Abstract
Atherosclerotic plaques are chronic inflammatory lesions composed of dysfunctional endothelium, smooth muscle cells, lipid-laden macrophages, and T lymphocytes. This study analyzed atherosclerotic tissue specimens for expression of CD1 molecules, a family of cell surface proteins that present lipid antigens to T cells, and examined the possibility that CD1+ lipid-laden macrophages might present antigen to T cells. Immunohistochemical studies using a panel of specific monoclonal antibodies demonstrated expression of each of the four previously characterized human CD1 proteins (CD1a, -b, -c, and -d) in atherosclerotic plaques. Expression of CD1 was not observed in normal arterial specimens and appeared to be restricted to the CD68+ lipid-laden foam cells of atherosclerotic lesions. CD1 molecules colocalized in areas of the arterial wall that also contained abundant T lymphocytes, suggesting potential interactions between CD1+ cells and plaque-infiltrating lymphocytes in situ. Using CD1-expressing foam cells derived from macrophages in vitro, we demonstrated the ability of such cells to present lipid antigens to CD1 restricted T cells. Given the abundant T cells, CD1+ macrophages, and lipid accumulation in atherosclerotic plaques, we propose a potential role for lipid antigen presentation by CD1 proteins in the generation of the inflammatory component of these lesions.
Atherosclerosis and its sequelae, including heart disease and stroke, are a major cause of morbidity and the leading cause of mortality in the United States, and their incidence continues to rise worldwide. 1 Most concepts of disease pathogenesis in atherosclerosis have focused on the accumulation of lipid and the proliferation of smooth muscle cells in the development of arterial wall lesions and on the role of thrombosis in acute arterial occlusion. However, more recently investigators have begun to appreciate a component of chronic inflammation within atherosclerotic lesions, consistent with the involvement of the immune system in the pathogenesis of this disease. Thus, the cellular components of atherosclerotic plaques include not only dysfunctional endothelium, monocyte-derived lipid laden foam cells, and smooth muscle cells, but also various subsets of activated lymphocytes. 2,3 T lymphocytes are present throughout the life of the lesion and produce a variety of cytokines locally. These mediators can in turn contribute to the differentiation and recruitment of surrounding cells, resulting in the evolution of atherosclerotic lesions and the occurrence of long-term complications with their associated morbidity and mortality. 2-7
Several lines of clinical and experimental evidence suggest a role for the cellular immune system in the development of atherosclerosis. In humans, the development of accelerated coronary arteriosclerosis in heart transplant patients supports the involvement of the immune system in arterial disease. 8-12 In mice a similar process has been shown to be associated with mismatching at major and minor histocompatibility loci, 13 implying that activation of T cells may initiate or accelerate lesion formation. Studies of atherosclerosis in apolipoprotein E (ApoE)-deficient mice also indicate a role for lymphocytes, since crossing these animals with recombinase activating gene-1 (RAG-1)-deficient animals, which lack T and B lymphocytes, results in a 40% reduction in lesion formation in animals maintained on a standard laboratory mouse diet. 14 Although this partial attenuation of atherogenesis by the immunodeficiency state can be overcome by feeding a high fat diet, these findings nonetheless point to a potentially important role for the cellular immune response in accelerating arterial lesion formation. The inhibition of atherosclerosis in low-density lipoprotein (LDL) receptor-deficient and C57BL/6 hyperlipidemic mice seen upon treatment with monoclonal antibodies against lymphocyte surface molecules such as CD4 and CD40 further support this concept. 15,16 Likewise, apoE-deficient mice also lacking the interferon γ receptor have a substantial reduction in atherosclerotic lesion size, lipid content, and cellularity. Thus, this important T-cell-derived cytokine is implicated in the progression of this disease. 17
Atherosclerotic lesions are characterized by the abundant expression of major histocompatibility complex (MHC) class I and II molecules on macrophages, endothelia, and smooth muscle cells. 4,15-19 These molecules may present foreign or autologous peptide antigens to T cells present within atherosclerotic plaques. 20-23 In this regard, it is extremely provocative that several investigators have demonstrated the presence of chlamydia species and cytomegalovirus within human atherosclerotic lesions, suggesting potential foreign microbial targets that may drive the activation of infiltrating T cells. 24-26 Alternatively, T cells within atherosclerotic plaques could also react with self-antigens and thus generate an autoimmune response with inflammatory changes characteristic of these lesions. The possibility that T cell recognition of altered self-antigens could contribute to atherosclerosis has been suggested by Stemme et al, who demonstrated that T cell clones derived from atherosclerotic plaques recognize oxidized LDL in a MHC class II-dependent manner. 23
Recent work has identified a novel mechanism for antigen presentation in which T cells recognize specific lipids and glycolipids in the context of non-MHC-encoded CD1 molecules. 27-29 Both foreign microbial or synthetic lipids and glycolipids are presented to CD1-restricted T cells. 30-32 In addition, a substantial number of CD1-reactive T cells have been isolated that recognize CD1 molecules in the absence of deliberately added exogenous lipid antigens. 33-36 Endogenous glycolipids have been demonstrated to enhance CD1 restricted T cell responses 37 and autologous cellular lipids have been eluted from CD1 molecules. 38 These data suggest that CD1-restricted T cells not only respond to exogenous lipids, but may also recognize endogenous self-lipids. These findings raise the possibility that CD1 molecules could present native or altered lipid epitopes derived from the abundant lipid deposits in atherosclerotic plaques and, thus, drive a T-cell-dependent inflammatory reaction in this disease.
As a step toward evaluating the possible role of antigen presentation by CD1 molecules in the pathogenesis of atherosclerosis, we have examined the expression of CD1 proteins in atherosclerotic plaques in human pathological specimens. We observed the expression of all four previously characterized human CD1 proteins (CD1a, -b, -c, and -d) within atherosclerotic lesions. These proteins were expressed strongly and specifically by lipid-laden macrophages (foam cells) and not by other cells in the lesions. CD1+ monocyte-derived foam cells generated in vitro were able to present lipid antigens to CD1-restricted T cells, suggesting that this mechanism for T cell activation may be active for the phenotypically similar cells observed within atherosclerotic plaques in vivo. Given the abundance of potential lipid antigens within atherosclerotic lesions, our results suggest that lipid antigen presentation by CD1 proteins could contribute to the activation of T cells within plaques, and thus play a role in the cascade of inflammatory processes that influence the progression of this disease.
Materials and Methods
Tissue Specimens and Antibodies
Tissues from atherosclerotic lesions were obtained from material removed and discarded during carotid endarterectomy procedures. Surgical specimens of human carotid atheroma and aorta were obtained by protocols approved by the Human Investigations Internal Review Board at the Brigham and Women’s Hospital. Monoclonal antibodies (mAbs) were either generated as mouse ascites fluids or purified from culture supernatants by protein G affinity column chromatography (Pharmacia, Uppsala, Sweden), or obtained commercially as purified immunoglobulins. Previously unpublished mAbs F10/21A3 and BCD1b2.1 were produced from mice immunized with GM-CSF- and interleukin (IL)-4-activated monocytes as previously described. 39 Monoclonal antibodies CD1d51.1.3, CD1d27.1, and CD1d68.3 were produced by fusion of spleen cells from mice immunized with CD1d-mouse IgG2a fusion proteins. 40 The specificities of anti-CD1 antibodies were confirmed by FACS analysis of CD1a, -b, -c, and -d transfected C1R cells. 36,40 Antibodies tested included: OKT6 (IgG1, anti-CD1a), 36 10D12 (IgG1, anti-CD1a), 41 BCD1b2.1 (IgG1, anti-CD1b; SM Behar and SA Porcelli, unpublished), 4A7.6.5 (IgG2a, anti-CD1b), 41 10C3 (IgG1, anti-CD1c≫CD1b), 42 F10/21A3 (IgG1, anti-CD1c; SM Behar and SA Porcelli, unpublished), CD1d51.1.3 (IgG2b, anti-CD1d > -b ≫-c), 40 CD1d27.1 (IgG1, anti-CD1d; SA Porcelli, unpublished), CD1d68.3 (IgG1, anti-CD1d; SA Porcelli, unpublished), MT310 (IgG1, anti-CD4; Dako, Carpinteria, CA), DK25 (IgG1, anti-CD8; Dako), KP1 (anti-CD68, Dako), W6/32 (IgG2a, anti-HLA-A, -B, -C), 43 L243 (IgG2a, anti-HLA-DR), 44 3C10 (IgG2b, anti-CD14), 45 IC3/1 (IgG1, anti-ICAM-3), 46 LM2/1 (IgG1, anti-Mac-1), 47 and YZI (IgG1, anti-complement receptor 1). 48 Non-binding isotype-matched controls were ascites fluids or purified immunoglobulins generated from mouse myeloma cell lines P3X63Ag8 (P3; IgG1), MPC11 (IgG2b), and RPC5.4 (IgG2a), all obtained from the American Type Culture Collection (ATCC, Manassas, VA).
Immunohistochemistry and Flow Cytometry
Serial cryostat sections (5 μm thickness) were air-dried onto glass microscope slides and fixed in acetone at −20°C for 5 minutes. Sections preincubated with phosphate buffered saline (PBS) containing 0.3% hydrogen peroxide were incubated with primary or control antibody diluted in PBS with 5% horse serum. This was followed by incubation with biotinylated secondary antibody for 45 minutes, and then by avidin-biotin-peroxidase complex (Vectastain ABC kit, Vector Laboratories, Burlingame, CA). Antibody binding was visualized with 3-amino-9-ethyl carbazole, with counterstaining using Gill’s hematoxylin (Sigma Chemical, St. Louis, MO). For double immunostaining, sections were first stained with anti-CD1a mAb followed by biotinylated anti-mouse IgG, avidin-biotin-peroxidase complex, and 3-amino-9-ethyl carbazole, as above. After development of the first immunostain (red color), sections were subjected to treatment with an avidin/biotin blocking kit (Vector Laboratories) according to the manufacturer’s recommendations, and further blocked with 5% normal horse serum for 20 minutes. Tissue sections were then processed for detection of the second antigen using anti-human CD68 mAb (clone KP1, DAKO), followed by biotinylated horse anti-murine secondary antibody and streptavidin coupled to alkaline phosphatase followed 30 minutes later by Fast Blue substrate.
Flow cytometry was performed as described, 49 with gating on viable cells according to forward and side scatter and exclusion of propidium iodide-stained cells.
T Cell Proliferation Assays
T cell lines DN1 (CD4−CD8−; M. tuberculosis mycolic acid-specific and CD1b-restricted) and CD8.1 (CD4−CD8+, M. tuberculosis phospholipid-specific and CD1c-restricted) have been described. 30,50-52 Maintenance of T cell lines and T cell proliferation assays were performed as previously described. 51 To induce antigen-specific proliferation, DN1 T cells were stimulated with either a sonicate of M. tuberculosis (strain H37Ra; DIFCO, Detroit, MI) or with purified mycolic acids (Sigma). A total lipid extract of M. tuberculosis (strain H37Ra) was used to stimulate CD8.1 T cells as previously described. 52 Serial dilutions of the antigens were performed as described in Results and in figure legends. All cultures contained 50,000 T cells and 50,000 irradiated monocyte-derived antigen-presenting cells per well.
Preparation of Cytokine-Activated Monocytes and Foam Cells
Monocytes were isolated from leukocyte concentrates of normal donors by plastic adherence 53 and incubated in medium alone or in medium containing either 100 μg/ml oxidized LDL or 100 μg/ml acetylated LDL (both from Biotechnologies Inc., Stoughton, MA) for 7 days at 37°C in a 5% CO2 incubator. Cells were collected by centrifugation and recultured in fresh medium on day 5. Medium for incubations was RPMI-1640 (Gibco BRL, Gaithersburg, MD) with 10% fetal calf serum (FCS, Hyclone, Logan, UT) with or without a combination of 300 U/ml of GM-CSF (Immunex, Seattle, WA) and 200 U/ml IL-4 (Schering Corp., Kenilworth, NJ). Foam cells were analyzed on day 7 by Nile Red staining according to the method of Greenspan et al. 54 Quantitative fluorescence analysis was performed with a FACSort flow cytometer (Becton-Dickinson, Mountain View, CA) using 488-nm excitation wavelength and 515–545 nm detectors. Qualitative analysis was carried out by fluorescence microscopy of Nile Red-stained cells in PBS on glass slides and cover slips using a Nikon Optiphot 2 fluorescent microscope with 470- to 490-nm excitation filters and a 505-nm dichroic mirror and 520- to 560-nm visualization filters.
Results
Expression of CD1 Proteins in Atherosclerotic Lesions
Monoclonal antibodies specific for each of the four currently defined human CD1 proteins were tested for staining of frozen sections from a total of 14 atherosclerotic plaques and 6 non-atherosclerotic arteries used as normal controls (Figure 1) ▶ . The latter included normal human aorta and carotid arteries. Lesions studied were predominantly advanced carotid plaques with characteristic fibrous caps, lipid-laden macrophages, intimal hyperplasia, and lymphocytes distributed throughout the lesions. Reactivity of CD1-specific mAbs was noted exclusively in the intima where gross changes associated with the atherosclerotic process were visible, but not in adjacent areas of the media, which generally had grossly normal architecture and cellularity.
Figure 1.
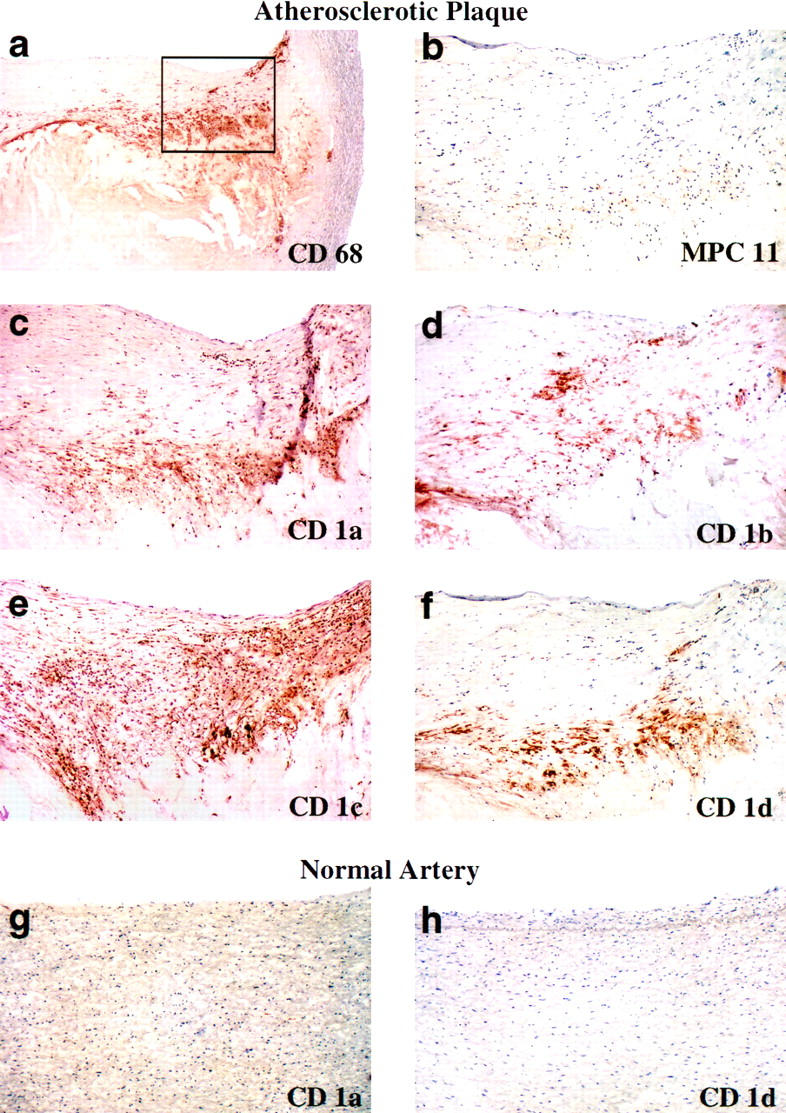
Expression of CD1a, -b, -c, and -d molecules in human atherosclerotic plaques. Serial frozen sections from human atherosclerotic carotid arteries were stained with a mAb against the macrophage marker CD68 or with mAbs specific for CD1a, -b, -c, or -d. (a) Intimal hyperplasia, fibrous cap formation and a macrophage-rich lipid-laden core are seen in a low power (×40) view of a section stained with antibody to the macrophage marker CD68. (b–f) Higher power (×100) views of macrophage-rich areas (corresponding to area enclosed in box shown in a) demonstrate staining of CD1a, -b, -c and -d molecules. Antibodies used were: a, KP1 (anti-CD68), b, MPC-11 (nonbinding control), c, OKT6 (anti-CD1a), d, 4A7.6.5 (anti-CD1b), e, 10C3 (anti-CD1c≫CD1b), and f, CD1d51.1.3 (anti-CD1d > CD1b ≫ CD1c). Undiseased arterial tissue, including human aorta and carotid arterial tissue, demonstrated little or no staining with any of these antibodies; see (g), OKT6, (h), CD1d51.1.3, and additional data not shown. For each CD1 isoform at least two different antibodies (see Materials and Methods) were used with similar results (data not shown). Staining was performed on a total of 14 atherosclerotic plaques and 6 normal arterial controls, and the results shown are representative of the findings for all samples examined.
In every specimen of atherosclerotic tissue studied, we observed immunoreactive CD1a, -b, -c, and -d (Figure 1, c–f) ▶ , whereas these proteins were not detected in similarly processed specimens of normal arterial tissue (Figure 1, g and h) ▶ . No reactivity was seen using non-binding isotype matched control mAbs (Figures 1b and 2d) ▶ ▶ , demonstrating that reactivity with anti-CD1 mAbs was specific and was not due to binding to Fc receptors present on cells within the lesions. Expression of CD1a, -b, -c, and -d occurred, for the most part, in the same regions of the atherosclerotic plaques. However, subtle differences did exist, with the expression of CD1c generally appearing more diffuse within the lesions. CD1 molecules were expressed predominantly in areas that also had a relatively high density of CD3+ lymphocytes (Figure 2, a and b) ▶ , indicating close contact and possible interactions between T cells and cells expressing CD1 molecules.
Figure 2.
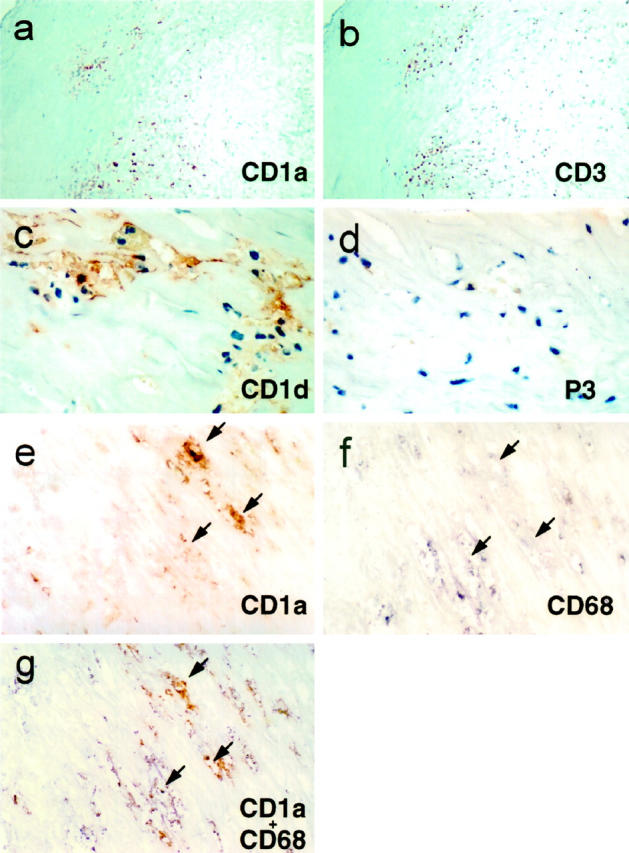
Expression of CD1 by foam cells and in T cell rich areas of atherosclerotic plaques. a and b: Serial sections through an atherosclerotic plaque demonstrated staining for CD1 (a, ×100 view of anti-CD1a mAb OKT6 staining) that colocalized in T-cell-rich areas of the atherosclerotic plaque (b, ×100 view of anti-CD3 mAb OKT3 staining). c and d: High-power (original magnification, ×400) views of a section from a typical atherosclerotic plaque revealed CD1d staining predominantly in large lipid-laden cells with vacuolated cytoplasm characteristic of foam cells (c, mAb CD1d68.3), whereas a serial section from the same plaque showed absence of staining using an isotype-matched negative control antibody (d, mAb P3). e–g: serial sections of another atherosclerotic plaque stained with mAb against CD1a (e, mAb OKT6, red reaction product), the macrophage marker CD68 (f, mAb KP1, blue reaction product), or double-stained to reveal CD1 expression in CD68+ foam cells (g, stained with OKT6 revealed by the red reaction product and KP1 revealed as the blue reaction product; original magnification, ×400). Arrows are provided as references points for serial sections e, f, and g (note colocalization). Similar results were seen in sections stained for CD1b (data not shown).
Identification of CD1+ Cells in Atherosclerotic Lesions as Lipid-Laden Macrophages
Staining of adjacent serial sections of the same plaque with anti-CD68, a marker for macrophages, revealed that CD1 expression occurred predominantly in macrophage-rich areas of the lesion (Figures 1, a–f, and 2, e and f ▶ ▶ ). High-power views of atherosclerotic lesions demonstrated that CD1 expression was restricted to the peripheral regions of large irregular cells containing abundant lipid inclusions (Figure 2, c and e) ▶ . The appearance of these cells was characteristic of the macrophage-derived lipid-laden foam cells commonly found in mature atherosclerotic plaques. To verify that CD1 expression was restricted to macrophage-derived foam cells, we performed double staining of individual sections with mAbs specific for CD68 and either CD1a or CD1b. High-power views of these sections revealed that all CD1+ cells were also co-stained for CD68, and that all of these double-labeled cells had vacuolated cytoplasmic inclusions (Figure 2g) ▶ . These findings demonstrated the macrophage origin of the CD1+ foam cells in atherosclerotic lesions.
Generation of CD1+ Monocyte-Derived Foam Cells in Vitro
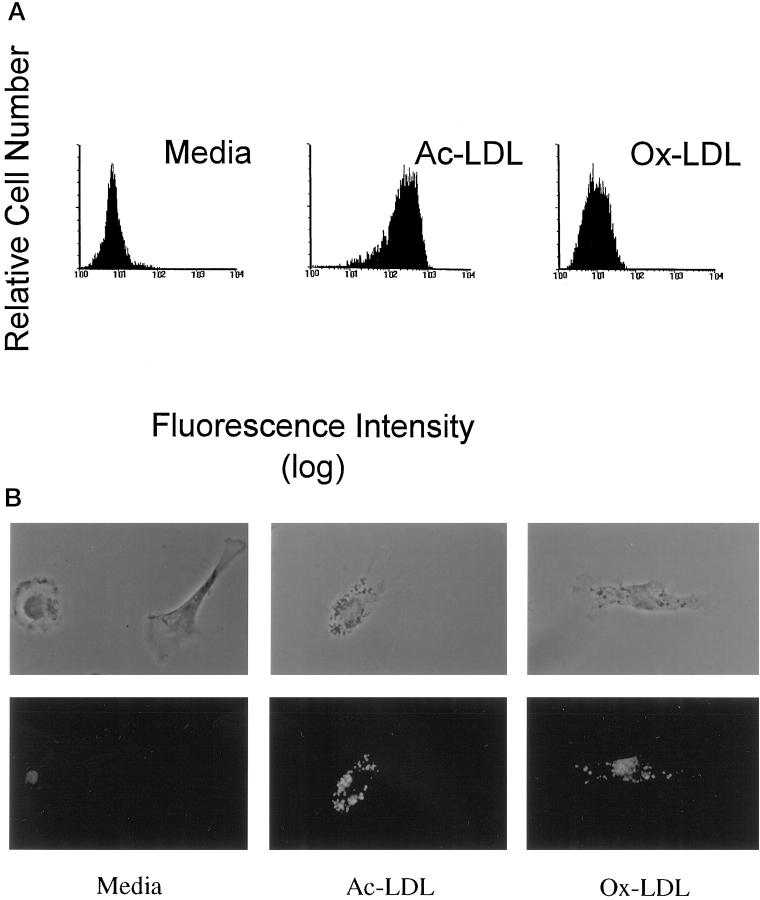
The expression of CD1 molecules by lipid-laden foam cells suggested that these cells might present lipid antigens to T cells present in atherosclerotic plaques. Because the relatively small number of such cells in the available tissue specimens precluded their direct isolation for in vitro functional analyses, we produced monocyte-derived foam cells in vitro to assess their ability to act as antigen-presenting cells for CD1-restricted T cell responses. Peripheral blood monocytes were cultured in media containing GM-CSF and IL-4 to augment the expression of CD1 molecules (CD1a, -b, and -c). To induce foam cell formation, some cultures were incubated with medium containing either acetylated LDL or oxidized LDL. In contrast to untreated control cells, staining of these LDL-loaded cells with the lipid stain Nile Red demonstrated lipid accumulation characteristic of foam cells (Figure 3, a and b) ▶ .
Figure 3.
Generation of lipid-laden foam cells in vitro. Freshly isolated monocytes accumulated intracellular lipid droplets when incubated in culture in the presence of GM-CSF, IL-4 and exogenous acetylated or oxidized LDL. a: Intracellular lipid accumulation was stained with Nile red and quantitative analysis of fluorescently labeled cells was performed by flow cytometry. b: Fluorescence microscopy of monocytes cultured with GM-CSF and IL-4 plus exogenous acetylated LDL (Ac-LDL) or oxidized LDL (Ox-LDL) and stained with Nile Red revealed intracellular lipid droplets typical of foam cells (original magnification, ×400). The top row of images shows the cells under phase contrast illumination, and the bottom row shows fluorescence of the same cells. Monocytes treated with GM-CSF and IL-4 in the absence of exogenously modified LDL, indicated as “media,” are shown for comparison.
Direct observation by fluorescence microscopy confirmed the presence of intracytoplasmic lipid droplet accumulation in cells incubated with either oxidized (Ox-LDL) or acetylated LDL (Ac-LDL) (Figure 3b) ▶ . Acetylated LDL-treated cells were brighter, with distinct lipid droplets present diffusely throughout the cytoplasm, consistent with a foam cell phenotype. Oxidized LDL-treated cells were less bright, with hazy Nile Red staining in a perinuclear distribution in addition to the cytoplasmic droplet appearance, suggesting lipid accumulation in additional compartments in these cells. In contrast, when compared to these cells, GM-CSF and IL-4-treated monocytes (G/4) cultured in the absence of modified LDL treatment gave only weak fluorescence (Figure 3b) ▶ .
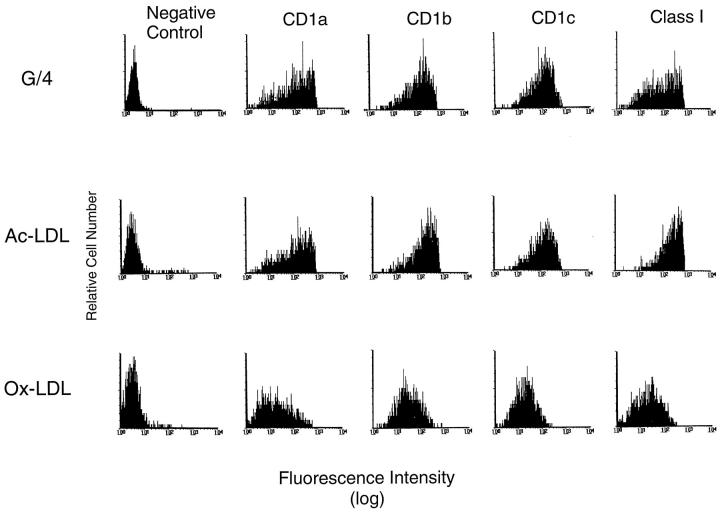
CD1a, -b, and -c proteins were expressed on monocyte-derived foam cells from cultures treated with either oxidized or acetylated LDL in the presence of GM-CSF and IL-4, as determined by flow cytometry (Figure 4) ▶ . These cells also expressed a variety of cell surface proteins typically found on macrophages, including MHC class I and class II molecules, CD11b (Mac1), complement receptor 1 (CR-1), CD14, and ICAM 3 (Figure 4 ▶ , Table 1 ▶ , and additional data not shown). Overall, this cell-surface phenotype showed strong similarities to that described previously for foam cells in atherosclerotic lesions in vivo, 4,15-19,55-58 as summarized in Table 1 ▶ . Interestingly, incubation with oxidized LDL consistently reduced the expression of most of the cell surface molecules studied when compared to acetylated LDL-treated monocytes or cytokine-activated controls (Figure 4 ▶ , Table 1 ▶ , and additional data not shown). These in vitro findings suggested that lipid accumulation resulting from uptake of certain types of lipoprotein particles (eg, oxidized LDL) could be responsible for further differentiation and phenotypic modulation of CD1+ foam cells within atherosclerotic lesions in vivo.
Figure 4.
Expression of CD1 proteins by in vitro-generated lipid-laden foam cells. Cytokine-treated foam cells expressed high levels of CD1a, -b, and -c as assessed by FACS analysis. Cells were stained with antibodies with the specificities indicated at the top of each column. Antibodies used were P3 (nonbinding negative control), OKT6 (anti-CD1a), 4A7.6.5 (anti-CD1b), F10/21A3 (anti-CD1c), and W6/32 (anti-MHC class I, positive control). Further characterization of cytokine-treated foam cells in a separate experiment revealed that these cells expressed the macrophage markers CD11b (Mac1) and complement receptor 1 (CR-1) but expressed only low levels of CD14 when compared to macrophages cultured in medium alone, as shown in Table 1 ▶ .
Table 1.
Comparison of Surface Phenotypes of Foam Cells of Atherosclerotic Plaques in Vivo and Cultured Monocyte-Derived Cell Populations
| Foam cells | Ac-LDL | Ox-LDL | G/4 | Mac | |
|---|---|---|---|---|---|
| CD1a | ++* | ++ | + | ++ | − |
| CD1b | ++* | ++ | + | ++ | − |
| CD1c | ++* | ++ | + | ++ | − |
| CD1d | ++* | + | + | + | + |
| MHC II | ++ 4,18,19 | ++ | + | ++ | + |
| Mac-1 | ++ 58 | ++ | ++ | ++ | ++ |
| CD14 | + 55 | + | + | + | ++ |
| CR1 | ++ 56,57 | ++ | + | ++ | + |
The phenotype of foam cells shown in this table is a summary of previously published immunohistochemical studies on atherosclerotic lesions (references as indicated in table) and results from the present study (indicated by asterisks; data from Figures 1 and 2 ▶ ▶ ). For foam cells, + indicates definite staining by immunohistochemistry, and ++ indicates strongly detected staining indicative of high expression. Monocytes were cultured with GM-CSF and IL-4 in combination with either oxidized LDL (Ox-LDL) or acetylated LDL (Ac-LDL) for 7 days and stained with mAbs against various cell surface markers known to be expressed on foam cells in atherosclerotic lesions and analyzed by FACS. These were compared to macrophages grown in medium alone (Mac) or in combination with GM-CSF and IL-4 in the absence of exogenous addition of modified LDL (G/4). Positive staining is indicated as + (corresponding to a mean fluorescence intensity (MFI) between channels 10 and 100 by FACS) or ++ (MFI between >100 and 500). Absence of significant staining above background levels in FACS is indicated by the − symbol.
T Cell Recognition of Lipid Antigens Presented by CD1+ Foam Cells
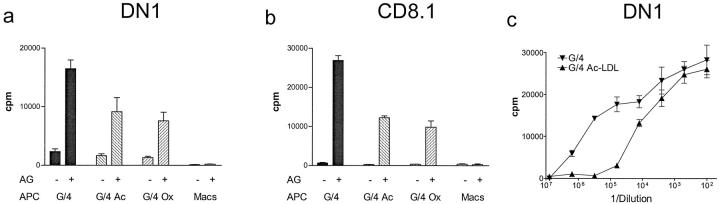
CD1-expressing monocyte-derived foam cells were tested in T cell proliferation assays for their ability to present known bacterial lipid antigens to established CD1b- and CD1c-restricted T cell lines. Foam cells generated in vitro by loading with either oxidized or acetylated LDL were capable of inducing antigen-dependent proliferation of the mycolic acid-specific CD1b-restricted T cell line DN1 (Figure 5a) ▶ . Similarly, the CD1c-restricted T cell line CD8.1 was also induced to proliferate by presentation of mycobacterial phospholipid antigens by both types of in vitro-derived foam cells (Figure 5b) ▶ . As expected, neither T cell line showed responses to the specific lipid antigens in the presence of cultured macrophages, which lack expression of the relevant CD1b and -c antigen-presenting proteins (Figure 5, a and b) ▶ .
Figure 5.
Antigen presentation by CD1+ in vitro-generated lipid-laden foam cells. Foam cells generated in vitro using acetylated or oxidized LDL were used as antigen-presenting cells in T cell proliferation assays with the CD1b restricted T cell line DN1 (specific for mycobacterial mycolic acids, a), or the CD1c-restricted T cell line CD8.1 (specific for a component of the phospholipid fraction of M. tuberculosis, b. M. tuberculosis phospholipid and mycolic acid antigens were used at 5 and 10 μg/ml respectively. c: Comparison of antigen dose responses of DN1 stimulated by CD1+ monocyte-derived cells derived in the absence (G/4) or presence (G/4 Ac-LDL) of acetylated LDL. T cell proliferation (counts per minute (CPM) of 3H-thymidine incorporated) was assessed over a range of dilutions of M. tuberculosis whole bacterial sonicate (expressed as dilution of 5.9 μg/ml bacterial sonicate on the x-axis) in the presence of both lipid-loaded and non-lipid-loaded monocyte-derived CD1+ antigen-presenting cells. All assays were performed in triplicate and mean values and standard deviations are shown.
Cells treated with acetylated or oxidized LDL showed reduced efficiency of antigen presentation by CD1 molecules (Figure 5, a and b) ▶ , and we observed similar reductions in antigen-presenting function when GM-CSF plus IL-4-treated monocytes were exposed to crude lipids extracted directly from atherosclerotic plaques (data not shown). This reduction of antigen-presenting capacity was particularly pronounced at low concentrations of the exogenously added bacterial lipid antigen (Figure 5c) ▶ . This observation provided additional evidence that accumulation of certain forms of lipid may induce further differentiation of foam cells, resulting in effects on their antigen-presenting function. Nevertheless, CD1+ foam cells generated by either method activated lipid antigen-specific CD1-restricted T cells, indicating that they retained the capacity to present lipid antigens to T cells through the CD1 pathway.
Discussion
Recent evidence favoring an inflammatory and possibly even infectious component in the etiology of atherosclerosis has heightened interest in the potential role of T cell activation within the arterial lesions in this disease. This study demonstrated the expression of CD1 molecules in atherosclerotic plaques, thus potentially implicating this family of lipid antigen-presenting molecules in the development or progression of this disease. We detected expression of all four previously characterized human CD1 proteins in these lesions by immunohistochemistry and showed that this expression was confined to the macrophage-derived foam cells present within atherosclerotic plaques. To assess the functional consequences of this expression, we established an in vitro system for the generation of CD1+ monocyte-derived foam cells. These cells shared phenotypic similarities with foam cells in atherosclerotic plaques, and in the presence of lipid antigens they activated CD1-restricted T cells in vitro.
The expression of CD1 proteins on foam cells in atheroma agrees with the known distribution of these molecules on cells derived from the myelomonocytic lineage. 27 However, whereas previous studies have frequently shown the pronounced expression of CD1a, -b, and -c on dendritic cells in various tissues, these molecules have generally not been detected on resident tissue macrophages. 27 Information on the expression of the fourth CD1 protein, CD1d, is currently limited. Studies on the homologue of this protein in the mouse have indicated its expression on a variety of hematopoietic cell types, 59,60 but have not established its expression by resident tissue macrophage populations. Thus, the finding of strong expression of all four known human CD1 proteins on the macrophage-derived foam cells of atheromatous plaques was an unexpected, novel result of this study. Besides the thymus, 61 the atherosclerotic plaque is to our knowledge the only tissue thus far demonstrated to express all four of these human CD1 molecules in vivo.
The mechanisms that induce the expression of CD1 proteins in atherosclerotic lesions are unknown. CD1 proteins, with the exception of low levels of CD1d, have not generally been detected on circulating myelomonocytic cells in vivo. 27 Thus, the expression of CD1 proteins that we have observed on foam cells suggests that the local environment of the atherosclerotic plaque may contain factors that increase transcription or translation of these proteins. Leading possibilities for such inducing factors are the cytokines produced within these lesions, which include interferon-γ (IFNγ), GM-CSF, tumor necrosis factor-α (TNFα), tumor necrosis factor-β (TNFβ), and interleukin-2 (IL-2). 3,7,21 Among these, GM-CSF is the only cytokine currently known to induce CD1 proteins on monocyte-derived cells. 27,51 The possibility of other inducing factors within these lesions remains to be investigated. These could potentially include other secreted proteins as well as signals mediated through receptors interacting with either cell-bound or matrix-associated factors present within the plaques.
Studies on the antigen-presenting function of CD1 proteins suggest that CD1 restricted T cells can respond to foreign as well as potential endogenous lipid antigens. It is now well established that these molecules can present a variety of foreign lipid and glycolipid antigens found in the cell walls of pathogenic mycobacteria to human T cells. Several of the lipid antigens presented by the human CD1b protein have been isolated and structurally characterized. 30-32 These studies reveal the existence of a potentially broad range of CD1-presented lipid antigens, including molecules as disparate in structure as the mycolic acids and phosphatidylinositol-containing lipoglycans. 30-32 In addition, CD1d proteins can present glycosylated ceramide-like and glycosylphosphatidylinositol (GPI)-containing lipids to T cells. 37,62 The mechanism of this lipid antigen presenting function of CD1 proteins has been illuminated by the solution of the crystal structure of a mouse CD1 protein, which reveals a large hydrophobic antigen-binding cavity that is well suited to function as a lipid binding site. 63
Several observations suggest that CD1 proteins may also be capable of binding and presenting normal endogenous cellular lipids to T cells. For example, the mouse CD1d protein produced in Drosophila cells that was studied by X-ray crystallography contained additional electron dense material within its putative lipid-binding cavity. 63 Based on the linear and unbranched appearance of this density, it was suggested to most likely represent a bound cellular lipid, presumably inserted during synthesis and assembly of the protein. Consistent with this possibility, mouse CD1 proteins expressed in mammalian cells and purified on affinity columns were shown to contain detectable amounts of bound cellular GPI, 38 and another study has shown that a synthetic form of a mammalian GPI can be recognized by CD1-restricted murine T cells. 37 More recently, evidence has been obtained that suggests that gangliosides found in mammalian brain tissue may be presented to human CD1b-restricted T cells. 64 These findings indicate that normal or structurally altered endogenous lipids could serve as targets for CD1-restricted T cells, particularly in inflammatory lesions where CD1 molecules are up-regulated.
Although our data do not yet establish a functional role for CD1 proteins expressed on foam cells in atheroscerotic plaques, several features of these lesions support this possibility. T cells within atheroma appear to be chronically activated 19,21,65,66 and frequently express markers of activation including HLA-DR, CD26, and VLA-1. 65 Immunohistochemical and PCR analyses demonstrate that T cells within these lesions actively transcribe and secrete cytokines, including IFNγ and IL-2. 3,7,21 Other studies suggest that T cells within atherosclerotic plaques proliferate, as might be expected after antigen recognition. 67 Plaque T cells are predominately CD45RA low and CD45RO high, which is characteristic of the memory phenotype typically displayed by antigen-selected T cells. 65 Although none of these findings directly demonstrates that ongoing antigen presentation occurs within lesions, together they form a strong circumstantial argument in favor of this hypothesis.
Potential targets that may contribute to the activation of plaque-infiltrating T cells include infectious organisms that have been identified in these lesions, such as cytomegalovirus or chlamydia, and also normal or chemically modified self-antigens. Presentation of such antigens may occur through the MHC class I or II pathways, as these molecules are expressed by cells within the lesions. In fact, studies directly examining the specificity of plaque-infiltrating T cells have demonstrated that a fraction of these may recognize oxidized LDLs presented by MHC class II proteins, whereas others appear to respond to heat shock proteins. 21,23,68 However, previous studies have not considered the possibility that antigen presentation by CD1 might account for some component of T cell activation in atheroma. Clearly, as CD1 is now recognized to function in the presentation of lipid antigens and because atheroma contain an abundance of chemically altered lipids, it now appears reasonable to consider and carefully assess this possibility.
Using foam cells generated in vitro, we demonstrated the capability of such cells to present defined exogenous foreign lipid antigens to CD1-restricted T cells. These in vitro cultured foam cells, similar to their in vivo counterparts, 4,15-19,55-58 developed typical lipid inclusions and expressed MHC class I and II, Mac-1, CR-1, CD14, and CD1 proteins. Our results demonstrated that these cells are capable of presenting lipid antigens by either the CD1b or CD1c antigen presentation pathways. Although our experiments have not yet directly demonstrated lipid antigen presentation within atherosclerotic plaques, the large accumulations of normal and altered lipids within the same cells that express the CD1 proteins in these lesions clearly suggests this possibility. Future studies should help determine if direct recognition of foreign or endogenous lipids is a common feature of plaque-infiltrating T cells. And our initial studies on in vitro cultured foam cells suggest they may be suitable for use to test for CD1-restricted antigen recognition by these cells.
In summary, the present data suggest a potential role for CD1-restricted antigen presentation in atherosclerosis. Atheroma are inflammatory lesions, and the progression of these lesions is likely to be driven and regulated by the cells within the lesions. The activation of T cells within atherosclerotic plaques likely contributes to the development and evolution of these lesions, and macrophage-derived foam cells probably play a central role in this process. Our finding that these cells express the CD1 system of lipid antigen-presenting molecules, coupled with the abundance of abnormal lipids within atherosclerotic lesions, points to a novel potential mechanism for sustained T cell activation. Further development of the in vitro system described here may allow direct analysis of this component of atherosclerosis and may lead to new strategies for prevention and therapeutic intervention in this disease.
Acknowledgments
We thank Evan Beckman for assistance in cultivation of DN1 T cells and for providing Mycobacterium tuberculosis sonicates, Sam Behar for assisting in the generation of antibodies, Chris Dascher and Gerald Watts for helping to characterize antibodies against CD1d, Otto Majdic and Walter Knapp for providing mAb 10C3 ascites fluid, and Lloyd Klickstein for IC3/1, LM2/1, and YZI antibodies. We are very grateful to Daniel Olive for making available to us the hybridomas used to produce mABs 4A7.6.5 and 10D12. Carotid enderacterectomy specimens were generously provided by the Vascular Surgery Department at Brigham and Women’s Hospital per Internal Review Board-approved protocols. Assistance with phase contrast microscopy was kindly provided by Nancy Kedersha.
Footnotes
Address reprint requests to Dr. Agustín Melián, Division of Rheumatology, Immunology and Allergy, Department of Medicine, Brigham and Women’s Hospital, Boston, MA 02115. E-mail amelian@bics.bwh.harvard.edu.
Supported by grants from the National Institutes of Health K11AIO13858 (NIH/NIAID) to A. M., AI40135 (NIH/NIAID) to S. P., and R37-HL34636 to P. L. Additional support was provided by the American Heart Association/Bugher Foundation fellowships to A. M. and by a grant from the American Cancer Society to S. P.
References
- 1.Murray CJ, Lopez AD: Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997, 349:1269-1276 [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Jonasson L, Seifert PS, Stemme S: Immune mechanisms in atherosclerosis. Arteriosclerosis 1989, 9:567-578 [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Hansson GK: Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest 1991, 64:5-15 [PubMed] [Google Scholar]
- 4.Xu QB, Oberhuber G, Gruschwitz M, Wick G: Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol 1990, 56:344-359 [DOI] [PubMed] [Google Scholar]
- 5.Emeson EE, Robertson AL, Jr: T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol 1988, 130:369-376 [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson GK, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G: Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis 1988, 72:135-141 [DOI] [PubMed] [Google Scholar]
- 7.Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362:801-809 [DOI] [PubMed] [Google Scholar]
- 8.Thomson JG: Production of severe atherosclerosis in a transplanted human heart. Lancet 1969, 2:1088-1092 [DOI] [PubMed] [Google Scholar]
- 9.Schroeder JS, Hunt SA: Cardiac transplantation: where are we? N Engl J Med 1986, 315:961-963 [DOI] [PubMed] [Google Scholar]
- 10.Griepp RB, Stinson EB, Bieber CP, Reitz BA, Copeland JG, Oyer PE, Shumway NE: Control of graft arteriosclerosis in human heart transplant recipients. Surgery 1977, 81:262-269 [PubMed] [Google Scholar]
- 11.Taylor DO, Ibrahim HM, Tolman DR, Hess ML: Accelerated coronary atherosclerosis in cardiac transplantation. Transplant Rev 1991, 5:165-174 [Google Scholar]
- 12.Libby P, Salomon RN, Payne DD, Schoen FJ, Pober JS: Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc 1989, 21:3677-3684 [PubMed] [Google Scholar]
- 13.Koulack J, McAlister VC, MacAulay MA, Bitter-Suermann H, MacDonald AS, Lee TD: Importance of minor histocompatibility antigens in the development of allograft arteriosclerosis. Clin Immunol Immunopathol 1996, 80:273-277 [DOI] [PubMed] [Google Scholar]
- 14.Dansky HM, Charlton SA, Harper MM, Smith JD: T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA 1997, 94:4642-4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emeson EE, Shen ML, Bell CG, Qureshi A: Inhibition of atherosclerosis in CD4 T-cell-ablated and nude (nu/nu) C57BL/6 hyperlipidemic mice. Am J Pathol 1996, 149:675-685 [PMC free article] [PubMed] [Google Scholar]
- 16.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P: Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 1998, 394:200-203 [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C: IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 1997, 99:2752-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonasson L, Holm J, Skalli O, Gabbiani G, Hansson GK: Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest 1985, 76:125-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE: Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest 1989, 61:166-170 [PubMed] [Google Scholar]
- 20.Wick G, Kleindienst R, Schett G, Amberger A, Xu Q: Role of heat shock protein 65/60 in the pathogenesis of atherosclerosis. Int Arch Allergy Immunol 1995, 107:130-131 [DOI] [PubMed] [Google Scholar]
- 21.Wick G, Schett G, Amberger A, Kleindienst R, Xu Q: Is atherosclerosis an immunologically mediated disease? Immunol Today 1995, 16:27-33 [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Dietrich H, Steiner HJ, Gown AM, Shoel B, Mikuz G, Kaufman SH, Wick G: Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb 1992, 12:789-799 [DOI] [PubMed] [Google Scholar]
- 23.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK: T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 1995, 92:3893-3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam E, Melnick JL, Probtsfield JL, Petrie BL, Burek J, Bailey KR, McCollum CH, DeBakey ME: High levels of cytomegalovirus antibody in patients requiring vascular surgery for atherosclerosis. Lancet 1987, 2:291-293 [DOI] [PubMed] [Google Scholar]
- 25.Benditt EP, Barrett T, McDougall JK: Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci USA 1983, 80:6386-6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thom DH, Wang SP, Grayston JT, Siscovick DS, Stewart DK, Kronmal RA, Weiss NS: Chlamydia pneumoniae strain TWAR antibody and angiographically demonstrated coronary artery disease. Arterioscler Thromb 1991, 11:547-551 [DOI] [PubMed] [Google Scholar]
- 27.Porcelli SA: The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol 1995, 59:1-98 [DOI] [PubMed] [Google Scholar]
- 28.Melian A, Beckman EM, Porcelli SA, Brenner MB: Antigen presentation by CD1 and MHC-encoded class I-like molecules. Curr Opin Immunol 1996, 8:82-88 [DOI] [PubMed] [Google Scholar]
- 29.Porcelli SA, Segelke BW, Sugita M, Wilson IA, Brenner MB: The CD1 family of lipid antigen-presenting molecules. Immunol Today 1998, 19:362-368 [DOI] [PubMed] [Google Scholar]
- 30.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB: Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 1994, 372:691-694 [DOI] [PubMed] [Google Scholar]
- 31.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzacaro RJ, Soriano T, Bloom BR, Brenner MB, Kronnenberg M, Brennan PJ, Modlin RL: CD1-restricted T cell recognition of microbial lipoglycan antigens (see comments). Science 1995, 269:227-230 [DOI] [PubMed] [Google Scholar]
- 32.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, Besra GS, Porcelli SA: Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 1997, 278:283-286 [DOI] [PubMed] [Google Scholar]
- 33.Beckman EM, Melian A, Behar SM, Sieling PA, Chatterjee D, Furlong ST, Matsumoto R, Rosat JP, Modlin RL, Porcelli SA: CD1c restricts responses of mycobacteria-specific T cells: evidence for antigen presentation by a second member of the human CD1 family. J Immunol 1996, 157:2795-2803 [PubMed] [Google Scholar]
- 34.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR: CD1 recognition by mouse NK1+ T lymphocytes. Science 1995, 268:863-865 [DOI] [PubMed] [Google Scholar]
- 35.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D: CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med 1995, 182:993-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA: Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature 1989, 341:447-450 [DOI] [PubMed] [Google Scholar]
- 37.Schofield L, McConville MJ, Hansen D, Campell AS, Fraser-Reid B, Grusby MJ, Tachado SD: CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 1999, 283:225-229 [DOI] [PubMed] [Google Scholar]
- 38.Joyce S, Woods AS, Yewdell JW, Bennink JR, DeSilva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR: Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 1998, 279:1541-1544 [DOI] [PubMed] [Google Scholar]
- 39.Behar SM, Porcelli SA, Beckman EM, Brenner MB: A pathway of costimulation that prevents anergy in CD28-T cells: B7-independent costimulation of CD1-restricted T cells. J Exp Med 1995, 182:2007-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Exley M, Garcia J, Balk SP, Porcelli S: Requirements for CD1d recognition by human invariant V α24+ CD4−CD8− T cells. J Exp Med 1997, 186:109-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olive D, Dubreuil P, Mawas C: Two distinct TL-like molecular subsets defined by monoclonal antibodies on the surface of human thymocytes with different expression on leukemia lines. Immunogenetics 1984, 20:253-264 [DOI] [PubMed] [Google Scholar]
- 42.Boumsell L: Leukocyte Typing IV. Edited by Knapp W. Oxford, Oxford University Press, 1989, pp 251–254
- 43.Brodsky FM, Parham P: Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol 1982, 128:129-135 [PubMed] [Google Scholar]
- 44.Lampson LA, Levy R: Two populations of la-like molecules on a human B cell line. J Immunol 1980, 125:293-299 [PubMed] [Google Scholar]
- 45.Van Voorhis WC, Steinman RM, Hair LS, Luban J, Witmer MD, Koide S, Cohn ZA: Specific antimononuclear phagocyte antibodies: application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med 1983, 158:126-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Fougerolles AR, Springer TA: Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med 1992, 175:185-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller LJ, Schwarting R, Springer TA: Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol 1986, 137:2891-2900 [PubMed] [Google Scholar]
- 48.Changelian PS, Fearon DT: Tissue-specific phosphorylation of complement receptors CR1 and CR2. J Exp Med 1986, 163:101-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panchamoorthy G, McLean J, Modlin RL, Morita CT, Ishiwaka S, Brenner MB, Band H: A predominance of the T cell receptor V γ 2/V Δ2 subset in human mycobacteria-responsive T cells suggests germline gene encoded recognition. J Immunol 1991, 147:3360-3369 [PubMed] [Google Scholar]
- 50.Stenger S, Mazzacaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, Modlin RL: Differential effects of cytolytic T cell subsets on intracellular infection. Science 1997, 276:1684-1687 [DOI] [PubMed] [Google Scholar]
- 51.Porcelli S, Morita CT, Brenner MB: CD1b restricts the response of human CD4–8- T lymphocytes to a microbial antigen. Nature 1992, 360:593-597 [DOI] [PubMed] [Google Scholar]
- 52.Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling PA, Frederique D, Modlin RL, Porcelli SA, Furlong ST, Brenner MB: CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. J Immunol 1999, 162:366-371 [PubMed] [Google Scholar]
- 53.Anegon I, Grolleau D, Soulillou JP: Regulation of HILDA/LIF gene expression in activated human monocytic cells. J Immunol 1991, 147:3973-3980 [PubMed] [Google Scholar]
- 54.Greenspan P, Mayer EP, Fowler SD: Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 1985, 100:965-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poston RN, Hussain IF: The immunohistochemical heterogeneity of atheroma macrophages: comparison with lymphoid tissues suggests that recently blood-derived macrophages can be distinguished from longer-resident cells. J Histochem Cytochem 1993, 41:1503-1512 [DOI] [PubMed] [Google Scholar]
- 56.Seifert PS, Hansson GK: Complement receptors and regulatory proteins in human atherosclerotic lesions. Arteriosclerosis 1989, 9:802-811 [DOI] [PubMed] [Google Scholar]
- 57.Saito E, Fujioka T, Kanno H, Hata E, Ueno T, Matsumoto T, Takahashi Y, Tochihora T, Yasugi T: Complement receptors in atherosclerotic lesions. Artery 1992, 19:47-62 [PubMed] [Google Scholar]
- 58.Mehrabian M, Demer LL, Lusis AJ: Differential accumulation of intimal monocyte-macrophages relative to lipoproteins and lipofuscin corresponds to hemodynamic forces on cardiac valves in mice. Arterioscler Thromb 1991, 11:947-957 [DOI] [PubMed] [Google Scholar]
- 59.Brossay L, Jullien D, Cardell S, Sydova BC, Burdin N, Modlin RL, Kronneberg M: Mouse CD1 is mainly expressed on hemopoietic-derived cells. J Immunol 1997, 159:1216-1224 [PubMed] [Google Scholar]
- 60.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A: CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol 1998, 160:3121-3127 [PubMed] [Google Scholar]
- 61.Calabi F, Milstein C: A novel family of major histocompatibility complex-related genes not mapping to chromosome 6. Nature 1999, 323:540-543 [DOI] [PubMed] [Google Scholar]
- 62.Spada FM, Koezuka Y, Porcelli SA: CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med 1998, 188:1529-1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA: Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 1997, 277:339-345 [DOI] [PubMed] [Google Scholar]
- 64.Shamshieu A, Donda A, Carena I, Mori L, DeLibero G: Self glycolipids as T cell autoantigens. Eur J Immunol 1999, 29:1667-1675 [DOI] [PubMed] [Google Scholar]
- 65.Stemme S, Holm J, Hansson GK: T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb 1992, 12:206-211 [DOI] [PubMed] [Google Scholar]
- 66.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 1989, 135:169–175 [PMC free article] [PubMed]
- 67.Rekhter MD, Gordon D: Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol 1995, 147:668-677 [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Q, Kleindienst R, Waitz W, Dietrich H, Wick G: Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J Clin Invest 1993, 91:2693-2702 [DOI] [PMC free article] [PubMed] [Google Scholar]