Abstract
To define the unique contributions of the α subunit cytoplasmic tails of the α1β1 and α2β1 integrin to epithelial differentiation and branching morphogenesis, a variant NMuMG cell line lacking α1β1 and α2β1 integrin expression was stably transfected with the full-length α2 integrin subunit cDNA (X2C2), chimeric cDNA consisting of the extracellular and transmembrane domains of the α2 subunit and the cytoplasmic domain of the α1 subunit (X2C1), or α2 cDNA truncated after the GFFKR sequence (X2C0). The X2C2 and X2C1 transfectants effectively adhered, spread, and formed focal adhesion complexes on type I collagen matrices. The X2C0 transfectants were less adherent to low concentrations of type I collagen, spread less well, and formed poorly defined focal adhesion complexes in comparison to the X2C2 and X2C1 transfectants. The X2C2 and X2C1 transfectants but not the X2C0 transfectants proliferated on collagen substrates. Only the X2C2 transfectants developed elongate branches and tubules in three-dimensional collagen gels and migrated on type I collagen. These findings suggest a unique role for the α2 integrin cytoplasmic domain in postligand binding events and cooperative interactions with growth factors that mediate epithelial differentiation and branching morphogenesis. Either intact α1 or α2 integrin subunit cytoplasmic domain can promote cell cycle progression.
The α2β1 integrin, a collagen/laminin receptor, is expressed at high levels by most normal epithelial cells, and its expression is required for normal epithelial organization, including the formation of branching glands and ducts of the breast. 1-10 In many epithelial malignancies, α2β1 integrin expression is diminished or lost in a manner that correlates with the loss of epithelial differentiation and tumor progression. 2,3,11-14 The important role that the α2β1 integrin plays in normal epithelial differentiation has been substantiated by a number of in vitro “gain-of-function” and “loss-of-function” models. 4,5,15,16
For example, when a full-length α2 integrin cDNA was introduced into a poorly differentiated, tumorigenic murine breast cancer cell line that expressed no detectable α2 integrin subunit but high levels of the α1 integrin subunit, reexpression of the α2β1 integrin resulted in dramatic phenotypic alteration from a fibroblastoid, spindle-shaped, non-contact-inhibited cell to an epithelioid, polygonal-shaped, and contact-inhibited cell in culture. 15 Although the adhesion to collagen (mediated by the α1β1 integrin) of the parental and control cells was comparable to the adhesion of the α2 transfectants, only the α2 transfectants formed organized structures, including alveolar-like and elongated multilayered duct-like structures in three-dimensional floating collagen gels. These findings suggested that expression of the α2β1 integrin, but not the α1β1 integrin, supported epithelial differentiation and glandular morphogenesis in vitro.
The α1β1 and α2β1 integrins also appear to play distinct roles in other cell types. When cultured in three-dimensional collagen gels, primary fibroblasts that express both the α1β1 and α2β1 integrins down-regulate collagen biosynthesis and up-regulate metalloproteinase expression. 17-19 Ligation of the α1β1 integrin results in down-regulation of collagen gene expression, whereas ligation of the α2β1 integrin up-regulates matrix metalloproteinase-I gene expression. 17 In several cell types the α1β1 integrin has been shown to promote cell cycle progression and prevent apoptosis, and the α2β1 integrin fails to promote cell survival or cell cycle progression. 20
To begin to explore the mechanisms underlying these dramatic differences in postligand binding events mediated by the two collagen/laminin receptors, we have focused on the cytoplasmic tails of the α2 and α1 integrin subunits. We have reexpressed either the full-length α2 integrin subunit (X2C2), a chimeric integrin α chain composed of the extracellular and transmembrane domains of the α2 subunit fused to the cytoplasmic domain of α1 subunit (X2C1), or a truncated α2 subunit in which the cytoplasmic domain terminates following the conserved GFFKR motif (X2C0). Our findings reveal similarities in the ability of the α1 and α2 integrin cytoplasmic domains to mediate cell adhesion, spreading, focal adhesion complex formation, and proliferation. In addition, our findings demonstrate that an intact α subunit cytoplasmic domain is required for cell cycle progression. They furthermore reveal a unique role for the α2 integrin cytoplasmic domain in mediating branching morphogenesis by epithelial cells in three-dimensional collagen gels.
Materials and Methods
Cell Culture and Transfection
The murine NMuMG cell line and subclones were maintained in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% fetal bovine serum and insulin (5 μg/ml). NMuMG subclones were derived by limiting dilution techniques. The full-length human α2 integrin (X2C2) cDNA, the α2 cDNA lacking the cytoplasmic tail of the α2 integrin beyond the GFFKR sequence (X2C0), and the chimeric α2 cDNA containing the extracellular domain of α2 and the cytoplasmic tail of α4 (X2C4) in the expression vector pFneo were generous gifts from Dr. Martin Hemler (Harvard Medical School, Boston, MA). 21,22 The chimeric integrin cDNA containing the extracellular domain of α2 and the cytoplasmic tail of α1 was constructed by replacing the α4 cytoplasmic tail of the X2C4 construct with the α1 cytoplasmic tail. The α1 cytoplasmic tail was generated by annealing two complementary oligonucleotides representing base pairs 3916 to 3961 of the α1 integrin sequence. 23 The full-length, chimeric, and deletion mutant cDNA constructs of the α2 subunit were subcloned into the expression vector pSRα (a gift from Dr. Andre Shaw, Washington University School of Medicine, St. Louis, MO), which contains a cytomegalovirus promoter. All constructs were transfected into the NMuMG-3 clonal cell line by calcium phosphate transfection methodology. Clonal cell lines were selected and maintained in geneticin (850 μg/ml) and evaluated by Southern blot analysis for integration site determination to ensure that distinct clones were evaluated.
Immunoblot and Flow Cytometric Analysis
Immunoblot analysis was carried out by lysing confluent cell cultures in sodium dodecyl sulfate (SDS) sample buffer (0.1% SDS, 150 mmol/L NaCl, 5 mmol/L EDTA, 10 mmol/L Tris-HCl (pH 7.4), 1% Triton X-100, 0.5% deoxycholic acid, 100 μg/ml aprotinin, 50 μg/ml leupeptin, and 5 mmol/L phenylmethylsulfonyl fluoride). Total protein concentration was determined by the Pierce protein assay (Pierce, Rockford, IL). Equivalent amounts of protein lysate were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto Immobilon-P transfer membrane (Millipore, Bedford, MA). Immunoblots were blocked in 5% dried milk in Tris-buffered saline (TBS) containing 0.5% Tween 20 and incubated overnight with an appropriate dilution of primary antibody at 4°C. Secondary antibody incubation was performed with horseradish peroxidase-conjugated goat anti-mouse IgG (Amersham Life Science, Arlington Heights, IL) for 2 hours at room temperature. The ECL Chemiluminescence System (Amersham Life Science, Arlington Heights, IL) was used for visualization.
Flow cytometric analysis was performed on adherent cells harvested with 2 mmol/L EDTA in phosphate-buffered saline (PBS) (pH 7.45). Single cells (1 × 106) in PBS containing 1.5% horse serum were incubated with the appropriate monoclonal or polyclonal antibodies at either 5 μg/ml or at the saturating concentration recommended by the manufacturer for 45 minutes at 4°C. Cells were washed three times and incubated with 2 μg/ml of a secondary goat anti-mouse or donkey anti-rat antibody coupled to fluorescein (Tago, Birmingham, CA) for 45 minutes at 4°C, washed twice, and resuspended in PBS. Fluorescein-labeled cells were analyzed using a FACScan instrument (Becton Dickinson, Mountain View, CA).
Monoclonal antibodies (mAbs) against the extracellular domain of the murine α1 integrin (Pharmingen, San Diego, CA); the murine α6 integrin subunit, GoH3, (Immunotech, Westbrook, ME); the murine β1 integrin subunit, 9EG7 (Sigma Immunochemical, St. Louis, MO); and the murine α5 integrin subunit, 5H10–27 (Pharmingen, San Diego, CA); were used for flow cytometric analysis. Polyclonal antisera directed against a portion of the carboxyl terminus of the murine β4 integrin subunits or against a portion of the murine c-Met receptor were obtained from Chemicon (Temecula, CA) or Santa Cruz Biotech (Santa Cruz, CA), respectively. The polyclonal antiserum against the cytoplasmic domain of the murine α2 integrin has been described previously. 24 A polyclonal antiserum against the extracellular domain of the murine α2 integrin was prepared using standard procedures 24 by immunization of rabbits with recombinant murine α2 integrin I-GST fusion protein that was expressed, purified, and characterized as recently described for the analogous human protein. 25
Adhesion and Spreading Assays
Adhesion and spreading assays were carried out as described in detail. 26 In adhesion assays, cells (2 × 10 4 cells/ml) were allowed to adhere to either type I collagen or fibronectin (Sigma Chemical, St. Louis, MO) at the designated concentration for 1 hour at 37°C. Nonadherent cells were removed by washing three times. Cell spreading was quantitated by counting the number of spread versus nonspread cells after 1 hour of adhesion to collagen type I (20 μg/ml).
Morphogenesis in Gels of Type I Collagen
Glandular morphogenesis in floating gels of type I collagen was carried out as described in detail. 26 The morphology was photographed at ×200 magnification.
Measurement of Contractile Forces in Collagen Gels
Formation of collagen gels was carried out by resuspending cells (1 × 10 6 cells/ml) in DMEM with monomeric collagen (1 mg/ml) solubilized in 0.02 mol/L acetic acid at 4°C (Upstate Biotechnology, Lake Placid, MA). Polymerization of the collagen gels was carried out by neutralizaing the pH with 0.1 NaOH before the mixture of cells and collagen was cast in Teflon wells and the gels were incubated in a 37°C humidified incubator with 5% C02. Collagen polymerization was complete after 15–30 minutes. The cylindrical walls surrounded by a central mandrel containing polymerized collagen gel and cells formed a cell populated matrix (cpm) ring. After 1 hour of incubation, the mandrel was removed from the cpm ring. The cpm ring was then looped over horizontal bars of a triangular hook connected to an isometric force transducer (model 52-9545; Harvard Apparatus, South Natick, MA) by a gold chain. The opposite end of the ring was also looped over another horizontal bar. An analog-to-digital signal converter (CIO-DAS 1602/16; Computer Boards, Mansfield, MA) attached to a personal computer translated the voltage signal from the isometric force to a digital signal for recording. The sample was submerged into 50 ml HEPES-buffered DMEM supplemented with 10% calf serum in a thermoregulated organ bath (Harvard Apparatus) at physiological temperature (37°) and pH (7.4). The baseline force development by the collagen matrix contraction was monitored for 20–30 hours. After baseline force development, the force generated in response to calf serum (20%) stimulation was measured over the subsequent 50–100 minutes. After force levels stabilized, the model tissues were exposed to cytochalasin D (2 μm).
Growth on Type I Collagen and Fibronectin
Before growth analysis, cells were serum-starved in DMEM containing 0.4% serum and insulin (5 μg/ml) for 48 hours, followed by DMEM-containing insulin (5 μg/ml) only for 18 hours, before being plated on type I collagen (25 μg/ml) or fibronectin (25 μg/ml). Cells (4 × 104/well) were plated on 30-mm plates in MEBM containing bovine pituitary extract (13 μg/ml), hydrocortisone (0.5 μg/ml), human epidermal growth factor (EGF) (10 μg/ml), and insulin (5 μg/ml) (Clonetics, San Diego, CA). The medium was changed every 3 days after the initial plating. On days 3, 6, 9, 12, and 15, the cells from triplicate wells were detached with 0.025% trypsin and counted in a hemacytometer.
Cell Migration on Type I Collagen
Cell migration assays were performed using a modification of the protocol previously described. 26 Cells were serum-starved as described for the proliferation assays. Briefly, 12-mm transwell chambers (Costar, Cambridge, MA) containing polycarbonate membrane with 12-μm pores were coated overnight at 4°C with collagen type I at 25 μg/ml (Sigma Chemical). The filters were washed with PBS and air-dried. The bottom chamber was filled with RPMI 1640 medium with 1% bovine serum albumin and Mg2+ (2 mM). Cells were placed in the top chamber at 1.5 × 10 5 cells/ml in PBS with Mg2+ (2 mM) and allowed to migrate for 4 hours at 37°C in a humidified incubator. In experiments in which EGF was included, EGF (10 ng/ml) was placed in either the lower chamber only or both the upper and lower chambers as a control for chemotaxis. Cells remaining on the upper surface of the transwell filter were removed by mechanical scraping. Cells migrating to the lower filter surface were fixed and stained with Gill’s hematoxylin and eosin solution (Sigma Diagnostics, St. Louis, MO). The number of cells migrating to the lower surface was determined by counting the number of cells in 10 random fields (×400, high-power field). All experiments were repeated a minimum of three times. Migration was determined by averaging the cell count from at least three separate experiments.
Results
NMuMG, an immortalized but nonmalignant mammary epithelial cell line, undergoes striking glandular morphogenesis that recapitulates normal mammary differentiation with the development of branching ducts and glands when cultured in three-dimensional collagen gels. 27 Previous work from our laboratory as well as a number of other laboratories demonstrated high α2β1 integrin expression by epithelial cells of the normal mammary gland and suggested an important role for the α2β1 integrin in normal branching morphogenesis of mammary epithelial cells, as well as other cell types. 4-6,15 It thus seemed likely that the NMuMG cells also expressed high levels of the α2β1 integrin. Preliminary analysis demonstrated that the NMuMG cell line expressed high levels of the α2β1 integrin protein (data not shown).
Further evaluation of the NMuMG cells in culture revealed variability in cellular morphology. Many NMuMG cells were round or polygonal with epithelioid features; rare cells were spindle-shaped with fibroblastoid features. Before further analysis, clonal sublines of NMuMG reflecting these different phenotypes were derived by limiting dilution subcloning techniques. Three clonal cell lines, designated NMuMG-1, NMuMG-2, and NMuMG-3, were selected for further analysis. As shown in Figure 1,A, D, and E ▶ , the NMuMG-1 subclone exhibited an epithelioid morphology and expressed high levels of the α2β1 integrin. In contrast, the NMuMG-3 subclone exhibited fibroblastoid features and had undetectable levels of the α2β1 integrin (Figure 1, C, D, and E) ▶ . The NMuMG-2 subclone showed features intermediate between those of NMuMG-1 and NMuMG-3 and expressed intermediate levels of the α2β1 integrin (Figure 1, B, D, and E) ▶ .
Figure 1.
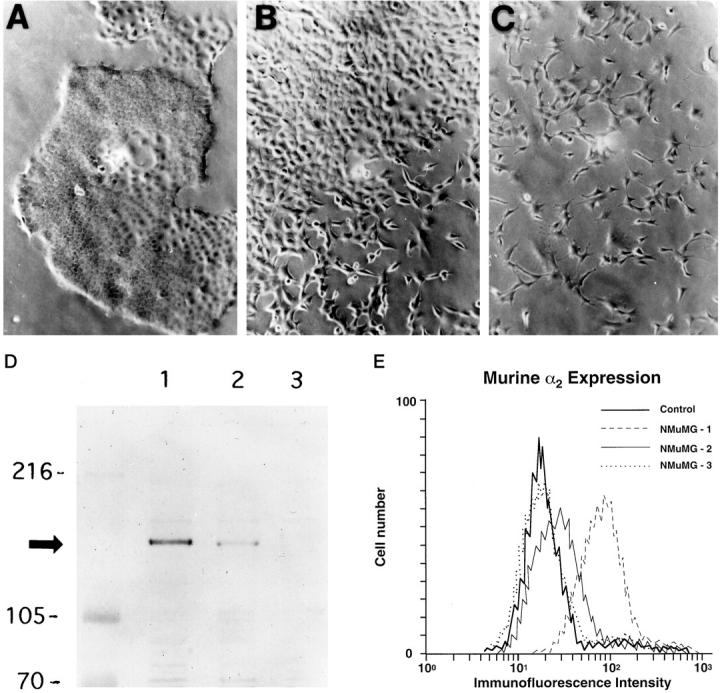
A: Clonal cell lines of the NMuMG cells were derived by limiting dilution subcloning techniques. The growth characteristics in routine culture of the three subclones designated NMuMG-1 (A), NMuMG-2 (B), and NMuMG-3 (C) are demonstrated (×200). The NMuMG-1 subclone exhibited epithelioid morphology (A); the NMuMG-3 subclone exhibited fibroblastoid features (C); the NMuMG-2 subclone had intermediate features between NMuMG-1 and NMuMG-3 (B). D: Immunoblot analysis of cell lysates of NMuMG-1, NMuMG-2, and NMuMG-3 subclones, using a polyclonal antipeptide antibody specific for a portion of the carboxyl terminus of the mouse α2 integrin subunit demonstrated different levels of α2 integrin subunit expression. E: Cell surface expression of the murine α2 integrin subunit by the NMuMG-1, NMuMG-2, NMuMG-3 subclones was assessed by flow cytometric analysis. The control represents NMuMG-1 stained with secondary antibody only.
We compared the ability of the NMuMG subclones to develop elongate branches and glands in three-dimensional collagen gels. As shown in Figure 2 ▶ , cells derived from the NMuMG-1 and NMuMG-2 subclones formed complex branches and glandular structures when grown for 3 days in three-dimensional collagen gels. In contrast, NMuMG-3 cells failed to develop branches or extensions but remained as single isolated cells or small clusters of cells.
Figure 2.
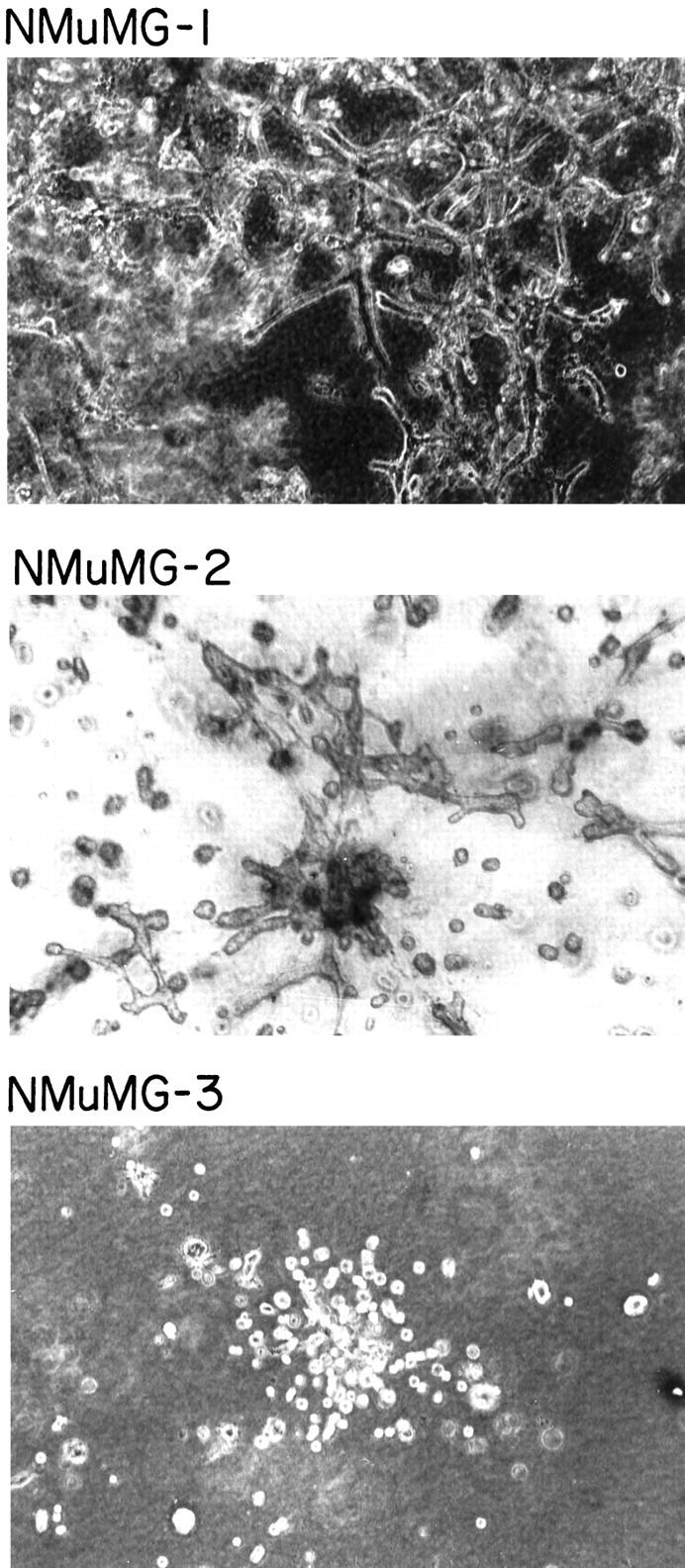
Branching morphogenesis of the NMuMG subclones in three-dimensional collagen gels. The ability of the NMuMG subclones NMuMG-1, NMuMG-2, and NMuMG-3 to develop elongate branches in three-dimensional collagen gels was evaluated. Magnification ×200.
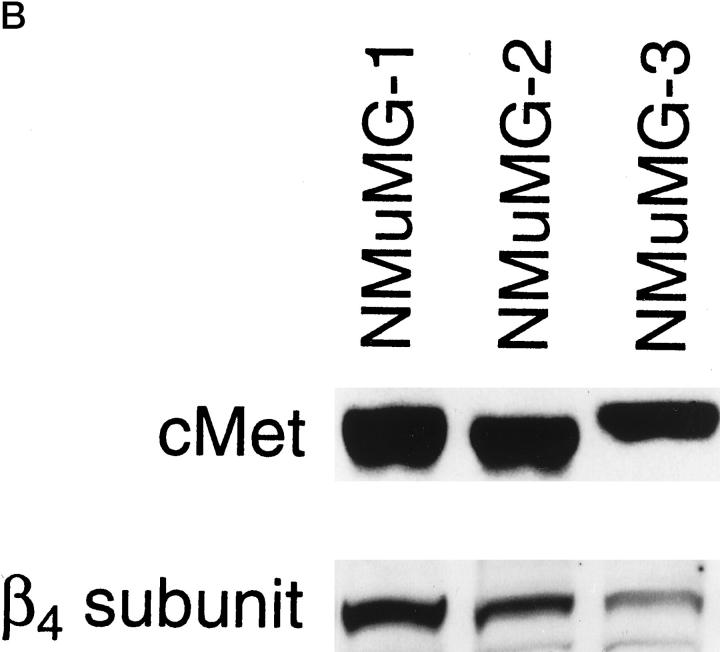
Our previous work suggested that the interplay of multiple cell adhesion receptors may contribute to complex morphological mammary cell differentiation. 28,29 We therefore evaluated the expression of other integrins by the three NMuMG subclones. We compared the expression levels of the α1, α5, α6, and β1 integrin subunits by NMuMG-1, NMuMG-2, and NMuMG-3 cells by flow cytometric analysis (Figure 3A) ▶ . All three subclones expressed intermediate to high levels of the α5, α6, and β1 integrin subunits. None of the subclones expressed detectable levels of the α1 integrin subunit. The β4 integrin subunit was expressed by all three NMuMG subclones, as demonstrated by Western blot analysis. NMuMG-1 expressed the highest levels of the β4 subunit; NMuMG-3 expressed the lowest level of β4 subunit; NMuMG-2 expressed intermediate levels of the β4 subunit (Figure 3B) ▶ . A correlation between expression of the α2β1 and α6β4 integrins has previously been recognized. 28,29 Because an earlier investigation established the important role of the hepatocyte growth factor (HGF)/cMet receptor interaction in branching morphogenesis of the NMuMG cell line, 27 we assessed cMet expression by the NMuMG subclones (Figure 3B) ▶ . There was high and equivalent levels of cMet expression by the NMuMG-1 and NMuMG-2 subclones. NMuMG-3 cells expressed slightly lower levels of the cMet receptor, as determined by Western blot analysis. All three cell lines expressed comparable levels of cytokeratin and E-cadherin (data not shown).
Figure 3.
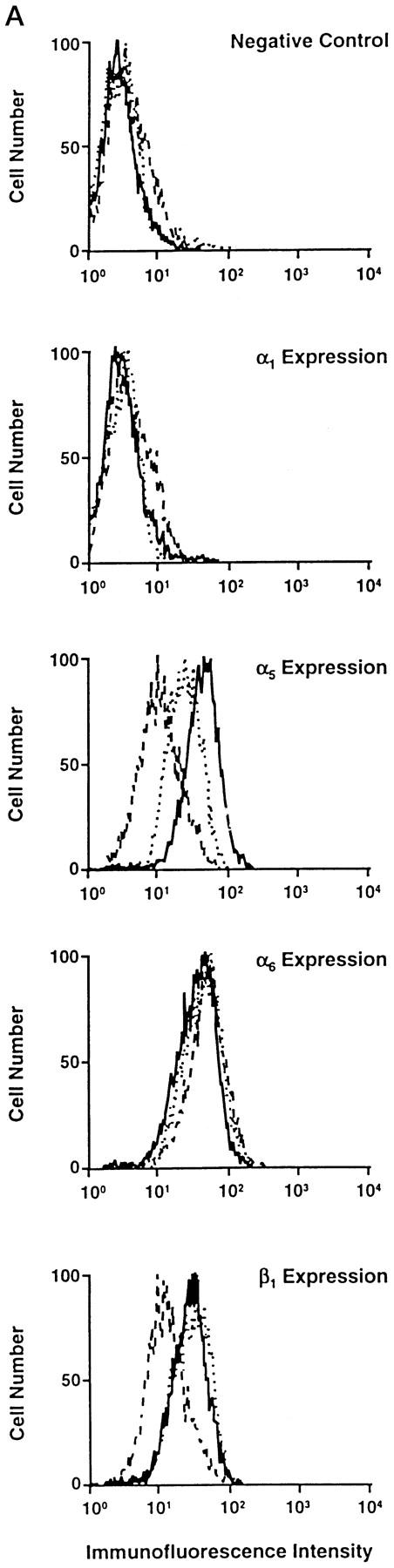
Expression of other integrin subunits and the c-Met receptor by the NMuMG subclones. A: Cell surface expression of the murine α1, α5, α6, and β1 integrin subunits by NMuMG-1 (——), NMuMG-2 (·····), and NMuMG-3 (– – –) cells was determined by flow cytometric analysis. B: Immunoblot analysis of cell lysates of NMuMG-1, NMuMG-2, NMuMG-3 subclones using a polyclonal antiserum against a portion of the carboxyl terminus of the murine β4 integrin subunit or against the murine c-Met receptor compared the levels of the β4 integrin subunit and the c-Met receptor.
To confirm the role of the α2β1 integrin in branching morphogenesis by NMuMG cells, we reexpressed the full-length human α2 integrin subunit in the NMuMG-3 cell line and evaluated the morphological response. After transfection and selection in geneticin, multiple clonal cell lines were isolated and evaluated for expression of the human α2 integrin subunit by flow cytometric analysis. Clonal cell lines (X2C2#1 and X2C2#4) expressing the full-length human α2 integrin subunit (X2C2) at levels comparable to that of the high-level expression of the murine α2 subunit by the NMuMG-1 subclone were selected for study (Figure 4, A and B) ▶ . Vector-only transfected control cells, like parental NMuMG-3 cells, did not express detectable levels of the α2β1 integrin (Figure 4, A and B) ▶ .
Figure 4.
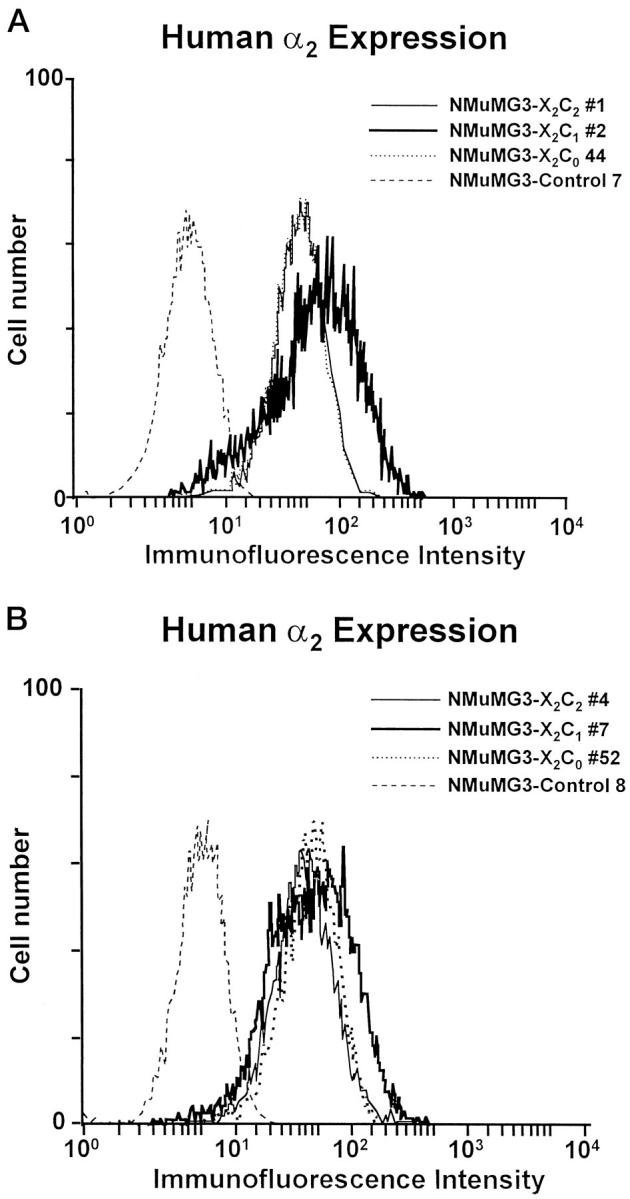
Cell surface expression of the extracellular domain of the human α2 integrin subunit by the X2C2, X2C1, X2C0, and vector-only transfectants. A: Cell surface expression of the α2 subunit extracellular domain by the X2C2-transfected clone NMuMG-3-X2C2#1, the X2C1-transfected clone NMuMG-3-X2C1#2, and the X2C0-transfected clone NMuMG-3-X2C0#44 was compared with that of the vector-only control cells (Control 7) by flow cytometric analysis. B: Cell surface expression of the X2C2 subunit by the X2C2-transfected clone NMuMG-3-X2C2#4, of the X2C1 subunit by the X2C1-transfected clone NMuMG-X2C1#7, and of the X2C0 subunit by the X2C0-transfected clone NMuMG-X2C0#52 was compared to the vector-only control cells (Control 8) by flow cytometric analysis.
The ability of the NMuMG-3 cell line reexpressing the full-length α2β1 integrin to branch and form elongated structures in three-dimensional collagen gels was compared to the ability of the parental cells and control transfectants to branch (Figure 5) ▶ . Reexpression of the α2β1 integrin restored the ability of the NMuMG-3 subclone to form branches in three-dimensional collagen gels. We have now demonstrated in several different model systems, using both benign and malignant breast epithelium, the important role of α2β1 in epithelial cell branching morphogenesis.
Figure 5.
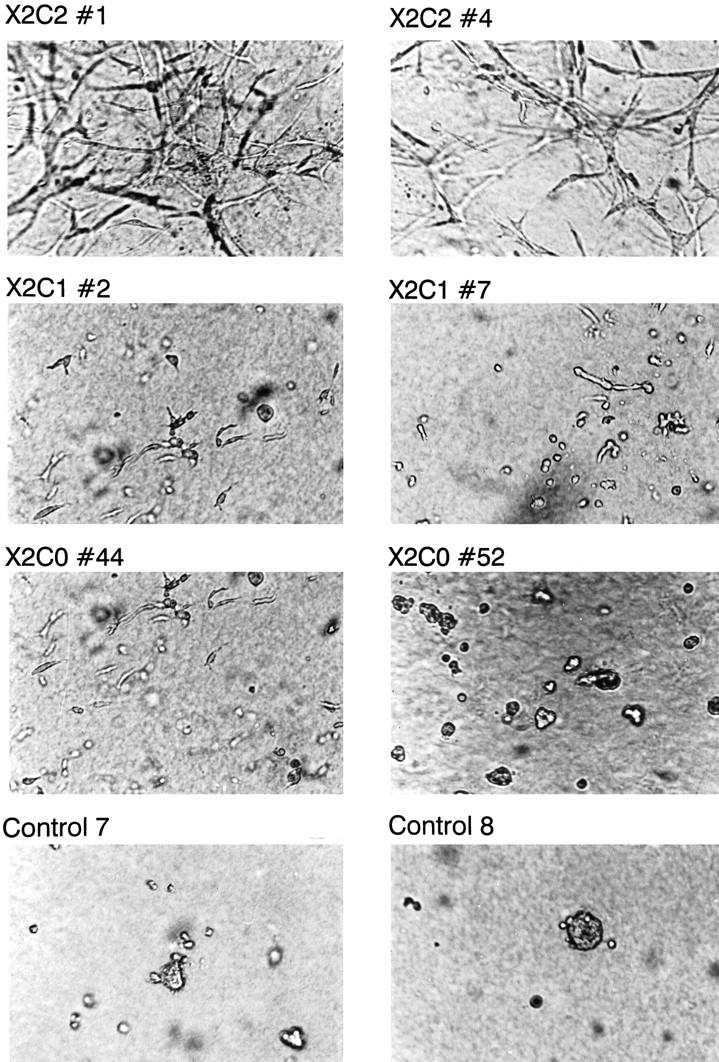
Expression of the X2C2, X2C1, and X2C0 integrin subunits modulates the ability of the NMuMG cells to undergo branching morphogenesis in three-dimensional collagen gels. Branching of X2C2 transfectants X2C2#1 and X2C2#4, X2C1 transfectants X2C1#2 and X2C1#7, X2C0 transfectants X2C0#44 and X2C0#52, and vector-only control cells (Controls 7 and 8) were analyzed in three-dimensional gels of type I collagen.
To begin to understand the mechanism by which the α2 integrin subunit mediates branching morphogenesis in three-dimensional collagen gels, we focused on the role of cytoplasmic domains of the two collagen/laminin receptors, the α1β1 and α2β1 integrins. 8,30-33 We assembled a panel of NMuMG-3 clones expressing cDNA constructs encoding the full-length human α2 integrin subunit, X2C2 (as described above), a chimeric integrin subunit consisting of the extracellular and transmembrane domains of the α2 integrin fused to the cytoplasmic domain of the α1 integrin (X2C1), or an α2 integrin subunit truncated immediately distal to the highly conserved GFFKR sequence within the cytoplasmic domain of the integrin. At least six clonal cell lines were established from the transfection of each of the cDNA constructs.
The clonal cell lines were analyzed for cell surface expression of the X2C1 or X2C0 integrin subunits by flow cytometric analysis (Figure 4, A and B) ▶ . Two representative clonal cell lines expressing the chimeric X2C1 integrin subunit (X2C1#2 and X2C1#7) and two clonal lines expressing the truncated X2C0 integrin subunit (X2C0#44 and X2C0#52) at levels comparable to the expression of full-length X2C2 by the X2C2#1 and X2C2#4 clones were selected for further analysis. Because the levels of other integrin subunits varied slightly between the NMuMG-1 and NMuMG-3 subclones, we confirmed that the expression levels of the α1, α5, α6, β1, and β4 integrin subunits were similar among the evaluated clones (data not shown).
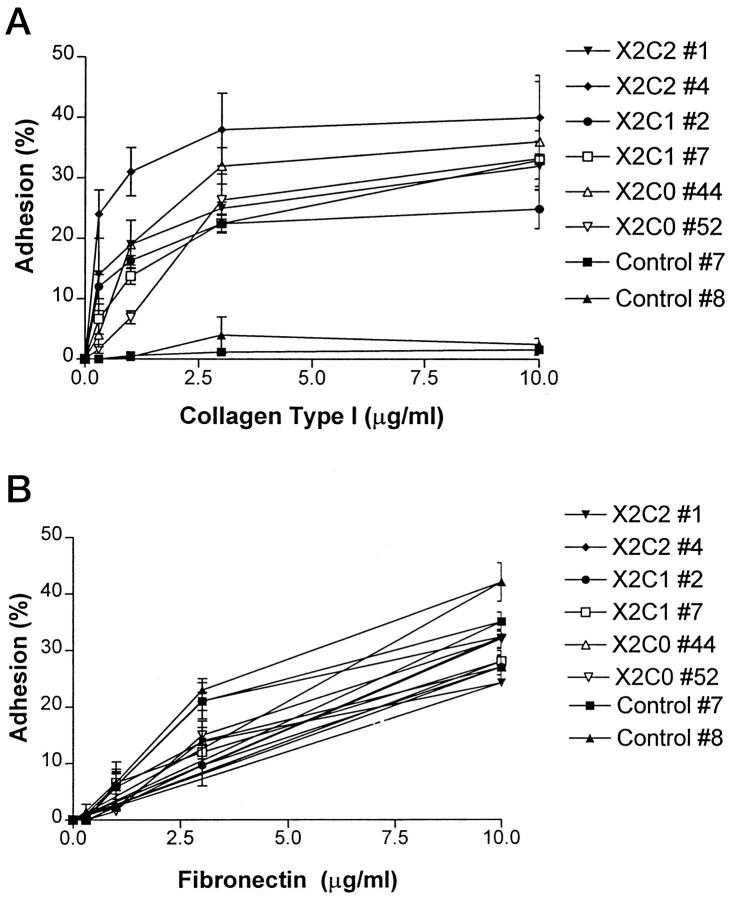
Adhesion to collagen type I and fibronectin substrates by clones expressing the X2C2, X2C1, X2C0 constructs as well as by the vector-only controls was analyzed. As shown in Figure 6A ▶ , cell lines expressing the X2C2, X2C1, or X2C0 subunit adhered to type I collagen in a Mg2+- and concentration-dependent manner. The X2C2-expressing clones X2C2#1 and X2C2#4, the X2C1-expressing clones X2C1#2 and X2C1#7, and the X2C0-expressing clone X2C0#52 all effectively and comparably adhered to type I collagen. The X2C0#44 clone adhered slightly less well to low concentrations of type I collagen, although the level of surface α2β1 integrin expression was similar. The vector-only control cells failed to adhere to type I collagen, consistent with the absence of both the α1β1 and α2β1 integrin collagen receptors. The X2C2, X2C1, and X2C0 transfectants, as well as the control cells, adhered to fibronectin substrates in a similar manner (Figure 6B) ▶ .
Figure 6.
Adhesion to collagen type I and fibronectin substrates. The X2C2 transfectants X2C2#1 and X2C2#4, the X2C1 transfectants X2C1#2 and X2C1#7, the X2C0 transfectants X2C0#44 and X2C0#52, and vector-only control cells were assayed for adhesion to substrates composed of the indicated concentrations of type I collagen (A) or fibronectin (B) in the presence of 2 mmol/L Mg2+. Results are presented as mean ± SD.
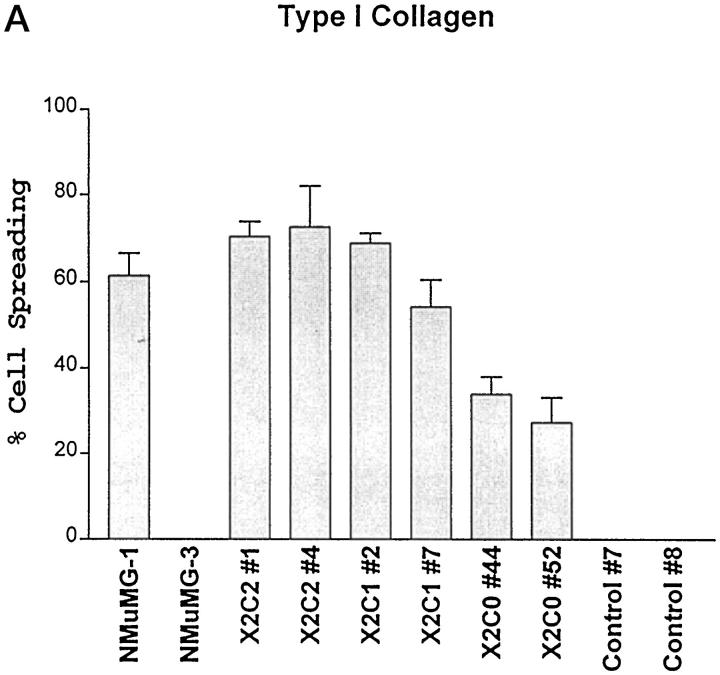
To further evaluate the role of the cytoplasmic tails of the α1 and α2 integrin subunits in cell-matrix interactions, the ability of the cells to spread after adhesion to type I collagen was analyzed. The X2C2-expressing clones, X2C2#1 and X2C2#4, adhered, spread, and adopted a polygonal morphology after 1 hour of adhesion to type I collagen (Figure 7A) ▶ . The cells expressing full-length α2 integrin subunit exhibited well-defined membrane extensions and lamellipodia (Figure 7B) ▶ . Although the extent of adhesion of the X2C1- and X2C0-expressing clones was similar to that of the X2C2 transfectants, a lower percentage of X2C1-expressing NMuMG cells spread when attached to type I collagen substrates for the same time period (Figure 7A) ▶ . The X2C1-expressing cells formed short, rudimentary extensions and filopodia and developed a few well-defined lamellipodia after 1 hour of adherence (Figure 7B) ▶ . The X2C0 transfectants spread poorly. These findings suggest that the cytoplasmic tails of the α1 and α2 integrins serve different roles after adhesion to type I collagen and that the α cytoplasmic domain is required for rapid spreading.
Figure 7.
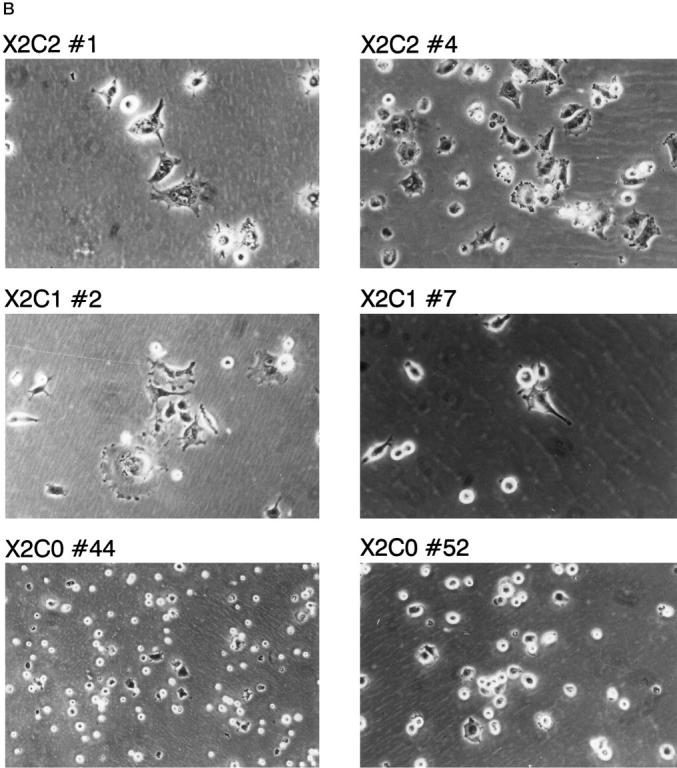
Spreading on type I collagen substrates. A: Spreading on type I collagen substrates. The ability of the X2C2, X2C1 transfectants and the X2C0 transfectants to spread after 1 hour on type I collagen substrates in the presence of 2 mmol/L Mg2+ was analyzed quantitatively. The number of adherent and spread versus adherent and nonspread cells was quantitated at ×200, using an inverted phase-contrast microscope. The percentage (mean ± SD) of spread cells from at least three separate experiments is shown. B: Morphology of spread cells on type I collagen matrices. The X2C2, X2C1, and X2C0 transfectants were allowed to adhere and spread for 1 hour on type I collagen matrices in 2 mmol/L Mg2+. Adherent cells were evaluated microscopically (×200). X2C2 transfectants demonstrated a flattened, polygonal morphology. The X2C1 transfectants formed microspikes and filopodia. The X2C0 transfectants spread poorly.
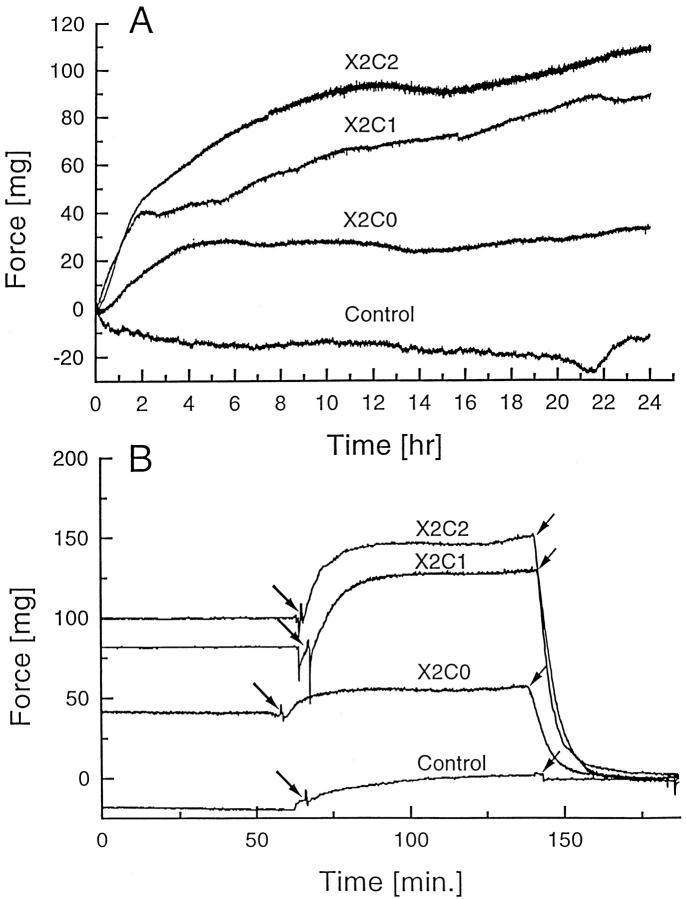
Both the α1β1 and α2β1 integrins have been shown to play an important role in collagen gel contraction. 18,19,22 We have evaluated the ability of the X2C2-, X2C1-, and X2C0-expressing clones to stiffen a collagen matrix and develop basal force within collagen gels. Reconstituted tissue rings were formed with the X2C2#1, X2C1#2, and X2C0#52 transfectants or control cells as described in Materials and Methods. Within an hour after collagen gelation, before gel compression or remodeling, the reconstituted tissue rings were mounted on isometric force transducers. Force development from the collagen gels containing either the X2C2, X2C1, X2C0 transfectants or control cells was monitored continuously for 24 hours (Figure 8A) ▶ . Collagen gels containing X2C2 and X2C1 transfectants developed the highest contractile force. Collagen gels containing the X2C0 transfectants also developed contractile force, albeit significantly less than that produced by the X2C2 and X2C1 transfectants. In contrast, the rings containing control cells produced no detectable force. Hence, the presence of the extracellular and transmembrane domains of the α2 subunit, coupled to an intact β1 subunit, is sufficient for low basal force development in the absence of a corresponding α cytoplasmic domain. Integrin subunits containing the extracellular and transmembrane domains of the α2 subunit and either the α2 or the α1 cytoplasmic domain can generate comparable force that is substantially greater than the force generated in the absence of a cytoplasmic tail. Control cells fail to develop detectable force.
Figure 8.
Force development in three-dimensional collagen gels. A: Force development during matrix compression. Model tissue rings formed of collagen gels containing the X2C2#1, X2C1#2, and X2C0#52 transfectants or vector-only control cells were mounted on a force transducer system within 1 hour after collagen gelation. At this time the cells adhere to the collagen but have not yet begun to exert significant contractile force. The model tissues were maintained at 37°C in the presence of 10% fetal calf serum, and the force exerted by the model tissues was recorded continuously for 24 hours. B: Acute force response to calf serum. After the period of tissue “development” and force generation shown above, cells were exposed to 20% calf serum (large arrows), and the force was monitored. After force levels had stabilized to the maximum levels (approximately 2.5 hours), the model tissues were exposed to 2 μmol/L cytochalasin D (small arrows), which caused a rapid drop in force.
Model tissues respond to mitogenic agonists such as calf serum or thrombin by activating myosin light chain kinase and developing readily measured contractile force. 34 After a 24-hour period of force development (Figure 8A) ▶ , the collagen tissues were exposed to 20% calf serum (Figure 8B) ▶ . Tissues containing the X2C2#1 and X2C1#2 transfectants generated comparable force in response to calf serum. In contrast, collagen gels containing either the X2C0#52 transfectants or control transfectants generated only minimal additional force in response to calf serum. After the model tissues had reached maximum force levels in response to calf serum, disruption of the actin cytoskeleton with 2 μmol/L cytochalasin D abolished the increase in force, which occurred both during tissue compression (Figure 8A) ▶ and in response to calf serum (Figure 8B) ▶ . Force generation diminished to the zero force level. The failure of cytochalasin D to reverse the small force increase elicited by the addition of calf serum to gels containing control cells suggests that the small force was unlikely to have resulted from myosin-dependent contraction. Indeed, 2 μmol/L CD not only should abolish actin-myosin interactions, but should also interfere with integrin-dependent interaction of cells with the matrix, which also involves actin filaments. It is unlikely that the small cytochalasin D-resistant force increase shown by the control cells resulted from any type of cellular response. On the contrary, it is more likely to have been caused by some technical artifact in this measurement. The presence of this potential artifact complicates the interpretation of the similar small force increase shown by the X2C0 transfectants in response to calf serum (CS). It is possible that this acute response to CS is smaller than what appears in Figure 8B ▶ , and may even be zero. The affect of cytochalasin D on this tissue confirms that the substantial basal force developed during the period of tissue compression is actin-dependent, as expected.
To determine whether the cytoplasmic tail of the α2 subunit confers specific signals that lead to α2β1 integrin-mediated branching morphogenesis, the ability of clonal NMuMG-3 cell lines expressing either the X2C1 subunit or the X2C0 subunit to develop elongated branches and tubules in three-dimensional collagen gels was compared to the ability of the X2C2-expressing clones. X2C2-transfected cell lines formed an extensive network of well-defined branches and tubules in three-dimensional collagen gels at 72 hours, as shown above (Figure 5) ▶ . In contrast, X2C1- and X2C0-transfected cell lines exhibited poorly organized rudimentary extensions or formed small grape-like clusters (Figure 5) ▶ . The latter phenotype was characteristic of cells expressing the X2C0 subunit. Vector-only control cells remained as individual cells or rounded cell aggregates. Proliferation by the vector-only control cells and to a lesser extent by the X2C0-expressing clones in three-dimensional collagen matrices appeared to be reduced in comparison to that of cells expressing either the X2C2 or the X2C1 subunit (Figure 5) ▶ . These findings establish an independent role for the cytoplasmic domain of the α2 integrin subunit in mediating downstream signals distinct from those mediated by the cytoplasmic domain of the α1 subunits in determining the three-dimensional organization of glandular structures.
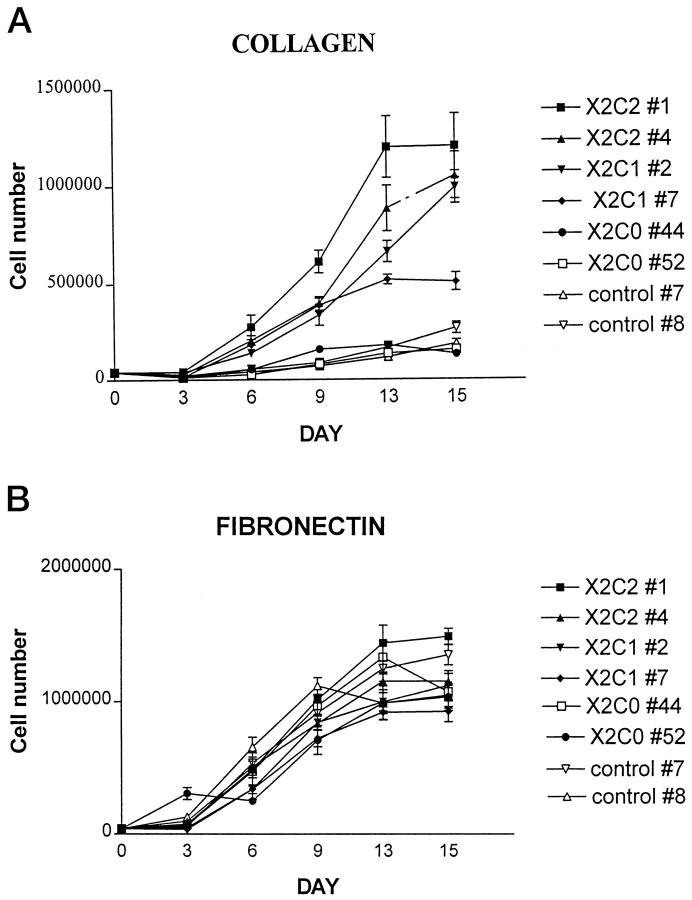
Because of the apparent differences in proliferation in collagen gels noted above, we quantitatively evaluated the proliferation of the transfectants on collagen and fibronectin substrates. The X2C2 and X2C1 transfectants proliferated at comparable rates, as shown in Figure 9A ▶ . In contrast, the X2C0 transfectants and the vector-only transfected control cells failed to grow on type I collagen substrates. All transfectants and the vector-only control cells grew at similar rates on fibronectin (Figure 9B) ▶ . These results suggest that the cytoplasmic tail of the integrin α subunit is required for signals that mediate cell cycle progression.
Figure 9.
Adhesion-dependent growth of α2 transfectants on type I collagen (A) and fibronectin (B). The rate of cell proliferation was determined by plating serum-starved cells at low density on either collagen type I (25 μg/ml) or fibronectin (25 μg/ml) in MEBM media containing bovine pituitary extract, hydrocortisone, human epithelial growth factor, and insulin, in triplicate. Cell numbers were determined every 3 days for 15 days. Data are presented as mean ± SD.
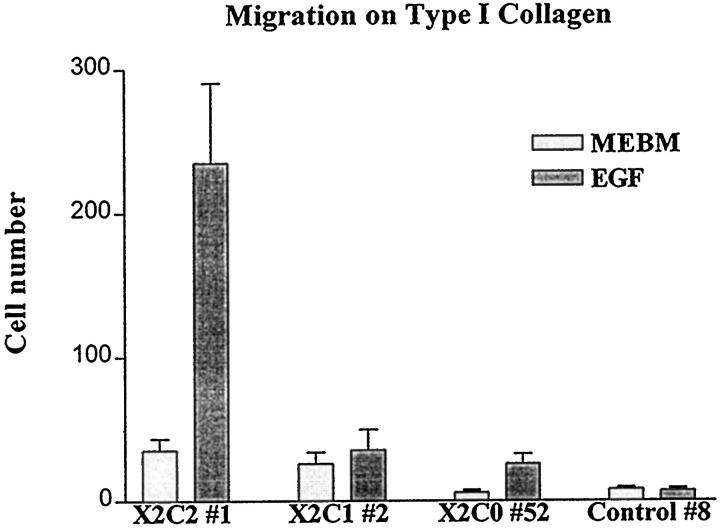
To begin to address the differences in the ability of the cytoplasmic domains of the α1 and α2 integrin subunits to signal branching morphogenesis, we focused on the ability of the X2C2 and X2C1 transfectants to respond to growth factors. In experiments carried out in serum-free media, branching morphogenesis of the X2C2 transfectants was dependent on growth factors including EGF and bovine pituitary extract (data not shown). We therefore evaluated the ability of the X2C2, X2C1, X2C0, and control transfectants to migrate in chemotaxis assays in response to EGF (10 μg/ml). As shown in Figure 10 ▶ , the X2C2 transfectants migrated in response to EGF on type I collagen. In contrast, the X2C1, X2C0, and control transfectants failed to migrate in response to EGF on type I collagen. The transfectants failed to migrate when identical concentrations of EGF were placed in both the upper and lower chambers, documenting the chemotactic nature of migration. The transfectants all expressed comparable levels of the EGF receptor (data not shown). We proposed that one potential mechanism for specificity mediated by the α1β1 and α2β1 integrins is the ability of the cytoplasmic tail of the α2versus the α1 integrin subunit to respond to distinct signaling pathways that activate complex cellular processes such as migration and branching morphogenesis.
Figure 10.
Chemotaxis of the α2 transfectants on type I collagen in response to epidermal growth factor. X2C2, X2C1, X2C0, and control transfectants were plated on the upper surface of the transwell filter coated with type I collagen (25 μg/ml). Migration through the pores of the transwell filter was stimulated with either no additional growth factors or epidermal growth factor (10 ng/ml). Cell migration was allowed to proceed for 4 hours in a 5% C02 humidified chamber at 37°. The number of cells attached to the lower surface of the transwell filter was quantitated microscopically. Results are presented as the mean ± SD of at least three separate experiments.
Discussion
Evidence from a number of laboratories suggests that different integrins may mediate distinct “postreceptor ligand occupancy” events that determine cellular differentiation, alteration in gene expression, and the regulation of tumor progression. 35-39 The signaling pathways activated by integrin receptor occupancy and clustering have been extensively studied in the last few years. Multiple pathways, including the ras/raf/erk, the rho/rac/cdc42, the Gi-linked pathways, and the PI 3 kinase pathway, mediate “inside-out” and “outside-in” signaling. 40-46 Although a great deal has been learned about overall signaling pathways mediated by the β1 integrin family, the mechanisms by which specific α and β heterodimers alter cellular differentiation and gene expression in contrast to distinct effects of other integrins have not been resolved.
Our previous studies using complementary “gain of function” and “loss of function” models indicated that the α2β1 integrin is required for epithelial differentiation and morphogenesis of breast glands and tubules in vitro and that the diminished α2β1 integrin expression contributes to motility and the invasive behavior of tumor cells in vitro. 4,5,15,47 We have also shown that reexpression of the α2β1 integrin in a poorly differentiated, invasive breast carcinoma cell line greatly diminishes, but does not completely abrogate, the malignant potential in vivo. 15 This critical observation that α2β1 integrin expression is required for maintenance of the differentiated epithelial phenotype and glandular differentiation in vitro has been confirmed by others. The α2β1 integrin appears to play a similar role in the morphological differentiation of colonic epithelial cells and renal tubular epithelial cells. 5,48
The present study describes a model system, an immortalized but nonmalignant epithelial cell line that fails to express either the α1β1 or α2β1 integrin, to evaluate the role of collagen receptors in epithelial differentiation. The results reveal that both the α1 and α2 integrin cytoplasmic domains can effectively support adhesion, spreading, collagen gel contraction, and growth. In the presence of a truncated α subunit lacking a cytoplasmic tail, cells effectively adhere and attempt to spread but fail to proliferate when attached to type I collagen. Cells expressing either the X2C2 or the X2C1 integrin subunits develop contractile force in collagen matrices both during an initial period of slow matrix compression and subsequently in an acute response to the addition of calf serum. In contrast, model tissues of collagen-containing control cells, lacking either the α1 or α2 integrin subunits, exert no detectable force on the collagen matrix in either compression or acute response phase. Collagen gels containing the X2C0 transfectants, which express the α2 extracellular and transmembrane domains but lack the cytoplasmic domain of either the α1 or α2 integrin, also produce a significant level of basal force during tissue compression, although the force generated by the X2C0 cells is substantially less than that seen with the X2C2 and X2C1 transfectants. Unlike the X2C2 or the X2C1 cells, the X2C0 cells exert little or no additional force in response to calf serum. Collagen gels containing the control cells generate no detectable force on the collagen matrix in either the compression or the acute response to serum phase. These findings suggest that the slow development of force during tissue compression does not require the α integrin cytoplasmic domain, but that the cytoplasmic domain is required for the acute response to serum.
The clones expressing the X2C0 integrin subunit and vector-only control cells appeared to have a defect in cellular proliferation on collagen relative to cells expressing either the X2C2 or X2C1 subunits. No growth defect was apparent under standard culture conditions. The vector-only control cells exhibited the most severe proliferative defect. The inability of the control cells to grow on collagen is not surprising, because the cells do not adhere to the substrate. However, an alternative explanation is required for the diminished growth of cells expressing the X2C0 construct, because they effectively adhere to the collagenous substrates. The differences in cell proliferation between the X2C0-, the X2C2-, and the X2C1-expressing cells suggest that an intact α subunit cytoplasmic domain is required to fully support the integrin-mediated signals leading to the cell cycle progression of NMuMG cells.
These findings are surprising in light of the results of Wary et al. 20 In their study, ligation of the α1β1 integrin, which was associated with shc in caveoli, led to activation of the ras/MAP kinase cascade, to cell cycle progression, and to prevention of apoptosis. In contrast, they observed no association of the α2β1 integrin with shc. In addition, the α2β1 integrin failed to activate the ras/MAP kinase cascade, to promote cell cycle progression, or to prevent apoptosis. However, we have observed that in some cell types, notably mammary epithelium, the α2β1 integrin can support cell proliferation. Perhaps the α2 integrin subunit can associate with shc in some cell types and thereby activate the ras/MAP kinase cascade. A more likely explanation, however, is that the α2β1 integrin mediates signals that promote cell cycle progression via alternative pathways. These findings do suggest that other signaling molecules may stimulate growth and cell cycle progression mediated via the α2β1 integrin.
The cytoplasmic tail of the α2 integrin subunit uniquely confers the ability to branch in three-dimensional collagen gels. The cytoplasmic tail of the α1 integrin subunit could not support the formation of extensive elongated branches. In recent studies, transfectants expressing the full-length human α1 integrin subunit (X1C1) also failed to form elongate branches in three-dimensional collagen gels (data not shown). Thus more complex downstream events, beyond adhesion to collagen and the formation of focal adhesion complexes, are responsible for the morphogenetic activity. In other systems using recombinant basement membrane instead of collagen, the α6β4 integrin can mediate branching morphogenesis. 49 Earlier work by our laboratory demonstrated cross-talk between the α2β1 and α6β4 integrins. In a malignant mammary epithelial cell line, reexpression of the α2β1 integrin resulted in the up-regulation of expression of both the α6 and β4 integrin subunits. 28 In recent work by O’Connor et al, reexpression of the β4 integrin subunit by a highly malignant breast epithelial cell resulted in increased chemotactic migration on collagen that was inhibited by an anti-β1 integrin subunit monoclonal antibody but not by an anti-α6 integrin subunit monoclonal antibody. 50 The importance of the β1 integrin family of cell adhesion receptors for mammary epithelial organization is also supported by the works of Weaver and colleagues. 51 The migration assays suggest that one mechanism for specificity mediated by the α1 and α2 subunit cytoplasmic domains is the ability of the α2, but not the α1 integrin subunit to respond to distinct signaling pathways, such as those initiated by EGF. Alternatively, the cytoplasmic domain of the α2 integrin subunit may participate in transdominant stimulation or inhibition of other integrin subunits in a manner similar to that recently described for the α3 integrin subunit by Hodivila-Dilke et al. 52
Our findings extend earlier observations regarding the function of the α2 cytoplasmic tail. Chan et al 22 demonstrated that the cytoplasmic tails of the α4 or α5 integrin subunits could substitute for the α2 integrin subunit tail in promoting adhesion to type I collagen when chimeric integrin subunits were expressed in either K562 cells (a hematopoietic progenitor cell line) or RD cells (a rhabdomyosarcoma cell line). In contrast, RD cells expressing either the full-length α2 integrin subunit, X2C2, or a chimeric integrin subunit consisting of the extracellular and transmembrane domains of the α2 subunit and the cytoplasmic domain of α5 subunit contracted collagen gels, whereas RD cells expressing the chimeric subunit consisting of the extracellular and transmembrane domains of the α2 subunit and the cytoplasmic domain of the α4 subunit failed to contract collagen gels. Although these important findings first suggested distinct roles for the cytoplasmic tails of integrin subunits in mediating integrin function, the α2 integrin subunit is not expressed by normal skeletal muscle cells in vivo, and the α4 and α5 integrin subunits mediate adhesion to fibronectin and V-CAM. Thus the studies do not address the apparent redundancy of the two collagen/laminin receptors α1β1 and α2β1 and do propose a model of specificity for the distinct cytoplasmic domains.
In summary, our work provides compelling new data on the ability of the α1β1 and the α2β1 integrins to support epithelial differentiation and complex processes such as morphogenesis and cell migration. Integrin subunits containing either the α1 or α2 cytoplasmic domain, but not tailless α subunits, can mediate the formation of well-defined focal adhesion complexes, generation of optimal contractile force in three-dimensional collagen gels, and progression through the cell cycle. These roles of the α subunit cytoplasmic tails were not heretofore appreciated. Despite these shared functions, some notable differences were also apparent. For example, the α2 cytoplasmic domain but not the α1 cytoplasmic domain effectively supported branching morphogenesis of mammary epithelial cells and migration on type I collagen in response to EGF. These findings indicate that the α1 and α2 integrin cytoplasmic domains, in response to, and in concert with, distinct growth signals, exert profoundly different influences on cell phenotype.
Footnotes
Address reprint requests to Dr. Mary M. Zutter, Washington University School of Medicine, Department of Pathology, Box 8118, 660 S. Euclid Ave, St. Louis, MO 63110.
Supported in part by National Institutes of Health grants HL40506 and CA70275.
References
- 1.Zutter MM, Mazoujian G, Santoro SA: Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol 1990, 137:863-870 [PMC free article] [PubMed] [Google Scholar]
- 2.Zutter MM, Santoro SA: Widespread histologic distribution of the α2β1 integrin cell surface collagen receptor. Am J Pathol 1990, 137:113-120 [PMC free article] [PubMed] [Google Scholar]
- 3.Zutter MM, Krigman HR, Santoro SA: Altered integrin expression in adenocarcinoma of the breast. Analysis by in situ hybridization. Am J Pathol 1993, 142:1439-1448 [PMC free article] [PubMed] [Google Scholar]
- 4.Keely PJ, Fong AM, Zutter MM, Santoro SA: Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense α2 integrin mRNA in mammary cells. J Cell Sci 1995, 108:595-607 [DOI] [PubMed] [Google Scholar]
- 5.Saelman EUM, Keely PJ, Santoro SA: Loss of MDCK cell α2β1 integrin expression results in reduced cyst formation, failure to hepatocyte growth factor/scatter factor-induced branching morphogenesis, and increased apoptosis. J Cell Sci 1995, 108:3531-3540 [DOI] [PubMed] [Google Scholar]
- 6.Berdichevsky F, Gilbert C, Shearer M, Taylor-Papadimitriou J: Collagen-induced morphogenesis of human mammary epithelial cells: the role of the α2β1 integrin. J Cell Sci 1991, 102:437-446 [DOI] [PubMed] [Google Scholar]
- 7.Santoro SA, Zutter MM: The α2β1 integrin: a collagen receptor on platelets and other cells. Thromb Haemost 74:813–821 [PubMed]
- 8.Santoro SA: Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell 1986, 46:913-920 [DOI] [PubMed] [Google Scholar]
- 9.Staatz WD, Rajpara SM, Wayner EA, Carter WG, Santoro SA: The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg2+-dependent adhesion of platelets to collagen. J Cell Biol 1989, 108:1917-1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchhofer D, Languino LR, Ruoslahti E, Pierschbacher MD: Alpha2β1 integrins from different cell types show different binding specificities. J Biol Chem 1990, 265:615-618 [PubMed] [Google Scholar]
- 11.Koretz K, Schlag P, Boumsell L, Moller P: Expression of VLA-α 2, VLA-α 6, and VLA-β 1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am J Pathol 1991, 138:741-750 [PMC free article] [PubMed] [Google Scholar]
- 12.Stallmach A, Von Lampe B, Matthes H, Bornhoft G, Riecken EO: Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malignant tumor transformation. Gut 1992, 33:342-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall PA, Coates P, Lemoine NR, Horton MA: Characterization of integrin chains in normal and neoplastic human pancreas. J Pathol 1991, 165:33-41 [DOI] [PubMed] [Google Scholar]
- 14.Bonkoff H, Stein U, Remberger K: Differential expression of α6 and α2 very late antigen integrins in the normal, hyperplastic and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol 1993, 24:243-248 [DOI] [PubMed] [Google Scholar]
- 15.Zutter MM, Santoro SA, Staatz WD, Tsung YL: Re-expression of the α2β1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA 1995, 92:7411-7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza B, Berdichevsky F, Kyprianou N, Taylor-Papadimitrious J: Collagen-induced morphogenesis and expression of the α2-integrin subunit is inhibited in c-erbB2-transfected human mammary epithelial cells. Oncogene 1993, 8:1797-1806 [PubMed] [Google Scholar]
- 17.Langholz O, Röckel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B: Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulation by α1β1 and α2β1 integrins. J Cell Biol 1995, 131:1903-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotwals PJ, Chi-Rosso G, Lindner V, Yang J, Ling L, Fawell SE, Koteliansky VE: The α1β1 integrin is expressed during neointima formation in rat arteries, and mediates collagen matrix reorganization. J Clin Invest 1996, 97:2469-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racin-Samson L, Rockey DC, Bissell DM: The role of α1β1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem 1997, 272:30911-30917 [DOI] [PubMed] [Google Scholar]
- 20.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG: The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 1996, 87:733-743 [DOI] [PubMed] [Google Scholar]
- 21.Takada Y, Hemler ME: The primary structure of the VLA-2/collagen receptor α2 subunit (platelet GP IA): homology to other integrins and the presence of a possible collagen-binding domain. J Cell Biol 1989, 109:397-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan BMC, Kassner PD, Schiro JA, Byers R, Kupper TS, Hemler ME: Distinct cellular functions mediated by different VLA integrin α subunit cytoplasmic domains. Cell 1992, 68:1051-1060 [DOI] [PubMed] [Google Scholar]
- 23.Briesewitz R, Epstein MR, Marcantonio EE: Expression of native and truncated forms of the human integrin α1 subunit. J Biol Chem 1993, 268:2989-2996 [PubMed] [Google Scholar]
- 24.Wu JE, Santoro SA: Complex patterns of expression suggest extensive roles for the α2β1 integrin in murine development. Dev Dyn 1994, 199:292-314 [DOI] [PubMed] [Google Scholar]
- 25.Dickeson SK, Walsh JJ, Santoro SA: Contributions of I and EF hand domains to the divalent cation-dependent collagen binding activity of the α2β1 integrin. J Biol Chem 1997, 272:7661-7668 [DOI] [PubMed] [Google Scholar]
- 26.Santoro SA, Zutter MM, Wu JE, Staatz WD, Saelman EUM, Keely PJ: Analysis of collagen receptors. Methods Enzymol 1995, 245:147-183 [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W: Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol 1995, 131:1573-1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun H, Santoro SA, Zutter MM: Down-stream events in mammary gland morphogenesis mediated by re-expression of the α2β1 integrin: the role of the α6 and β4 integrin subunits. Cancer Res 1998, 58:2224-2233 [PubMed] [Google Scholar]
- 29.Zutter MM, Sun H, Santoro SA: Altered integrin expression and the malignant phenotype. The contribution of multiple integrated integrin receptors. J Mamm Gland Biol Neonate 1998, 3:191-200 [DOI] [PubMed] [Google Scholar]
- 30.Ignatius MJ, Large TH, Houde M, Tawil JW, Barton A, Esch F, Carbonetto S, Reichardt LF: Molecular cloning of the rat integrin α1-subunit: a receptor for laminin and collagen. J Cell Biol 1990, 111:709-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignatius MJ, Reichardt LF: Identification of a neuronal laminin receptor: an Mr 200K/120K integrin heterodimer that binds laminin in a divalent cation-dependent manner. Neuron 1988, 1:713-725 [DOI] [PubMed] [Google Scholar]
- 32.Tawil NJ, Houde M, Blacher R, Esch F, Reichardt LF, Turner DC, Carbonetto S: α1β1 integrin heterodimer functions as a dual laminin/collagen receptor in neural cells. Biochemistry 1990, 29:6540-6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall DR, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH: The α1β1, and α6β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol 1990, 110:2175-2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodney MS, Elson EL: Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem 1993, 268:23850-23855 [PubMed] [Google Scholar]
- 35.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ: Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 1997, 137:231-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dedhar S, Argraves WS, Suzuki S, Ruoslahti E, Pierschbacher MD: Human osteosarcoma cells resistant to detachment by an Arg-Gly-Asp-containing peptide overproduce the fibronectin receptor. J Cell Biol 1987, 5:1175-1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichardt LF, Tomaselli KJ: Extracellular matrix molecules and their receptors: function in neural development. Annu Rev Neurosci 1991, 14:531-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werb Z, Tremble PM, Behrendsten O, Crowley E, Damsky CH: Signal transduction through the fibronectic receptor induces collagenase and stromelysin gene expression. J Cell Biol 1989, 109:877-899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damsky CH, Werb Z: Signal transduction by integrin for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol 1992, 4:772-781 [DOI] [PubMed] [Google Scholar]
- 40.Schwartz MA, Schaller MD, Ginsberg MH: Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995, 11:549-599 [DOI] [PubMed] [Google Scholar]
- 41.Clark EA, Brugge JS: Integrins and signal transduction pathways: the road taken. Science 1995, 268:233-239 [DOI] [PubMed] [Google Scholar]
- 42.Dedhar S, Hanningan GE: Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol 1996, 8:657-669 [DOI] [PubMed] [Google Scholar]
- 43.McNamee HP, Ingber DE, Schwartz MA: Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol 1993, 121:673-678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapron-Bras C, Fitz-Gibbon L, Jeevaratnam P, Wilkins J, Dedhar S: Stimulation of tyrosine phosphorylation and accumulation of GTP-bound p21ras upon antibody-mediated α2β1 integrin activation in T-lymphoblastic cells. J Biol Chem 1993, 268:20701-20704 [PubMed] [Google Scholar]
- 45.Chen Q, Kinch MS, Lin TH, Burridge K, Juliano R: Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem 1994, 269:26602-26605 [PubMed] [Google Scholar]
- 46.Clark EA, Hynes RO: Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem 1996, 271:14814-14818 [DOI] [PubMed] [Google Scholar]
- 47.Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV: Cdc42 and Rac1 induce integrin-mediated cell motility, and invasiveness through PI(3)K. Nature 1997, 390:632-636 [DOI] [PubMed] [Google Scholar]
- 48.Del Buono R, Pignatelli M, Bodmer WF, Wright NA: The role of the arginine-glycine-aspartic acid-directed cellular binding to type I collagen and rat mesenchymal cells in colorectal tumour differentiation. Differentiation 1991, 46:97-103 [DOI] [PubMed] [Google Scholar]
- 49.Stahl S, Weitzman S, Jones JCR: The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci 1997, 55–63 [DOI] [PubMed]
- 50.O’Connor KL, Shaw L, Mercurio A: Release of cAMP gating by the α6β4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol 1998, 143:1749-1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weaver VM, Peterson O, Wang C, Larabell P, Damsky C, Bissell M: Reversion of the malignant phenotype of human breast cells in three dimensional culture and in vivo by integrin. J Cell 1997, 231–245 [DOI] [PMC free article] [PubMed]
- 52.Hodivala-Dilke KA, DiPersio C, Kreidberg J, Hynes RO: Novel roles for α3β1 integrin as a regulator for cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J Cell Biol 142:1357–1360 [DOI] [PMC free article] [PubMed]