Abstract
Melanocytic nevus cells in the dermis adopt many morphological features of Schwann cells. These differentiation-related changes typically are not observed in melanomas. However, nevus cells do not fully recapitulate a Schwann cell phenotype, because they lack expression of mature myelin-associated proteins. In this study, melanocytic nevi and malignant melanomas were examined by immunohistochemistry for expression of low-affinity nerve growth factor receptor (p75NGFR), neural cell adhesion molecule (CD56/N-CAM), and growth-associated phosphoprotein-43 (GAP-43). These three proteins define the earliest stages of Schwann cell development but are not expressed in myelinated Schwann cells or normal melanocytes. p75NGFR was expressed in 25 of 25 (100%) and CD56/N-CAM and GAP-43 in 23 of 25 (92%) nevi, predominantly in type C nevus cells and nevic corpuscles. Most (84%) of the nevi expressed all three proteins. In primary invasive and metastatic melanoma, expression of each of the three proteins was limited to ≤20% of lesions but was not observed in any melanoma in situ (χ2 P < 0.0001). None of the melanomas expressed all three proteins (ANOVA P < 0.0001). These data confirm and extend earlier studies by showing that terminal differentiation of melanocytes in the dermis recapitulates some aspects observed in the earliest stages of Schwann cell development and that invasive melanomas follow a divergent pathway. Studying these early differentiation events may help to identify specific defects in the relevant signaling pathways and establish tenable targets for therapy of advanced-stage melanoma.
Tumor progression in cutaneous malignant melanoma occurs as a multistep process beginning with the loss of cell cycle control and the dysregulated proliferation of intraepidermal melanocytes, followed by additional genetic perturbations that permit tumor cells to invade and migrate through the dermis, gain access to vascular spaces, and eventually metastasize. 1 Critical to the later stages of this process is the interaction between invasive melanoma cells and the dermis. Consequently, to understand these interactions in melanoma, it is necessary first to explore the usual relationship that occurs between the dermis and nonmalignant melanocytes.
Under normal circumstances, intraepidermal melanocytes proliferate infrequently unless subjected to chronic UV exposure. 2-4 If proliferation continues, melanocytes may aggregate to form a melanocytic nevus. 5,6 These nevomelanocytes or “nevus cells” may acquire the ability to migrate from the epidermis into the dermis, where they are subjected to cellular and stromal interactions characteristic of this location. It is generally believed that the normal orderly sequence of growth of melanocytic nevi begins with limited and controlled proliferation of cells in the epidermis, followed by penetration into the dermis, where the nevus cells withdraw from the cell cycle, undergo a final terminal differentiation, and become senescent. 5,6 This differentiation or “maturation” sequence in the dermis results in identifiable changes in morphology, gene expression, and biochemical function of the nevus cells. 6 Morphologically, nevus cells situated in the epidermis and superficial dermis are rounded or “epithelioid” in appearance, are functionally equivalent to epidermal melanocytes in their ability to synthesize melanin, and are termed “type A” nevus cells. As nevus cells migrate into deeper portions of the dermis, however, they undergo a transition whereby they produce less melanin, withdraw from the cell cycle, and become smaller, forming so-called type B nevus cells. With further penetration into the dermis, the cells produce little if any melanin, continue to diminish in size, and adopt a fusiform morphology representing that of “type C” nevus cells. In long-standing intradermal nevi, type C areas may be the predominant component and likely represent a final stage of cellular senescence before involution. 5-7
Type C nevus cells in many ways morphologically resemble neural crest-derived Schwann cells of the peripheral nervous system. 6 First, the deeper dermal type C areas of nevi often contain a complex network of small (<1 μm) axons around which the nevus cells aggregate, giving the appearance of axon ensheathment at the level of light microscopy. 8,9 Furthermore, type C cells can produce structures that recapitulate the morphology of Wagner-Meissner corpuscles, specialized tactile sensory organs formed by Schwann cells in the dermis of acral skin. 6 We have shown that, like Wagner-Meissner corpuscles, “nevic corpuscles” are invested by numerous small axons. 10,11 However, type C nevus cells are not identical to mature myelinated Schwann cells, because these two cell types maintain significant differences that relate to their ultimate function. For example, although type C nevus cells are tightly apposed to small nerves, they do not truly ensheath them, as do unmyelinated Schwann cells. 8 In addition, type C nevus cells lack the myelin figures observed in myelinated Schwann cells; correspondingly, most proteins related to full myelin production have not been demonstrated in type C nevus cells. 8,12,13 However, type C nevus cells and nevic corpuscles express the Schwann cell-related antigen AHMY-1, identified as an epitope shared by myelin proteins P0, P1, and myelin basic protein (MBP), indicating that type C nevus cells and nevic corpuscles undergo partial activation of myelination programs. 14
We hypothesize that because type C nevus cells and nevic corpuscles have no known similar physiologically relevant function (unlike Schwann cells), the development of Schwann cell characteristics is an example of transdifferentiation in which these cells access an alternative differentiation program. As melanocytes, Schwann cells, and neurons all descend from the same neural crest progenitor, it is plausible that each of these cell types retains a degree of plasticity between the differentiation pathways that ultimately determines cellular function and can be reactivated by specific signals. 15,16 Further support for the idea that type C nevus cells and Schwann cells use overlapping differentiation programs is the fact that Schwann cells can have melanocytic properties, including melanin production. 17
In contrast to the observed morphological and biochemical changes indicative of terminal differentiation from the proliferation-competent type A to the proliferation-incompetent type C nevus cell, invasive melanoma cells do not typically undergo a similar transition. Instead, invasive melanoma cells continue to proliferate and may even adopt a phenotype that includes the expression of some proteins more typical of neurons than of Schwann cells. 10,18-20 Together, these observations strongly suggest that there is divergence between the neural crest differentiation programs activated by normal melanocytes and by invasive melanoma cells on entry into the dermis.
The purpose of the present study was twofold. First, given the similarities between Schwann cells and type C nevus cells and nevic corpuscles, it was determined whether unique markers of early Schwann cell differentiation are expressed in vivo during nevus cell differentiation in the dermis, because other studies have shown that proteins associated with mature Schwann cells, including products of many of the myelin-associated genes, are not expressed in nevi. 8,12-14,21,22 Expression of the low-affinity nerve growth factor receptor (p75NGFR), neural cell adhesion molecule (CD56/N-CAM), and growth-associated phosphoprotein-43 (GAP-43) was evaluated because these proteins define the unmyelinated and premyelinating/promyelinating stages of Schwann cell development but are not expressed by mature myelinated Schwann cells or by normal melanocytes. 23,24 Second, expression of these same proteins in vivo was evaluated during the histologically recognizable stages of tumor progression in cutaneous malignant melanoma. These studies may lead to the recognition of specific defects in the signal transduction pathways that control differentiation and thus may help to establish targets for novel therapies to alter the biological behavior of otherwise late-stage melanomas.
Materials and Methods
Tissues
All tissues were obtained from the archives of formalin-fixed, paraffin-embedded tissues at the Department of Pathology, New York Presbyterian Hospital–Cornell Medical Center. The study included 25 benign melanocytic nevi (BMN) and 50 malignant melanomas representing histologically recognizable stages of tumor progression, including 10 melanomas in situ (MISs); 30 primary invasive melanomas (PIMs); and 10 metastatic melanomas (METs). The BMN were selected for the presence of prominent type C areas and nevic corpuscles. For the PIM group, lesions were further characterized as superficially invasive (<1.0 mm thick, n = 13) or deeply invasive (>1.0 mm thick, n = 17). Desmoplastic and neurotropic melanomas were not included in the study, because in our patient population, this rare variant of melanoma represented fewer than 1% of primary invasive melanomas accessioned. All tissues were cut at 5-μm thickness, mounted on positively charged glass microscope slides (ProbeOn Plus; Fisher Scientific, Pittsburgh, PA), deparaffinized, rehydrated through a graded series of ethanol, and submitted to appropriate epitope retrieval methods (described below) before immunohistochemistry.
Immunohistochemistry
The primary antibodies used in this study previously were characterized as follows. The monoclonal anti-p75NGFR antibody (clone ME82–11; Boehringer-Mannheim, Indianapolis, IN) was raised against membrane fragments of the melanoma cell line WM-9. 25 This antibody reacts with human p75NGFR and has been well characterized with respect to specificity and tissue distribution. The well-characterized monoclonal anti-CD56/N-CAM (clone 123C3; NeoMarkers, Fremont, CA) was raised against the membrane fraction of a small cell lung carcinoma and recognizes native (150 kd) and glycosylated (185 kd) isoforms of human CD56/N-CAM. 26 The monoclonal anti-GAP-43 (clone 5E7; Oncogene Research Products, Cambridge, MA) is one of several well-characterized antibodies raised against rat full-length GAP-43, which also reacts with human GAP-43, given the highly conserved peptide sequence between vertebrates. 27 Each of these antibodies labels unmyelinated Schwann cells but not mature myelinated Schwann cells of the peripheral nervous system or melanocytes.
Before immunohistochemistry, tissues were treated with an appropriate epitope retrieval method to optimize immunoreactivity. For the antibodies specific for p75NGFR (final concentration 0.5 μg/ml) and GAP-43 (final concentration 1 μg/ml), tissues were first digested with pepsin (Sigma Chemical Co., St. Louis, MO; 0.25% w/v in TBS: 150 mM NaCl, 50 mM Tris·HCl, pH 2.0) for 5 minutes at 45°C. For the antibody specific for CD56/NCAM (final concentration 4 μg/ml), tissues were subjected to heat-induced epitope retrieval 28 by immersion of the tissue sections in 10 mM Na·citrate (pH 6.0) for 20 minutes at 95°C, followed by cooling in the same buffer for an additional 20 minutes at room temperature (rt).
Immunohistochemistry was performed as described previously. 29 Briefly, tissue sections were incubated with primary antibody for 1 hour at rt (anti-p75NGFR and anti-GAP-43) or for 16 hours at 4°C (anti-CD56/N-CAM). After appropriate washes, the primary antibodies were localized with biotinylated secondary horse anti-mouse immunoglobulin (Vector Laboratories, Burlingame, CA) for 8 minutes at 45°C, followed by a streptavidin alkaline phosphatase detection system (Boehringer-Mannheim) for 10 minutes at 45°C, and finally by chromogen (Vector Red; Vector Laboratories) for 10 minutes at rt. All tissues were counterstained with hematoxylin, permanently mounted, and viewed with a standard light microscope. A tissue was considered to be positively labeled if lesional melanocytes contained visually detectable red chromogen in a membrane and/or cytoplasmic pattern. The distribution of immunolabeled cells within a given lesion was also noted (ie, intraepidermal and/or intradermal). For immunolabeled BMN, the specific cell types labeled (ie, type A, B, and C nevus cells; nevic corpuscles) were noted.
Small cutaneous nerves served as internal positive controls in each tissue section, because all three proteins are expressed in unmyelinated Schwann cells. As a negative control, selected tissue sections were incubated with a monoclonal mouse IgG of irrelevant specificity (anti-measles virus).
Statistical Analysis
Statistical comparisons were made between diagnostic groups for the expression of a given protein, using the χ 2 test. To evaluate the diagnostic groups for the usual expression phenotype for all three proteins, analysis of variance (ANOVA) was performed together with post hoc analysis, using the Scheffé test. Comparisons of expression between superficially and deeply invasive PIMs were made with the t-test. A result was considered significant at or below a P value of 0.05. All analyses were performed with Statview software (version 4.5; Abacus Concepts, Berkeley, CA).
Results
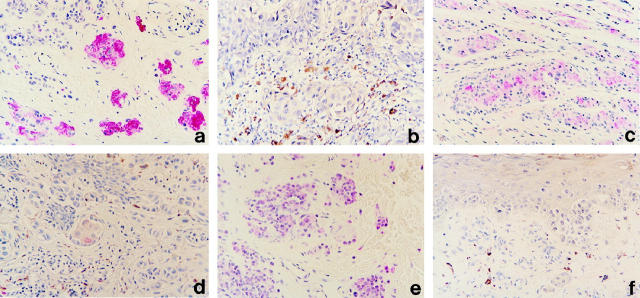
Protein expression of p75NGFR, CD56/N-CAM, and GAP-43 in representative melanocytic lesions is shown in Figure 1 ▶ , and all results are summarized in Table 1 ▶ . p75NGFR was detected in 100% (25/25) of the BMN studied, with labeling occurring exclusively in the type C nevus cells and in nevic corpuscles (Figure 1a) ▶ . Almost all of the type C nevus cells and nevic corpuscles were labeled. In contrast to BMN, only 12% (6/50) of melanomas expressed p75NGFR (χ2 P < 0.0001) (Figure 1b) ▶ . Most of the labeled melanomas were PIMs (5/30). In these lesions, expression was restricted to small foci, accounting for less than 10% of the tumor cells, and usually was limited to the invasive component of the lesion. For PIMs that contained labeled intraepidermal melanoma cells, the invasive dermal component always was labeled as well. PIMs that expressed p75NGFR were more deeply invasive (mean depth 1.78 mm ± 0.57 mm SE) than those that lacked expression (mean depth 0.82 mm ± 0.14 mm SE) (t-test P = 0.020). None of the MIS (0/10) and only 10% of the MET (1/10) expressed detectable levels of p75NGFR. For the one MET that expressed p75NGFR, less than 10% of the tumor cells were labeled.
Figure 1.
Protein expression of p75NGFR, CD56/N-CAM, and GAP-43 in BMN and PIM. a and b: p75NGFR in BMN (a) and PIM (b). Note expression in nevic corpuscles and lack of expression in melanoma cells. c and d: CD56/N-CAM in BMN (c) and PIM (d). Similar to p75NGFR, note expression in most nevic corpuscles, but only rare labeled melanoma cells. e and f: GAP-43 in BMN (e) and PIM (f). The greatest level of labeling is seen in type A and B nevus cells, but there is no labeling in melanoma cells. Immunohistochemistry was performed as described in the text: Vector Red chromogen, hematoxylin counterstain.
Table 1.
Summary of Results
| Lesion | p75NGFR | CD56/N-CAM | GAP-43 |
|---|---|---|---|
| BMN | 25/25 (100%)* | 23/25 (92%)* | 23/25 (92%)† |
| MIS | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) |
| PIM | 5/30 (17%) | 5/30 (17%) | 6/30 (20%) |
| MET | 1/10 (10%) | 1/10 (10%) | 0/10 (0%) |
Results are expressed as number of lesions expressing the protein/number of lesions studied in that diagnostic group (and the corresponding percentage positive).
*Labeling occurred exclusively in type C nevus cells and nevic corpuscles.
†Labeling occurred in types A, B, and C nevus cells and nevic corpuscles, but the greatest intensity of labeling occurred in types A and B nevus cells.
The expression of CD56/N-CAM in BMN was similar to that observed for p75NGFR in that labeling was common in BMN but uncommon in melanomas. For BMN, 92% (23/25) of the lesions contained CD56/N-CAM. Like p75NGFR, expression occurred exclusively in the type C nevus cells and in nevic corpuscles (Figure 1c) ▶ . Unlike BMN, however, only 12% (6/50) of melanomas expressed CD56/N-CAM (χ2P < 0.0001) (Figure 1d) ▶ . Most of the labeled melanomas were PIMs (5/30). In these lesions, expression was always limited to small foci of tumor cells in the invasive dermal component of the lesion. Unlike p75NGFR, there was no significant difference in the mean depth of invasion between those PIMs that expressed CD56/N-CAM and those that did not (t-test P = 0.406). None of the MISs (0/10) and only 10% of the METs (1/10) expressed detectable levels of CD56/N-CAM. For the one MET that expressed CD56/N-CAM, approximately half of the tumor cells were labeled.
GAP-43 was expressed in 92% (23/25) of the BMN studied. Like the other two markers of early Schwann cell differentiation, GAP-43 was expressed in type C nevus cells and in nevic corpuscles; however, the intensity of immunolabeling was considerably less and occurred in a smaller percentage of cells (<50%) than that observed for p75NGFR or CD56/N-CAM. However, 64% (16/25) of the BMN also demonstrated stronger immunolabeling in the type A and type B nevus cells (Figure 1e) ▶ , a feature never observed for p75NGFR or CD56/N-CAM. This phenotype was confirmed using a different monoclonal antibody (clone 1G7; Novocastra Laboratories, Newcastle upon Tyne, England) raised against a recombinant prokaryotic fusion protein containing the human complete GAP-43 molecule. For this validation, 13 selected BMN from the group evaluated with 5E7 were studied with 1G7. Using this antibody, 92% (12/13) of the original BMN expressed GAP-43 in a distribution identical to that observed for 5E7.
Unlike BMN, only 12% (6/50) of melanomas expressed GAP-43 (χ2P < 0.0001) (Figure 1f) ▶ . All of the melanomas that expressed GAP-43 were PIMs (6/30); none of the MISs (0/10) or METs (0/10) were labeled. In PIMs, expression of GAP-43 was limited to small foci representing less than 10% of the tumor cells and generally occurred in the invasive dermal component of the lesions. Two of the PIMs, however, expressed GAP-43 only in the intraepidermal component of the lesions, a feature never observed for p75NGFR or CD56/N-CAM. There was no significant difference in the mean depth of invasion between those PIMs that expressed GAP-43 and those that did not (t-test P = 0.369).
It is of interest that none of the PIMs, MISs, or METs expressed all three of the early Schwann cell differentiation-related proteins, but 3/6 of the PIMs that expressed GAP-43 also expressed either CD56/N-CAM or p75NGFR. In contrast, 84% (21/25) of the BMN expressed all three of the early Schwann cell-related proteins (ANOVA P < 0.0001 overall; Scheffé post hoc analysis P < 0.0001 between BMN and each melanoma diagnostic category, ie, MIS, PIM, MET). Control tissues incubated with a mouse monoclonal antibody of irrelevant specificity (anti-measles virus) were uniformly negative.
Discussion
In this report, we demonstrate that Type C nevus cells and nevic corpuscles express three proteins that also define early stages of Schwann cell differentiation. These findings provide confirmatory evidence that nevus cell differentiation is at least in part related to Schwann cell differentiation and extend earlier studies by more precisely defining the stage of Schwann differentiation recapitulated by melanocytes during their normal orderly process of terminal differentiation in the dermis. Furthermore, this differentiation program does not occur during tumor progression in malignant melanomas.
p75NGFR is one of the earliest proteins to be expressed by migrating neural crest stem cells, even before they are committed to entering the Schwann cell differentiation program. 23,24 p75NGFR is a membrane-associated receptor tyrosine kinase (RTK) that serves as a major receptor for the neurotrophins. p75NGFR functions in association with a family of other RTKs encoded by the Trk family of related genes 30,31 and may also cooperate with other growth factors to act as a Schwann cell survival factor. 32 The expression of p75NGFR remains high in precursor Schwann cells as they proliferate and establish initial connections with axons. High levels of p75NGFR also persist indefinitely in unmyelinated Schwann cells, but expression ceases on exit from the cell cycle and acquisition of the myelinated phenotype. 23
Unlike Schwann cells, however, a role for p75NGFR in melanocyte differentiation remains obscure. The gene for p75NGFR is positioned on chromosome 17q21-ter. 33 Aberrations involving this chromosomal region are rare in melanoma, 1 and the expression of p75NGFR is infrequent in primary invasive lesions. 13,34 We also found that unlike benign nevi in which expression was usual in type C nevus cells and nevic corpuscles, p75NGFR was found in only a minority of invasive melanomas and metastatic melanomas. The primary melanomas that expressed p75NGFR were predominantly deeply invasive lesions in which labeling was limited to small foci of tumor cells. Despite the fact that p75NGFR is not expressed in melanoma in situ and is expressed in low frequency in primary invasive and metastatic melanomas, it is up-regulated in approximately half of immortalized cell lines established from melanoma metastases because of increased transcription of the gene. 35 In fact, the monoclonal anti-p75NGFR antibody used in this study was raised against the membrane fraction from a metastatic melanoma cell line. 25 The biological significance of this observation is unclear because p75NGFR was detected in only 10% of the in vivo metastases included in this study. It has been suggested that p75NGFR may contribute to metastatic competence or organ tropism. 36-38 It is of particular interest that the expression of p75NGFR has been described in desmoplastic and neurotropic melanomas, 39,40 rare subtypes of primary melanoma believed to recapitulate perineurial, endoneurial, or Schwann cell-like characteristics. 41 As mentioned above, these melanoma variants were not included in the present study, because in our patient population, they were extremely rare tumors representing fewer than 1% of all primary invasive melanomas accessioned and thus were not representative of the usual differentiation-related changes in primary melanomas.
The second protein that defines early stages of Schwann cell development is CD56/N-CAM. CD56/N-CAM is a member of the immunoglobulin superfamily that exists in at least eight isoforms. 42 CD56/N-CAM mediates Ca2+-independent homophilic intercellular attachments and likely functions in cell migration during embryogenesis as well as contributing to the induction and maintenance of differentiation in some cell types. 42,43 The 150-kd isoform of CD56/N-CAM is expressed in precursor and unmyelinated Schwann cells and has been identified in Wagner-Meissner corpuscles. 44 Like p75NGFR, the expression of CD56/N-CAM is extinguished by Schwann cells with a mature myelinated phenotype. 23 The gene for CD56/N-CAM is positioned on chromosome 11q23, a site rarely deleted in melanomas. 1,45 In this study, the pattern of expression of CD56/N-CAM was similar to that observed for p75NGFR in that it was present in type C nevus cells and nevic corpuscles, but usually absent in melanomas.
The third protein that defines early stages of Schwann cell differentiation is GAP-43. Concomitant with axonal contact, migrating precursor Schwann cells begin to express GAP-43 (also known as B50, F1, neuromodulin, and pp46). 23,24 GAP-43 is a highly conserved protein among vertebrates and has been demonstrated in both precursor Schwann cells and mature unmyelinated Schwann cells of the peripheral nerves in humans. 46 Although the precise range of functions of this protein remains unknown, GAP-43 is regulated by protein phosphorylation and is a natural substrate for protein kinase C (PKC). 27,47,48 In its active phosphorylated state, GAP-43 can bind actin and mediate the formation of filopodia and changes in cell shape. 49 In this capacity, GAP-43 may influence the plasticity and motility of the unmyelinated Schwann cell, whose function is to associate with multiple small axons. Another potentially important role for GAP-43 is its ability to reduce intracellular levels of the second messenger cAMP. 47 GAP-43 is expressed in high levels in precursor and unmyelinated Schwann cells, but the level of expression falls as cells reach the promyelinating stage of differentiation. 23 Because many of the early myelin genes are positively regulated by cAMP, 23,50-52 accordingly, it is believed that GAP-43 may serve a major role in maintaining the unmyelinated phenotype.
In this study, GAP-43 expression was observed in type A, B, and C nevus cells as well as in nevic corpuscles, although the apparent level of expression was low relative to that observed for p75NGFR and CD56/N-CAM. Because the antibodies used in this study react with both phosphorylated and unphosphorylated forms of GAP-43, it is unknown whether the low levels of expression in nevi are reflective of the level of biological activity. The gene for GAP-43 is positioned on chromosome 3, a site having few documented aberrations in melanoma. 1,53 Similar to p75NGFR and CD56/N-CAM, GAP-43 typically was not expressed in melanomas.
Based on our data, it can be concluded that melanocytic nevi and malignant melanomas in vivo differ significantly with respect to the expression of proteins related to early differentiation events in Schwann cells. Our data also show that among melanocytic nevi, the expression of Schwann cell-related proteins is dependent on the phenotype of the nevus cells (ie, expression occurs predominantly in type C nevus cells and nevic corpuscles) and that expression of p75NGFR and CD56/N-CAM in nevi is apparently more abundant than is GAP-43. In normal Schwann cell differentiation, the expression of GAP-43 is down-regulated before the reduction of p75NGFR protein levels as cells enter the promyelinating stage of development and before the expression of the major myelin protein genes. 23 Thus, based on the observed protein expression pattern, type C nevus cells and nevic corpuscles most closely recapitulate the phenotype of cells at the premyelinating/promyelinating Schwann cell border, a stage of Schwann cell development defined by the down-regulation of GAP-43, early expression of myelin-associated proteins, and withdrawal from the cell cycle. Additional support for this conclusion comes from independent studies that noted the expression of a myelin protein-related epitope shared by P0, P1, and myelin basic protein in type C nevus cells and nevic corpuscles, but the lack of expression of proteins characteristic of fully myelinated Schwann cells such as P2, the proteolipid protein (PLP), myelin-associated glycoprotein (MAG), CD57, and CD9. 8,12-14,22
Our data show that terminal differentiation of melanocytes in the dermis recapitulates aspects observed in the early stages of Schwann cell development. In contrast, most melanomas follow a divergent differentiation pathway that is not used by nevus cells or Schwann cells. It should be noted that the expression of these proteins observed in only a few invasive and metastatic melanomas likely represents additional perturbations secondary to tumor progression because melanomas in situ universally lacked expression. Alternatively, it is possible that the low frequency of expression of these proteins in melanoma represents an incomplete or failed attempt at normal differentiation programs in the dermis. Thus, it is possible that the reported expression of p75NGFR in desmoplastic and neurotropic melanomas 39,40 is related to partial activation of a more nevus-like or Schwann cell-like differentiation pathway in these tumors. This is an attractive hypothesis, because it has been shown by others that these rare types of melanoma may have a less aggressive clinical course when compared to other melanomas of identical stage and depth of invasion. 54,55 It also seems plausible that the phenotype shared by nevus cells and Schwann cells may include, in addition to p75NGFR, CD56/N-CAM, and GAP-43, other components of the signaling pathways that control differentiation. Further study of these pathways may identify in melanomas specific defects that disrupt the normal melanocyte differentiation program and may establish tenable targets for chemotherapy of advanced-stage lesions.
Footnotes
Address reprint requests to Dr. Jon A. Reed, Section of Dermatopathology, Department of Pathology, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030.
References
- 1.Albino AP, Reed JA, McNutt NS: Molecular biology of cutaneous malignant melanoma. Cancer. Principles and Practice of Oncology. Edited by VT DeVita Jr, S Hellman, and SA Rosenberg. New York, Lippincott-Raven, 1997, pp 1935–1946
- 2.Quevedo WC, Jr, Szabo G, Virks J, Sinesi J: Melanocyte populations in UV-irradiated human skin. J Invest Dermatol 1965, 45:295-298 [DOI] [PubMed] [Google Scholar]
- 3.Mishima Y, Tanay A: The effect of α-methyldopa and ultraviolet irradiation on melanogenesis. Dermatologica 1968, 136:105-114 [DOI] [PubMed] [Google Scholar]
- 4.Rosdahl IK: Melanocyte mitosis in UVB-irradiated mouse skin. Acta Dermatol Venereol 1978, 58:217-221 [PubMed] [Google Scholar]
- 5.Lund HZ, Stobbe GD: The natural history of the pigmented nevus. Factors of age and anatomic location. Am J Pathol 1949, 25:1117-1147 [PMC free article] [PubMed] [Google Scholar]
- 6.Imber MJ, Mihm MC, Jr: Benign melanocytic tumors. Farmer ER Hood AF eds. Pathology of the Skin. 1990, :663-683 Appleton and Lange Norwalk CT [Google Scholar]
- 7.Goovaerts G, Buyssens N: Nevus cell maturation or atrophy. Am J Dermatopathol 1988, 10:20-27 [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Hirose T, Hasegawa T, Seki K, Nakanishi H, Hizawa K: Ultrastructural heterogeneity of acquired intradermal melanocytic nevus cells. Ultrastruct Pathol 1996, 20:255-261 [DOI] [PubMed] [Google Scholar]
- 9.Brocker EB, Magiera H, Herlyn M: Nerve growth and expression of receptors for nerve growth factor in tumors of melanocyte origin. J Invest Dermatol 1991, 96:662-665 [DOI] [PubMed] [Google Scholar]
- 10.Prieto VG, McNutt NS, Lugo J, Reed JA: The intermediate filament peripherin is expressed in cutaneous melanocytic lesions. J Cutan Pathol 1997, 24:145-150 [DOI] [PubMed] [Google Scholar]
- 11.Prieto VG, McNutt NS, Lugo J, Reed JA: Differential expression of the intermediate filament peripherin in cutaneous neural lesions and neurotized melanocytic nevi. Am J Surg Pathol 1997, 21:1450-1454 [DOI] [PubMed] [Google Scholar]
- 12.Gray MH, Smoller BR, McNutt NS, Hsu A: Neurofibromas and neurotized melanocytic nevi are immunohistochemically distinct neoplasms. Am J Dermatopathol 1990, 12:234-241 [DOI] [PubMed] [Google Scholar]
- 13.Argenyi ZB, Rodgers J, Wick M: Expression of nerve growth factor and epidermal growth factor receptors in neural nevi with nevic corpuscles. Am J Dermatopathol 1996, 18:460-464 [DOI] [PubMed] [Google Scholar]
- 14.Aso M, Hashimoto K, Eto H, Fukaya T, Ishihara M, Shimao S, Shimizu Y: Expression of Schwann cell characteristics in pigmented nevus. Cancer 1988, 63:938-943 [DOI] [PubMed] [Google Scholar]
- 15.Le Douarin NM, Ziller C: Plasticity in neural crest cell differentiation. Curr Opin Cell Biol 1993, 5:1036-1043 [DOI] [PubMed] [Google Scholar]
- 16.Murphy M, Reid K, Dutto R, Brooker G, Bartlett PF: Neural stem cells. J Invest Dermatol Symp Proc 1997, 2:8-13 [DOI] [PubMed] [Google Scholar]
- 17.Carbonell AL, Boya J, Garcia-Maurino JE: Presence of melanin in normal human Schwann cells. Histol Histopathol 1992, 7:329-332 [PubMed] [Google Scholar]
- 18.Khare VK, Albino AP, Reed JA: The neuropeptide/mast cell secretagogue substance P is expressed in cutaneous melanocytic lesions. J Cutan Pathol 1998, 25:2-10 [DOI] [PubMed] [Google Scholar]
- 19.Lammerding-Koppel M, Noda S, Blum A, Schaumburg-Lever G, Rassner G, Drews U: Immunohistochemical localization of muscarinic acetylcholine receptors in primary and metastatic malignant melanomas. J Cutan Pathol 1997, 24:137-144 [DOI] [PubMed] [Google Scholar]
- 20.Dhillon AP, Rode J, Leathem A: Neurone specific enolase: an aid to the diagnosis of melanoma and neuroblastoma. Histopathology 1982, 6:81-92 [DOI] [PubMed] [Google Scholar]
- 21.Van Paesschen M-A, Goovaerts G, Buyssens N: A study of the so-called neurotization of nevi. Am J Dermatopathol 1990, 12:242-248 [DOI] [PubMed] [Google Scholar]
- 22.Si Z, Hersey P: Expression of the neuroglandular antigen and analogues in melanoma, CD9 expression appears inversely related to metastatic potential of melanoma. Int J Cancer 1993, 54:37-43 [DOI] [PubMed] [Google Scholar]
- 23.Zorick TS, Lemke G: Schwann cell differentiation. Curr Opin Cell Biol 1996, 8:870-876 [DOI] [PubMed] [Google Scholar]
- 24.Mirsky R, Jessen KR: Schwann cell development, differentiation and myelination. Curr Opin Neurobiol 1996, 6:89-96 [DOI] [PubMed] [Google Scholar]
- 25.Ross AH, Grob P, Bothwell M, Elder DE, Ernst CS, Marano N, Ghrist CC, Herlyn M, Atkinson B, Koprowski H: Characterization of nerve growth factor receptor in neural crest tumors using monoclonal antibodies. Proc Natl Acad Sci USA 1984, 81:6681-6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schol DJ, Mooi WJ, van der Gugten AA, Wagenaar SSc, Hilgers J: Monoclonal antibody 123C3, identifying small cell carcinoma phenotype in lung tumours, recognizes mainly, but not exclusively, endocrine and neuron-supporting normal tissues. Int J Cancer Suppl 2 1988, 2:34–40 [DOI] [PubMed]
- 27.Meiri KF, Bickerstaff LE, Schwob JE: Monoclonal antibodies show that kinase C phosphorylation of GAP-43 during axonogenesis is both spatially and temporally restricted in vivo. J Cell Biol 1991, 112:991-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckstead JH: Improved antigen retrieval in formalin-fixed paraffin-embedded tissue. Appl Immunohistochem 1994, 2:274-281 [Google Scholar]
- 29.Reed JA, Manahan LJ, Park C-S, Brigati DJ: Complete one-hour immunocytochemistry based on capillary action. Biotechniques 1992, 13:434-443 [PubMed] [Google Scholar]
- 30.Chao MV, Hempstead BL: p75 and trk: a two-receptor system. Trends Neurosci 1995, 18:321-326 [PubMed] [Google Scholar]
- 31.Kaplan DR, Miller FD: Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol 1997, 9:213-221 [DOI] [PubMed] [Google Scholar]
- 32.Barbacid M: Structural and functional properties of the trk family of neurotrophin receptors. Ann NY Acad Sci 1995, 766:442-458 [DOI] [PubMed] [Google Scholar]
- 33.Rettig WL, Thomson TM, Spengler BA, Biedler JL, Old LJ: Assignment of human nerve growth factor receptor gene to chromosome 17 and regulation of receptor expression in somatic cell hybrids. Somat Cell Mol Genet 1986, 12:441-447 [DOI] [PubMed] [Google Scholar]
- 34.Thompson SJ, Schatteman GC, Gown AM, Bothwell MA: A monoclonal antibody against nerve growth factor receptor. Immunohistochemical analysis of normal and neoplastic human tissue. Am J Clin Pathol 1989, 92:415-423 [DOI] [PubMed] [Google Scholar]
- 35.Papandreou C, Bogenrieder T, Loganzo F, Chao MV, Nanus DM, Albino AP: Mutation and expression of the low affinity neurotrophin receptor in human malignant melanoma. Melanoma Res 1996, 6:373-378 [DOI] [PubMed] [Google Scholar]
- 36.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA: Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science 1994, 265:1596-1599 [DOI] [PubMed] [Google Scholar]
- 37.Marchetti D, Menter D, Jin L, Nicolson GL: Nerve growth factor effects on human and mouse melanoma cell invasion and heparanase production. Int J Cancer 1993, 55:692-699 [DOI] [PubMed] [Google Scholar]
- 38.Menter DG, Herrmann JL, Nicolson GL: The role of trophic factors and autocrine/paracrine growth factors in brain metastasis. Clin Exp Metastasis 1995, 13:67-88 [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto S, Odland PB, Piepkorn M, Bothwell M: Evidence that the p75 neurotrophin receptor mediates perineural spread of desmoplastic melanoma. J Am Acad Dermatol 1996, 35:725-731 [DOI] [PubMed] [Google Scholar]
- 40.Kanik AB, Yaar M, Bhawan J: p75 nerve growth factor receptor staining helps identify desmoplastic and neurotrophic melanoma. J Cutan Pathol 1996, 23:205-210 [DOI] [PubMed] [Google Scholar]
- 41.Barnhill RL: Tumors of melanocytes. Barnhill RL eds. Textbook of Dermatopathology. 1998, :537-591 McGraw-Hill New York [Google Scholar]
- 42.Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM: Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science 1987, 236:799-806 [DOI] [PubMed] [Google Scholar]
- 43.Miettinen M, Cupo W: Neural cell adhesion molecule distribution in soft tissue tumors. Hum Pathol 1993, 24:62-66 [DOI] [PubMed] [Google Scholar]
- 44.Mechtersheimer G, Staudter M, Moller P: Expression of the natural killer cell-associated antigens CD56 and CD57 in human neural and striated muscle cells and their tumors. Cancer Res 1991, 51:1300-1307 [PubMed] [Google Scholar]
- 45.Nguyen C, Mattei MG, Mattei JF, Santoni MJ, Goridis C, Jordan BR: Localization of the human NCAM gene to band q23 of chromosome 11: the third gene coding for a cell interaction molecule mapped to the distal portion of the long arm of chromosome 11. J Cell Biol 1986, 102:711-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis R, Stewart HJS, Hall SM, Wilkin GP, Mirsky R, Jessen KR: Gap-43 is expressed by nonmyelin-forming Schwann cells of the peripheral nervous system. J Cell Biol 1992, 116:1455-1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strittmatter SM, Valenzuela D, Kennedy TE, Neer EJ, Fishman MC: G0 is a major growth cone protein subject to regulation by GAP-43. Nature 1990, 344:836-841 [DOI] [PubMed] [Google Scholar]
- 48.Oehrlein SA, Parker PJ, Herget T: Phosphorylation of GAP-43 (growth-associated protein of 43 kd) by conventional, novel, and atypical isotypes of the protein kinase C gene family: differences between oligopeptide and polypeptide phosphorylation. Biochem J 1996, 317:219-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Q, Dent EW, Meiri KF: Modulation of actin filament behavior by GAP-43 (neuromodulin) is dependent on the phosphorylation status of serine 41, the protein kinase C site. J Neurosci 1997, 17:3515-3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosse F, Zoidl G, Wilms S, Gillen CP, Kuhn HG, Muller HW: Differential expression of two mRNA species indicates a dual function of peripheral myelin protein PMP22 in cell growth and myelination. J Neurosci Res 1994, 37:529-537 [DOI] [PubMed] [Google Scholar]
- 51.Suter U, Snipes GJ, Schoener-Scott R, Welcher AA, Sangeeta P, Lupski JR, Murphy RA, Shooter EM, Patel PI: Regulation of tissue-specific expression of alternative peripheral myelin protein-22 (PMP22) gene transcripts by two promoters. J Biol Chem 1994, 269:25795-25808 [PubMed] [Google Scholar]
- 52.Kuhn G, Lie A, Wilms S, Muller HW: Coexpression of PMP22 gene with MBP and P0 during de novo myelination and nerve repair. Glia 1993, 8:256-264 [DOI] [PubMed] [Google Scholar]
- 53.Kosik KS, Orecchio LD, Bruns GA, Benowitz LI, MacDonald GP, Cox DR, Neve RL: GAP-43: its deduced amino acid sequence and chromosomal localization in mouse and human. Neuron 1988, 1:127-132 [DOI] [PubMed] [Google Scholar]
- 54.Skelton HG, Smith KJ, Laskin WB, McCarthy WF, Gagnier JM, Graham JH, Lupton GP: Desmoplastic malignant melanoma. J Am Acad Dermatol 1995, 32:717-725 [DOI] [PubMed] [Google Scholar]
- 55.Baer SC, Schultz D, Synnestvedt M, Elder DE: Desmoplasia and neurotropism. Prognostic variables in patients with stage I melanoma. Cancer 1995, 76:2242-2247 [DOI] [PubMed] [Google Scholar]