Abstract
Mice deficient in either or both mouse α2-macroglobulin (MAM) and murinoglobulin-1 (MUG1) were generated and proved phenotypically normal under standard conditions. Acute pancreatitis was induced with a diet deficient in choline and methionine, supplemented with ethionine. The mortality was less than 25% in wild-type mice, as opposed to at least 56% in knockout mice, and was highest (70%) in MAM−/− mice, with earliest onset at 2 days. Plasma amylase and lipase levels were increased, but pancreatic tissue appeared histologically variable in individual mice. The clinical symptoms were most severe in MAM−/− mice and, surprisingly, were not aggravated in the double knockout mice, suggesting that the lack of proteinase inhibition capacity was not the major problem. Therefore, we analyzed the expression of 21 different cytokines and polypeptide factors in the pancreas of all experimental groups of mice. Interleukin-1-receptor antagonist mRNA was consistently induced by the diet in the pancreas of MAM−/− mice, and transforming growth factor-β, tumor necrosis factor-α, tumor necrosis factor-β, β-lymphotoxin, and interferon-γ mRNA levels were also increased. The data demonstrate the important role of α2-macroglobulin (A2M) in acute pancreatitis as both a proteinase inhibitor and a cytokine carrier. Mice deficient in MAM and/or MUG thus offer new experimental models for defining in vivo the role of the macroglobulins in pancreatitis and in other normal and pathological processes.
Mouse α2-macroglobulin (MAM) and murinoglobulin (MUG) are molecularly characterized members of the α2-macroglobulin (A2M) family. 1-5 MAMs are all broad-spectrum inhibitors of proteinases, including pancreatic trypsin and chymotrypsin, that act by trapping or covalent tagging after proteolytic cleavage of the bait region. 1,6 The expression of the cryptic receptor binding domain allows A2M-proteinase complexes to bind to the A2M receptor, 7 molecularly identified as the multifunctional lipoprotein receptor-related protein. 8 Elimination is efficient and keeps circulating levels of A2M-proteinase complexes in plasma low, even in severe cases of acute pancreatitis. 9-13
The original function of A2M as a proteinase scavenger was complemented by a role as a binding protein for various growth factors, polypeptide hormones, and cytokines. The number of different cytokines that bind to A2M is steadily increasing and includes transforming growth factor-β (TGF-β), interleukin-1 (IL-1), IL-2, IL-6, IL-8, platelet-derived growth factor (PDGF), tumor necrosis factor-α (TNF-α), basic fibroblast growth factor (bFGF), interferon-γ, activin, inhibin, epidermal growth factor, vascular epidermal growth factor, nerve growth factor-β, brain-derived neurotrophic factor, neurotrophin-3, neurotrophin-4, and ciliary neurotrophic factor, among others. 14-17 Major differences in binding mode and affinity to particular conformers of A2M, ie, native or activated A2M, were noted, and binding to activated A2M necessarily implicates only the rapid elimination of the bound cytokine or factor as noted above for A2M-proteinase complexes. Because most interactions were demonstrated in vitro, their physiological meaning in vivo is largely unexplored.
The binding of A2M to both proteinases and cytokines suggests an important role for A2M in many diseases and processes. In this report we concentrated on the role of A2M in a particular condition, ie, acute pancreatitis. In humans, acute pancreatitis typically varies in intensity from mild self-limiting or edematous pancreatitis to severe, acutely hemorrhagic necrotizing disease, with multiple organ failure and fatal outcome. High plasma levels of pancreatic enzymes, ie, amylase, lipase, and trypsin(ogen), were described. 9,18-20 Although diagnostically valuable, levels of circulating pancreatic enzymes have limited value in assessing the etiology or severity of acute pancreatitis. 21-23 Attention has been given to the levels and the role of the circulating proteinase inhibitors, ie, α2-macroglobulin (A2M), α1-proteinase inhibitor, and α1-antichymotrypsin, among others. 10,11,24 Plasma A2M levels allow differentiation between mild and complicated attacks. 12
In addition to proteinase inhibitors, inflammatory cytokines act as pathogenic mediators of acute pancreatitis. Death in most patients is caused not by pancreatic inflammation per se, but rather by multiorgan failure, to which pro-inflammatory cytokines could contribute. Studies with patients and in animal models demonstrated production or overproduction of different cytokines, ie, interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor-α (TNF-α). 25-29 Transforming growth factor-β (TGF-β) has been implicated in the disease process and in regeneration from acute pancreatitis. 30-32 Cytokine production not only correlated to disease severity, but involvement of the pancreas preceded that of other organs, indicating that they participate in or even determine on the progression from initial, local pancreatic inflammation to the multiorgan process. 33
The implication of these two completely different classes of proteins, ie, proteinases and cytokines, in the pathology of acute pancreatitis, in which both bind to A2M, suggested a moderating role for A2M in acute pancreatitis. The role of A2M in acute pancreatitis was tested in mice with a targeted inactivation of the A2M gene. 34 Mice, as opposed to humans, also express murinoglobulins (MUG), single-chain variants typical for rodents, and one, MUG1, is transcriptionally important. 2,5,35 We report here the inactivation of this gene and the generation of doubly deficient MAM−/−MUG1−/− mice. Because the MAM and MUG1 genes proved to be closely spaced, recombination by breeding was circumvented by generating embryonal stem (ES) cell lines with both MAM and MUG1 genes targeted. The singly and doubly deficient mice were viable, fertile, and phenotypically normal. Acute pancreatitis was induced with a diet deficient in choline and methionine and supplemented with ethionine (the CDE diet). 36 Surprisingly, the high susceptibility and mortality of MAM-deficient mice were not made worse by the extra deficiency in MUG1, thereby excluding that lack of proteinase inhibitor capacity was the major problem. RNA protection assays identified expression of different cytokines and polypeptide factors in the pancreas; in particular, IL-1 receptor antagonist (IL-1Ra) correlated most closely with clinical findings. These mice, deficient in either MAM or MUG1 or in both inhibitors, provide interesting models for defining the role of proteinases and cytokines not only in the etiology of acute pancreatitis, but also in other diseases and conditions.
Materials and Methods
MUG1 and MAM/MUG1 Gene Targeting
The MUG1 construct was based on a 7.5-kb NheI-BamHI genomic clone comprising exons 18 to 25 of the MUG1 gene. 5 A 0.9-kb ScaI-KpnI fragment with exon 18 and part of exon 19 was replaced by a 1.8-kb cassette containing the phosphoglycerate kinase gene promoter driving the hygromycinB phosphotransferase cDNA 37 (Figure 1) ▶ . Before electroporation into ES cells, the construct was linearized with SalI. For the double knockout, the MUG1 gene was targeted in ES cell lines that contained a recombined MAM gene 34 using a construct producing neomycin resistance 4 (Figure 1) ▶ . ES cells (line E14, 129/ola) 38 grown on mitomycin STO feeders with single or double antibiotic resistance were positively selected in medium containing either hygromycinB (100 μg/ml) or neomycin (400 μg/ml) or both. Colonies were picked, expanded, frozen, and genotyped by Southern blotting with probes located outside, at either end of the MUG1 fragment (Figure 1) ▶ : a 0.4-kb HindIII/NheI genomic DNA fragment at the 5′ end (L probe); a 0.6-kb BamHI/ClaI genomic DNA fragment located outside the 3′ end (R probe); a 1.8-kb BglII fragment of the PGK-hygromycin gene; a 1.1-kb BglII fragment of the PGK-neomycin gene. 4,34,37 Homozygous deficient mice were obtained and genotyped by Southern blotting or polymerase chain reaction (PCR) as described. 34 PCR mixtures contained 0.1 μmol/L of each primer, 200 μmol/L of each dNTP, 50 mmol/L KCl, 10 mmol/L Tris-HCl, 1.5 mmol/L MgCl2, and 1.25 units of Taq DNA polymerase, in a total volume of 50 μl. Wild-type MUG1 allele was amplified with a forward primer located in exon 17 (position 1986–2004) and a reverse primer in exon 18 (position 2098–2119). 5 Targeted alleles were amplified with a reverse primer located in exon 19 (position 2439–2457) in combination with forward primers either in the hygromycin gene (5′ GATGTGGAATGTGTGCGA 3′) or in the neomycin gene (5′ GTCAAGAAGGCGATAGAAGGCGAT 3′). Diagnostic amplicons were, respectively, 1.9 kb for wild-type and 0.2 and 1 kb for recombined MUG1 alleles.
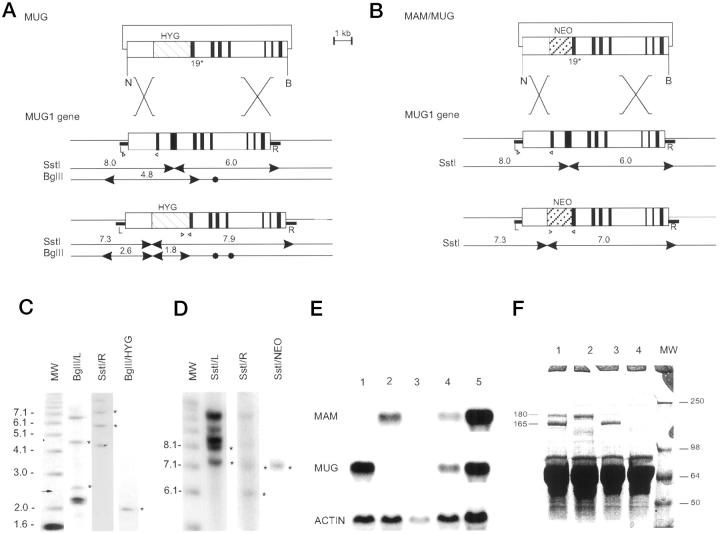
Figure 1.
Generation of mice deficient in MUG and in both MAM and MUG. A: Recombinant DNA construct (top) based on the MUG1 gene (middle) and predicted structure of the targeted MUG1 gene (bottom). The positive selection marker is represented by a hatched box marked HYG. NheI (N) and BamHI (B) restriction sites are marked by arrowheads for diagnostic fragments and closed circles for irrelevant sites. R and L represent genomic DNA probes. B: Recombinant DNA construct (top) to target the MUG1 gene in ES cells with a targeted MAM gene. 34 Structure of the MUG1 gene (middle) and recombinant MUG1 gene (bottom) with positive selection marker represented by a hatched box marked NEO. Diagnostic SstI restriction fragments are indicated. C and D: Southern blots of ES cell lines with recombined MUG1 and MAM/MUG1 genes, respectively, digested with SstI or BglII and hybridized with DNA probes, ie, R, L, HYG, or Neo, as indicated. Standards on the right are in kilobasepairs and asterisks denote diagnostic bands described in the text. E: Northern blot of liver RNA from mice with genetic status MAM−/− (Lane 1), MUG1−/− (Lane 2), MAM−/−MUG1−/− (Lane 3), MAM+/−MUG1+/− (Lane 4), and wild-type (Lane 5). Blot was sequentially hybridized with cDNA probes specific for MAM, MUG1, and actin. F: SDS-PAGE of plasma from wild-type mouse (Lane 1), MAM−/− mouse (Lane 2), MUG1−/− mouse (Lane 3), and MAM−/−MUG−/− mouse (Lane 4). Indicated are the 165-kd MAM subunit and the 180-kd MUG1 subunit with apparent MW of markers (Lane MW) shown in kilodaltons.
Analysis of mRNA
Total liver RNA was extracted with Trizol reagent (Gibco BRL, Glasgow, UK) and analyzed by Northern blotting. 34 Probes were generated by PCR amplification, corresponding to position 1188–1758 of the MAM cDNA 3 and to position 1777–2262 of the MUG1 cDNA. 2 RNA protection assays were performed on total pancreas RNA with commercially available reagents (Riboquant Multiprobe RNase Protection Assay, Pharmingen, San Diego, CA). Autoradiographs taken for different exposure times on Hyperfilm MP were scanned and quantitated densitometrically (Amersham-Pharmacia, Uppsala, Sweden).
Diet-Induced Acute Pancreatitis
Choline- and methionine-deficient powder (CDE diet, Tecklad Harlan CPB, AD Zeist, The Netherlands) was supplemented with D,L-ethionine (final concentration 0.5% w/w). Mice had free access to water and either regular chow or to CDE powder for 2 to 5 days. Mice were killed with chloroform and pancreata were isolated. Pancreatic tissue was fixed in 4% paraformaldehyde for 3 hours and embedded in paraffin, and thin sections were stained with hematoxylin and eosin. Serum amylase and lipase levels and blood glucose levels were determined using clinical kits or analyzers. Immunohistochemistry for IL-1Ra was performed on dewaxed sections (5 μm). Biotinylated antibody to mouse IL-1Ra (R&D Systems, Abingdon, UK) was reacted overnight at room temperature, followed by inactivation of endogenous peroxidase and incubation with streptavidin-biotin complex. Reaction was visualized with diaminobenzidine and H2O2. All numerical data are expressed as means ± SE and analyzed by two-tailed Student’s t-test.
Analysis of Binding of IL-1Ra to Mouse and Human A2M and Murinoglobulin
Recombinant IL-1Ra (R&D Systems) was incubated with purified MAM or human A2M or added to mouse plasma or serum, separated by nondenaturing rate electrophoresis and denaturating sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting. The presence of endogenous mouse IL-1Ra was also analyzed on samples of mouse plasma and serum taken from wild-type C57Bl/6 mice and from MAM−/−, MUG1−/−, and MAM−/−MUG1−/− mice.
Samples of purified human and mouse A2M (4 μg) and plasma and serum samples (1 or 2 μl) were diluted and incubated in phosphate buffered saline containing a mixture of proteinase inhibitors, and in specified experiments, with extra addition of recombinant IL-1Ra, and with or without extra addition of 5 mmol/L calcium ions. Samples were incubated for 30 minutes at room temperature and products were subjected to nondenaturating rate electrophoresis in homogenous (6%) or gradient (4–20%) Tris-glycine PAGE. After electrophoretic transfer to nitrocellulose membranes (Hybond-C, Amersham, Uppsala, Sweden) and inactivation with fat-free milk, sequential incubation was done with a biotinylated antibody to IL-1Ra (R&D Systems) and with streptavidin-peroxidase for detection of immune complexes with the enhanced chemiluminescence system (Amersham).
In other experiments, similar samples were transferred and blots were analyzed by ligand blotting by incubating the membranes with recombinant IL-1Ra (0.01–0.1 μg/ml) for 1 hour. After washing, detection of IL1-Ra binding to immobilized proteins was performed with the antibody to IL-1Ra Ab as described above.
Results
Generation of MUG1 Knockout Mice
In a 7.5-kb genomic clone comprising exons 18 to 25 of the MUG1 gene from the 129 mouse strain (129/J library, 5) we replaced a 0.9-kb ScaI-KpnI fragment containing exon 18 and 108 bp of exon 19 with a 1.8-kb XhoI-ClaI cassette encoding the hygromycin B phosphotransferase gene. 37 Before electroporation into ES cells, the construct was linearized with SalI (Figure 1A) ▶ . Selection in hygromycin containing medium yielded 428 colonies that were genotyped by Southern blotting (SstI digestion and hybridization with the L probe). The 19 ES clones with correct hybridization pattern (Figure 1A, C) ▶ were expanded and genotyped with the three probes (L, R, and hyg; see Figure 1, A and C ▶ ). Eventually, five ES cell lines with the expected restriction and hybridization patterns (overall frequency of recombination, 1.2%) were injected into C57Bl blastocysts and resulted in coat-color chimeric offspring. Only one line transmitted the targeted MUG1 gene through the germline. From a total of 195 offspring, 44 were homozygous (22.6%), 104 heterozygous (53.3%), and 47 wild-type (24.1%) mice, establishing a normal mendelian inheritance pattern of the targeted MUG1 gene. Homozygous MUG1-deficient breeding pairs produced litters of normal size with normal frequency. At this writing, the oldest MUG1−/− mice approach 18 months of age and still appear healthy in our open animal house.
Proof of MUG1 mRNA deficiency was obtained on liver RNA from wild-type, heterozygous, and homozygous MUG-deficient mice. The 5-kb MUG1 mRNA was absent in the liver of MUG1−/− mice (Figure 1E) ▶ even after overexposure for 7 days (results not shown). Subsequent hybridization of the same membrane with the MAM cDNA probe revealed the typical 5-kb mRNA in all mice (Figure 1E) ▶ .
Plasma proteins were analyzed by rocket immunoelectrophoresis (results not shown) and by SDS-PAGE (Figure 1F) ▶ . Plasma of MUG1−/− mice was devoid of MUG1 protein, whereas plasma levels of MAM were normal (Figure 1F ▶ , lane 3). Inactivation of the MUG1 gene was complete and not replaced by expression of other murinoglobulin isoforms, confirming that only the MUG1 gene is transcriptionally active. 2,5,35
Generation of MAM/MUG1 Double Knockout Mice
To generate double MAM/MUG1 knockout ES cells, a MUG1 construct with a neomycin resistance gene (Figure 1B) ▶ was electroporated into an ES cell line with one MAM allel targeted with a hygromycin resistance gene. 34 Double selection in medium containing both neomycin and hygromycin, yielded 572 resistant ES cell colonies, analyzed by Southern blotting (SstI restriction and hybridization with L probe; Figure 1 ▶ ). The 17 ES cell lines with a targeted MUG1 gene were expanded and re-analyzed with the three probes (Figure 1B, D) ▶ , resulting in eight ES cell lines with a correctly targeted MUG1 gene and with one copy of the targeted MAM gene. 4 This equaled a 1.4% frequency of recombination.
Of three cell lines yielding germline chimeras, two produced pups with both recombinant alleles. Crossing and genotyping of offspring over several generations demonstrated that the MUG1 and the MAM gene were in linkage disequilibrium. This was confirmed by crossings of single knockout mice, from which double MAM/MUG1 deficient mice were rarely obtained, following chromosomal recombination events (5.3% recombination frequency). Matings of double heterozygous MAM+/−MUG1+/− mice eventually resulted in mice homozygous for both inactivated gene (results not shown).
Proof of deficiency of MAM and MUG in the homozygous double transgenic mice was obtained by Northern blotting of liver mRNA and by plasma protein electrophoresis as above. Liver of double heterozygous MAM+/−MUG1+/− mice contained about half the mRNA levels relative to wild-type mice (Figure 1E) ▶ , and the 5-kb MAM and MUG mRNA transcripts were completely absent in the liver of the double knockout mice (Figure 1E) ▶ . Electrophoresis of plasma proteins demonstrated the presence or absence of the 165- and 35-kd subunits of MAM 3 and/or of the 180-kd subunit of MUG1 5 (Figure 1F) ▶ .
The MAM−/−MUG1−/− mice produced litters of normal size with a normal frequency, establishing a mouse strain that is devoid of all A2M family members. This is experimental proof that these proteinase inhibitors are not vitally needed, opposite to what we inferred from the lack of humans deficient in A2M ( Ref. 1, and references therein). Individual doubly deficient MAM−/−MUG1−/− mice have been under observation for over 15 months and appear normal and healthy in open animal housing conditions.
Single and double knockout mice were back-crossed into the C57Bl mouse strain for at least 6 generations. To exclude the possibility that the whole breeding colony would be derived from only a few mice, six couples per generation were used. Mice with a C57Bl background of at least 98.5% were obtained, which alleviated problems with differences in genetic background between experimental and control mice (C57Bl) in subsequent experiments.
Experimental Induction of Edematous Pancreatitis
Edematous pancreatitis was first experimentally attempted by induction with cerulein, following a regime of 7 injections every hour. 39,40 Amylase and lipase levels in plasma were significantly increased in all genetically modified mice relative to wild-type C57Bl mice (results not shown) but during and following the experiment, all mice appeared healthy and behaved normally. Macroscopically, the pancreas appeared somewhat swollen and enlarged, but histologically, no major signs of necrosis were noted. Only the appearance of vacuoles in the cytoplasm of acinar cells was different, relative to animals receiving vehicle only. Subsequent studies were therefore performed with another model of induction of acute experimental pancreatitis, ie, by using a diet deficient in choline and methionine and supplemented with ethionine. 36
Experimental Induction of Acute Hemorrhagic Pancreatitis
Mortality
In preliminary experiments, the mortality of MAM−/− mice resulting from the CDE diet was compared in mice with a mixed 129 × C57Bl background 34 and after backcrossing into the C57Bl background. Mortality was 60% in MAM−/−MUG1−/− mice in the C57Bl strain after 5 days of CDE diet, whereas in the mixed 129 × C57Bl background, mortality was only 23%. This demonstrated the importance of the genetic background, although this back-crossing was time-consuming and expensive. To exclude the influence of the C57Bl genetic background in the following experiments, mice were also backcrossed to the FVB mouse strain for 6 generations. Because preliminary experiments revealed no difference between the C57Bl and FVB mouse strain, the data reported here were all obtained from mice back-crossed into C57Bl.
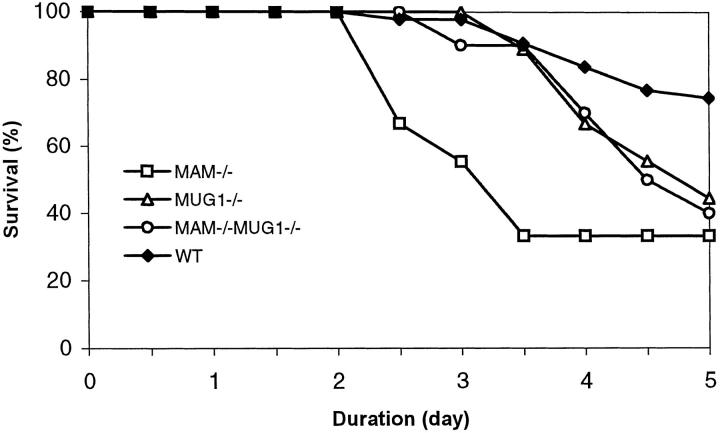
The CDE diet was tested in a total of 6 independent experiments spread over a period of 10 months. Each test lasted from 2 to 5 days and resulted in an overall mortality rate of 27% in wild-type C57Bl mice and at least 56% in all of the deficient mice (Figure 2) ▶ . Surprisingly, mortality commenced most early in the MAM−/− mice, in all experiments. Typically, after 5 days on the CDE diet, nearly 70% of the MAM−/− mice had succumbed (Figure 2) ▶ . MUG1−/− mice were more sensitive to the diet than nontransgenic mice but less than MAM−/− mice, whereas the combined deficiency of both inhibitors in MAM−/−MUG1−/− mice did not aggravate mortality, and even alleviated the effect as far as time of onset was concerned (Figure 2) ▶ .
Figure 2.
Mortality during the CDE diet. Survival of female mice with different genetic status on the CDE diet. Data are combined from all experiments performed (n = 61 for C57Bl, n = 18 for MAM−/−, n = 9 for MUG1−/−, n = 10 for MAM−/−MUG1−/−).
Plasma Levels of MAM, MUG, Glucose, Amylase, and Lipase
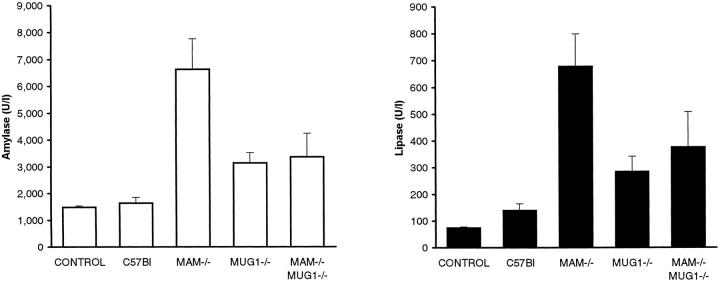
Because amylase and lipase levels are the highest between 60 and 72 hours, 41 mice were given the CDE diet for 66 hours to measure enzyme and cytokine levels. Enzymatic activity of amylase and lipase increased in plasma of all mice, confirming as expected the development and course of acute pancreatitis. After 66 hours of diet, concentrations of amylase and lipase peaked in the MAM knockout mice, to 4 and 10 times higher levels, respectively, relative to nontransgenic C57Bl mice (P < 0.01; Figure 3 ▶ ). Levels of both enzymes were higher in MAM−/− mice than in MUG1−/− mice (P < 0.05) and, in complete accord with the mortality data, the amylase level was also significantly higher than in doubly deficient MAM−/−MUG1−/− mice (P < 0.05; Figure 3 ▶ ). These effects were reversible since feeding regular chow following a 3-day CDE diet, resulted in complete normalization of amylase and lipase activities in plasma of all mice, including MAM−/− mice, after 4 days (results not shown).
Figure 3.
Amylase and lipase activity in plasma of mice on CDE diet. Plasma amylase (left) and lipase (right) activities (mean ± SE) for 5 groups of 8 female mice of genetic status indicated. Control mice are pooled values from C57Bl, MAM−/−, MUG1−/−, and MAM−/−MUG1−/− mice on regular chow. Blood samples were taken after 66 hours on the CDE diet.
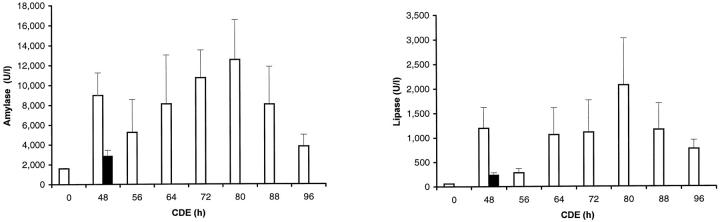
In a second type of experiment, a group of 23 MAM−/− mice were subjected to the CDE diet. Mice in this group were not analyzed at random, but at regular time points those mice that appeared most affected by the diet were sacrificed. The selection was objectively based on loss of body weight, lack of activity in the home cage, cleanliness of the coat, and general condition, judged by the vigor by which mice tried to escape handling needed for examination and weighing trice daily. Under such nonrandom conditions, the highest values of amylase and lipase were observed on day 4 of the diet (Figure 4) ▶ . High activity of both enzymes was conspicuously evident after 48 hours with values much higher than in a group of MAM−/− mice, all analyzed nonselectively after 48 hours on the same CDE diet (Figure 4) ▶ . Importantly, these very high levels of amylase and lipase activity coincided with the onset of mortality in the MAM−/− mice (Figure 2) ▶ .
Figure 4.
Amylase and lipase activity in plasma of mice on CDE diet. Amylase and lipase activities (mean with SEM) in MAM−/− mice subjected to the CDE diet, whereby sick animals were selected at each time point (nonrandom experiment as described and discussed in text). After 48 hours, the corresponding values in MAM−/− mice randomly analyzed are shown for comparison (filled blocks).
Plasma levels of MAM and MUG were essentially unchanged by the CDE diet, in respectively MUG1−/− and MAM−/− mice, indicating that increased consumption of the inhibitors by proteinase trapping during the acute phase was not evident (results not shown). In addition, MUG or MAM mRNA transcripts were not changed by the CDE diet (results not shown). These findings were indications that lack of proteinase inhibition capacity was not a prime cause of disease and mortality. The endocrine function of the pancreas was unaffected, because plasma glucose levels were not significantly affected by the CDE diet (results not shown).
Histology of the Pancreas
After 3 days of the CDE diet, pancreatic morphology was largely unaffected and normal, with minimal differences between the 4 groups. Occasionally, minor areas of the pancreas were necrotic and showed signs of infiltration by immune cells (Figure 5, A and B ▶ , and Table 1 ▶ ). The exception was a pronounced vacuolization of acinar cells in the MAM−/− mice (Figure 5C) ▶ , not evident in mice of the other experimental groups.
Figure 5.
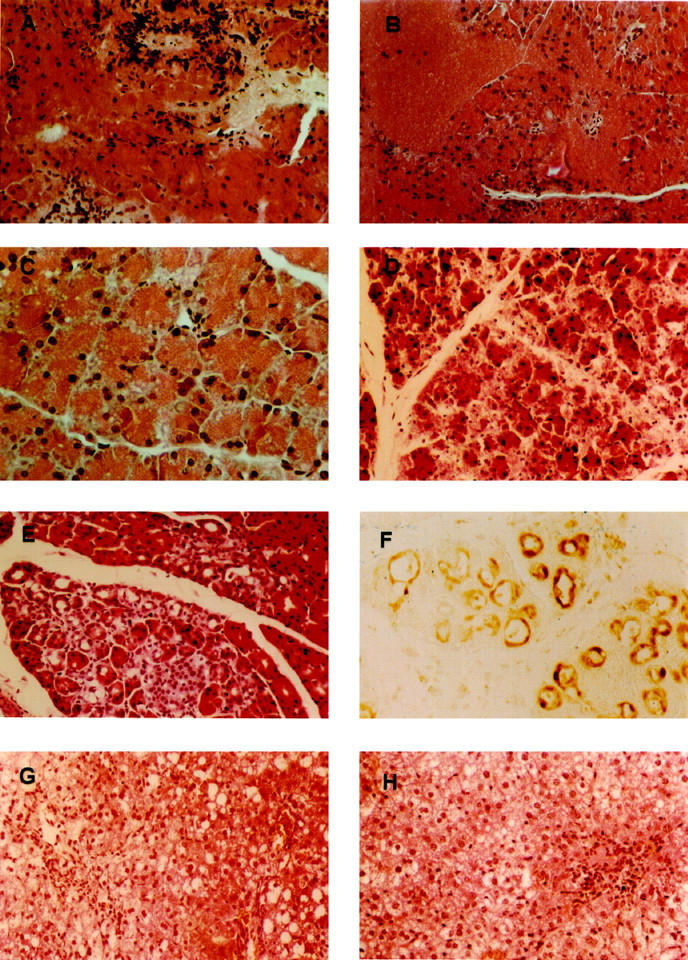
Histopathology of pancreas and liver of mice on CDE diet. Pancreas of a C57Bl (A) and MAM−/− (B, C) mouse after 66 hours with necrotic area and inflammatory infiltration (A, B) and vacuolization (C). Pancreas of a C57Bl (D) and MAM−/− (E) mouse after 120 hours with necrotic acini (D) and acini with an enlarged lumen (E). Liver of a C57Bl (G) and MAM−/− (H) mouse after 120 hours with pronounced vacuolization and local inflammatory infiltration. All sections are stained with hematoxylin/eosin. F: Immunostaining for IL-1Ra in pancreas of a MAM−/− mouse after 120 hours.
Table 1.
Semiquantitative Analysis of the Pancreas of C57BI, MAM−/−, MUG1−/−, and MAM−/−MUG1−/− Mice
| Genotype | 66 hours of CDE diet | 120 hours of CDE diet | ||
|---|---|---|---|---|
| Score of necrosis | Number of mice (percentage) | Score of necrosis | Number of mice (percentage) | |
| C57BI | 0 | 3 (38) | 0 | 4 (22) |
| 1 | 4 (50) | 1 | 4 (22) | |
| 2 | 1 (12) | 2 | 3 (17) | |
| 3 | 0 (0) | 3 | 1 (6) | |
| 4 | 0 (0) | 4 | 6 (33) | |
| MAM−/− | 0 | 0 (0) | 0 | 1 (17) |
| 1 | 2 (40) | 1 | 2 (33) | |
| 2 | 2 (40) | 2 | 1 (17) | |
| 3 | 1 (20) | 3 | 2 (33) | |
| 4 | 0 (0) | 4 | 0 (0) | |
| MUG1−/− | 0 | 4 (57) | 0 | 1 (17) |
| 1 | 3 (43) | 1 | 2 (33) | |
| 2 | 0 (0) | 2 | 0 (0) | |
| 3 | 0 (0) | 3 | 0 (0) | |
| 4 | 0 (0) | 4 | 3 (50) | |
| MAM−/−MUG1−/− | 0 | 2 (33) | 0 | 0 (0) |
| 1 | 4 (67) | 1 | 0 (0) | |
| 2 | 0 (0) | 2 | 2 (33) | |
| 3 | 0 (0) | 3 | 0 (0) | |
| 4 | 0 (0) | 4 | 4 (67) |
The following score of necrosis was used: 0, normal morphology; 1, appearance of vacuoles; 2, minor areas of necrosis; 3, many areas of necrosis; 4, necrosis over the whole pancreas.
After 5 days of CDE diet, more mice (Table 1) ▶ showed massive necrosis of the pancreatic acini with inflammatory infiltration and hemorrhage, although in some mice there was still no evidence of histological alteration of the pancreas (Figure 5, D and E ▶ , and Table 1 ▶ ). The degree of necrosis was not markedly different in the MAM−/− mice relative to wild-type C57Bl mice on the same diet for the same duration, a finding that indicated that necrosis and mortality did not correlate. Obviously, this outcome was distorted in the sense that only the surviving mice, which were fewer in number in the MAM−/− group, were examined. Nevertheless, in all experiments taken together, no correlation was discerned between the amylase and lipase activities in the plasma and the degree of necrosis of the pancreas (results not shown).
One of the remarkable morphological features in severely affected pancreata after 5 days of CDE diet in MAM−/− mice (5 of 7) and MAM−/−MUG1−/− mice (5 of 10) was the appearance of acini with an enlarged lumen (Figure 5, E and F) ▶ . This might result from atrophy of the acinar cells or might indicate acino-ductular metaplasia, a phenotypic trait much rarer in nontransgenic C57Bl mice (2 of 29 mice) or in MUG1−/− mice (1 of 6). The CDE diet also affected the liver, resulting in hepatic cell necrosis, formation of large vacuoles, and inflammatory infiltration, observed in all mice (Figure 5, G and H) ▶ . These lesions became apparent after 2 days of CDE diet and remained unchanged thereafter. In addition, 4 days of regular chow after 3 days of CDE diet restored liver morphology to a nearly normal microscopic appearance (results not shown). The histology of the lungs from mice that received the diet between 48 and 96 hours was completely normal (results not shown).
Expression of Cytokines in the Pancreas
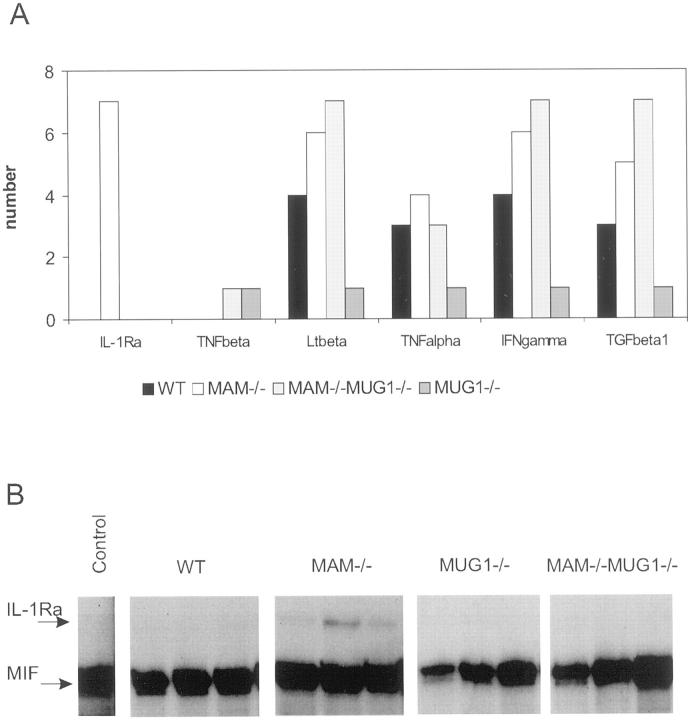
RNA protection assays covering 21 different cytokines and polypeptide hormones revealed that mRNA coding for IL-1Ra, TNF-β, Lt-β, TNF-α, IFN-γ, and TGF-β1 were detectable in the pancreas of some or all of the mice in the four experimental groups analyzed after 3 days of CDE diet. No factor was, however, expressed in all mice of all groups; eg, TGF-β1 was expressed in 5 of 8 MAM−/−MUG1−/− mice, whereas TNF-β mRNA was seen in only 1 of these mice (Figure 6A) ▶ . The least responsive mice were the MUG1−/− mice, in which up-regulation of none of the 21 markers was evident except in 1 mouse, whereas Lt-β, TNF-α, IFN-γ, and TGF-β1 mRNA was present in most of the pancreata of MAM−/− and MAM−/−MUG1−/− mice. Had we considered only these data, we would have concluded that MAM regulates cytokine expression in acute pancreatitis. Wild-type mice, however, with the lowest mortality rate, reacted more than the MUG1−/− mice, demonstrating that the correlation between cytokine expression and the presence of MAM is not very clear, at least not at the mRNA level. Therefore, cytokine expression needs to be analyzed in more detail on the protein level to determine whether MAM binds and regulates cytokine levels not only in vitro, but also in vivo.
Figure 6.
Cytokine RNA protection assay and western blotting for IL-1Ra. A: Frequency histogram of expression of mRNA of different cytokines in the pancreas of mice with genetic status indicated (n = 8 for C57Bl and MAM−/− MUG1−/− mice, n = 7 for MAM−/− and MUG1−/− mice) after 3 days of CDE diet. Expression of 15 other mRNA species analyzed was negligible. B: Representative examples of RNase Protection Assay gels of IL-1Ra mRNA in the pancreas of mice with indicated genetic status after 3 days of CDE diet. MIF denotes macrophage inhibitory factor, used as internal standard in the assay.
The only cytokine exclusively expressed in the pancreas of MAM−/− mice, albeit at variable levels, was IL-1Ra (Figure 6B) ▶ . Immunohistochemically, IL-1Ra protein was visualized only in the cells of acini with an enlarged lumen and was absent in normal acini (Figure 5F) ▶ . The local production of IL-1Ra within the acini is consistent with the fact that other cytokines are also expressed in the pancreatic parenchyma. 32,33
Because no data are available in the literature about the binding of MAM to IL-1Ra, Western blotting and ligand blotting were performed. Western blotting did not reveal the binding of endogenous IL-1Ra to MAM or MUG or other high-molecular-weight proteins in plasma or serum (results not shown). To overcome the presumed problem of detection limits, we attempted to demonstrate binding of additionally added recombinant IL-1Ra to MAM by Western blotting. This also proved negative, however, even when nondenaturing rate electrophoresis in 6% gels was used as separation medium before transfer (results not shown). Ligand blotting with recombinant IL-1Ra also did not indicate appreciable binding of IL-1Ra to either purified mouse A2M or human A2M (results not shown).
Discussion
Mice deficient in either MAM 34 or MUG, or in both proteinase inhibitors, were generated and proved viable and fertile, bearing litters of normal size with normal frequency and with normal sex ratios among the pups. Mice up to 2 years of age did not show any phenotypic abnormality. The suggestion that redundancy in the A2M family, ie, that functional backup by murinoglobulin blurred the picture of the MAM−/− mice, 34 is thereby rejected. The double knockout mice, completely deficient in proteinase inhibitors of the A2M family, prove that these inhibitors are not essential for either embryonic development or adult life. This fact contradicts the hypothesis of the essential nature of A2M in humans based on the long evolutionary history of A2M, their conservation in all higher species, and the lack of functional deficiencies of A2M in humans ( Ref. 1 and references therein). The functional reason for a strict evolutionary conservation of A2M and structural complexity must, then, be sought in other than normal conditions, ie, under physiological stress.
In acute pancreatitis, many enzymes are released in the blood and proteinases must be rapidly inhibited, a process A2M was thought to control. 10-12,24 The severity of acute pancreatitis is determined by the degree of local inflammation in the pancreas, which is variable, whereas death is caused in most patients by multiorgan system failure. Both phenomena must be related, and pro-inflammatory cytokines, ie, IL-1, IL-6, IL-8, and TNF-α, were shown to be produced during acute pancreatitis. 25-29 A2M is not only a wide-spectrum proteinase inhibitor but also a major carrier of cytokines, and the MAM and MUG1 knockout mice were therefore ideal experimental models. Earlier experiments with MAM−/− mice had provided indications that MAM plays a crucial role in pancreatitis, but the mixed genetic background was recognized as a potential problem. 34 The MAM−/−, MUG1−/−, and MAM−/−MUG1−/− mice were therefore backcrossed to the C57Bl strain for 6 generations and all experiments were performed with mice against this homogeneous genetic background.
In this study, we demonstrated that A2M limits the severity of acute pancreatitis, probably not only as a proteinase inhibitor, but also as a potent carrier of biologically active polypeptides. The evidence for the first part of this conclusion is immediate: deficiency in MAM or MUG increased the mortality resulting from the CDE diet from 27% to, respectively, 67% and 56% after 5 days. The lack of synergy in mice lacking both proteinase inhibitors and the difference in mortality between MAM−/− and MUG1−/− mice argued strongly against a deficit in proteinase inhibition capacity as the only or even the major cause, because their spectra of proteinase inhibition are comparable. 42-44
MAM−/− mice started to die as early as the second and third days of the regime, defining a critical window in which mortality was 7 times higher than in wild-type mice. Deficiency of MUG and of MAM and MUG combined, although eventually increasing mortality significantly and to nearly the same level, showed remarkable differences in kinetics. This warranted the conclusion that A2M contributed strongly to, or even determined, the outcome of this critical period at 2 to 3 days of CDE diet. The nonrandom type of experiment corroborated this conclusion. A large group of MAM−/− mice and analysis of only the sick mice at each time point (see Results) demonstrated that high amylase and lipase levels at 48 hours of diet coincided with onset of lethality in random experiments.
The difference in mortality between MAM−/− and MUG−/− mice can be explained by the differential cytokine or polypeptide carrier characteristics of MAM and MUG. As reviewed in the introduction, the diversity and number of polypeptide hormones, growth factors, and cytokines identified as binding to A2M are steadily increasing. We demonstrated differential binding to MAM, but not to MUG, of TGF-β1 and TGF-β2. 45 Comparative or differential binding characteristics of other factors to MAM and MUG are lacking. Analysis of binding of these cytokines and other biologically active factors to MAM and MUG is a future direction of research to which these mice can contribute considerably. They provide a physiological model to test the importance of the binding of A2M to cytokines in vivo, which until now had been demonstrated only in vitro.
Because the severity of pancreatitis is determined not only by the initial damage in the pancreas but also by the further local or multiorgan systemic immune reaction, cytokines were analyzed by RNA protection assays. The presence of Lt-β, TNF-α, IFN-γ, and TGF-β1 mRNA in the pancreata of most MAM−/− and MAM−/−MUG1−/− mice and in only one pancreas of MUG1−/− mice suggested a role for MAM in the regulation of cytokine expression. The data obtained from the wild-type mice, however, did not support this conclusion. Detailed analysis of cytokine expression on the protein level will be necessary to answer the question whether MAM is indeed a cytokine scavenger.
The consistent expression of IL-1Ra mRNA in all MAM−/− mice and its absence in the other knockout and wild-type mice was surprising and puzzling. IL-1Ra has been shown to be protective in this experimental model of pancreatitis. 26,46-47 A variety of exogenous stimuli results in the local production of IL-1 and IL-1Ra, with appearance of IL-1 before IL-1Ra within a short time interval. In addition, IL-1 is itself a weak inducer of IL-1Ra production by monocytes. Thus, it was hypothesized that IL-1Ra occurs as part of a physiological regulatory response to limit the proinflammatory effect of IL-1, influenced by the production of other cytokines. 48 Assuming that expression of IL-1Ra is a local protective reaction in the pancreas of mice severely affected by pancreatitis, then one implication could be that in MAM−/− mice, which were demonstrated to be the most affected by the diet, pancreatic expression of IL-1Ra would be higher compared to less affected mice. If IL-1Ra plays a protective role, absence of IL-1Ra should increase the severity of pancreatitis, which can be analyzed in double MAM/IL-1Ra knockout mice. 49 Alternatively, MAM could transport noxious factors, like TNF-α, IL-1, and IL-6, and protective factors, like IL-1Ra, out of and away from the intoxicated pancreas. In the absence of MAM these factors may accumulate and amplify ongoing pancreatic disease. Immune infiltration, local and distant production of other factors, and, indeed, other effects might positively or negatively affect progression of the process and explain the observed variability in patients and models alike. This hypothesis is based on the fact that A2M binds many cytokines and growth factors although, to our knowledge, binding of IL-1Ra to A2M has not been experimentally demonstrated and the attempt described here was negative. In contrast, binding of TGF-β, TNF-α, IL-1, IL-6, and IL-8 to A2M was demonstrated. 14-17 They are all factors proven to act on and even determine the progression of acute pancreatitis, suggesting that the high mortality of the MAM−/− mice can be explained by the absence of an effective cytokine scavenger.
Histological variability of the pancreas of mice on the CDE diet ranged from normal to massive necrosis of the acini, inflammatory infiltration, and hemorrhage. We did not observe a close correlation between amylase and lipase concentrations and the degree of pancreatic necrosis. Similarly, in the human condition, the value and meaning of amylase and lipase profiles are still debated. 21-23 A remarkable morphological feature in severely affected pancreata of MAM−/− and MAM−/−MUG1−/− mice deserves attention. The occurrence of ductular structures is observed, similar in appearance to those in transgenic mice overexpressing TGF-β 50 or a dominant-negative TGF-β receptor. 51 We noted increased levels of TGF-β in plasma of mice on the CDE diet, but these were not markedly different in MAM−/− mice (results not shown).
In conclusion, we demonstrated that the A2M family of proteinase inhibitors is not essential for life and established the direct contribution of A2M to the pathology and mortality of acute pancreatitis as postulated. 34 The definite involvement of A2M in acute pancreatitis is clear, although cytokines and growth factors need to be further analyzed at the protein level. Based on the data presented here and data from literature, we postulate that the high mortality of the MAM−/− mice can be explained by both the proteinase- and cytokine-binding activity of A2M. In this report we concentrated on pancreatitis, but there are many other areas, eg, sepsis, immunology, and lung pathology, in which these single and double knockout mice are now being used and can yield important information. The recent and most interesting finding that the A2M gene is associated with sporadic Alzheimer’s disease 52 suggests another area in which these mice might become experimentally useful.
Acknowledgments
We thank K. Crassaerts, P. Holemans, and N. Caluwaerts for expert technical assistance and S. Gonias for stimulating discussions.
Footnotes
Address reprint requests to Fred Van Leuven, Ph.D., Dr.Sc., Experimental Genetics Group, CME/VIB/KU Leuven, Campus Gasthuisberg O&N 6, B-3000 Leuven, Belgium. E-mail: fredvl@med.kuleuven.ac.be.
Supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (FWO), by NFWO-Lotto, by the Interuniversity Network for Fundamental Research (IUAP) of the Belgian Government, and by the Special Biotechnology Program of the Flemish Government (IWT/VLAB/COT-008). L. U. and L. O. were post-doctoral research fellows of the Special Research Fund of the K. U. Leuven.
L. Umans and L. Serneels contributed equally to this work.
References
- 1.Van Leuven F: Human a2-macroglobulin: structure and function. Trends Biochem Sci 1982, 7:185-187 [Google Scholar]
- 2.Overbergh L, Torrekens S, Van Leuven F, Van den Berghe H: Molecular characterization of the murinoglobulins. J Biol Chem 1991, 266:16903-16910 [PubMed] [Google Scholar]
- 3.Van Leuven F, Torrekens S, Overbergh L, Lorent K, De Strooper B, Van den Berghe H: The primary structure of mouse a2-macroglobulin, deduced from protein sequencing of the isolated subunits and from molecular cloning of the cDNA. Eur J Biochem 1992, 210:319-327 [DOI] [PubMed] [Google Scholar]
- 4.Umans L, Serneels L, Hilliker C, Stas L, Overbergh L, De Strooper B, Van Leuven F, Van den Berghe H: Molecular cloning of mouse gene coding for alpha-2-macroglobulin. Genomics 1992, 22:519-529 [DOI] [PubMed] [Google Scholar]
- 5.Overbergh L, Hilliker C, Lorent K, Van Leuven F, Van den Berghe H: Identification of four genes coding for isoforms of murinoglobulin, the monomeric mouse a2-macroglobulin. Genomics 1994, 22:530-539 [DOI] [PubMed] [Google Scholar]
- 6.Barret AJ, Starkey PM: The interaction of a2-macroglobulin with proteinases: characterization and specificity of the reaction and a hypothesis concerning its molecular mechanism. Biochem J 1994, 133:709-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Leuven F, Cassiman JJ, Van den Berghe H: Primary amines inhibit recycling of α2M receptors in fibroblasts. Cell 1980, 20:37-43 [DOI] [PubMed] [Google Scholar]
- 8.Herz J, Hamann U, Rogne S, Mylkbost O, Gausepohl H, Stanley KK: Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggests a physiological role as a lipoprotein receptor. EMBO J 1988, 7:4119-4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conn M, Goldenberg A, Conception L, Mandeli J: The effect of ERCP on circulating pancreatic enzymes and pancreatic protease inhibitors. Am J Gastroenterol 1991, 86:1011-1014 [PubMed] [Google Scholar]
- 10.Dubick MA, Mar G, Mayer AD, Majumdar APN, McMahon M, Geoakas MC: Digestive enzymes and protease inhibitors in plasma from patients with acute pancreatitis. Pancreas 1987, 2:187-194 [DOI] [PubMed] [Google Scholar]
- 11.Banks RE, Evans SW, Alexander D, Van Leuven F, Wigher JT, McMahon MJ: Alpha-2-macroglobulin state in acute pancreatitis: raised values of alpha-2-macroglobulin-protease complexes in severe and mild attacks. Gut 1991, 32:430-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson C, Heads A, Shenkin A, Imrie CW: C-reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg 1989, 76:177-181 [DOI] [PubMed] [Google Scholar]
- 13.Gross V, Leser HG, Heinisch A, Scholmerich J: Inflammatory mediators and cytokines. New aspects of the pathophysiology and assessment of the severity of acute pancreatitis? Hepatogastroenterology 1993, 40:522-530 [PubMed] [Google Scholar]
- 14.Lamarre J, Wollenberg GK, Gonias SL, Hayes M: Biology of disease cytokine binding and clearance properties of proteinase-activated a2-macroglobulins. Lab Invest 1991, 65:3-14 [PubMed] [Google Scholar]
- 15.James K: Interactions between cytokines and α2-macroglobulin. Immunol Today 1990, 11:163-166 [DOI] [PubMed] [Google Scholar]
- 16.Borth W: a2-Macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J 1992, 6:3345-3353 [DOI] [PubMed] [Google Scholar]
- 17.Chu CT, Pizzo S: Biology of disease: a2-macroglobulin, complement and biologic defense: antigens, growth factors, microbial proteases and receptor ligation. Lab Invest 1994, 71:792-812 [PubMed] [Google Scholar]
- 18.Geokas MC, Rinderknecht H, Broderick JW, Largman C: Studies on the ascites fluid of acute pancreatitis in man. Dig Dis Sci 1978, 23:182-188 [DOI] [PubMed] [Google Scholar]
- 19.Ventrucci M, Gullo L, Danielle C: Comparative study of serum pancreatic isoamylase, lipase, and trypsin like immunoreactivity in pancreatic disease. Digestion 1978, 28:114-121 [DOI] [PubMed] [Google Scholar]
- 20.Robert J, Roth M, Rohner A, Meyer P: The diagnostic and prognostic contribution in acute pancreatitis of 6 enzymes determined in blood and peritoneal fluid. Eur Surg Res 1984, 16:23-24(suppl. 1)6698068 [Google Scholar]
- 21.Moossa AR: Diagnostic tests and procedure in acute pancreatitis. N Engl J Med 1984, 311:639-643 [DOI] [PubMed] [Google Scholar]
- 22.Gumaste VV: Diagnostic tests for acute pancreatitis. Gastroenterologists 1994, 2:119-130 [PubMed] [Google Scholar]
- 23.Pezilli R, Billi P, Barakat B, Miglio F: Lipase-amylase ratio does not determine the etiology of acute pancreatitis: another myth bites the dust. J Clin Gastroenterol 1998, 26:34-38 [DOI] [PubMed] [Google Scholar]
- 24.McMahon MJ, Bowen M, Mayer AD, Cooper EH: Relation of a2-macroglobulin and other antiproteases to the clinical feature of acute pancreatitis. J Biol Chem 1984, 260:775-781 [DOI] [PubMed] [Google Scholar]
- 25.Gross V, Andreessen R, Leser HG, Ceska M, Liehl M, Lausen M, Farthmann EH, Scholmerich J: Interleukin-8 and neutrophil activation in acute pancreatitis. Eur J Clin Invest 1992, 22:200-203 [DOI] [PubMed] [Google Scholar]
- 26.Norman JG, Fink G, Franz M, Guffey J, Carter G, Davison B, Sexton C, Glaccum M: Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Ann Surg 1996, 223:163-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norman JG, Fink G, Messina J, Carter G, Franz MG: Timing of tumor necrosis factor antagonism is critical in determining outcome in murine lethal acute pancreatitis. Surgery 1996, 120:515-521 [DOI] [PubMed] [Google Scholar]
- 28.Inagaki T, Hoshino M, Hayakawa T, Ohara H, Yamada T, Yamada H, Iida M, Nakazawa T, Ogasawara T, Uchida A, Hasegawa C, Miyaji M, Takeuchi T: Interleukin-6 is a useful marker for early prediction of acute pancreatitis. Pancreas 1997, 14:1-8 [DOI] [PubMed] [Google Scholar]
- 29.Rau B, Steinbach G, Gansauge F, Mayer JM, Grunert A, Beger GH: The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut 1997, 41:832-840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menke A, Yamamuchi H, Gress TM, Adler G: Extracellular matrix is reduced by inhibition of transforming growth factor β1 in pancreatitis in rat. Gastroenterology 1997, 113:295-303 [DOI] [PubMed] [Google Scholar]
- 31.Riesle E, Friess L, Zhao L, Wagner M, Uhl W, Backazo K, Gold LI, Kore M, Buchler MW: Increased expression of transforming growth factor β after acute oedematous pancreatitis in rats suggests a role in pancreatitic repair. Gut 1997, 40:73-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friess H, Lu Z, Riessle E, Uhl W, Brundler AM, Horvath L, Gold L, Korc M, Buchler M: Enhanced expression of TGF-βs and their receptors in human acute pancreatitis. Ann Surg 1998, 227:95-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman JG, Fink G, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG: Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci 1997, 42:1783-1788 [DOI] [PubMed] [Google Scholar]
- 34.Umans L, Serneels L, Stas L, Overbergh L, Van Leuven F, Van den Berghe H: Targeted inactivation of the mouse alpha-2-macroglobulin gene. J Biol Chem 1995, 270:19778-19785 [DOI] [PubMed] [Google Scholar]
- 35.Lorent K, Overbergh L, Delabie J, Van Leuven F, Van den Berghe H: Distribution of mRNA coding for alpha-2-macroglobulin, the murinoglobulins, the alpha-2-macroglobulin receptor and the alpha-2-macroglobulin receptor-associated protein during mouse embryogenesis and in adult tissues. Differentiation 1994, 55:213-223 [DOI] [PubMed] [Google Scholar]
- 36.Lombardi B, Estes LW, Longnecker DS: Acute hemorrhagic pancreatitis (massive necrosis) induced in mice by DL-ethionine fed with a choline deficient diet. Am J Pathol 1975, 79:465-480 [PMC free article] [PubMed] [Google Scholar]
- 37.Te Riele H, Robanus Maandag E, Larke A, Hooper M, Berns A: Consecutive inactivation of both alleles of the pim-1 proto-oncogene by homologous recombination in embryonic stem cells. Nature 1990, 348:649-651 [DOI] [PubMed] [Google Scholar]
- 38.Hooper M, Hardy K, Handyside A, Hunter S, Monk M: HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature 1987, 326:292-295 [DOI] [PubMed] [Google Scholar]
- 39.Lampel M, Kern HF: Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secratogogue. Virchows Arch A Pathol Anat Histol 1977, 373:97-117 [DOI] [PubMed] [Google Scholar]
- 40.Niederau C, Liddle RA, Ferrel LD, Grendell JH: Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorraghic pancreatitis in mice. J Clin Invest 1986, 78:1056-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niederau C, Brinsa R, Niederau M, Lüthen R, Strohmeyer G, Ferrel LD: Effects of C1-esterase inhibitor in three models of acute pancreatitis. Int J Pancreatol 1995, 17:189-196 [DOI] [PubMed] [Google Scholar]
- 42.Saito A, Sinohara S: Murinoglobulin, a novel protease inhibitor from murine plasma: isolation, characterization and comparison with murine a-macroglobulin and human a-macroglobulin. J Biol Chem 1985, 260:775-781 [PubMed] [Google Scholar]
- 43.Abe K, Yamamoto K, Sinohara H: Proteinase spectrum of mouse murinoglobulin and a-macroglobulin. J Biochem (Tokyo) 1989, 106:564-568 [DOI] [PubMed] [Google Scholar]
- 44.Miyake Y, Shinomura M, Ito T, Yamamoto K, Abe K, Amemiya S, Sinohara H: Hamster a-macroglobulin and murinoglobulin: comparison of chemical and biological properties with homologs from other mammals. J Biochem (Tokyo) 1993, 114:513-521 [DOI] [PubMed] [Google Scholar]
- 45.Webb D, Wen J, Lysiak JJ, Umans L, Van Leuven F, Gonias SL: Murine a-macroglobulins demonstrate divergent activities as neutralizers of transforming growth factor-β and as inducers of nitric oxide synthesis. J Biol Chem 1996, 271:24982-24988 [DOI] [PubMed] [Google Scholar]
- 46.Norman JG, Franz MG, Fink GS, Messina J, Fabri PJ, Gower WR, Carey LC: Decreased mortality of severe acute pancreatitis after proximal cytokine blockade. Ann Surg 1995, 221:625-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fink G, Yang J, Carter G, Norman J: Acute pancreatitis-induced enzyme release and necrosis are attenuated by IL-1 antagonism through indirect mechanism. J Surg Res 1997, 67:94-97 [DOI] [PubMed] [Google Scholar]
- 48.Arend P: Interleukin-1 receptor antagonist. Adv Immunol 1993, 54:167-227 [DOI] [PubMed] [Google Scholar]
- 49.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y: Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med 1998, 187:1463-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bockman DE, Merlino G: Cytological changes in the pancreas of transgenic mice overexpressing transforming growth factor α. Gastroenterology 1992, 103:1883-1892 [DOI] [PubMed] [Google Scholar]
- 51.Bottinger E, Jakubczak JL, Roberts JL, Mumy M, Hemmati P, Bagnal K, Merlino G, Wakefield LM: Expression of a dominant-negative TGF-β type II receptor in transgenic mice reveals essential roles for TGF-β in regulation of growth and differentiation in the exocrine pancreas. EMBO J 1997, 10:2621-2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blacker D, Wilcox MA, Laird NM, Rodes L, Horvath S, Go RCP, Perry R, Watson B, Basset SS, McInnis MG, Albert MS, Hyman BT, Tanzi RE: Alpha-2-macroglobulin is genetically associated with Alzheimer disease. Nat Genet 1998, 19:357-360 [DOI] [PubMed] [Google Scholar]