Abstract
PGP9.5 is a neurospecific peptide that functions to remove ubiquitin from ubiquitinated proteins and prevents them from targeted degradation by proteasomes. Using the serial analysis of gene expression method (SAGE), we observed that the PGP9.5 transcript was highly expressed in primary lung cancers and lung cancer cell lines but was not detectable in the normal lung. Here we examined the expression of PGP9.5 protein in normal lung epithelium, lung tumor cell lines, and 98 resected primary non-small-cell lung carcinomas (NSCLCs). We found PGP9.5 reactivity in normal lung in a pattern compatible with K-cells of the diffuse neuroendocrine system. However, the PGP9.5 was present in both small-cell lung cancer (SCLC) and NSCLC cell lines (22/24) independent of neuronal differentiation. In primary NSCLCs, 54% (53/98) of the cases had positive PGP9.5 staining, and the expression of protein was strongly associated with pathological stage of the cancer. It was present in 44% (29/66) of stage I NSCLCs and in 75% (24/32) of stage II and IIIA NSCLCs (p = 0.0032). These results suggest that the increased expression of PGP9.5 is specifically associated with lung cancer development and may serve as a potential marker for the detection of lung cancer.
Lung cancer is the second most common malignancy worldwide and is the leading cause of cancer death in men. 1 Accumulating evidence indicates that a series of genetic changes in dominant oncogenes such as myc and ras are involved in the pathogenesis of human lung cancer. 2,3 Several other candidate oncogenes have also been implicated. 4,5 It is now clear that the accumulation of multiple genetic changes in a tumor leads to major differences involving altered expression of many genes. 6 Recently, using the serial analysis of gene expression (SAGE) method, we showed that the PGP9.5 (protein gene product 9.5) gene had no detectable expression in normal lung tissues but was frequently overexpressed in primary non-small-cell lung tumors. 7
PGP9.5 is a ubiquitin hydrolase widely expressed in neuronal tissues at all stages of neuronal differentiation. 8,9 Ubiquitination of cellular proteins and targeting them for subsequent degradation via ubiquitin-mediated proteolysis is potentially an important mechanism that regulates cell cycle genes. 10,11 In tumors, increased deubiquitination of cyclins by PGP9.5 could contribute to the uncontrolled growth of somatic cells. 12
To better characterize the role of PGP9.5 in lung cancer, we first studied PGP9.5 expression in normal lung and a panel of lung cancer cell lines with defined neuroendocrine (NE) differentiation. Next, we examined the expression of PGP9.5 in 98 resected primary non-small-cell lung cancers (NSCLCs), using immunohistochemistry and correlated PGP9.5 expression in tumors with the clinicopathological features of affected patients.
Materials and Methods
Tissue Specimens
All lung cancer cell lines were obtained from the American Type Culture Collection (ATCC) and propagated according to the provided instructions. 13 Formalin-fixed and paraffin-embedded tumor samples from consecutive patients who had undergone resections of NSCLCs with curative intent were retrieved from the Surgical Pathology files of the Johns Hopkins Hospital (JHH). Information regarding tumor stage, tumor recurrence, and patient survival was obtained from the medical records, including the JHH Tumor Registry files.
Northern Blot Analysis
Cell lines used for Northern blot analysis were collected after trypsinization and lysed immediately in Trizol reagent (GIBCO BRL, Gaithersburg, MD). Normal lung total RNA was extracted by the GuSCN method and purified by CsCl gradient ultracentrifugation as described. 7 Ten micrograms of RNA was separated on a 1.5% denaturing agarose gel and transferred to Gene Screen membrane (DuPont, Boston, MA). A PGP9.5 cDNA probe was isolated from an EST clone (no. 268107) obtained from Genome Systems (Huntsville, AL). Northern blot hybridization using the PGP9.5 cDNA probe was performed as described. 7
Western Blot Analysis
Twenty micrograms of cell lysates was separated on a 4–20% sodium dodecyl sulfate gradient gel and transferred to a polyvinylidene difluoride membrane (Micron Separations, Westborough, MA). After the nonspecific sites were blocked by incubation in phosphate-buffered saline + 5% nonfat dry milk (NFDM), the blot was incubated with the polyclonal rabbit antiserum against PGP9.5 (Biogenesis, Sandown, NH) at 1:400 dilution for 2 hours at room temperature. After washing, an ECL kit (Amersham, Arlington Heights, IL) was used to visualize the antibody binding to PGP9.5 protein.
Immunohistochemical Analysis
Six-μm sections were made from paraffin tissue blocks, and the slides were dried at 60°C for 30 minutes, treated with xylenes, and then dehydrated in alcohol. Endogenous peroxidase was blocked with 0.3% H2O2. Microwave treatment was performed for 4 minutes in Antigen Retrieval Glyca solution (Biogenex, San Ramon, CA), because it has been shown that the immunoreactivity of PGP9.5 was markedly enhanced by this method. 14 After blocking with normal goat serum, the slides were incubated with the polyclonal rabbit antiserum against PGP9.5 (Biogenesis) at 1:1000 dilution for 2 hours at room temperature. Vectastain ABC Kit and DAB Substrate Kit (Vector, Burlingame, CA) were used to visualize the antibody binding, and the sections were counterstained with hematoxylin.
Immunohistochemical staining for PGP9.5 was interpreted by an experienced pathologist (W.H.W.). For control studies, HeLa and H157 lung cancer cell lines were used as negative and positive controls. The PGP9.5 status of these two cell lines was confirmed by Northern and Western analysis 7 (data not shown). Optimized conditions were then used for the immunostaining of primary lung cancer specimens. In all cases, small nerves in the tissue sections served as a positive internal control, and desmoplastic stroma served as a negative internal control for PGP9.5 staining. Only cytoplasmic staining above background levels was regarded as specific staining. All immunohistochemical slides were initially reviewed to assess the range of this PGP9.5 immunoreactivity with respect to intensity and distribution of staining. Two patterns were recognized: 1) the tumor was uniformly nonimmunoreactive; or 2) moderate to strong immunoreactivity approaching the intensity seen in the internal control (ie, nerves) was uniformly present throughout most (ie, >70%) of the tumor. Accordingly, tumors were subsequently scored as either negative or positive, based on the total absence or generalized presence of specific staining. To correlate the distribution of the scattered PGP9.5-positive cells in the normal bronchial epithelium with the distribution of the neuroendocrine K-cells, lung sections from two independent cases were incubated with a monoclonal antibody against chromogranin (Boehringer Mannheim, Indianapolis, IN; 1:2000 dilution).
Statistical Analysis
The χ 2 test was used to examine the association between the PGP9.5 expression status and clinicopathological features.
Results
Expression of PGP9.5 Protein in Normal Lung Tissues
We have previously observed that the PGP9.5 message was frequently detected in lung carcinomas, but was not detectable in normal lung by Northern analysis or reverse transcription-polymerase chain reaction methods. 7 This result suggested that PGP9.5 protein expression could potentially be used as a marker for lung cancer. To better characterize the cell type origin and the timing of PGP9.5 expression, an immunohistochemical approach was used to localize PGP9.5 protein expression in the normal lung and the tumor samples. As shown in Figure 1A ▶ , the majority of the normal lung epithelial cells were negative by PGP9.5 staining. In sections of nonneoplastic lung, PGP9.5 staining was restricted to nerves and a few isolated cells scattered throughout the bronchial epithelium. The positive staining of neurons is consistent with the fact that PGP9.5 is a neuron-specific peptide widely expressed in neuronal tissue. 8 The PGP9.5-positive cells scattered throughout the nonneoplastic bronchial epithelium were reminiscent of the neuroendocrine K-cells, based on their morphological appearance and pattern of distribution. 15
Figure 1.
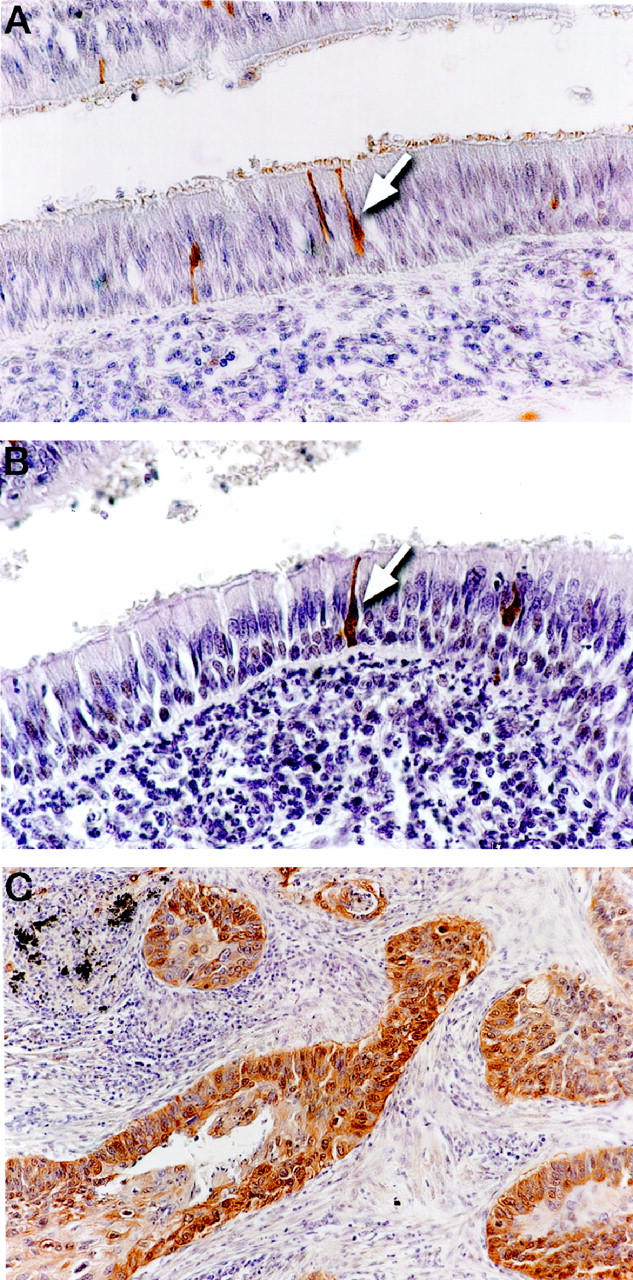
Immunohistochemical staining of lung tumor and adjacent lung tissue for PGP9.5 and chromogranin. A: PGP9.5 staining is present in isolated cells dispersed throughout the normal bronchial mucosa (arrow). B: Chromogranin staining is present in the compatible population of cells as seen in A (arrow). C: Example of PGP9.5 staining in lung cancer.
To determine the histological origin of these scattered PGP9.5-positive cells, we stained parallel sections from two cases with chromogranin, a well-characterized marker of neuroendocrine differentiation. 16 As shown in Figure 1B ▶ , chromogranin-positive cells showed the same location and tissue distribution as the PGP9.5-positive cells. This result suggests that, in nonneoplastic lung, PGP9.5 protein expression is restricted to cells of the dispersed NE system.
PGP9.5 Expression Is Independent of hASH1 Status in Lung Cancers
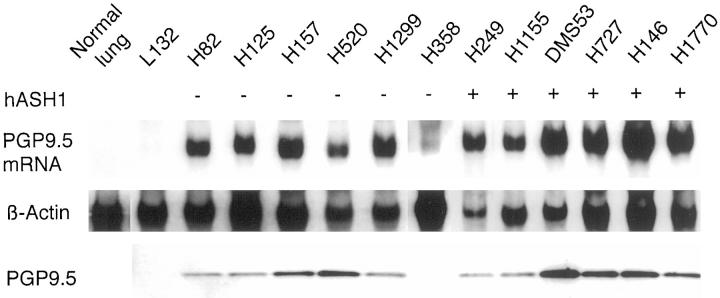
Because a majority of small-cell lung cancers and a portion of the NSCLCs often exhibit features of NE differentiation, we next tested whether PGP9.5 overexpression was associated with lung cancers of NE lineage. A panel of established lung cancer cell lines with hASH1 status was used for this study. hASH1 is a transcription factor essential for the NE development of the human lung. 17 As shown in Figure 2 ▶ (upper panel), PGP9.5 message was abundantly detected in both small-cell lung cancer (SCLC) and NSCLC cell lines regardless of hASH1 status. Similarly, the PGP9.5 protein was detected in nearly all-cell lines that expressed the gene (Figure 2 ▶ , lower panel). Although there was a faint message in H358 by Northern analysis, no PGP9.5 protein was detectable for this sample. Furthermore, no PGP9.5 product was obtained by reverse transcription-polymerase chain reaction in this case, indicating that the message was probably rearranged or unstable and could not serve as a template for protein translation. Thus the expression of PGP9.5 appears to be present in NSCLC in the absence of NE differentiation, as well as SCLC frequently derived from NE cells of the lung.
Figure 2.
PGP9.5 expression in lung cancer cell lines with defined hASH1 status. PGP9.5 transcript was not detectable in either normal lung or an embryonic lung cell line (L132) but was expressed in all lung cancer cell lines. The hASH1 status is indicated at the top. H358 had a weak PGP9.5 message compared to other cell lines. The lower panel shows a Western blot using the anti-PGP9.5 antibody. Note that all tumor cell lines except H358 had PGP9.5 protein. The β-actin control was used to normalized sample loading. The histological subtypes of the cell lines are: L132, embryonic lung; H82, H249, DMS53, and H146, small cell carcinoma; H125, adenocarcinoma; H157 and H520, squamous cell carcinoma; H1299 and H1155, large cell carcinoma; H358, bronchoalveolar carcinoma; H727, carcinoid; H1770, neuroendocrine carcinoma.
Association of PGP9.5 Expression with Pathological Stage in NSCLC
To seek the role of PGP9.5 expression in NSCLC, we examined the presence of PGP9.5 protein in the tumors of 98 NSCLC patients, using immunohistochemistry. The clinicopathological features of the patients and the results of PGP9.5 staining are shown in Table 1 ▶ . Overall, 54% (53/98) of NSCLCs were positive by PGP9.5 staining, and the intensity of the staining was uniform and consistent for almost all cases (Figure 1C) ▶ . Seventy-two percent (26/36) of squamous cell carcinomas were stained positive for PGP9.5, whereas only 41% (22/54) of adenocarcinomas had positive PGP9.5 staining (p = 0.0066). These results are consistent with previous reports which showed that squamous cell carcinomas were more likely to be PGP9.5-positive than adenocarcinomas of the lung. 18,19 However, when the clinical stage of the patients was considered, the PGP9.5-positive rate for stage II and IIIA patients was significantly higher than those with stage I disease (p = 0.0074) (Table 1) ▶ .
Table 1.
Clinicopathological Features and PGP9.5 Expression in Primary NSCLC
| Clinicopathological feature | Variable | No. of cases | PGP9.5 expression | p value* | |
|---|---|---|---|---|---|
| + | − | ||||
| Sex | Male | 61 | 37 (61%) | 24 (39%) | |
| Female | 37 | 16 (43%) | 21 (57%) | 0.14 | |
| Histology | Adenocarcinoma | 55 | 22 (41%) | 32 (59%) | |
| Squamous cell carcinoma | 36 | 26 (72%) | 10 (28%) | <0.01† | |
| Others | 8 | 5 (63%) | 3 (37%) | ||
| Tumor stage | I | 66 | 29 (44%) | 37 (56%) | |
| II and IIIA | 32 | 24 (75%) | 8 (25%) | <0.01 | |
| Vital status | Alive | 26 | 11 (42%) | 15 (58%) | |
| Dead | 72 | 42 (58%) | 30 (42%) | 0.24 | |
| Median follow-up time | 41 months | ||||
| Total | 98 | 53 (54%) | 45 (46%) |
*χ 2 test.
†PGP9.5 expression in adenocarcinoma versus squamous cell carcinoma.
Discussion
PGP9.5 was first isolated as a specific cytoplasmic marker for neurons and NE cells. 20,21 About 10–15% NSCLCs demonstrate NE features by electron microscopy or immunohistochemistry, despite the absence of NE features by light microscopy. Therefore, it was possible that PGP9.5 expression in these cancers may have simply reflected their NE status. Indeed, PGP9.5 had been used as a marker of NE differentiation for lung tumors. However, discordant staining patterns between PGP9.5 and other neuroendocrine markers have often been observed. 18,19 In our study, hASH1 was used as a marker of neuroendocrine differentiation, because homozygous disruption of the hASH1 gene in mouse prevents the development of pulmonary NE cells. 17 However, most lung tumor cell lines expressed high levels of PGP9.5, regardless of hASH1 gene expression. Therefore, our results suggested that expression of PGP9.5 in lung cancers is independent of NE differentiation.
Functionally, PGP9.5 belongs to the ubiquitin carboxyl-terminal hydrolase (UCH) family. 8 The UCH family has conserved Cys and His domains common to yeast and mammalian ubiquitin hydrolases. 22,23 It is becoming increasingly clear that these enzymes are a part of the cellular proteolytic pathway that regulates many cellular processes, including cell cycle progression and cell death. 12 Several other studies have shown that some UCH family members are overexpressed in different tumors and can potentially be oncogenic. For example, the tre-2 gene was identified as one of the UCH family proteins. The expression of tre-2 is not detectable in normal tissues but becomes elevated in a variety of tumor cells. 24 Another UCH family gene, Unph, was isolated as a human homolog of the murine Unp protooncogene. 25 Overexpression of murine Unp gene leads to oncogenic transformation of NIH3T3 cells, and the expression level of this gene is also elevated in lung tumors. 25,26 The results of our current study also suggest that the expression of PGP9.5 in lung cancer may play a causative role in the oncogenic transformation of human lung epithelial cells, because 1) PGP9.5 shares conserved domains with the members of ubiquitin hydrolase family, several of which are potential oncogenes; 2) PGP9.5 expression is not present in the normal lung epithelium, but becomes activated sometimes during the course of neoplastic transformation; and 3) expression of PGP9.5 is closely associated with advanced stages of NSCLC. Although further studies will be needed to elucidate the downstream target of deubiquitination by PGP9.5 and to better understand the molecular mechanism of PGP9.5 overexpresson in lung cancer development, the frequent occurrence of PGP9.5 expression in NSCLC makes it a potential marker for the detection of this disease.
Acknowledgments
We thank Dr. Stephen Baylin for helpful discussions about the project and Mrs. Janice Pulley for assistance with the manuscript preparation.
Footnotes
Address reprint requests to Dr. Jin Jen, Department of Otolaryngology–Head and Neck Surgery, Division of Head and Neck Cancer Research, Johns Hopkins University School of Medicine, 824 Ross Research Building, 720 Rutland Avenue, Baltimore, MD 21205-2196. E-mail: jenjin@jhmi.edu.
Supported in part by National Institutes of Health grant CA06973, Lung SPORE CA58184, and an award to J. Jen from the V Foundation.
References
- 1.American Cancer Society: Cancer Facts and Figures—1997. Atlanta, American Cancer Society, 1997
- 2.Rodenhuis S, van de Wetering ML, Mooi WJ, Evers SG, van Zandwijk N, Bos JL: Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med 1987, 317:929-935 [DOI] [PubMed] [Google Scholar]
- 3.Nau MM, Brooks BJ, Battey J, Sausville E, Gazdar AF, Kirsch IR, McBride OW, Bertness V, Hollis GF, Minna JD: L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature 1985, 318:69-73 [DOI] [PubMed] [Google Scholar]
- 4.Hibi K, Takahashi T, Sekido Y, Ueda R, Hida T, Ariyoshi Y, Takagi H, Takahashi T: Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer. Oncogene 1991, 6:2291-2296 [PubMed] [Google Scholar]
- 5.Hibi K, Yamakawa K, Ueda R, Horio Y, Murata Y, Tamari M, Uchida K, Takahashi T, Nakamura Y, Takahashi T: Aberrant upregulation of a novel integrin α subunit gene at 3p21.3 in small cell lung cancer. Oncogene 1994, 9:611-619 [PubMed] [Google Scholar]
- 6.Kinzler KW, Vogelstein B: Landscaping the cancer terrain. Science 1998, 280:1036-1037 [DOI] [PubMed] [Google Scholar]
- 7.Hibi K, Liu Q, Beaudry GA, Madden SL, Westra WH, Wehage SL, Yang SC, Heitmiller RF, Bertelsen AH, Sidransky D, Jen J: Serial analysis of gene expression in non-small cell lung cancer. Cancer Res 1998, 58:5690-5694 [PubMed] [Google Scholar]
- 8.Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J: The neuron-specific protein PGP9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 1989, 246:670-673 [DOI] [PubMed] [Google Scholar]
- 9.Schofield JN, Day IN, Thompson RJ, Edwards YH: PGP9.5, a ubiquitin C-terminal hydrolase; pattern of mRNA, and protein expression during neural development in the mouse: Brain Res Dev Brain Res 1995, 85:229-238 [DOI] [PubMed] [Google Scholar]
- 10.Hochstrasser M: Ubiquitin-dependent protein degradation. Annu Rev Genet 1996, 30:405-439 [DOI] [PubMed] [Google Scholar]
- 11.Diehl JA, Zindy F, Sherr CJ: Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev 1997, 11:957-972 [DOI] [PubMed] [Google Scholar]
- 12.Spataro V, Norbury C, Harris AL: The ubiquitin-proteasome pathway in cancer. Br J Cancer 1998, 77:448-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelps RM, Johnson BE, Ihde DC, Gazdar AF, Carbone DP, McClintock PR, Linnoila RI, Matthews MJ, Bunn PA, Jr, Carney D, Minna JD, Mulshine JL: NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem Suppl 1996, 24:32-91 [DOI] [PubMed] [Google Scholar]
- 14.McQuaid S, McConnell R, McMahon J, Herron B: Microwave antigen retrieval for immunocytochemistry on formalin-fixed, paraffin- embedded post-mortem CNS tissue. J Pathol 1985, 176:207-216 [DOI] [PubMed] [Google Scholar]
- 15.McDowell EM, Barrett LA, Trump BF: Observations on small granule cells in adult human bronchial epithelium and in carcinoid and oat cell tumors. Lab Invest 1976, 34:202-206 [PubMed] [Google Scholar]
- 16.Wilson BS, Lloyd RV: Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol 1984, 115:458-468 [PMC free article] [PubMed] [Google Scholar]
- 17.Borges M, Linnoilla RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, Baylin SB, Ball DW: An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997, 386:852-855 [DOI] [PubMed] [Google Scholar]
- 18.Dhillon AP, Rode J, Dhillon DP, Moss E, Thompson RJ, Spiro SG, Corrin B: Neural markers in carcinoma of the lung. Br J Cancer 1985, 51:645-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Addis BJ, Hamid Q, Ibrahim NB, Fahey M, Bloom SR, Polak JM: Immunohistochemical markers of small cell carcinoma and related neuroendocrine tumours of the lung. J Pathol 1987, 153:137-150 [DOI] [PubMed] [Google Scholar]
- 20.Jackson P, Thompson RJ: The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J Neurol Sci 1981, 49:429-438 [DOI] [PubMed] [Google Scholar]
- 21.Day IN, Thompson RJ: Molecular cloning of cDNA coding for human PGP9.5 protein. A novel cytoplasmic marker for neurones and neuroendocrine cells. FEBS Lett 1987, 210:157-160 [DOI] [PubMed] [Google Scholar]
- 22.Baker RT, Tobias JW, Varshavsky A: Ubiquitin-specific proteases of Saccharomyces cerevisiae: Cloning of Ubp2 and Ubp3, and functional analysis of the Ubp gene family. J Biol Chem 1992, 267:23364-23375 [PubMed] [Google Scholar]
- 23.Papa FR, Hochstrasser M: The yeast DOA4 gene encodes a deubiquitinating enzyme related to a product of the human tre-2 oncogene. Nature 1993, 366:313-319 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Hillova J, Mariage-Samson R, Onno M, Huebner K, Cannizzaro LA, Boghosian-Sell L, Croce CM, Hill M: A novel transcriptional unit of the tre oncogene widely expressed in human cancer cells. Oncogene 1992, 7:733-741 [PubMed] [Google Scholar]
- 25.Gray DA, Inazawa J, Gupta K, Wong A, Ueda R, Takahashi T: Elevated expression of Unph, a proto-oncogene at 3p21.3, in human lung tumors. Oncogene 1995, 10:2179-2183 [PubMed] [Google Scholar]
- 26.Gupta K, Chevrette M, Gray DA: The Unp proto-oncogene encodes a nuclear protein. Oncogene 1994, 9:1729-1731 [PubMed] [Google Scholar]