Abstract
Keratinocyte growth factor (KGF) and hepatocyte growth factor (HGF) are known mitogens for normal alveolar Type 2 cells in vitro and in vivo. We wished to determine whether these two growth factors are involved in lung repair after epithelial cell necrosis by determining the levels of each factor in lung lavage fluid collected serially after bleomycin-induced injury, and how these values relate specifically to proliferation of bronchiolar and alveolar epithelial cells. Rats received an intratracheal injection of 1 unit bleomycin in 0.5 ml water and were killed at intervals up to 4 weeks with 1 μCi/g tritiated thymidine injected 1 hour before death. Early necrosis of bronchiolar epithelial (BR) cells and Type 1 alveolar epithelium was followed by an increase in inflammatory cell numbers and high protein levels in bronchoalveolar lavage (BAL) fluids. In addition, the levels of KGF and HGF, measured by enzyme-linked immunosorbent assay in BAL, increased as early as 3 days and peaked at 7–14 days, when KGF was measured at 160 pg/ml (n = 50) and HGF reached 460 pg/ml (n = 40). Both values dropped sharply after 2 weeks. Epithelial cell proliferation was quantitated as percentage of labeled cells in autoradiographs of methacrylate sections. Labeling of BR cells predominated in the first week and peaked at 7% at 3 days. Type 2 cell proliferation was delayed somewhat but occurred in 3 to 10 days with a peak of 7% labeled cells at 1 week. The results demonstrate that both HGF and KGF are present in the lung in greatly increased amounts soon after bleomycin-induced epithelial cell necrosis. These high levels are associated with both BR and alveolar epithelial cell proliferation.
Widespread necrosis of alveolar and bronchiolar epithelial cells in the lung is often followed by a delayed, sometimes abnormal epithelial regenerative pattern while the fibrotic process predominates. This type of response is seen clinically in examples of fibrosing alveolitis and in various experimental models of this disease. 1-3 Although many studies concentrate on the role of macrophage activation and its cytokine secretions in the evolution of pulmonary fibrosis, there is also evidence that the regulation of epithelial cell regeneration is important in the pathogenesis. Rapid epithelial proliferation can restore the surface layer and prevent fibroblast migration to the air spaces and is associated with control of underlying fibroblasts. 3,4
Identification of molecules that induce pulmonary epithelial cell proliferation is therefore important. Several recent studies have indicated that two cytokines have this potential, namely hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF), also known as fibroblast growth factor-7. These molecules stimulate normal alveolar Type 2 cell proliferation in vivo and in vitro. 5-7 They also appear to promote bronchiolar cell proliferation 7,8 and have been shown to be essential for normal lung development. 9 The role of KGF and HGF in repair is less certain; for example, when KGF is administered after lung injury induced by bleomycin, there is no change in outcome and no accelerated repair. 10 However, if KGF is given before injury is induced, a protective effect is seen; this has been shown in several models of lung injury. 10-12 This leads to the question of what role HGF and KGF play in vivo in stimulating Type 2 cells and bronchiolar epithelial cells to proliferate after injury. We studied this relationship by using intratracheal bleomycin in rats to induce injury to these two pulmonary epithelial cell populations. Subsequently, we measured levels of KGF and HGF in lung lavage fluids at several time points from initial injury to fibrotic repair and correlated levels of these factors specifically with the proliferative responses of both bronchiolar and alveolar epithelial cell populations.
Materials and Methods
Male Sprague-Dawley rats weighing 180 to 200 g were injected intratracheally (i.t.), while under mild barbiturate-induced anesthesia, with 5 U/kg bleomycin (Bristol-Myers Squibb Co., Evansville, IN) in 0.5 ml sterile water. Animals were killed by intraperitoneal (i.p.) barbiturate overdose in groups of 4 at 0, 3, 7, 10, 14, and 28 days after i.t. bleomycin. Each rat received an i.p. injection of tritiated thymidine (3HT) at 1 μm Ci/g 1 hour before death. Two control animals per group received 0.5 ml water only (i.t.) and were killed at the same time intervals.
On each animal, the chest was opened and the lungs allowed to collapse. A tracheotomy was performed and the lungs lavaged twice with 5 ml of Tris-based saline solution containing 10 μl per ml of protease inhibitor cocktail (Sigma, St. Louis, MO). After lavage, the 2 samples per animal, about 9 ml, were pooled then 5 μl of 20% bovine serum albumin and 5 μl of Tween ∼20 were added per milliliter of fluid. An aliquot was taken to count cells by hemocytometer and the remainder was centrifuged to separate the fluid component, which was divided into several aliquots and frozen at −70°C. One aliquot was used later to measure protein content 13 and the remainder to measure growth factors by enzyme-linked immunosorbent assay (ELISA).
Lung Tissue
After lavage, the left lobes of lung were tied off, removed, and frozen for later measurement of total hydroxyproline. 14 The right lobes were re-inflated with buffered formaldehyde overnight, then 6 random tissue blocks approximately 5 × 5 × 8 mm were cut for processing and embedded in glycol methacrylate. Three random blocks per rat were sectioned at a thickness of 0.75 μm and used for autoradiography. 15 On each of the 3 sections, the overall percentage of 3HT-labeled cells was determined after counting 1500 cells per slide. The initial field was set at the pleural surface about midlobe and the field moved systematically back and forth across the lobe until 1500 cells were recorded. The percentages of labeled cells per animal and per group at each time were then calculated. Subsequently, using the same sections, 1000 bronchial and bronchiolar (BR) epithelial cells were identified and the number labeled was recorded. Also, 1000 alveolar Type 2 epithelial cells were identified by their shape, location and lamellar body contents; the number incorporating 3HT was recorded. To obtain enough BR and Type 2 cells, usually the whole sections had to be counted and the numbers then used to calculate the percentages of labeled BR and alveolar epithelial cells at each time interval after administration of bleomycin. In lavaged lungs, any remaining alveolar macrophages were usually round and free from the alveolar wall. These cells were very rarely labeled.
ELISA
The bronchoalveolar lavage (BAL) fluid was used to measure KGF and HGF using standard ELISA methods. Samples of 100 μl of BAL were used for duplicate plate readings at each time interval per animal per group. The antibodies used and the growth factor standards were obtained from R & D Systems (Minneapolis, MN). These agents were designed specifically to measure human growth factors, and we obtained reproducible linear standard values using ELISA on human HGF and KGF diluted in our BAL solution. A protein in the rat lung BAL fluids also bound to each human antibody during a 2-hour incubation period and the ELISA showed linear results on dilution. Measurements were expressed as pg/ml BAL and both assays were sensitive to a level of 5 pg/ml.
In a subsequent test, we obtained an ELISA kit for rat HGF (Institute of Immunology, Tokyo). We ran one set of the above BAL fluids and the growth factor controls with 22-hour incubation at room temperature using instructions provided by the manufacturer. The level of rat HGF was quantitated by ELISA. Samples of lavage fluid were also incubated with antibody to rat HGF to test whether it neutralized the factor measured by ELISA.
Results
Lung Injury
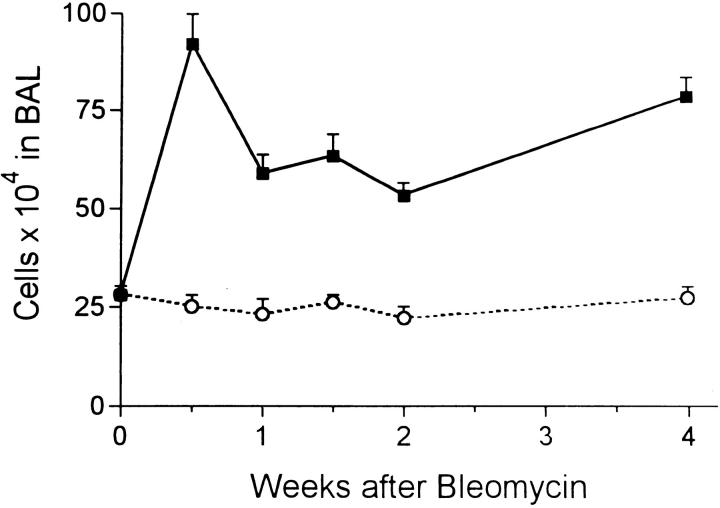
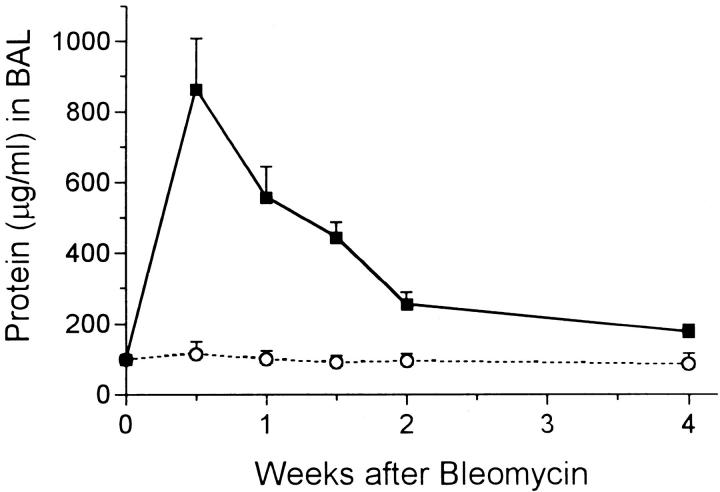
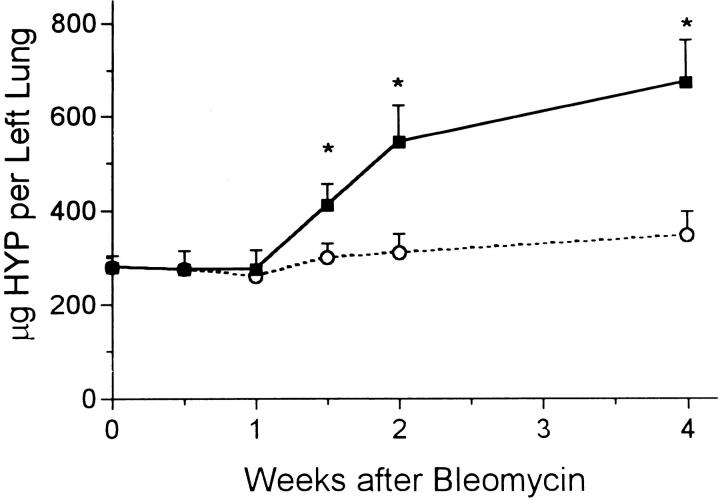
Bleomycin induced immediate lung injury and an inflammatory response as previously described. 2,4 Epithelial cell necrosis at the bronchiolar and alveolar levels was seen and many inflammatory cells were observed nearby. The total number of these cells recovered by BAL was increased threefold at 3 days and continued at about double the normal value for the 4-week period (Figure 1) ▶ . The cytospin preparations showed these cells to be mixed alveolar macrophages and polymorphonuclear leukocytes. Further evidence of injury was found in the levels of protein in the BAL fluid. Control levels were always in the 80–100 μg/ml range, but 3 days after bleomycin, the alveolar protein level peaked at 8 times this value (Figure 2) ▶ . The protein level then fell steadily but did not reach the base value by the end of the 4-week experimental period. The amount of hydroxyproline measured in lung tissue rose steadily after 1 week and was more than twice the normal amount by 4 weeks (Figure 3) ▶ . From 2 to 4 weeks, evidence of pulmonary fibrosis was seen readily by light microscopy.
Figure 1.
Total number (mean ± SE) of cells recovered by BAL at intervals after bleomycin (solid squares) or water (open circles). All experimental groups > control (P < 0.05) at same time point.
Figure 2.
Protein content in μg per ml BAL fluid after instillation of bleomycin (solid squares) or water (open circles). All experimental groups > control (P < 0.05) at same time.
Figure 3.
Total hydroxyproline content (μg) per left lobe of lung after bleomycin (solid squares) or water (open circles). *, P < 0.05 > control.
Autoradiography
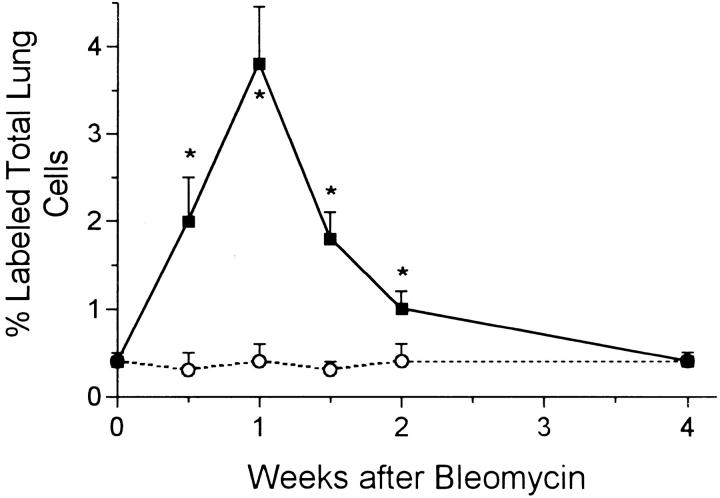
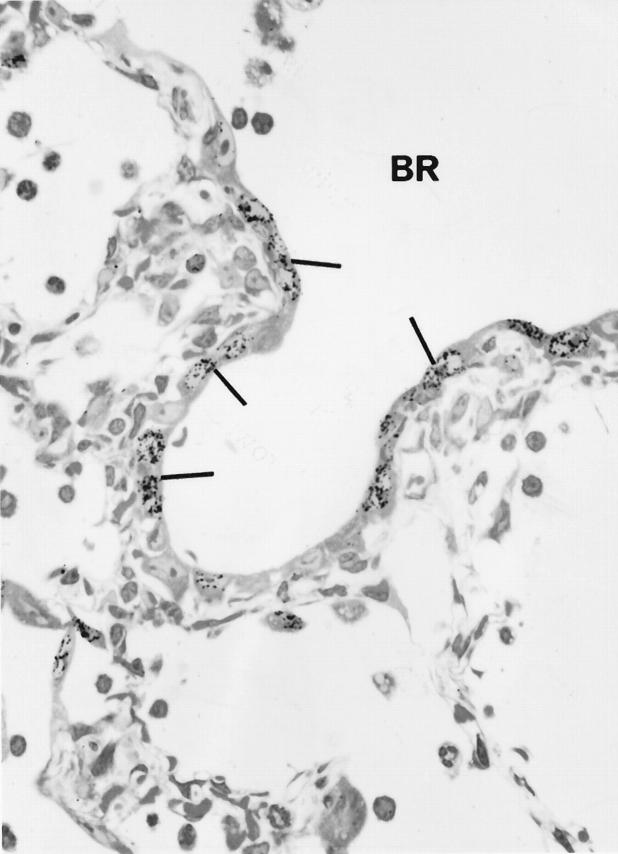
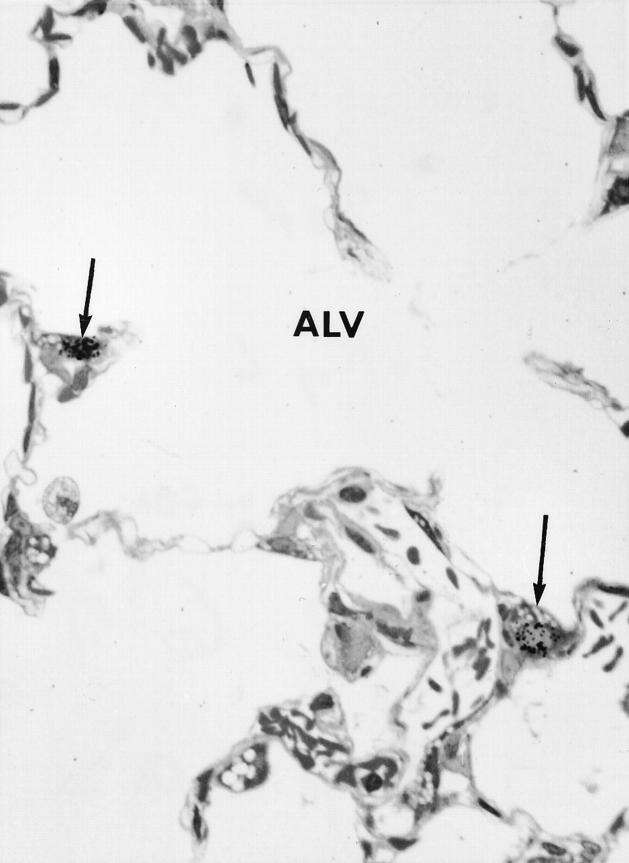
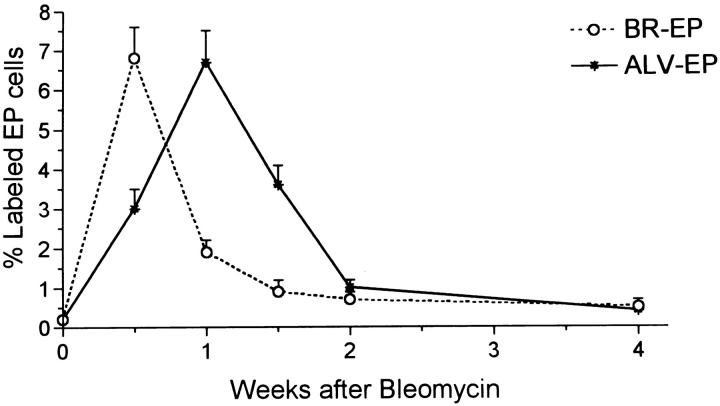
The percentage of 3HT-labeled lung cells in noninjected rats and in all of the water-injected controls was always around 0.3–0.4%. In bleomycin-injected rats, counting all cell types in the lung, the overall percentage of labeled cells rose to about 2% at day 3, then doubled again, peaking at 1 week (Figure 4) ▶ . The level then fell but did not return to the control range until 4 weeks. The vast majority of labeled cells in the first 10 days after bleomycin was administered were epithelial cells. Labeled BR epithelium was particularly prominent at 3 and 7 days, whereas Type 2 alveolar epithelium was most prominently labeled at 7 and 10 days (Figures 5 and 6) ▶ ▶ . When these epithelial types were identified and counted specifically at each time point, it was found that BR cells showed maximal labeling at 3 days and that labeling returned to normal by 2 weeks, whereas the rise in Type 2 cell labeling was somewhat slower and peaked at 7% of cells at 1 week before declining by 2 weeks (Figure 7) ▶ . Most of the 3HT labeling at 2 weeks and beyond was found in interstitial fibroblasts.
Figure 4.
Percentages of total lung cells that incorporated 3H thymidine at intervals after bleomycin (solid squares) or water (open circles). *, P < 0.05 > control.
Figure 5.
Autoradiograph of lung 3 days after bleomycin shows many labeled epithelial cells (arrows) of the bronchiolar epithelium (BR) × 950.
Figure 6.
Autoradiograph of an alveolar (ALV) region of lung 7 days after bleomycin shows 3H thymidine-labeled Type 2 cells (arrows) × 950.
Figure 7.
Percentages of bronchiolar epithelial cells (BR-EP) and alveolar epithelial cells (ALV-EP) labeled by 3H thymidine after bleomycin. Control values were always the same as for time 0.
Growth Factors in BAL
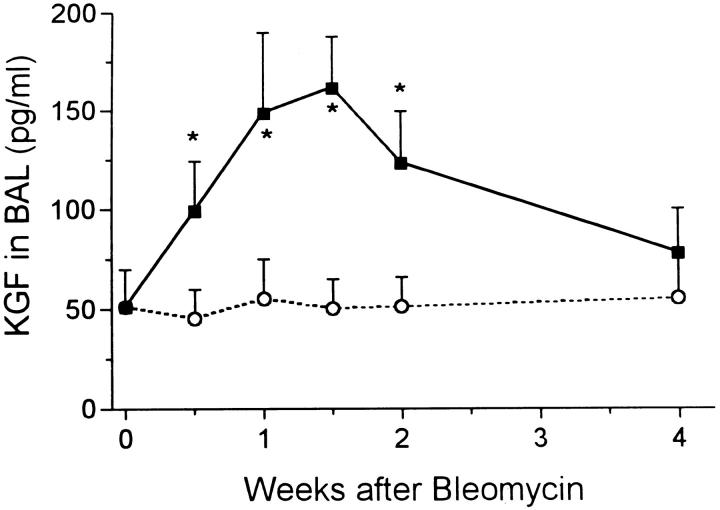
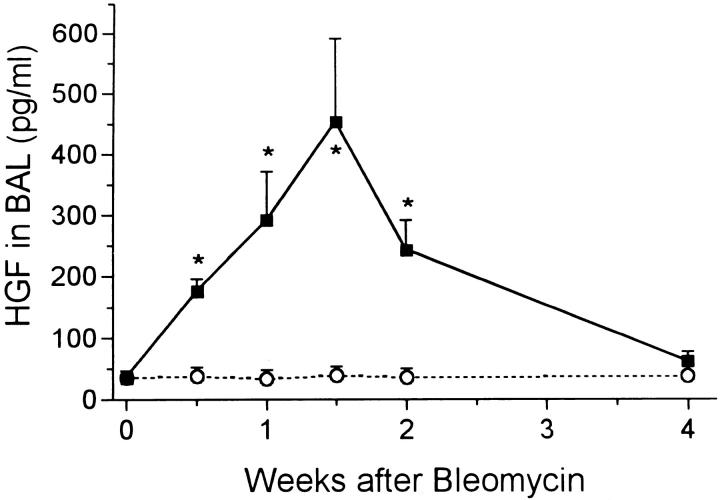
From each animal approximately 9 ml of fluid was recovered from the double lavage procedure. In the control rats, about 50 pg/ml of KGF was measured and the value was unchanged after water instillation. Bleomycin injection resulted in a rapid increase in KGF levels in lavage fluid, with peak levels occurring between 1 and 2 weeks at around 150 pg/ml (Figure 8) ▶ . The value returned to the normal range by 4 weeks. A much greater increase in HGF was induced by bleomycin treatment. The level rose from control value of 40 pg/ml to about 200 pg/ml at day 3 before peaking at 10 times normal at day 10 (Figure 9) ▶ . The HGF level had returned to normal by 4 weeks.
Figure 8.
Levels of KGF in BAL fluid at intervals after bleomycin (solid squares) and water (open circles). *, P < 0.05 > control.
Figure 9.
Levels of HGF in BAL fluid at intervals after bleomycin (solid squares) and water (open circles). *, P < 0.05 > control.
These results were obtained using the kit for human HGF. To confirm that we were measuring HGF in the rat lung, we subsequently ran these samples using a specific rat HGF ELISA kit. The values obtained and the shape of the graph were similar to that shown in Figure 9 ▶ (data not shown). Control levels of HGF were around 70 pg/ml BAL and rose after bleomycin to peak at 400 pg/ml on day 10. In addition, the HGF measured in human assay system was almost all neutralized when BAL samples were first incubated with the anti-rat HGF.
Discussion
The intratracheal instillation of bleomycin is a widely used model for the rapid induction of fibrosing alveolitis in experimental animals. The initial events involve necrosis of bronchiolar and alveolar Type 1 cells, and abnormal epithelial repair is a feature of the subsequent pathological changes that lead to pulmonary fibrosis. 1-4 These same features were observed in this study, in which the early epithelial cell injury was followed by an inflammatory response and the leakage of protein to the air spaces. The acute injury phase lasted 2 weeks, as indicated by the elevated protein levels in BAL fluid, and subsequently fibrosis could be seen in sections and measured biochemically. These results confirm the validity of this model system in the rat for the study of lung injury and repair.
Increased cell proliferation in the lung began quite soon after injury was induced. The autoradiographs indicated that DNA synthesis was 4 to 8 times normal over days 3 to 10 after administration of bleomycin, at which time the bulk of labeling was over the epithelial cell populations in the distal lung. It is well known that bleomycin is toxic to bronchiolar epithelial cells and Type 1 alveolar epithelium; several studies, including work from our laboratory, have reported altered Type 2 cell kinetics and differentiation after bleomycin. 2,4 In the present study, we concentrated specifically on the epithelial populations, independently identifying and counting BR and alveolar epithelial cells. The results demonstrate that, although injury occurs simultaneously in these cell populations at day 0, their regenerative patterns are not in step with one another. The BR epithelial cells show a very rapid repair pattern in which labeling peaks at 3 days and proliferation is near normal again by 10 days, whereas DNA synthesis by Type 2 cells increases more slowly, with a peak at 1 week and a return to normal by 2 weeks. The more rapid repair of the BR epithelium may explain the return to nearly normal morphology at this anatomical site, whereas the somewhat slower repair at the alveolar surface is associated with subsequent abnormal epithelial differentiation. 2,4
In a situation analogous to wound repair, rapid restoration of a normal pulmonary epithelium after acute injury is associated with an absence of fibrous scarring. 3,4 Factors that can promote accelerated repair have been sought and recent studies have shown that KGF can induce Type 2 cell division in culture and also when instilled to normal lung. 5,6 This mitogen is produced and secreted by fibroblasts, then binds exclusively to epithelial cells, 5,16 affecting various organs with a high epithelial cell component, including the lung. Most studies of KGF effects on lung cells have been in vitro or have involved injecting KGF in attempts to accelerate repair. In the latter cases, KGF has been found to be more beneficial when given before injury, implying a protective action on the alveolar epithelium, possibly by acting as an antioxidant. 10-12 There is little evidence of endogenous KGF production in the lung during injury or of how levels of this growth factor relate to the regeneration of various types of epithelium. In this report we show evidence that KGF is secreted rapidly after injury and that it can be detected in BAL fluid. After bleomycin-induced acute lung injury, the KGF level rose threefold, suggesting that the fibroblast production is up-regulated. This increase in KGF coincided with an increase in BR and alveolar epithelial cell proliferation, whereas higher than normal levels of KGF persisted even after the epithelial growth phase was over. Epithelial proliferation may have slowed as contact inhibition was reestablished, even though cytokine levels remained elevated.
A similar pattern of growth factor activity was seen when HGF was measured. Levels of this molecule in BAL were much higher and reached about 10 times control values within a few days after bleomycin-induced injury, then also stayed above normal when the phase of increased epithelial DNA synthesis was over. HGF has also been shown to be a mitogen for pulmonary epithelial cells 5,9 and is synthesized by mesenchymal cells, which include lung fibroblasts and endothelial cells. 7,17 As with KGF, there is little precise evidence to relate HGF’s presence in the lung with epithelial cell proliferation during repair because, in cases of lung injury where HGF has been measured, the proliferative status of the epithelium has not been clear. 18 In that study of patients with acute lung injury, levels of both HGF and KGF were found to be significantly raised in edema fluid, with HGF reaching much higher levels. In addition, the concentration of HGF in those patients was 7 times higher in edema fluid than in plasma, indicating a pulmonary origin for this growth factor. 18 The results of the present study show that two potent mitogens, KGF and HGF, are secreted and are present at high levels in the lung soon after epithelial cell necrosis. Although most attention to KGF and HGF has been directed at effects on epithelial cell proliferation, there is evidence that these factors may affect other properties of the reparative process. For example, KGF promotes cell migration and spreading of Type 2 cells, 19 which is essential if the regenerating epithelium is to cover the alveolar surface. In addition, KGF up-regulates the production of surfactant proteins in cultured cells 20 and may enhance alveolar fluid clearance after injury by up-regulating transepithelial ion transport. 21 In addition, these two growth factors may work in concert, as the administration of both at the same time further accelerates epithelial cell proliferation. 9
The present study shows that both HGF and KGF are present at high levels in the lung soon after injury at a time when epithelial cell proliferation occurs. Although most of our ELISA results were obtained using a detection system for human growth factors, there is >90% homology between the human and rat molecules, and we were able to measure a similarly increased level of HGF using a kit specific for rat HGF. We have not directly demonstrated a causal role for these cytokines in epithelial repair, but the fact that these two well known epithelial mitogens are up-regulated soon after injury is strong evidence of a causal relationship. There are few other known mitogens for Type 2 cells and, in an earlier study, it was shown that most epithelial proliferative activity in rat lung lavage fluid was due to HGF. 22 The fact that the level of protein in BAL fluid was maximal at 3 days, then fell sharply, while the levels of KGF and HGF continued to increase to their maximum levels at 1 to 2 weeks, indicates that these cytokines did not arise from leaked plasma proteins during early edema, but were more likely to have been produced in the lung.
The rapid production and secretion of epithelial growth factors soon after injury suggests that any delay in the epithelial proliferative response may be due to a failure to up-regulate receptors rather than to a deficiency of growth factor. The presence of high levels of endogenous growth factors may explain why the administration of additional growth factors does not accelerate repair. 10,12 Our results suggest that there may be enough HGF and KGF present so that any additional injections would be superfluous, although some studies show that growth factor injection before injury has some benefit, possibly due to DNA repair or to an unknown protective mechanism. 10-12 It appears, however, that acute injury such as that induced by bleomycin, although damaging to epithelial cells, does not inhibit the fibroblast from producing KGF, for example. Bleomycin administration results in the rapid induction of tumor necrosis factor-α, 23 which in turn can cause the up-regulation of KGF production by fibroblasts. 16 This suggests that after epithelial cell injury, when high levels of epithelial growth factors are present in the lung, any delay in epithelial cell proliferation may be due to the extent of damage, possibly including injury to progenitor cells such as the Type 2 epithelium. Such injury could prevent the up-regulation of receptors for HGF and/or KGF on these cells, thereby inhibiting their regenerative response.
Footnotes
Address reprint requests to Dr. I. Y. R. Adamson, Department of Pathology, University of Manitoba, D212–770 Bannatyne Avenue, Winnipeg, Manitoba, Canada R3E 0W3.
Supported by the Medical Research Council of Canada, grant MT 12972.
References
- 1.Crouch E: Pathobiology of pulmonary fibrosis. Am J Physiol 1990, 259:L159-L184 [DOI] [PubMed] [Google Scholar]
- 2.Adamson IYR: Drug-induced pulmonary fibrosis. Environ Health Perspect 1984, 55:25-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar RK, Lykke AWJ: Messages and handshakes: cellular interactions in pulmonary fibrosis. Pathology 1995, 27:18-26 [DOI] [PubMed] [Google Scholar]
- 4.Adamson IYR, Hedgecock C, Bowden DH: Epithelial cell-fibroblast interactions in lung injury and repair. Am J Pathol 1990, 137:385-392 [PMC free article] [PubMed] [Google Scholar]
- 5.Panos RJ, Rubin JS, Aaronson SA, Mason RJ: Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar Type II cells in fibroblast conditioned medium. J Clin Invest 1993, 92:969-977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulich TR, Yi ES, Longmuir K, Yin S, Blitz R, Morris CF, Housley RM, Pierce GF: Keratinocyte growth factor is a growth factor for Type II pneumocytes in vivo. J Clin Invest 1994, 93:1298-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin JS, Chan AM, Bottaro DP, Burgess WH, Taylor WG, Cech AC, Hirschfield DW, Wong J, Miki T, Finch PW, Aaronson SA: A broad spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci USA 1991, 88:415-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michelson PH, Tigue M, Sporn PHS, Panos RJ: Keratinocyte growth factor stimulates proliferation in bronchial epithelial cells in vitro. Am J Resp Crit Care Med 1997, 156:A428. [DOI] [PubMed] [Google Scholar]
- 9.Ohmichi H, Koshimizu U, Matsumoto K, Nakamura T: Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development 1998, 125:1315-1324 [DOI] [PubMed] [Google Scholar]
- 10.Deterding RR, Havill AM, Yano T, Middleton SC, Jacoby CR, Shannon JM, Simonet WS, Mason RJ: Prevention of bleomycin induced lung injury in rats by keratinocyte growth factor. Proc Assoc Am Phys 1997, 109:254-268 [PubMed] [Google Scholar]
- 11.Wu KI, Pollack N, Panos RJ, Sporn PHS, Kamp DW: Keratinocyte growth factor promotes alveolar epithelial cell DNA repair after H2O2 exposure. Am J Physiol Lung Cell Mol Physiol 1998, 14:L780-L787 [DOI] [PubMed] [Google Scholar]
- 12.Yi ES, Williams ST, Lee H, Malicki DM, Chin EM, Yin S, Tarpley J, Ulich TR: Keratinocyte growth factor ameliorates radiation- and bleomycin-induced lung injury and mortality. Am J Pathol 1996, 149:1963-1970 [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folin-phenol reagent. Eur J Biol Chem 1951, 193:265-275 [PubMed] [Google Scholar]
- 14.Woessner JF: The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys 1961, 93:440-447 [DOI] [PubMed] [Google Scholar]
- 15.Adamson IYR: Cellular Kinetics of the Lung. Witischi HP Brain JD eds. Toxicology of Inhaled Materials. 1985, :pp 289-317 Springer-Verlag, Berlin [Google Scholar]
- 16.Brauchle M, Angermeyer K, Hubner G, Werner S: Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene 1994, 9:3199-3204 [PubMed] [Google Scholar]
- 17.Adamson IYR, Young L, Bakowska J: Enhanced alveolar type II cell growth on a pulmonary endothelial matrix over fibroblasts. Am J Physiol 1997, 272:L413-417 [DOI] [PubMed] [Google Scholar]
- 18.Vergese GM, McCormick-Shannon K, Mason RJ, Mathay MA: Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Am J Resp Crit Care Med 1998, 158:386-394 [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Henke CA, Ingbar DH: Keratinocyte growth factor but not hepatocyte growth factor, stimulates alveolar epithelial cell migration. Am J Resp Crit Care Med 1997, 156:A179 [Google Scholar]
- 20.Sugahara K, Rubin JS, Mason RJ, Aronsen EL, Shannon JM: Keratinocyte growth factor increases in mRNAs for SP-A and SP-B in adult rat alveolar type II cells in culture. Am J Physiol 1995, 269:L344-L350 [DOI] [PubMed] [Google Scholar]
- 21.Borok Z, Danto SI, Dimen LL, Zhang XL, Lubman RL: Na(+)-K(+)-ATPase expression in alveolar epithelial cells: upregulation of active ion transport by KGF. Am J Physiol 1998, 274:L149-L158 [DOI] [PubMed] [Google Scholar]
- 22.Mason RJ, McCormick-Shannon K, Rubin JS, Nakamura T, Leslie CC: Hepatocyte growth factor is a mitogen for alveolar type II cells in rat lavage fluid. Am J Physiol 1996, 271:L46-L53 [DOI] [PubMed] [Google Scholar]
- 23.Sleijfer S, Vujaskovic Z, Limburg PC, Koops HS, Mulder NH: Induction of tumor necrosis factor-α as a cause of bleomycin-related toxicity. Cancer 1998, 82:970-974 [DOI] [PubMed] [Google Scholar]