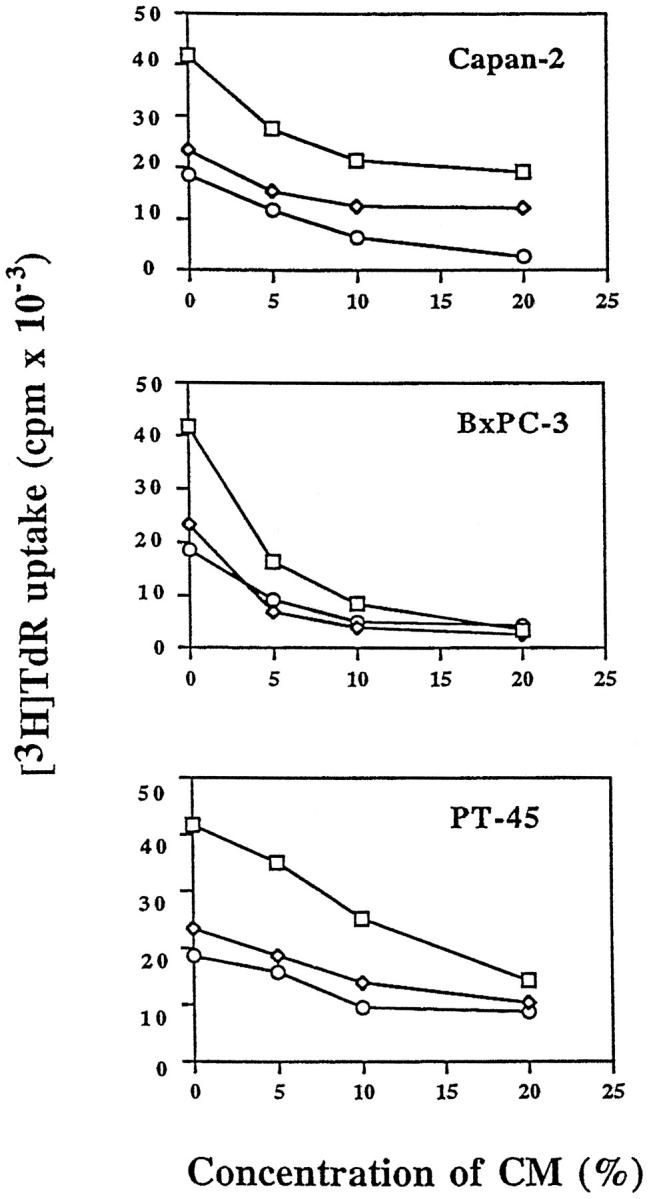
Inhibition of PBMC DNA synthesis by CM derived from pancreatic carcinoma cell lines Capan2, BxPC3, and PT45. The effects of 5× concentrated CM at different concentrations ranging from 5 to 20 vol% on [3H]TdR uptake of PBMCs derived from three normal donors are shown.
Figure 5.
Effects of neutralizing antibodies to IL-10 and TGF-β on the inhibition of PBMC proliferation caused by CM of pancreatic carcinoma cell lines Capan2, BxPC3, and PT45. The effects of anti-IL-10, anti-TGF-β, and anti-IL-10 plus anti-TGF-β are shown on anti-CD3-induced PBMC proliferation measured in the absence (control) and in the presence of 20 vol% CM derived from pancreatic carcinoma cell lines (all other conditions). Control cultures also received anti-IL-10 and anti-TGF-β antibodies. Column 1 (□) of all experimental groups shows PBMC proliferation in the presence of Capan2 CM; column 2 ( ) shows proliferation in the presence of BxPC3 CM; and column 3 (▨) shows proliferation in the presence of PT45 CM. Each column shows the mean ± SD of three independent experiments using three different normal donors. Results are expressed as a percentage of [3H]TdR uptake relative to that of cells grown in the absence of either neutralizing antibodies or CM. No effect was observed in the presence of a nonimmune rabbit antiserum used as the control (data not shown). Asterisks indicate statistically significant differences (P < 0.05 in Student’s t-tests) of data sets when compared to controls in the presence of anti-IL-10 and anti-TGF-β neutralizing antibodies.
Effects of Tumor-Derived IL-10 and TGFβ on Cytokine Production by PBMCs
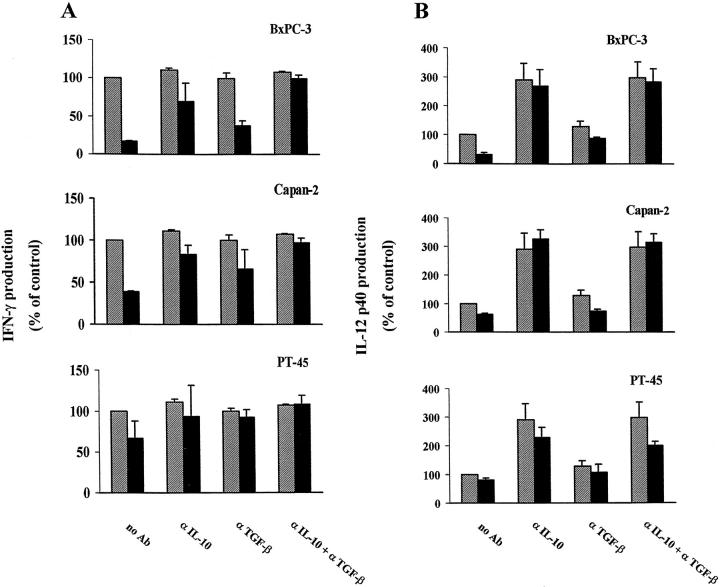
In certain parasitic diseases such as infection with Leishmania major, different patterns of cytokine production have been distinguished that correspond to the induction of functionally diverse T-cell subsets and seem to be associated with different clinical outcomes.42,43 Generally, predominance of the Th1 cytokine pattern (IFN-γ and IL-12) is associated with a vigorous cytotoxic T-cell response and favorable outcome, whereas the Th2 cytokine pattern (IL-4, IL-5, IL-10) is associated with chronic disease or disease progression. Upon antigenic stimulation, both IL-1044 and TGF-β45,46 have been shown to favor development of a Th2-like cytokine pattern and, thus, are likely to affect immune responses in vivo. Based on these findings, we asked whether IL-10 and TGF-β present in pancreatic carcinoma CM affected the patterns of Th1/Th2 cytokines produced by PBMC obtained from normal donors. Specifically, we tested whether pancreatic carcinoma cell CM down-modulated the production of the Th1-associated cytokines IFN-γ and IL-12 by PBMCs upon activation by either anti-CD3 antibody or SAC. As determined by cytokine-specific ELISA or RIA, CM from all three pancreatic carcinoma cell lines significantly (P < 0.05) reduced production of IFN-γ (Table 2)▶ and the p40 chain of IL-12 (Table 3)▶ by PBMCs from three normal donors. A combination of neutralizing antibodies to IL-10 and TGF-β abolished the inhibitory effect of all three pancreatic carcinoma CMs on both IFN-γ and IL-12p40 production in the three PBMC preparations, except in two PBMC preparations pretreated with PT45 CM (Figure 6)▶ . Optimal neutralization of the effect of pancreatic carcinoma CM on IFN-γ production was observed with a combination of anti-IL-10 and anti-TGF-β antibodies. The antibody to IL-10 had a comparatively stronger effect in neutralizing the IL-12 suppressive activity contained in Capan2 and BxPC3 CM and a weaker effect on PT45 CM. It should be noted that the anti-IL-10 antibody effectively reduced the effect of PT45 CM on IFN-γ production in 3/3 and on IL-12 production in 2/3 PBMC preparations, although PT45 did not produce IL-10 (see Table 1▶ ). In contrast to the anti-IL-10 antibody, the anti-TGF-β antibody only moderately antagonized down-regulation of IFN-γ and IL-12 production by Capan2 and BxPC3 CM. However, it was comparatively more effective in the case of PT45 CM, which produces TGF-β but not IL-10. These results are consistent with the view that production of either IL-10 alone or a combination of IL-10 and TGF-β by pancreatic carcinoma cells contributes to the inhibition of Th1-like responses in naive PBMCs.
Table 2.
Effects of Media Conditioned by Pancreatic Carcinoma Cells on IFN-γ Production by Normal PBMCs Stimulated with Anti-CD3 Antibody
| Donor | Medium control | IFN-γ (pg/ml)* | ||
|---|---|---|---|---|
| BxPC3 | Capan2 | PT45 | ||
| 1 | 2098 | 258 | 856 | 1233 |
| 2 | 1970 | 369 | 721 | 973 |
| 3 | 2237 | 427 | 896 | 2065 |
| Mean± SD | 2102 ± 134 | 351 ± 86† | 824 ± 65† | 1423 ± 570 |
* As determined by IFN-γ-specific ELISA.
† Statistically significant differences (P < 0.05 in Student’s t-tests) of data sets when compared to medium control.
Table 3.
Effects of Media Conditioned by Pancreatic Carcinoma Cells on IL-12 Production by Normal PBMCs Stimulated with SAC
| Donor | IL-12 (ng/ml)* | |||
|---|---|---|---|---|
| Control | BxPC3 | Capan2 | PT45 | |
| 1 | 9.9 | 4.0 | 5.4 | 7.0 |
| 2 | 13.2 | 4.2 | 7.9 | 10.4 |
| 3 | 12.8 | 5.0 | 9.0 | 11.9 |
| Mean± SD | 11.9 ± 1.8 | 4.4 ±0.5† | 7.4 ± 1.8† | 9.7 ± 2.5 |
* As determined by IL-12p40-specific RIA.
† Statistically significant differences (P < 0.05 in Student’s t-tests) of data sets when compared to medium control.
Figure 6.
Effects of neutralizing antibodies to IL-10 and TGF-β on the inhibition of IFN-γ (A) and IL-12p40 (B) production of normal PBMCs by pancreatic carcinoma CM. CMs were derived from Capan2, BxPC3, and PT45 cells, as indicated. Experimental conditions were as described in the legend to Figure 5▶ . No effect was observed in the presence of a nonimmune rabbit antiserum used as the control (data not shown). Each column shows the mean ± SD of three independent experiments using three different normal donors. Results are expressed as a percentage of cytokine production relative to that of cells grown in the absence of neutralizing antibodies (▨) or CM (▪).
PBMCs from Pancreatic Carcinoma Patients Display a Th2-Like Cytokine Expression Pattern
To address whether elevated TGF-β and IL-10 serum levels in pancreatic carcinoma patients may similarly affect the Th1/Th2-balance in vivo, we tested production of Th1 (IFN-γ, IL-12) and Th2 (IL-4) cytokines in PBMC preparations of six pancreatic carcinoma patients upon stimulation with either anti-CD3 antibody or SAC (Table 4)▶ . We observed a clear preference for production of the Th2 cytokine IL-4 but not the Th1 cytokines IFN-γ and IL-12 when compared to the cytokine patterns obtained when using PBMCs from six normal donors. As compared to normal controls, PBMC preparations from pancreatic carcinoma cells produced, on average, 5.4-fold higher levels of IL-4 upon stimulation with anti-CD3 antibody. In contrast, production of IFN-γ and IL-12 was 2.7- and 9.5-fold lower, respectively, than in controls; the differences in cytokine levels were all statistically significant (P < 0.05). These data show a preference for the development of a Th2-like response in PBMCs obtained from pancreatic carcinoma patients.
Table 4.
Determination of Th1 and Th2-Cytokine Patterns in PBMCs of Patients with Pancreatic Carcinoma
| Case | IFN-γ (pg/ml) | IL-12 (ng/ml) | IL-4 (pg/ml) |
|---|---|---|---|
| Normal donors | |||
| 1 | 1540 | 9.86 | 11.5 |
| 2 | 1600 | 10.85 | 9.6 |
| 3 | 1899 | 12.58 | 15.8 |
| 4 | 2120 | 11.30 | 9 |
| 5 | 2080 | 13.20 | 17.3 |
| 6 | 1343 | 10.24 | 6.8 |
| Mean± SD | 1764 ± 316 | 11.338 ± 1.314 | 11.7 ± 4.1 |
| Pancreatic carcinoma patients | |||
| 1 | 409 | 0.67 | 35.6 |
| 4 | 598 | 1.10 | 45.7 |
| 5 | 936 | 0.92 | 39.6 |
| 6 | 420 | 0.50 | 110.5 |
| 8 | 658 | 1.35 | 62.7 |
| 10 | 886 | 2.62 | 87.4 |
| Mean± SD | 651 ± 224* | 1.194 ± 0.761* | 63.6 ± 29.8* |
PBMCs from normal donors and pancreatic carcinoma patients were stimulated with anti-CD3 antibody (IFN-γ/IL-4) or SAC (IL-12), as described in Materials and Methods, followed by determination of the cytokine level by ELISA (IFN-γ/IL-4) or RIA (IL-12p40) in cell-free supernatants. Asterisks indicate statistically significant differences (P < 0.05 in Student’s t-tests) of data sets when compared with control data sets obtained using PBMCs from normal donors.
Discussion
The results of this study provide support for the idea that, in patients suffering from pancreatic carcinomas, elevated TGF-β and IL-10 serum levels affect systemic immunity in favor of a Th2-like phenotype. Overexpression of either one of these two cytokines in other tumor types has been recognized earlier. For example, elevated levels of TGF-β47 have been shown in the sera of breast carcinoma patients and of IL-1030 in the sera of melanoma patients. To our knowledge, this is the first report to demonstrate abnormally high levels of both IL-10 and TGF-β in the sera of patients with a solid malignancy. Although it was not possible to determine with certainty the cellular sources of serum IL-10 and TGF-β in the pancreatic tumor patients, we consider it likely that at least part of the overall serum cytokine activity originated in the tumor tissue itself. This conclusion is based on the demonstration of 1) TGF-β and IL-10 mRNA and protein expression in tumor tissues from pancreatic carcinoma patients, 2) coexpression and secretion of TGF-β and IL-10 by 2/3 pancreatic carcinoma cell lines included in this study, and 3) significant decreases in IL-10 serum levels from 428 to 258 pg/ml and from 127 to 52 pg/ml, respectively (results not shown), in two pancreatic carcinoma patients within 2 weeks after surgical intervention.
Earlier studies in syngeneic mouse tumor model systems have shown that both TGF-β and IL-10, when overexpressed by tumor cells, modulate the antitumor immune response with significant consequences for survival and growth of the tumor cells in the host. However, whereas TGF-β is generally considered to exert immunosuppressive effects enabling tumor cells to survive in the host, the immunomodulatory role of IL-10 as it relates to the antitumor immune response is less clear. Specifically, transfection of ultraviolet-induced mouse tumor cells with TGF-β1 has been shown to reduce the capacity of these cells to stimulate cytolytic T-cell responses and renders them tumorigenic in vivo.16 In contrast, high-level IL-10 expression in mouse mammary adenocarcinoma cells induced by transfection of an IL-10 transgene appears to augment tumor rejection.48,49 In vitro, TGF-β inhibits the growth of NK cells,34,41,50 T lymphocytes,38,51,52 and B lymphocytes,53 although TGF-β has also been shown to stimulate the growth and differentiation of activated T lymphocytes.54 IL-10 has been shown to inhibit the proliferation of T39 and B lymphocytes55 in certain experimental in vitro settings. However, the effect of tumor-derived IL-10 alone and the combined effect of IL-10 and TGF-β on human PBMC proliferation is poorly defined at present. We demonstrate that pancreatic carcinoma-derived TGF-β and IL-10 cooperatively inhibited the proliferation of PBMCs derived from normal donors. Although the contribution of either cytokine to the overall inhibition of DNA synthesis varied depending on the pattern of cytokine production by the three pancreatic carcinoma cell lines investigated, either IL-10 alone or IL-10 in combination with TGF-β was the main cytokine responsible for the PBMC growth-inhibitory activity present in pancreatic carcinoma CM. This conclusion is supported by the capacity of neutralizing antibodies to these two cytokines to reverse growth inhibition of PBMCs by pancreatic carcinoma CM. In contrast to their antiproliferative activity, we observed no significant effects of pancreatic carcinoma CM on LAK activity generated in PBMCs from normal donors. This result is consistent with earlier observations that TGF-β134 and IL-1025 only weakly inhibit the cytotoxic activity of LAK cells. Taken together, these results suggested that IL-10 and TGF-β produced by pancreatic carcinoma cells can influence the nature of the antitumor immune response by inhibiting the expansion of immune effector cells.
In addition to suppression of PBMC proliferation, we observed inhibitory effects of pancreatic carcinoma CM on PBMC cytokine production upon unspecific antigenic stimulation. We focused this investigation on the modulation of IL-12 and IFN-γ production, based on the relevance of these two cytokines to the development of T-lymphocyte-mediated adaptive immunity, which is thought to be important for immunological tumor rejection. As in the case of PBMC proliferation, we observed that TGF-β and IL-10 contained in Capan2 and BxPC3 CM cooperatively inhibited IL-12 and IFN-γ production induced by either SAC or anti-CD3 antibody. The contribution of TGF-β to the suppression of IL-12 production was marginal, as demonstrated by the comparably small effects of neutralizing anti-TGF-β antibody, whereas the anti-IL-10 antibody alone was sufficient to neutralize the inhibitory effect of Capan2 and BxPC3 CM on IL-12 production by PBMCs. Interestingly, anti-IL-10 treatment also reversed some of the effect of PT45 CM on IL-12 production by PBMCs, although PT45 cells did not express IL-10 mRNA or secreted IL-10 in measurable quantities. We consider the effect of the anti-IL-10 antibody in the absence of tumor-derived IL-10 to reflect the neutralization of PBMC-derived IL-10. This interpretation is supported by the finding that the anti-IL-10 antibody in the absence of pancreatic carcinoma CM increased IL-12 production four-fold, presumably by neutralizing IL-10 derived from PBMCs themselves. Note that treatment with the anti-IL-10 antibody did not completely restore IL-12 production to control levels in 2/3 PBMC preparations exposed to PT45 CM, suggesting the presence of yet another activity unrelated to either IL-10 or TGF-β that reduces IL-12 production by PBMCs. This activity is not likely to be prostaglandin E2 (PGE2), which has recently been reported to be produced by tumor cells56 and to induce Th2 cytokine patterns,57 as the pancreatic carcinoma CM used contained very low PGE2 levels (<25 pg/ml). At this concentration range PGE2 reportedly has no effect on cytokine production by PBMCs56 or CD4+ T cells.57
The strong inhibitory effects of pancreatic carcinoma-derived IL-10 on the production of the Th1 cytokines IL-12 and IFN-γ in naive PBMCs suggested that elevated serum levels of IL-10 in pancreatic carcinoma patients could also affect T-lymphocyte responses in PBMCs derived from these patients. As expected, we found a predominant Th2-like phenotype that develops upon stimulation of PBMCs from pancreatic carcinoma patients with either anti-CD3 antibody or SAC. Specifically, production of IL-12 and IFN-γ was significantly lower in all PBMC preparations from pancreatic carcinoma patients when compared to controls. This effect was most obvious in the case of IL-12 in that IL-12 levels amounted to less than 10% of controls in 5/6 PBMC preparations from tumor patients. Furthermore, the low levels of Th1 cytokine production in all six tumor patients were associated with significantly higher levels of the Th2 cytokine IL-4. These results demonstrate that PBMC preparations of pancreatic carcinoma patients are primed to develop a Th2-like rather than a Th1-like response upon antigenic stimulation. A preponderance of Th2 cytokine production has commonly been observed in mouse tumor models46 and has also recently been described in association with human tumors, including colorectal carcinoma,58 and in biopsy specimens of human basal cell carcinoma but not benign hyperproliferative lesions of the epidermis.59 Our findings are consistent with the conclusion that, in pancreatic carcinoma patients, tumor-derived TGF-β and IL-10 similarly contribute to systemic Th2-type immune responses. Experiments are currently under way to determine whether this observation extends to specific T-cell responses to recall antigens.
The functional consequences of a predominant Th2-like cytokine profile for antitumor immunity in pancreatic carcinoma patients are not known. In experimental models both Th1 (IL-2, IL-12) and Th2 (IL-4, IL-10) cytokines, when administered as vaccine adjuvants, have been shown to induce protective immune responses to malignant tumors.60-63 These results support the conclusion that both Th1 and Th2 cells participate in generating effective antitumor immunity. However, at least one study suggests that an excessive, vaccine-induced Th2 cytokine pattern is associated with poor protection against malignant tumors.64 In this study, the efficacy of different vaccine preparations containing a peptide sequence within the mammary mucin MUC1 were explored in a mouse tumor model consisting of human mucin (MUC1) expressed in murine BALB/c 3T3 cells. The capacity of vaccine preparations containing MUC1 expressed on tumor cells, MUC1-containing synthetic peptides, MUC1 fusion proteins, or natural mucin (HMFG) to induce protective immunity was tested. Immunization with whole cells expressing MUC1 induced predominantly Th1 immune responses associated with protective immunity. In contrast, vaccine preparations containing soluble synthetic or native materials provided poor protection associated with the development of prominent Th2 responses. It remains to be investigated whether aberrant Th2 immune responses induced by tumor-derived IL-10 and TGF-β in patients serve to protect tumor cells from effective antitumor immunity.
Acknowledgments
We thank Drs. D. Herlyn and G. Trinchieri for critical reading of the manuscript.
Footnotes
Address reprint requests to Dr. Graziella Bellone, Department of Clinical Physiopathology, University of Torino, Via Genova 3, 10126 Torino, Italy. E-mail: bellone@golgi.molinette.unito.it.
Supported in part by a grant from the Italian Association for Cancer Research to G.B. and National Institutes of Health grant (CA 25874) to U.R.
References
- 1.Traversari C, van der Bruggen P, Luescher IF, Lurquin C, Chomez P, Van Pel A, De Plaen E, Amar-Costesec A, Boon T: A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med 1992, 176:1453-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA: Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA 1994, 91:3515-3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde KH, Boon T: Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol 1994, 24:759-764 [DOI] [PubMed] [Google Scholar]
- 4.Sulitzeanu D: Immunosuppressive factors in human cancer. Adv Cancer Res 1993, 60:247-267 [DOI] [PubMed] [Google Scholar]
- 5.Massague J: The transforming growth factor-beta family. Annu Rev Cell Biol 1990, 6:597-641 [DOI] [PubMed] [Google Scholar]
- 6.Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS, Berger WH: Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res 1987, 47:707-712 [PubMed] [Google Scholar]
- 7.Baldwin RL, Friess H, Yokoyama M, Lopez ME, Kobrin MS, Buchler MW, Korc M: Attentuated ALK5 receptor expression in human pancreatic cancer: correlation with resistance to growth inhibition. Int J Cancer 1996, 67:283-288 [DOI] [PubMed] [Google Scholar]
- 8.Friess H, Yamanaka Y, Buchler M, Ebert M, Beger HG, Gold LI, Korc M: Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 1993, 105:1846-1856 [DOI] [PubMed] [Google Scholar]
- 9.Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA: Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Cancer Res 1992, 52:6949-6952 [PubMed] [Google Scholar]
- 10.Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A: Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol 1992, 148:1404-1410 [PubMed] [Google Scholar]
- 11.Jennings MT, Maciunas RJ, Carver R, Bascom CC, Juneau P, Misulis K, Moses HL: TGF beta 1 and TGF beta 2 are potential growth regulators for low grade and malignant gliomas in vitro: evidence in support of an autocrine hypotesis. Int J Cancer 1991, 49:129-139 [DOI] [PubMed] [Google Scholar]
- 12.Olofsson A, Miyazono K, Kanzaki T, Colosetti P, Engstrom U, Heldin CH: Transforming growth factor-beta 1, -beta 2, and -beta 3 secreted by a human glioblastoma cell line. Identification of small and different forms of large latent complexes. J Biol Chem 1992, 267:19482-19488 [PubMed] [Google Scholar]
- 13.Albino AP, Davis BM, Nanus DM: Induction of growth factor RNA expression in human malignant melanoma: markers of transformation. Cancer Res 1991, 51:4815-4820 [PubMed] [Google Scholar]
- 14.Rodeck U, Melber K, Kath R, Menssen HD, Varello M, Atkinson B, Herlyn M: Constitutive expression of multiple growth factor genes by melanoma cells but not normal melanocytes. J Invest Dermatol 1991, 97:20-26 [DOI] [PubMed] [Google Scholar]
- 15.Rodeck U, Bossler A, Graeven U, Fox F, Nowell P, Kari C: Transforming growth factor-beta production and responsiveness in normal human melanocytes and melanoma cells. Cancer Res 1994, 54:575-581 [PubMed] [Google Scholar]
- 16.Torre-Amione G, Beauchamp RD, Koeppen H, Park BH, Schreiber H, Moses HL, Rowley DA: A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance. Proc Natl Acad Sci USA 1990, 87:1486-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fakhrai H, Dorigo O, Shawler DL, Lin H, Mercola D, Black KL, Royston I, Sobol RE: Eradication of established intracranial rat gliomas by transforming growth factor beta antisense gene therapy. Proc Natl Acad Sci USA 1996, 93:2909-2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT: Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest 1993, 92:2569-2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrann M, Bodmer S, de Martin R, Siepl C, Hofer Warbinck R, Frei J, Hofer E, Fontana A: T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J 1987, 6:1633-1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruffini PA, Rivoltini L, Silvani A, Boiardi A, Parmiani G: Factors, including transforming growth factor beta, released in the glioblastoma residual cavity, impair activity of adherent lymphokine-activated killer cells. Cancer Immunol Immunother 1993, 36:409-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR: Interleukin-10. Annu Rev Immunol 1993, 11:165-190 [DOI] [PubMed] [Google Scholar]
- 22.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM: IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol 1993, 151:1224-1234 [PubMed] [Google Scholar]
- 23.Fernandez-Botran R, Sanders VM, Mosmann TR, Vitetta ES: Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med 1988, 168:543-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorentino DF, Bond MW, Mosmann TR: Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 1989, 170:2081-2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu DH, Moore KW, Spits H: Differential effects of IL-4 and IL-10 on IL2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int J Immunol 1992, 4:563-569 [DOI] [PubMed] [Google Scholar]
- 26.Gastl GA, Abrams JS, Nanus DM, Oosterkamp R, Silver J, Liu F, Chen M, Albino AP, Bander NH: Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer 1993, 55:96-101 [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura R, Modlin R, Wollman J, Dubinett SM: Human non-small-cell lung cancer cells express a type 2 cytokine pattern. Cancer Res 1995, 55:3847-3851 [PubMed] [Google Scholar]
- 28.Kruger-Krasagakis S, Krasagakis K, Garbe C, Diamantstein T: Production of cytokines by human melanoma cells and melanocytes. Recent Results Cancer Res 1995, 139:155-168 [DOI] [PubMed] [Google Scholar]
- 29.Lattime EC, Mastrangelo MJ, Bagasra O, Li W, Berd D: Expression of cytokine mRNA in human melanoma tissues. Cancer Immunol Immunother 1995, 41:151-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dummer W, Becker JC, Schwaaf A, Leverkus M, Moll T, Brocke EB: Elevated serum levels of interleukin-10 in patients with metastatic malignant melanoma. Melanoma Res 1995, 5:67-68 [DOI] [PubMed] [Google Scholar]
- 31.Warshaw AL, Fernandez-del Castillo C: Pancreatic carcinoma. N Engl J Med 1992, 326:455-465 [DOI] [PubMed] [Google Scholar]
- 32.Bellone G, Silvestri S, Artusio E, Tibaudi D, Turletti A, Geuna M, Giachino C, Valente G, Emanuelli G, Rodeck U: Growth stimulation of colorectal carcinoma cells via the c-kit receptor is inhibited by TGF-beta 1. J Cell Physiol 1997, 172:1-11 [DOI] [PubMed] [Google Scholar]
- 33.Behringer DM, Sunderer B, Andersson U, Kresin V, Mertelsmann R, Lindemann A: Simultaneous detection of cytokine and immunophenotype at the single cell level by immunoenzymatic double staining. Histochem J 1996, 28:461-466 [DOI] [PubMed] [Google Scholar]
- 34.Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U: Regulation of NK cell functions by TGF-beta 1. J Immunol 1995, 155:1066-1073 [PubMed] [Google Scholar]
- 35.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste-Amezaga M, Chan SH, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolfe SF, Trinchieri G: Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med 1992, 176:13871-13898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siepl C, Bodmer S, Frei K, Mac Donald HR, De Martin R, Hofer E, Fontana A: The glioblastoma-derived T cell suppressor factor/transforming growth factor-beta 2 inhibits T cell growth without affecting the interaction of interleukin 2 with its receptor. Eur J Immunol 1988, 18:593-600 [DOI] [PubMed] [Google Scholar]
- 37.Ruegemer JJ, Ho SN, Augustine JA, Schlager JW, Bell MP, McKean DJ, Abraham RT: Regulatory effects of transforming growth factor-beta on IL-2- and IL-4-dependent T cell-cycle progression. J Immunol 1990, 144:1767-1776 [PubMed] [Google Scholar]
- 38.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS: Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med 1986, 163:1037-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rousset F, Garcia E, Defrance T, Peonne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J: Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA 1992, 89:1890-1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Waal Malefyt R, Yssel H, de Vries JE: Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells specific inhibition of IL-2 production and proliferation. J Immunol 1993, 150:4754-4765 [PubMed] [Google Scholar]
- 41.Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, Lane HC, Fauci AS: Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol 1986, 136:3916-3920 [PubMed] [Google Scholar]
- 42.Liew FY: Induction and regulation of CD4+ T cell subsets. Ciba Found Symp 1994, 187:170-175 [DOI] [PubMed] [Google Scholar]
- 43.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P: IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol 1995, 154:5320-5330 [PubMed] [Google Scholar]
- 44.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A: IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991, 146:3444-3451 [PubMed] [Google Scholar]
- 45.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T: T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol 1994, 24:793-798 [DOI] [PubMed] [Google Scholar]
- 46.Maeda H, Shiraishi A: TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J Immunol 1996, 156:73-78 [PubMed] [Google Scholar]
- 47.Kong FM, Anscher MS, Murase T, Abbott BD, Iglehart JD, Jirtle RL: Elevated plasma transforming growth factor-beta 1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann Surg 1995, 222:155-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, di Pierro F, De Giovanni C, Musso T, Forni G: Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol 1995, 155:3112-3123 [PubMed] [Google Scholar]
- 49.Kundu N, Beaty TL, Jackson MJ, Fulton AM: Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. J Natl Cancer Inst 1996, 88:536-541 [DOI] [PubMed] [Google Scholar]
- 50.Ishizaka S, Yoshikawa M, Tsujii T: Immunoregulatory effects of transforming growth factor-beta in a prolonged period of culture. Cell Immunol 1992, 139:239-247 [DOI] [PubMed] [Google Scholar]
- 51.Kasid A, Bell GI, Director EP: Effects of transforming growth factor-beta on human lymphokine-activated killer cell precursors autocrine inhibition of cellular proliferation and differentiation to immune killer cells. J Immunol 1988, 141:690-698 [PubMed] [Google Scholar]
- 52.Fox FE, Ford HC, Douglas R, Cherian S, Nowell PC: Evidence that TGF-beta can inhibit human T-lymphocyte proliferation through paracrine and autocrine mechanisms. Cell Immunol 1993, 150:45-58 [DOI] [PubMed] [Google Scholar]
- 53.Kehrl JH, Taylor AS, Delsing GA, Roberts AB, Sporn MB, Fauci AS: Further studies of the role of transforming growth factor-beta in human B cell function. J Immunol 1989, 143:1868-1874 [PubMed] [Google Scholar]
- 54.Lee HM, Rich S: Co-stimulation of T cell proliferation by transforming growth factor-beta 1. J Immunol 1991, 147:1127-1133 [PubMed] [Google Scholar]
- 55.Itoh K, Hirohata S: The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol 1995, 154:4341-4350 [PubMed] [Google Scholar]
- 56.Huang M, Sharma S, Mao JT, Dubinett SM: Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol 1996, 157:5512-5520 [PubMed] [Google Scholar]
- 57.Katamura K, Shintaku N, Yamauchi Y, Fukui T, Ohshima Y, Mayumi M, Furusho K: Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J Immunol 1995, 155:4604-4612 [PubMed] [Google Scholar]
- 58.Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU: Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother 1996, 42:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamura M, Modlin RL, Ohmen JD, Moy RL: Local expression of antiinflammatory cytokines in cancer. J Clin Invest 1993, 91:1005-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tahara H, Zeh HJ, Storkus WJ, Pappo I, Watkins SC, Gubler U, Wolf SF, Robbins PD, Lotze MT: Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res 1994, 54:182-189 [PubMed] [Google Scholar]
- 61.Pericle F, Giovarelli M, Colombo MP, Ferrari G, Musiani P, Cavallo F, Di Pierro F, Novelli F, Forni G: An efficient Th2-type memory follows CD8+ lymphocyte-driven and eosinophil-mediated rejection of a spontaneous mouse mammary adenocarcinoma engineered to release IL-4. J Immunol 1994, 153:5659-5673 [PubMed] [Google Scholar]
- 62.Pardoll DM: Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol 1995, 13:399-415 [DOI] [PubMed] [Google Scholar]
- 63.Rodolfo M, Zilocchi C, Melani C, Capetti B, Arioli I, Parmiani G, Colombo MP: Immunotherapy of experimental metastases by vaccination with interleukin gene-transduced adenocarcinoma cells sharing tumor-associated antigens. Comparison between IL-12 and IL-2 gene-transduced tumor cell vaccines. J Immunol 1996, 157:5536-5542 [PubMed] [Google Scholar]
- 64.Apostolopoulos V, Xing PX, McKenzie IF: Murine immune response to cells transfected with human MUC1: immunization with cellular and synthetic antigens. Cancer Res 1994, 54:5186-5193 [PubMed] [Google Scholar]