Abstract
The β-thymosins comprise a family of structurally related, highly conserved acidic polypeptides, originally isolated from calf thymus. Recently, we have demonstrated the overexpression of thymosin β-10 (TB10) in rat thyroid transformed cell lines and in human thyroid carcinoma tissues and cell lines. To verify whether TB10 overexpression is a general event in the process of carcinogenesis, we have analyzed TB10 mRNA levels in human colon carcinomas, germ cell tumors of different histological types, breast carcinomas, ovarian carcinomas, uterine carcinomas, colon and esophageal carcinoma cell lines. Overexpression of the TB10 gene was detected in all of the neoplastic tissues and cell lines compared to the respective normal tissues. Moreover, the mouse model of skin carcinogenesis induced by the combined action of chemical carcinogens and phorbol esters was used to identify the stage of TB10 gene induction. The expression was almost undetectable in normal keratinocytes, its induction occurred even at the papilloma stage, however a further increased expression was observed in the carcinoma derived cell lines. Finally, immunohistochemical analysis of some breast, colon and ovary carcinoma samples by using specific anti-TB10 antibodies revealed the presence of the TB10 protein in all of the neoplastic tissues, but not in the respective normal tissues. Therefore the TB10 detection may be considered a potential tool for the diagnosis of several human neoplasias.
Thymosins comprise a family of small proteins originally isolated from calf thymus, 1 and divided into three classes (α, β, and γ) based on their isoelectric point. The β class comprises structurally related, highly conserved acidic polypeptides. At first, it was suggested that these proteins had some role in regulating the immune response. However, more recent studies suggest that the expression of thymosins is associated with growth and differentiation of many cell types. In human tissues, thymosin β-10 (TB10) is widely distributed along with another component of the class, the thymosin β-4 (TB4). 2 Both proteins have been shown to bind to and sequester G-actin. 3-4 TB10 expression is related to cell growth in proliferating tissues, and it is associated with rat and human brain development. 5-8 Previous reports indicated an enhanced TB10 expression in a variety of human tumors such as renal, medullary thyroid carcinomas and melanomas. 9-11 Recently, we have shown that the TB10 gene is highly expressed in human thyroid carcinoma cell lines and tissues whereas its expression is almost undetectable in normal thyroid. The expression was particularly abundant in the undifferentiated thyroid carcinomas. 12
To verify whether the TB10 gene induction is a general event in human neoplasias, we have analyzed TB10 mRNA levels in colon, breast, uterine, ovarian, germ-cell tumors, colon and esophageal carcinoma cell lines. TB10 overexpression was observed in all of the neoplastic tissues and cell lines. To further characterize the role of this protein as a progression marker, we have analyzed cell lines deriving from normal skin, and carcinogen-induced papillomas and carcinomas. A correlation was found between TB10 mRNA levels and the progression step of the carcinogenesis process. Immunohistochemical analysis on some carcinoma samples confirmed the data obtained at the RNA level. Therefore, TB10 detection may be considered as potential tool in the diagnosis of several human neoplasias.
Materials and Methods
Cell Lines and Tissues
The human colorectal and esophageal carcinoma cell lines, used in this study, were a kind gift of Dr. H. Yamasaki, (International Agency for Cancer Research, Lyon, France). The murine normal and neoplastic skin-derived cell lines were a kind gift of Dr. A. Balmain, (Beatson Institute for Cancer Research, Glasgow, Scotland). C5N is a nontumorigenic immortalized keratinocyte cell line; P1 and P6 derive from papilloma benign lesions obtained from DMBA/TPA treated mice; A5 and Car B derive from murine spindle cell skin carcinoma; B9 derives from squamous skin carcinoma. 13,14 All cell lines were cultured in DMEM containing 10% fetal calf serum (Life Technologies LTD, Paisley Scotland).
Breast, colon, and germ-cell tumor samples were collected at the Istituto Nazionale dei Tumori di Napoli, Naples, Italy. Ovarian and uterine carcinoma samples were collected at the Divisione di Ostetricia and Ginecologia of the Facoltà di Medicina e Chirurgia di Catanzaro, Catanzaro, Italy. They were immediately frozen in liquid nitrogen after the surgical removal.
RNA Extraction and Northern Blot Analyses
Total RNA was isolated from cultured cells by a modification of the guanidine thiocyanate method. 15 For Northern blot analysis, 20 μg of RNA per sample were electrophoresed on 1.2% agarose gels containing 2.2 mol/L formaldehyde. After the electrophoresis, gels were blotted onto Hybond nylon membranes (Amersham, Little Chalfont, UK). As a TB10 probe, a Sau3A TB10 cDNA fragment 178 bp long was used. A specific TB4 probe was generated by polymerase chain reaction (PCR) amplification of a cDNA fragment based on the human sequence. 16 A probe for the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to check for uniform RNA loading and integrity. Probes were radiolabeled by the random primer method of Feinberg and Vogelstein 17 and used to hybridize Northern blots. Hybridization was carried out under stringent conditions (50% formamide, 42°C), followed by washing in 0.1 × standard saline citrate at 60°C. Filters were exposed to films for 12 to 120 hours. The sizes of the RNAs were determined by comparison with rRNA standards.
Immunohistochemistry
Paraffin sections (5–6 μm) were deparaffinized and placed in a solution of absolute methanol and 0.3% hydrogen peroxide for 30 minutes, then washed first in distilled water, then in phosphate-buffered saline (PBS), before immunoperoxidase staining. The slides were then incubated overnight at 4°C in a humidified chamber with the antibodies diluted 1:100 in PBS. The slides were subsequently incubated with biotinylated goat anti-rabbit IgG for 20 minutes (Vectastain ABC kits, Vector Laboratories, Burlingame, CA) and then with premixed reagent ABC (Vector) for 20 minutes. For immunostaining, the slides were incubated in diaminobenzidine (DAB-DAKO) solution containing 0.06 mmol/L DAB and 2 mmol/L hydrogen peroxide in 0.05% PBS, pH 7.6, for 5 minutes. After chromogen development, the slides were washed, hematoxylin-counterstained, dehydrated with alcohol and xylene, and mounted with coverslips using a permanent mounting medium (Permount). Micrographs were taken on Kodak Ektachrome film with a photo Zeiss system. The antibodies used in this study were raised against the synthetic peptide TIEQEKRSEIS specific for the TB10 protein 18 and affinity-purified against the same synthetic peptide.
Results
TB10 Gene Is Overexpressed in Colon and Esophageal Carcinoma Cell Lines and in Surgically Removed Colon Carcinomas
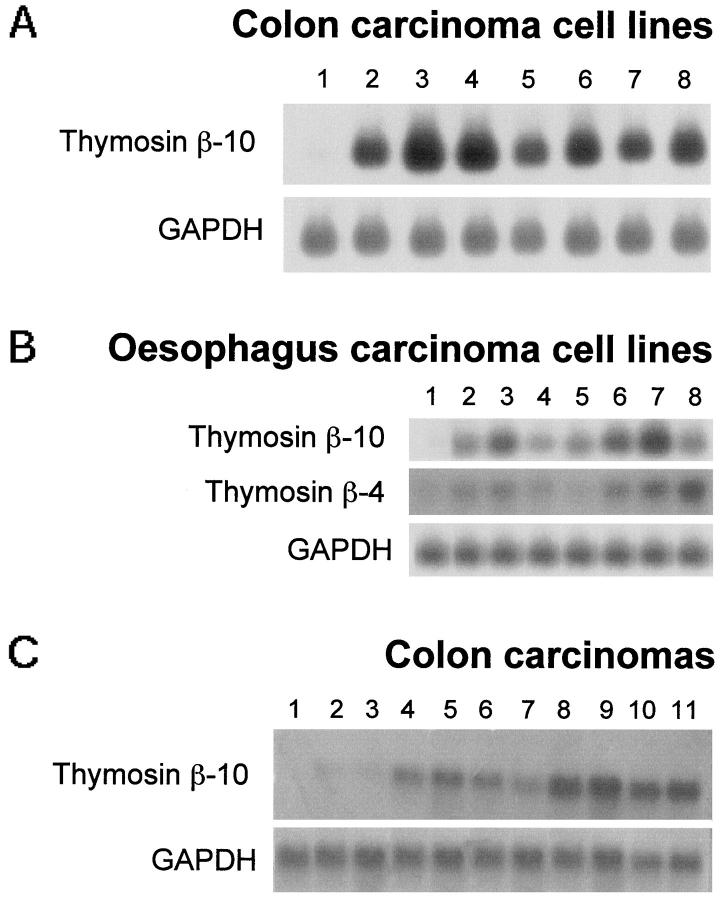
Seven colon carcinoma and seven esophageal cell lines were analyzed for TB10 gene expression. The results are shown in Figure 1, A and B ▶ .
Figure 1.
Analysis of the TB10 gene expression in human gastrointestinal tract-derived cell lines, normal mucosa, and colon carcinoma samples. Analysis of the TB4 gene expression in esophagus-derived cell lines. Twenty micrograms of total RNA were size-fractionated on a denaturing formaldehyde agarose gel, blotted onto Hybond-N nylon filters, and hybridized with TB10 cDNA and with a TB4-specific probe (B). A GAPDH probe was used to normalize results. RNA was extracted from the following sources: A, Lane 1, normal colonic mucosa; lane 2, Caco-3; lane 3, SW1417; lane 4, SW480; lane 5 , SW520; lane 6, LS 180; lane 7, Ω10F; lane 8, JVC. B, Lane 1, normal esophageal tissue; lane 2, TE 1; lane 3, TE 2; lane 4, TE 3; lane 5, TE 7; lane 6 , TE 8; lane 7, TE 10; lane 8, TE 13. C, lanes 1 and 2, normal colonic mucosa; lanes 3–11, colon carcinoma samples.
TB10 gene was abundantly expressed in all of the carcinoma cell lines (Figure 1A ▶ , lanes 2–8; Figure 1B ▶ , lanes 2–8), whereas it was not detectable in the normal colonic (Figure 1A ▶ , lane 1) and esophageal mucosa (Figure 1B ▶ , lane 1). However, no correlation was observed between the TB10 expression levels and the malignant phenotype of these cells, as evaluated by their ability to induce tumors into athymic mice. We have also analyzed TB4 gene expression in esophageal (Figure 1B) ▶ and colon (data not shown) carcinoma cell lines; it essentially parallels the TB10 gene expression.
Subsequently, the expression of the TB10 gene was evaluated in 9 human colon carcinoma samples by Northern blot (Figure 1C) ▶ . The level of the TB10-specific RNA transcript of 0.6 kb was found elevated in almost all of the neoplastic samples (Figure 1C ▶ , lanes 3–11) when compared with those detected in the normal tissue surrounding the lesion (Figure 1C ▶ , lanes 1 and 2).
TB10 Gene Expression in Human Germ Cell Tumors
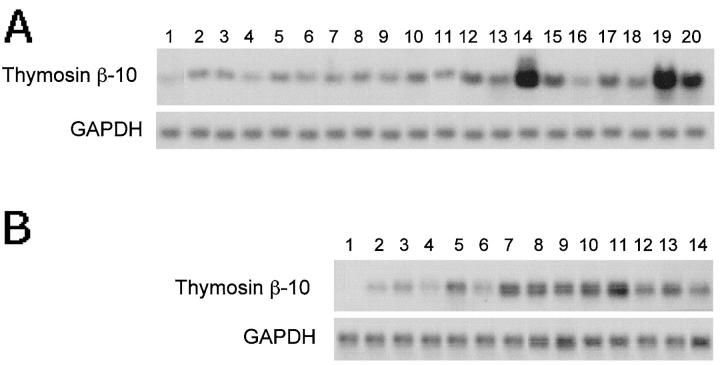
We analyzed TB10 gene expression in 32 human tumors of different histological types (15 seminomas and 17 nonseminomas) by Northern blot hybridization. The results are shown in Figure 2, A and B ▶ . No expression of TB10-specific transcript was detected in normal testis tissue (Figure 2A ▶ , lane 1, and Figure 2B ▶ , lane 1), whereas the neoplastic samples showed very high TB10 mRNA levels. On average, the expression levels observed in nonseminomas were higher than those detected in seminoma samples. The TB10 transcript was 0.6 kb in size, as in all of the other tissue examined. In the past, an additional band 0.75 kb long has been observed in rat mature testis, 18 but it was not detected in normal and neoplastic human samples, suggesting a species-specific pattern of TB10 gene expression in this tissue.
Figure 2.
Analysis of the TB10 gene expression in human germ-cell tumors. Twenty micrograms of total RNA were size-fractionated on a denaturing formaldehyde agarose gel, blotted onto Hybond-N nylon filters and probed with TB10 cDNA. A GAPDH probe was used to normalize results. RNA was extracted from seminoma and nonseminoma surgical samples. A: Lane 1, normal testis; lanes 2–11, seminomas; lanes 12–20, nonseminomas. B: Lane 1 , normal testis; lanes 2–6, seminomas; lanes 7–14, nonseminomas.
TB10 Gene Is Also Overexpressed in Breast, Ovarian, and Uterine Carcinomas
Ten human breast carcinomas, 13 ovarian carcinomas, and 16 uterine tumors were also analyzed for TB10 and TB4 gene expression. The results (apart from TB4 gene expression in ovarian carcinomas, which is not shown) are shown in Figure 3 ▶ . All of the tumor samples revealed a high expression of the TB10 and TB4 genes, whereas very low levels were observed in normal breast, ovary, and uterus. An additional band of approximately 2.5 kb was detected in 6 out of 10 breast carcinomas after hybridization with both probes. This band has never been observed in other normal and neoplastic tissues. Because it was detected with both the probes, we hypothesize that it might be the product of an unknown, closely related, β-thymosin gene. Interestingly, a transcript of a similar size was detected after serum stimulation of monkey kidney epithelial cells. 19
Figure 3.
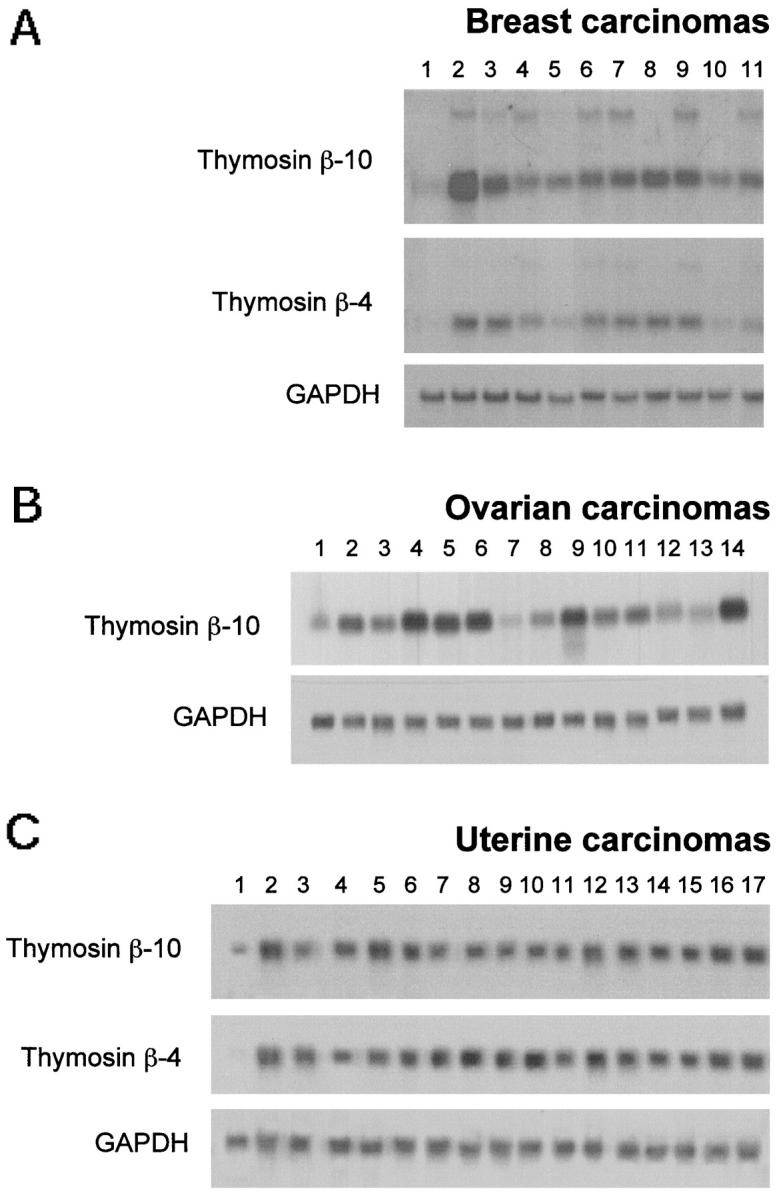
Analysis of the TB10 gene expression in human breast, ovarian, and uterine carcinomas. Twenty micrograms of total RNA were size-fractionated on a denaturing formaldehyde agarose gel, blotted onto Hybond-N nylon filters, and hybridized with TB10 cDNA and with a TB4-specific probe. A GAPDH probe was used to normalize results A: RNA was extracted from normal (lane 1) and carcinoma (lanes 2–11) breast tissues. B: RNA was extracted from normal (lane 1) and carcinoma (lanes 2–14) ovary tissues. C: RNA was extracted from normal (lane 1) and carcinoma (lanes 2–17) uterine tissues.
TB10 Expression as a Progression Marker in Mouse Skin Carcinogenesis
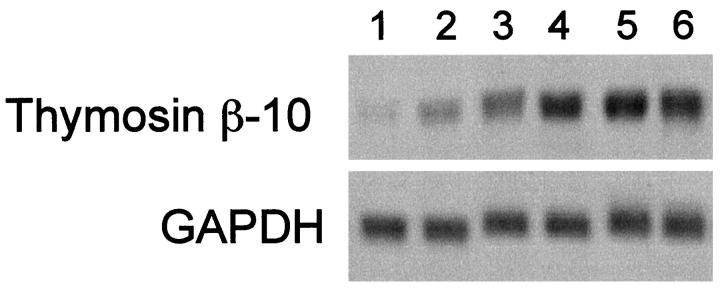
To investigate the TB10 gene expression in the process of carcinogenesis, we used the classical model of mouse skin carcinomas induced by the combined action of dimethyl-benzanthracene (DMBA) and 12-O-tetradecanoyl-phorbol-13-acetate (TPA). Cell lines were established from normal skin, benign papillomas, and malignant carcinomas. 14 The cell lines were as described in Materials and Methods. As shown in Figure 4 ▶ , TB10 gene expression was detectable at low levels in the normal keratinocytes (lane 1), whereas it was quite abundant in three carcinoma cell lines (lanes 4–6). Two papilloma cell lines showed intermediate levels (lanes 2 and 3).
Figure 4.
Analysis of the TB10 gene expression in normal and transformed keratinocytes. Twenty micrograms of total RNA were size-fractionated on a denaturing formaldehyde agarose gel, blotted onto Hybond-N nylon filters, and probed with TB10 cDNA. A GAPDH probe was used to normalize results. RNA was extracted from the following sources: Lane 1, C5N; lane 2, P1; lane 3, P6; lane 4, B9; lane 5, car B; lane 6, A5.
Detection of TB10 Protein in Neoplastic Tissues by Immnohistochemical Analysis
Immunohistochemical analysis can allow rapid and sensitive screening of histopathological tissues. Therefore, this technique was set up for the detection of TB10 protein using antibodies raised versus a peptide corresponding to the carboxyterminal region of the protein; this region is specific for each β-thymosin family member.
Paraffin-embedded tissues from ovary, breast, and colon carcinomas and the respective normal tissues were analyzed. A strong cytoplasmic staining was observed in all of the neoplastic tissues. Conversely, no or weak staining was observed in the normal tissues. The results are summarized in Table 1 ▶ . Some representative cases are shown in Figure 5 ▶ . Ovary (Figure 5, B and F) ▶ , breast (Figure 5D) ▶ , and colon (Figure 5H) ▶ carcinoma tissues showed immunostaining localized to cytoplasm, whereas a very weak staining was observed in normal breast, ovary, and colon tissues (Figure 5, C, E, and G ▶ , respectively), with the exception of blood vessels, which showed an intense TB10 positivity.
Table 1.
Thymosyn β-10 Protein Expression in Human Breast, Ovary, and Colon Carcinomas
| Histological specimens | No. of positive cases/No. of cases analyzed by immunohistochemistry |
|---|---|
| Normal breast | 0 /4 |
| Breast carcinoma | 5 /5 |
| Normal ovary | 0 /3 |
| Ovary carcinoma | 4 /4 |
| Normal colonic mucosa | 0 /4 |
| Colon carcinoma | 8 /8 |
Figure 5.
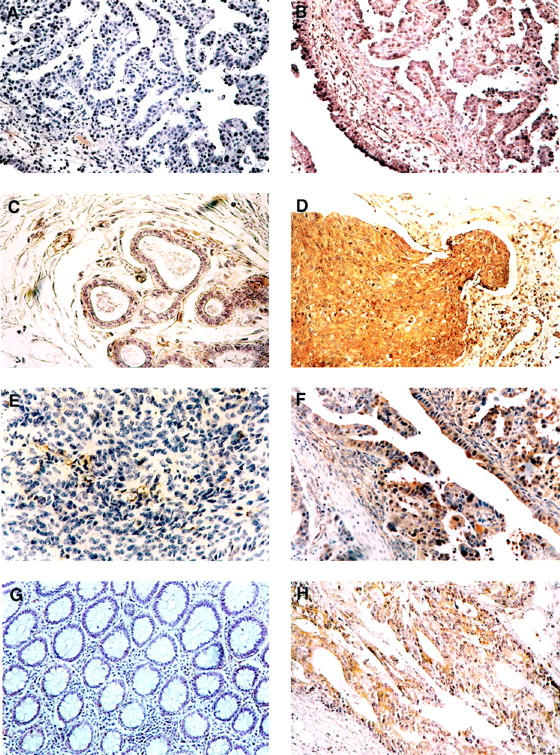
Immunohistochemical analysis of TB10 protein expression in breast, ovary, and colon neoplasms. Paraffin sections from ovarian, breast, and colon carcinomas and from the respective normal tissues were analyzed by immunohistochemistry using antibodies raised against a specific TB10 peptide and counterstained with hematoxylin. A: Immunostaining of an ovary carcinoma with the anti-TB10 antibody after preabsorption with the immunization peptide. No immunoreactivity is observed. B: Immunostaining of an another section from the same specimen as in A. A strong cytoplasmic staining can be observed in malignant cells. C: Immunostaining of a normal breast tissue. No immunoreactivity is observed in epithelial cells; a TB10 positivity can be observed in endothelial cells. D: Immunostaining of a breast carcinoma. A strong cytoplasmic staining can be observed in malignant cells. E: Immunostaining of a normal ovary tissue. No immunoreactivity is observed in epithelial cells; a TB10 positivity can be observed in endothelial cells. F: Immunostaining of an another sample of ovary carcinoma. A strong cytoplasmic staining can be observed in malignant cells. G: Immunostaining of a normal colon tissue. A very weak immunoreactivity can be observed. H: Immunostaining of a colon carcinoma. A strong cytoplasmic staining can be observed in malignant cells. Original magnifications, ×200.
The specificity of the reaction was demonstrated by the absence of staining when primary antibody was absent (not shown) or when it was pre-incubated with the peptide against which the antibodies were raised (Figure 5A) ▶ .
Discussion
We have recently demonstrated TB10 overexpression in human thyroid carcinoma-derived cell lines and tissues. 12 The highest TB10 levels were observed in the anaplastic carcinoma thyroid tumors and cell lines. 12 Moreover, in rat thyroid retrovirally transformed cells, TB10 gene was expressed at the highest level in the most malignant cell lines. Here we report TB10 gene overexpression in colon, breast, ovarian, and uterine carcinomas and in germ cell tumors compared to the respective normal tissues. In human neoplasias, increased TB10-specific mRNA levels were found in nonseminomas compared to the less aggressive seminomas. However, for the other neoplasias analyzed in this study, no positive correlation was found between TB10 mRNA levels and the degree of the malignant phenotype. Obviously, the analysis of gene expression in tumor samples by Northern blot analysis suffers from the contamination of the neoplastic cells by inflammatory cells, connective tissue, and normal epithelial cells. TB4 mRNA expression was analyzed in most of the neoplastic samples; it is analogous to the TB10 gene expression. In fact, TB4 was overexpressed in tumor and cell lines compared to the respective normal tissues.
The model of mouse skin carcinogenesis induced by chemical carcinogenesis was then used to verify the step of carcinogenesis at which the TB10 gene induction occurs. Very low TB10 expression was observed in normal cells, and an increased expression was already present at the preneoplastic papilloma stages. However, a further increase occurred at the carcinoma stages. Therefore, like the data that describe human melanoma tumors, 11 the TB10 gene expression associates with the degree of tumor progression in the experimental model of skin carcinogenesis.
Subsequently, we set up an immunohistochemical technique for the detection of the TB10 protein on paraffin sections, using antibodies generated against a carboxy-terminal peptide fragment specific for the TB10 protein. All of the neoplastic samples analyzed, originating from breast, ovary, and colon carcinomas, showed an intense cytoplasmic staining, whereas no or a very weak staining was observed in normal tissues. Moreover, the reaction appeared specific for the neoplastic cells. Therefore, the analysis of a large series of tumor samples associated with the clinical data are required to assess the potential of TB10 protein detection as a diagnostic and, perhaps, a prognostic tool for human neoplasias.
As far as the role of TB10 overexpression in human carcinogenesis is concerned, it is known that the β-thymosins bind and sequester G-actin, 3,4 which plays a pivotal role in maintaining the cellular structure. The dynamic equilibrium between monomeric and filamentous actin is certainly perturbed in transformed cells, and, because motility and adhesion processes are important in tumor progression and metastasis formation, the finding of elevated TB10 expression in malignant tumors may be correlated with the pattern of actin polymerization. It is known that overexpression of exogenous TB10 causes disassembly of actin stress fibers, 20 and, therefore, the changes observed in malignant growth may reflect the loss of filamentous actin with the corresponding increased size of G-actin pool.
Another recently discovered member of the β-thymosin family, the thymosin β-15 (TB15) gene, has been shown to be up-regulated in prostatic carcinoma cell lines that have metastasizing ability. This gene also inhibits actin polymerization and might thus increase cellular motility. 21 At variance with prostate carcinomas, TB15 appears to be induced at the early stages of breast carcinomas. In fact, it has been detected in in situ ductal carcinoma. 22 We have analyzed TB15 gene expression in our tumor samples; no detectable TB15 expression was observed by Northern blot using total RNA (data not shown). This suggests that the regulation of TB15 expression differs from that of TB4 and TB10.
In conclusion, the results of the present study prompt us to undertake further investigations to assess TB10 gene expression as a useful marker for diagnosis and prognosis of a large variety of human cancers.
Footnotes
Address reprint requests to Alfredo Fusco, Dipartimento di Biologia e Patologia Cellulare e Molecolare, Facoltà di Medicina e Chirurgia, Università di Napoli “Federico II”, via Pansini, 5, 80131 Napoli, Italy. E-mail: afusco@synapsis.it.
Supported in part by Ministero della Sanità, FSN1994, Rome, Italy.
References
- 1.Low TLK, Goldstein AL: Structure and function of thymosin and other thymic factors. Silber R Lobue J Gordon AS eds. The Year in Hematology. 1978, :pp 281-319 Plenum Press, New York [Google Scholar]
- 2.Horecker BL, Morgan JI: Ubiquitous distribution of thymosin β4 and related peptides in vertebrate cells and tissues. Lymphokines 1984, 9:15-35 [Google Scholar]
- 3.Safer D, Elzinga M, Nachmias VT: Thymosin β4, and Fx, an actin-sequestering peptide, are indistinguishable. J Biol Chem 1991, 266:4029-4032 [PubMed] [Google Scholar]
- 4.Yu FX, Lin SC, Morrison-Bogorad M, Atkinson MAL, Yin H: Thymosin β-10 and thymosin β-4 are both actin monomer sequestering proteins. J Biol Chem 1993, 268:502-509 [PubMed] [Google Scholar]
- 5.Lin SC, Morrison-Bogorad M: Developmental expression of mRNAs encoding Thymosin β-4 and β-10 in rat brain and other tissues. J Mol Neurosci 1990, 2:35-44 [DOI] [PubMed] [Google Scholar]
- 6.Hall K, Hempstead J, Morgan JI: Thymosin β-10 levels in developing human brain, and its regulation by retinoic acid in the HTB-10 neuroblastoma. Mol Brain Res 1990, 8:129-135 [DOI] [PubMed] [Google Scholar]
- 7.Lugo DI, Chen SC, Hall AK, Ziai R, Hempstead JL, Morgan JI: Developmental regulation of β-thymosins in the rat central nervous system. J Neurochem 1991, 56:457-461 [DOI] [PubMed] [Google Scholar]
- 8.Condon MR, Hall HK: Expression of thymosin β-4 and related genes in developing human brain. J Mol Neurosci 1992, 3:165-170 [DOI] [PubMed] [Google Scholar]
- 9.Hall AK: Differential expression of thymosin genes in human tumors and in the developing human kidney. Int J Cancer 1991, 48:672-677 [DOI] [PubMed] [Google Scholar]
- 10.Conlon JM, Grimelius L, Wallin G, Thim L: Isolation and structural characterization of Thymosin β-4 from human medullary thyroid carcinoma. J Endocrinol 1988, 118:155-159 [DOI] [PubMed] [Google Scholar]
- 11.Weterman MAJ, van Muijen GNP, Ruiter DJ, Bloemers HPJ: Thymosin β-10 expression in melanoma cell lines, and melanocytic lesions: a new progression marker for human cutaneous melanoma. Int J Cancer 1993, 53:278-284 [DOI] [PubMed] [Google Scholar]
- 12.Califano D, Monaco C, Santelli G, Giuliano A, Veronese ML, Berlingieri MT, de Franciscis V, Berger N, Trapasso F, Santoro M, Viglietto G, Fusco A: Thymosin β-10 gene overexpression correlated with the highly malignant neoplastic phenotype of transformed thyroid cells in vivo and in vitro. Cancer Res 1998, 58:823-828 [PubMed] [Google Scholar]
- 13.Haddow S, Fowlis DJ, Parkinson K, Akhurst RJ, Balmain A: Loss of growth control by TGF-β occurs at a late stage of mouse skin carcinogenesis and is independent of ras gene activation. Oncogene 1991, 6:1465-1470 [PubMed] [Google Scholar]
- 14.Stoler AB, Stenback F, Balmain A: The conversion of mouse skin squamous cell carcinomas to spindle cell carcinomas is a recessive event. J Cell Biol 1993, 122:1103-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. 1989. NY, Cold Spring Harbor Laboratory, Cold Spring Harbor
- 16.Gondo H, Kudo J, Whithe JW, Barr C, Selvanagayam P, Saunders GF: Differential expression of the human thymosin β4 gene in lymphocytes, macrophages, and granulocytes. J Immunol 1987, 139:3840-3848 [PubMed] [Google Scholar]
- 17.Feinberg AB, Vogelstein B: A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 1983, 132:613-619 [DOI] [PubMed] [Google Scholar]
- 18.Lin SC, Morrison-Bogorad M: Cloning and characterization of a testis-specific thymosyn β 10 cDNA. Expression in post-meiotic male germ cells. J Biol Chem 1991, 266:23347-23353 [PubMed] [Google Scholar]
- 19.McCreary V, Kartha S, Bell GI, Toback FG: Sequence of a human kidney cDNA clone encoding thymosin β-10. Biochem Biophys Res Commun 1988, 152:862-866 [DOI] [PubMed] [Google Scholar]
- 20.Yu FX, Lin SC, Morrison-Bogorad M, Yin HL: Effects of thymosyn β4 and thymosyn β10 on actin structures in living cells. Cell Motil Cytoskeleton 1994, 27:13-25 [DOI] [PubMed] [Google Scholar]
- 21.Bao L, Loda M, Janmey PA, Stewart R, Anand-Apte B, Zetter BR: Thymosin β-15: a novel regulator of tumor cell motility upregulated in metastatic prostate cancer. Nat Med 1996, 2:1322-1328 [DOI] [PubMed] [Google Scholar]
- 22.Gold JS, Bao L, Ghossoub RA, Zetter BR, Rimm DL: Localization and quantitation of expression of the cell motility-related protein thymosin β15 in human breast tissue. Mod Pathol 1997, 10:1106-1112 [PubMed] [Google Scholar]