Abstract
The nitric oxide (NO)-cGMP pathway has been proposed as a mechanism for relaxation of myometrium during pregnancy and as a modulator of labor. Carbon monoxide (CO), produced by hemeoxygenases (HO-1 and HO-2), also activates soluble guanylate cyclase to increase cGMP. A recent study reported a large increase in HO-1 and HO-2 proteins during pregnancy, suggesting that the HO-CO pathway may be important in the maintenance of uterine quiescence during pregnancy. In this study we used Western blotting, reverse transcription-polymerase chain reaction, and immunohistochemistry to determine HO-1 and HO-2 expression in nonpregnant, pregnant, and laboring myometrium. Immunolocalization of HO was also compared with endothelial and inducible nitric oxide synthases (eNOS and iNOS). In contrast to HO-1 protein, which was not detected in myometrium, HO-2 protein and mRNA were constitutively expressed, although there were no differences in expression between the groups. eNOS was expressed in endothelial cells but not in myometrial smooth muscle. iNOS protein was not detected in myometrium. These data do not support an up-regulation of HO-1 and HO-2 during pregnancy and are not consistent with a role for NO or a major role for CO in human myometrial quiescence. Our results are also in keeping with HO-2 being an noninducible protein.
Although spontaneous preterm birth (delivery before 37 weeks) accounts for 5 to 10% of all births, it is associated with up to 70% of neonatal deaths. It is also responsible for up to 75% of neonatal morbidity with considerable long-term morbidity in the child. 1-3 Drugs used to treat preterm labor, such as prostaglandin synthesis inhibitors, calcium channel antagonists and β-adrenoceptor agonists, have only short-lived effects or have potential side effects for mother and fetus. 4,5 Agents that have been used more recently, such as oxytocin receptor antagonists 6 and nitric oxide (NO) donors, 7 are currently the focus of intensive research, but early reports suggest that they will not confer major benefits. One reason there is no effective method of treating preterm labor is our limited knowledge of the mechanisms that control myometrial quiescence during pregnancy and its contractile state in normal and preterm labor. 8,9
NO, produced by the enzyme nitric oxide synthase (NOS), binds to the heme prosthetic moiety of the soluble guanylate cyclase, leading to increased cGMP production and smooth muscle relaxation. Carbon monoxide (CO) also binds to heme and activates soluble guanylate cyclase. 10 Discrepancies in localization of NOS and guanylate cyclase in the brain indicate that a substantial portion of guanylate cyclase may not serve as a target for NO. 11 CO is produced by heme oxygenase (HO), a microsomal enzyme that oxidatively cleaves heme, a pro-oxidant, to produce biliverdin and CO in the presence of NADPH-cytochrome P450 reductase and NADPH. 12 HO consists of two homologous isoenzymes: HO-1, which is inducible, and HO-2, which is constitutive. 13,14 HO-1 is expressed at high concentrations in the spleen and liver, where it is responsible for the destruction of heme from red blood cells. HO-1 can be induced by numerous stimuli including heme, human chorionic gonadotrophin, and oxidative stress. 15-21 HO-2 is not thought to be inducible and is widely distributed throughout the body. CO acts as a neurotransmitter, 11 inhibits platelet aggregation, 22 and is a vascular smooth muscle relaxant. 23 CO may also account for some of the NO-independent component of relaxation previously ascribed to endothelial-derived hyperpolarizing factor, as it hyperpolarizes smooth muscle cells by opening potassium channels. 24 Outside the brain, HO-2 has been localized by immunocytochemistry to endothelial cells, adventitial nerves, and vascular smooth muscle. It has been proposed that eNOS and HO-2 may have coordinated physiological roles. 25
Animal studies suggest that the NO-cGMP pathway may be involved in myometrial quiescence during pregnancy and in the control of labor. 26-31 However, human studies do not suggest a functional NOS in myometrium. 32 Myometrial cGMP has been reported to increase during guinea pig pregnancy independent of changes in NO synthesis, 33 and Acevedo et al recently reported a 16-fold increase in HO-1 and HO-2 proteins in pregnant compared to nonpregnant myometrium. 34 Furthermore, HO immunoreactivity was reported to show a similar pattern of expression to eNOS. 34 Activation of the HO-CO pathway by the HO inducer hemin completely inhibited spontaneous contractions, and progesterone induced both HO-1 and HO-2 mRNAs. These findings suggest that the HO-CO pathway may be important for uterine quiescence during pregnancy.
The aim of this study was to investigate HO-1 and HO-2 expression in nonpregnant, pregnant, and laboring myometrium using Western blotting, reverse transcription-polymerase chain reaction (RT-PCR), and immunohistochemistry. Immunolocalization of HO was also compared with that for eNOS and iNOS.
Materials and Methods
All chemicals were purchased from Sigma Chemical Company (Poole, UK) unless stated otherwise.
Tissue Collection
Myometrial tissue was obtained from pregnant women undergoing elective (nonlaboring, P, n = 10) or emergency (laboring, L, n = 10) lower uterine segment cesarean section. In the nonlaboring group the indications for delivery were previous cesarean section or breech presentation. In the laboring group the indication for delivery was failure to progress. All samples in the pregnant group were obtained at term (37–42 weeks’ gestation). Samples were also collected from five women not in labor undergoing elective lower uterine segment cesarean section preterm (PT; 32–33 weeks’ gestation). All biopsies were taken from the upper lip of the incision in the lower uterine segment. Myometrium was also collected from a group of women undergoing hysterectomy (nonpregnant, NP) for heavy menstrual bleeding, dysmenorrhea, or fibroids (n = 10). Care was taken to ensure myometrium was taken away from the site of the fibroids and was free of decidua and serosa. All myometrium used in this study was confirmed to be histologically normal. All tissues were snap-frozen in liquid nitrogen immediately after collection and stored at −70°C before protein/RNA extraction or cryostat sectioning. For cell culture studies, myometrium, also obtained from women undergoing hysterectomy, was collected into sterile Dulbecco’s modified Eagle’s medium (DMEM) containing 1% penicillin/streptomycin, 1% glutamine, and 1% Fungizone. All cell culture reagents were obtained from Gibco BRL (Paisley, UK). All women gave their written consent to participation in the study. The study was approved by the Joint Ethics Committee of Newcastle-on-Tyne Health Authority, the University of Newcastle, and the Yorkhill Hospital and Western Infirmary Ethical Committees.
Antibodies
HO-1 and HO-2 rabbit polyclonal antibodies were obtained from Stressgen Biotechnologies Corp. (Victoria, BC, Canada). The HO-1 antibody was raised against purified rat liver HO-1, and the HO-2 antibody was raised against purified native rat testis HO-2. Due to the highly conserved nature of HO-1 and HO-2 between species, these antibodies can be used to detect the human proteins. There is no cross-reaction between the antibodies, which were used for both Western blots and immunohistochemistry. The eNOS/type III mouse monoclonal antibody was purchased from Transduction Laboratories from a UK supplier (Affiniti, Exeter, UK). A 20.4-kd protein fragment corresponding to amino acids 1030–1209 of human eNOS was used as an immunogen. The iNOS antibody, a rabbit polyclonal antibody (C-19/SC649) raised against amino acids 1135–1153 mapping at the carboxy terminus of human iNOS, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Positive control for iNOS antibodies: A549 cells (a human lung airway epithelial cell line) treated with a cytokine cocktail that selectively induces iNOS were a gift from Dr. Simon Bartlett, King’s College, London. The cells were plated in 9-cm dishes and grown to confluence in DMEM containing 10% fetal calf serum. The cells were serum-deprived overnight before addition of the cytokine cocktail: 100 μmol/L lipopolysaccharide, 10 ng/ml interferon-γ, 10 ng/ml tumor necrosis factor-α, and 10 ng/ml interleukin-1β. Cells were incubated for 48 hours with cytokines, washed twice with ice cold phosphate-buffered saline (PBS), and lysed in 0.5 ml lysis buffer (50 mmol/L HEPES, pH 7.5, 2 mmol/L EDTA, 0.2% CHAPS, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 5 μg/ml chymostatin, 100 μg/ml antipain, 100 μg/ml soya bean trypsin inhibitor). Cells then underwent two freeze-thaw cycles and were spun at 13,000 rpm for 5 minutes at 4°C. The supernatant was removed and 30 μl of expanded ADP-sepharose (Pharmacia) were added and mixed continuously for 45 minutes at 4°C. The beads were spun briefly in a microcentrifuge, the supernatant discarded, and the beads washed with 250 μl of lysis buffer. This was repeated once more and then the beads were washed 3× in lysis buffer containing 0.5 mol/L NaCl followed twice more with standard lysis buffer. Finally, 200 μl of loading buffer was added to the pelleted beads, and the sample was boiled for 3 minutes and stored at −20°C until sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blots were performed.
Western Blotting
For the HO antibodies, tissue samples were ground to a fine powder in liquid nitrogen with a mortar and pestle and added to 4 volumes of cold lysis buffer consisting of 25 mmol/L Tris, 0.25 mol/L sucrose, 1 mmol/L EDTA, pH 7.6, and 50 μl/g tissue protease inhibitor cocktail (Sigma). Using a Polytron homogenizer at setting 10, the sample containers were surrounded by ice and homogenized for three 10-second intervals. The homogenate was spun at 5000 × g for 10 minutes at 4°C to remove debris and the resulting supernatant spun again at 50,000 × g for 20 minutes at 4°C to pellet the membranes. The supernatant containing the cytosolic fraction was divided into aliquots and stored at −70°C. The membrane pellet was resuspended in 25 mmol/L Tris, pH 7.6, and spun again at 50,000 × g for 20 minutes at 4°C. The supernatant was again removed and discarded and the membrane pellet resuspended in 25 mmol/L Tris, pH 7.6 (500 μl buffer per gram of starting material), and stored at −70°C. Protein concentrations of both the cytosol and membrane fractions were determined by the method of Lowry, using bovine serum albumin as a standard, and diluted to the required concentration.
Samples (membrane or cytosol) were mixed 1:1 with loading buffer (1.2 ml 1 mol/L Tris, pH 6.8, 2 ml glycerol, 4 ml 10% sodium dodecyl sulfate, 2 ml 1 mol/L dithiothreitol, 0.8 ml distilled water) with bromophenol blue added to give a deep blue color and boiled for 5 minutes before loading. Samples were separated on 10% sodium dodecyl sulfate polyacrylamide resolving gels with a 4% stacking gel using minigel kits (BioRad, Hemel Hempstead, UK) 35 at a constant current of 15 mA. Each well was loaded with 25 μg of protein. Molecular weight markers (Sigma, SDS-7B pre-stained, 33–205 kd range) were loaded beside the samples.
Protein was transferred overnight in buffer containing 25 mmol/L Tris, 19 mmol/L glycine, 20% methanol at a constant 30 V to BioBlot NC nitrocellulose membranes (Costar, Corning, NY). Filters were blocked for 1 hour at room temperature in TBSTB buffer (20 mmol/L Tris, pH 7.5, 0.5 mol/L NaCl, 0.4% Tween 20, 0.25% bovine serum albumin) containing 10% normal donkey serum. Both HO-1 and HO-2 antibodies were prepared at a concentration of 1:1000 in TBSTB containing 5% normal human serum and pre-absorbed for 1 hour at room temperature to reduce nonspecific binding before being used for immunodetection. Omission of this step resulted in many nonspecific bands appearing on the autoradiograph. The antibodies were added for 1 hour at room temperature. The filters were rinsed once, washed twice for 5 minutes in TBSTB, and then incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG (SAPU, Carluke, UK) diluted 1:2000 in TBSTB for 1 hour at room temperature. Blots were then rinsed again and washed twice in TBSTB followed by one 5-minute wash in distilled water. Proteins were detected using the Amersham enhanced chemiluminescence detection system and filters were exposed to Hyperfilm ECL (Amersham, Buckinghamshire, UK).
For the eNOS antibody the same procedure was followed with the following modifications. Samples (membranes or cytosol) were separated on 10% gels and 40 μg of protein were loaded into each well. Filters were blocked for 1 hour at room temperature in PBS/0.1% Tween 20/5% Marvel. The eNOS antibody was added at a dilution of 1:500 in PBS/0.1% Tween 20/1% Marvel for 1.5 hours at room temperature. The filters were washed for 5 × 5 minutes in PBS/0.1% Tween 20 and then incubated with horseradish peroxidase-conjugated donkey anti-mouse IgG (SAPU) diluted 1:2000 in PBS/0.1% Tween 20/1% Marvel for 1 hour at room temperature. Blots were then washed 5 × 5 minutes in PBS/0.1% Tween 20 followed by 1 × 5 minutes in distilled water. For detection of iNOS, the procedure for eNOS was followed except that the blocking buffer was TBSTB/5% donkey serum and the wash buffer was TBSTB. The antibody1:500 was first pre-absorbed for 1 hour at room temperature in TBSTB/5% normal human serum and then directly hybridized with the filters at room temperature for 1.5 hours. The secondary antibody was donkey anti-rabbit IgG-HRP (SAPU) diluted 1:2000 in wash buffer. Samples for iNOS were extracted from homogenized tissues with ADP-sepharose as described above.
RNA Extraction
Total RNA was prepared from using the RNAzol B method (Biogenesis, Poole, UK) from tissue samples which had been ground to a fine powder in liquid nitrogen with a mortar and pestle. Integrity of RNA was validated on agarose gels as previously described. 36
Amplification of HO-1 and HO-2
First-strand cDNA synthesis was performed using Superscript II reverse transcriptase (Gibco BRL, Paisley, UK). Approximately 10 μg of each purified RNA sample was reverse transcribed using oligo dT priming. Briefly, a 25-μl reaction was prepared by adding 5 μl 2.5 mmol/L dNTP mix (Promega, Southampton, UK), 5 μl 5× first-strand buffer (Gibco BRL), 2.5 μl 0.1 mol/L dithiothreitol, and 1 μl Superscript II (200 units). To this was added a mixture containing 1 μl (500 ng) of oligo dT primers (Gibco BRL) and 10.5 μl of RNA (∼10 μg), which had been first heated to 70°C for 10 minutes and cooled on ice. The resulting mix was incubated for 1 hour at 42°C. The left (5′-AAGATTGCCCAGAAAGCCCTGGAC-3′) and right (5′-AACTGTCGCCACCAGAAAGCTGAG-3′) primers were used to amplify a 399-bp region of HO-1 cDNA, whereas the left (5′-TTCCGCACTGGGCACAGGCCGTC-3′) and right (5′-ACCCACACCCACCCTCACTCCAG-3′) primers were used to amplify a 265-bp region of HO-2 cDNA. Both sets of primers were designed by the authors, synthesized commercially (Gibco, BRL) and obtained from published sequences of HO-1 37 and HO-2. 38 Amplification of the cDNA was carried out in a mixture containing 2.5 μl of the resultant cDNA products, 10 μl of 10× reaction buffer, 10 μl of left primer (1 μmol/l stock), 10 μl of right primer (1 μmol/l stock), 4 μl of dNTP mix, 57 μl of distilled water, 4.5 μl 25 mmol/L of magnesium chloride and 2 μl (1 Unit/μl) of Taq DNA polymerase (Cambio Ltd., Cambridge, UK). The mixture was then overlaid with mineral oil. For both HO-1 and HO-2 primers the reaction was amplified for 35 cycles as follows: 94°C, 1 minute; 60°C, 1 minute; 72°C, 1 minute. For detection of HO-1 transcripts, 5 μl of the end reaction mix were subjected to a second amplification step in fresh reaction mix for a further 15 cycles. Controls included omission of SuperScript and omission of the template in the first step.
Immunohistochemistry
Immunohistochemistry was performed using the Vectastain Universal kit (Vector Laboratories, Peterborough, UK). Sections (6 μm) were cut on a cryostat and mounted on glass slides that had been soaked in acetone for 5 minutes, soaked in 2% silane in acetone for 5 minutes, washed in water for 30 minutes, and air-dried. For HO antibodies, sections, which were used immediately following air-drying, were fixed in 1% paraformaldehyde for 5 minutes, then dehydrated in 100% ethanol for 5 minutes, then rehydrated in water for 2 × 5 minutes. These and all subsequent steps were performed at room temperature. Nonspecific binding sites were blocked with blocking agent (Biogenex, San Ramon, CA) for 15 minutes in a humidified chamber and washed in TBSTB for 5 minutes. Sections were then incubated with either the HO-1 or HO-2 antibodies for 45 minutes. Both were used at a concentration of 1:250 in TBSTB containing 5% normal human serum and, for the Western blots, were pre-absorbed for 1 hour in this buffer before immunodetection to reduce nonspecific binding. Omission of this step resulted in nonspecific binding of the antibodies. Sections were incubated with the primary antibody for 1 hour. Controls in which the primary antibody was omitted and replaced with pre-immune serum or in which secondary antibody was omitted were also included. After two 5-minute TBSTB washes, the biotinylated secondary antibody was added for 30 minutes at room temperature. Two more TBSTB washes were performed and endogenous peroxidase activity then quenched by incubating the sections in 1% (v/v) hydrogen peroxide in methanol for 15 minutes. The remaining steps were performed according to the instructions supplied with the kit. Immunoreactive proteins were detected with Fast diaminobenzidine tablets (Sigma). Sections were counterstained in Harris’s hematoxylin (BDH, Poole, UK) and mounted with synthetic resin.
The procedure for the eNOS antibody was the same as for the HO antibodies, with the following modifications. Sections were fixed in acetone for 5 minutes followed by immersion in ethanol for 5 minutes, water for 5 minutes, then PBS for 5 minutes. Sections were blocked with the blocker supplied with the kit for 30 minutes at 37°C and washed in PBS 2 × 5 minutes. Then the primary antibody (diluted in 1:200 in blocking buffer) was added for 90 minutes at 37°C. Following two 5-minute PBS washes, the secondary antibody was added for 30 minutes at 37°C. The remaining steps were performed as above. The procedure for the iNOS antibody was the same as the eNOS antibody, except that the blocking buffer was 10% horse serum/10% human serum in PBS and the primary antibody was diluted 1:250 in blocking buffer.
Induction of HO-1 in Myometrial Cell Cultures
Tissue samples (1 cm3) were cut into small pieces and then incubated for approximately 2 hours at 37°C in DMEM/penicillin/streptomycin/Fungizone containing 10 mg/ml Dispase, 300 U/ml type II collagenase, 2 U/ml type 1 elastase, 30 U/ml type IV Dnase, and 1 mg/ml fatty acid-free bovine serum albumin. The mixture was triturated periodically. Any remaining undigested tissue was allowed to settle and the single cells in the supernantant were then pelleted by centrifugation and resuspended in DMEM/penicillin/sreptomycin/Fungizone containing 20% heat-inactivated fetal bovine serum. The remaining undigested tissue was subjected to a further incubation in the original digestion mix and the cells obtained were combined with the first batch of isolated cells. Cells were plated at a density of 1.5 × 10 4 cells/cm 2 in 75 cm 2 flasks, allowed to grow to confluence, and passaged using standard methods at a ratio of 1:3. At confluence the medium was removed and fresh medium was added containing either 50 μmol/L sodium arsenite (NaArs), 100 μmol/L cadmium chloride (CdCl2), 100 μmol/L diethylmaleate (DEM), 20 μ mol/L H202, or medium alone. Cells were incubated for 8 hours at 37°C. At the end of the incubation the cells were scraped from the flasks, pelleted by centrifugation, and lysed in the same buffer used for whole tissue extraction. Samples were frozen and thawed three times, membranes were prepared, and HO-1 was detected as described for Western blots of tissue samples. For immunohistochemical detection of HO-1, duplicate flasks were set up as for CdCl2 stimulation; cells were scraped from one of the flasks and used to prepare cytospin preparations. Experiments were performed with three separate cell preparations. Cytospin preparations of CdCl2-stimulated cultured myometrial cells were prepared by scraping cells from culture flasks and adjusting the cell concentration to 12,000 cells per 100 μl. One hundred microliters of each cell suspension were spun onto slides using a Shandon Cytospin 3 centrifuge at 1500 rpm for 7 minutes. Unstimulated cells were used as a control.
Results
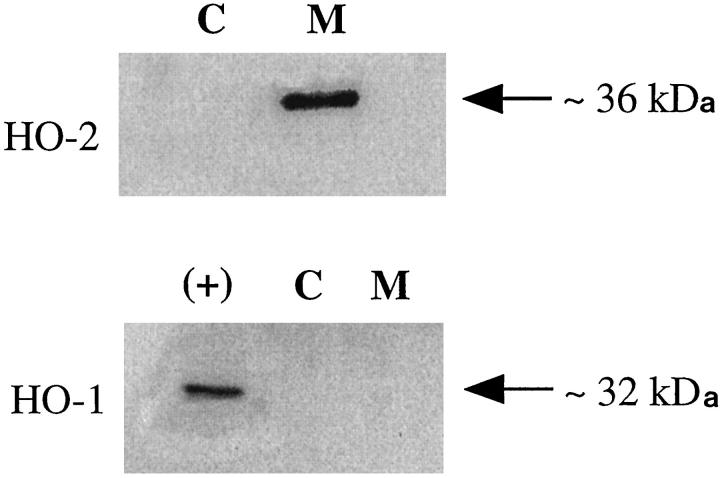
Western blot analysis of both the membrane and cytosolic fraction of nonpregnant myometrial preparations showed that HO-2 protein (Figure 1 ▶ upper panel) was located in the membrane fraction (M). No HO-2 protein was detected in the cytosol (C). The size of the HO-2 was estimated at 36 kd, which is in agreement with the size reported for other human tissues. In contrast, HO-1 (Figure 1 ▶ lower panel) was undetectable in either the membrane (M) or cytosolic fraction (C) in nonpregnant myometrial tissue.
Figure 1.
Western blot analysis for HO-2 (upper panel) and HO-1 (lower panel) in human nonpregnant myometrium. Each lane was loaded with 25 μg of protein. A band of 36 kd was detected in the membrane fraction (M) with the HO-2 antibody. No HO-2 protein was detectable in the cytosolic fraction (C). HO-1 protein was undetectable in both the membrane (M) and cytosolic fractions (C). A positive control (+) for HO-1 (recombinant rat HO-1) confirmed the HO-1 antibody’s specificity.
Figure 2a ▶ shows representative Western blot analysis of HO-2 (upper panel) and HO-1 (lower panel) in human nonpregnant myometrium (NP), pregnant term nonlaboring myometrium (P), pregnant term laboring myometrium (L), and pregnant preterm nonlaboring myometrium (PT). A band of 36 kd was detected in the membrane fraction (M) of all of the samples with the HO-2 antibody. There was no difference in the apparent molecular weight of HO-2 between the groups. Scanning densitometric analysis of all of the samples performed showed there was no quantitative difference in intensity of the bands between nonpregnant, term nonlaboring, laboring, or preterm nonlaboring myometrium (Figure 2b) ▶ . HO-1 protein was undetectable in all of the samples. When total homogenates of tissue (up to 100 μg of protein loaded), rather than membrane preparations, were separated on gels, the findings were similar (data not shown).
Figure 2.
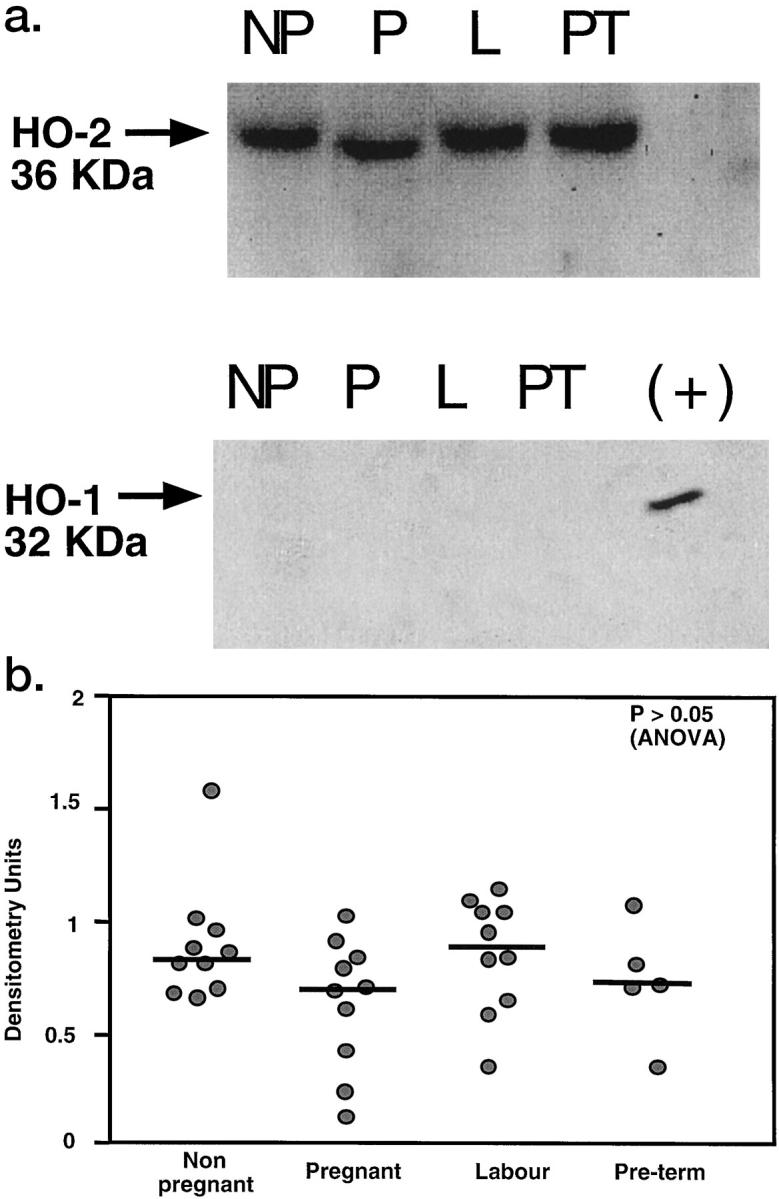
a: Western blot analysis for HO-2 (upper panel) and HO-1 (lower panel) in human nonpregnant myometrium (NP), pregnant term nonlaboring myometrium (P), pregnant term laboring myometrium (L), and preterm laboring myometrium (PT). Each lane was loaded with 25 μg of membrane protein. A band of 36 kd was detected in the membrane fraction (M) of all of the samples with the HO-2 antibody. No HO-2 was detectable in the cytosolic fraction (C) of any of the samples. A positive control (+) for HO-1 (recombinant rat HO-1) confirmed the HO-1 antibody’s specificity. HO-1 was undetectable in all of the samples. b: Scanning densitometric analysis of autoradiographs obtained from non-pregnant (n = 10), pregnant term nonlaboring (n = 10), term laboring (n = 10), and preterm nonlaboring (n = 5) myometrium. Data are shown as a scattergram and bars show median values.
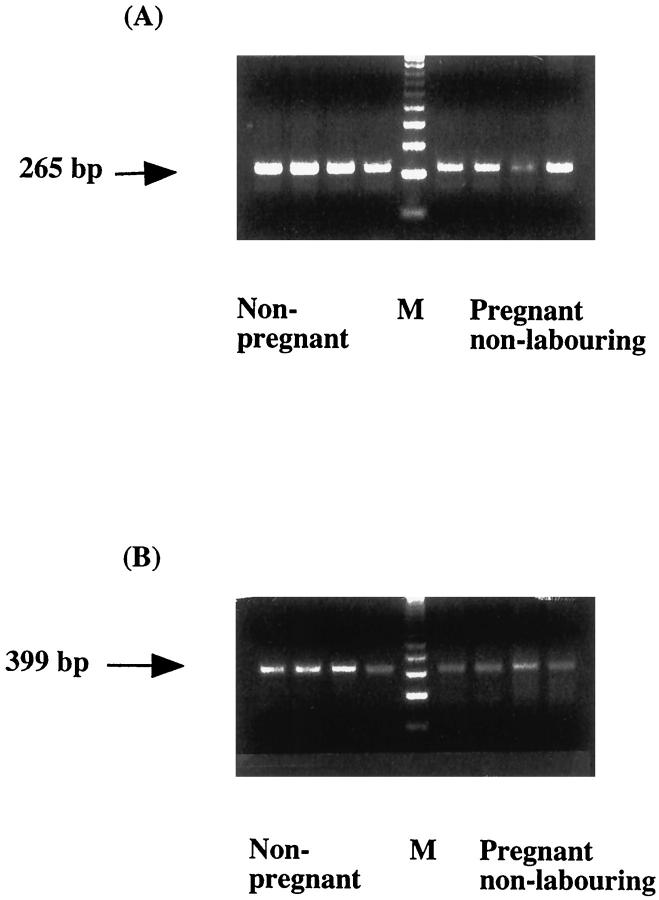
Figure 3A ▶ shows the RT-PCR results for HO-2. This is a nonquantitative technique and thus, no interpretations can be made of the intensity of bands between groups. A band of the predicted molecular weight (265 bp) was identified in both nonpregnant and pregnant myometrium. This is in agreement with the Western blotting and immunohistochemical data and shows that both mRNA and protein for HO-2 are present in the tissues. In contrast, after the first 35 cycles of PCR only a very faint band for HO-1 was present in all of the samples (not shown). However, when 5 μl of the end reaction mix was subjected to a second amplification step in fresh reaction mix for an additional 15 cycles, a band for HO-1 (399 bp) was detectable in all of the samples (Figure 3B) ▶ . These results suggest that HO-1 mRNA is present in both nonpregnant and pregnant myometrium but at very low levels.
Figure 3.
RT-PCR amplification of HO-2 (A) and HO-1 (B) mRNA. Controls where SuperScript II was omitted and where template was omitted in the first step resulted in no bands (not shown). M, molecular weight marker (123-bp ladder).
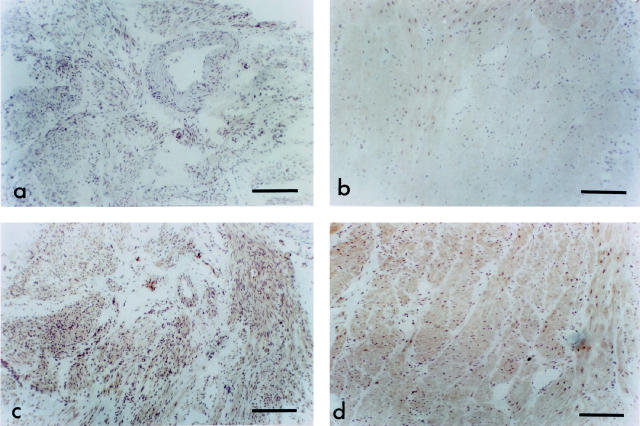
In nonpregnant myometrium (Figure 4a) ▶ and pregnant myometrium (Figure 4b) ▶ there was virtually no immunostaining for HO-1. In contrast, consistent with the Western blot studies, HO-2 protein was evident in both nonpregnant (Figure 4c) ▶ and pregnant (Figure 4d) ▶ myometrium. The endothelium of some blood vessels also expressed HO-2. There were no overall differences in intensity of HO-2 immunostaining between the nonpregnant and pregnant myometrium. There was no immunostaining with control sections.
Figure 4.
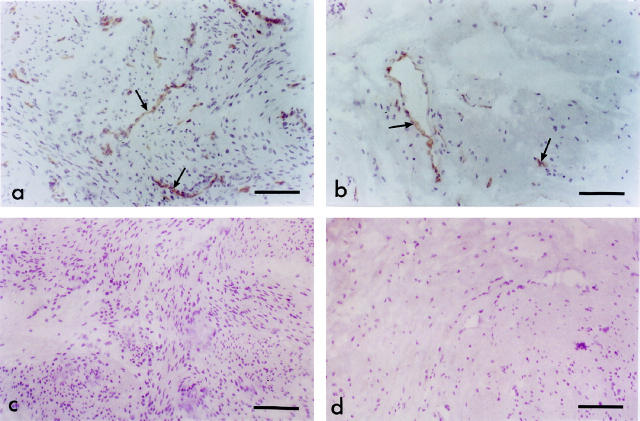
Immunostaining for HO proteins. HO-1 immunostaining is virtually absent from nonpregnant (a) and pregnant (b) myometrium. In nonpregnant myometrium HO-2 had a similar distribution of staining (c) as in pregnant myometrium (d). Staining was evident on muscle and on endothelium of some blood vessels. There were no obvious differences in intensity of immunostaining between the nonpregnant and pregnant myometrium. Scale bar, 200 μm.
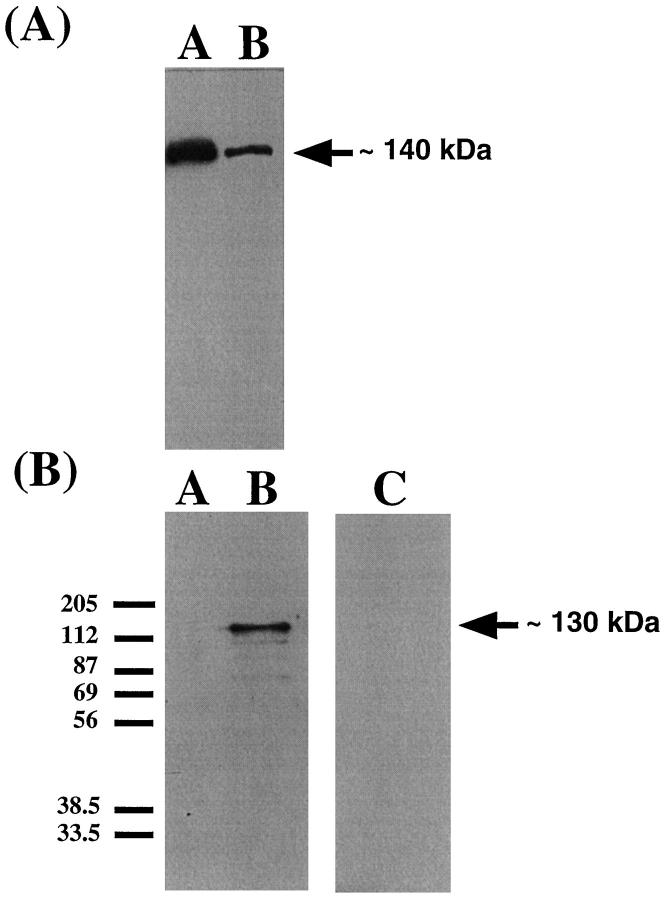
The Western blot with the eNOS antibody (Figure 5A) ▶ showed an eNOS-immunoreactive band at approximately 140 kd in membrane preparations of term placental villous tissue and membrane preparations of nonpregnant myometrium. The eNOS band in placenta reflects the eNOS found in endothelium and syncytiotrophoblast. Because subsequent immunohistochemistry results showed that in myometrium eNOS expression is restricted to vascular endothelium, the less abundant eNOS band in nonpregnant myometrium sample reflects the eNOS in endothelium alone. The Western blots with the iNOS antibodies are shown in Figure 5B ▶ . An iNOS immunoreactive band at approximately 130 kd was apparent in cytokine-stimulated cells only. No iNOS protein was detectable in nonpregnant myometrium.
Figure 5.
A: Western blot for eNOS showing an immunoreactive band at approximately 135 kd in a membrane preparation of term placental villous tissue (Lane A) and a membrane preparation of nonpregnant myometrium (Lane B). Forty micrograms of membrane protein were loaded in each lane. B: Western blot for iNOS. Lane A, ADP-sepharose extracted proteins from unstimulated A549 cells; no band was evident. Lane B, cytokine cocktail-stimulated cells. An iNOS immunoreactive band at approximately 130 kd is clearly identifiable in the stimulated cells. Twenty microliters of sample were loaded in each lane. Lane C: ADP-sepharose extracted proteins from 2 g nonpregnant myometrium. No iNOS was detectable.
There was no eNOS detectable in myometrial muscle of nonpregnant (Figure 6a) ▶ or pregnant myometrium (Figure 6b) ▶ . eNOS was detected in endothelium of blood vessels in both groups. There was no difference in eNOS expression between non-pregnant and pregnant myometrium. iNOS was absent in both nonpregnant (Figure 6c) ▶ and pregnant (Figure 6d) ▶ myometrium. There was no immunostaining with control sections.
Figure 6.
Immunostaining for NOS proteins. eNOS immunostaining is present on vascular endothelial cells of nonpregnant (a) and pregnant (b) myometrium. eNOS is undetectable on muscle. There were no differences in immunostaining between nonpregnant and pregnant myometrium. iNOS immunostaining was absent in both nonpregnant (c) and pregnant (d) myometrium. Scale bars, 100 μm (a and b) and 130 μm (c and d).
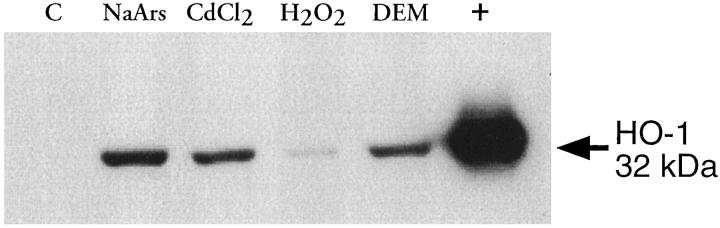
The Western blot in Figure 7 ▶ shows conclusively that when HO-1 was present in cells, it could also be extracted and detected by the methods described in this study. Unstimulated human myometrial cells did not express HO-1 protein when grown in culture; this is in agreement with the in vivo findings. However, exposure of the cells to agents known to induce HO-1 in other cell types resulted in induction of HO-1 protein in all samples. Similarly, cytospin preparations (Figure 8) ▶ showed that when HO-1 protein is induced in cells it can be detected using the same immunohistochemical methods described for the tissue sections.
Figure 7.
Immunodetection of HO-1 in cultured human myometrial cells. Cells were treated with either 50 μmol/L sodium arsenite (NaArs), 100 μmol/L cadmium chloride (CdCl2), 20 μmol/L hydrogen peroxide (H202), 100 μmol/L diethylmaleate (DEM), or medium alone.
Figure 8.
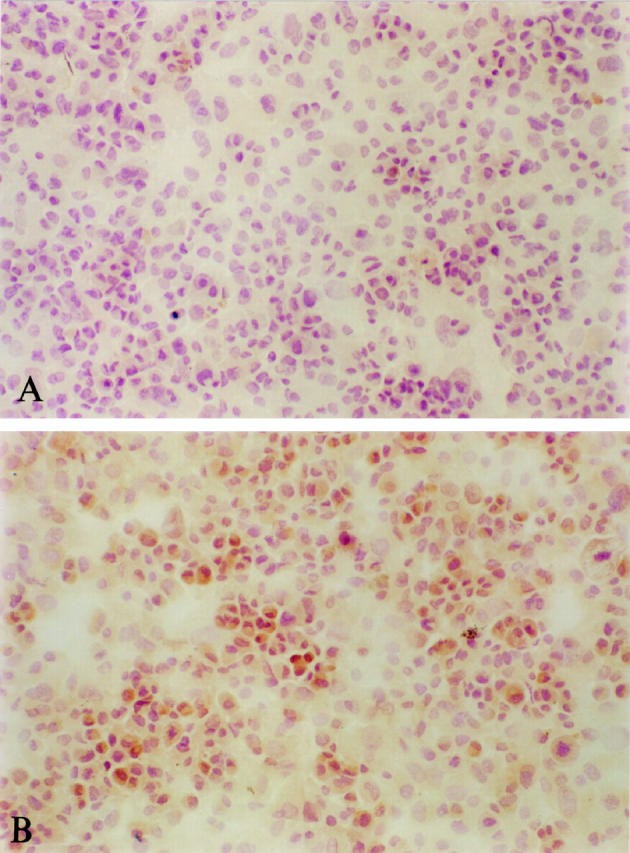
Immunostaining for HO-1 in cytospin preparations of myometrial cells. A: Untreated cells. B: Cells treated with 100 μmol/L CdCl2. Replacement of primary antibody with rabbit serum resulted in a result similar to that shown in A.
Discussion
In this study we have demonstrated the presence of HO-2 protein and mRNA in human myometrium. There was no quantitiative difference in the amount of HO-2 protein in nonpregnant, nonlaboring, laboring, and preterm myometrium. No HO-1 protein was detectable by Western blotting in any of the groups studied. This was confirmed with immunohistochemistry data, which showed little or no HO-1 protein in any of the myometrial samples studied. We were able to detect mRNA for HO-1, but only after a second round of PCR, suggesting very low levels of message in the tissue. Finally, although eNOS was detectable on the endothelium of blood vessels in nonpregnant myometrium, no eNOS was detectable on smooth muscle. In pregnant myometrium an identical pattern was seen. These results suggest that HO and NOS are not up-regulated during human pregnancy and therefore are unlikely to be involved in uterine quiescence.
The presence of HO-2 mRNA and protein in myometrium and our observation that no changes in expression were apparent in pregnancy or labor are consistent with HO-2 being a noninducible protein. Our Western blotting data differ from one previous study of HO in human myometrium, 34 which detected almost no HO-2 protein in nonpregnant myometrium and a 16- to 17-fold up-regulation during pregnancy from almost undetectable levels. In agreement, we did not detect any differences in HO-2 protein between nonlaboring and laboring myometrium. Our data show HO-2 immunostaining was localized to muscle and some endothelial cells. Acevedo 34 reported a similar pattern of immunohistochemical staining for HO-2 in the three groups of myometrium studied, despite the very striking differences found in the Western blots. Our RT-PCR experiments showed the presence of mRNA for HO-2 in all of the groups studied. In contrast, Acevedo and Ahmed did not find mRNA by RT-PCR in nonpregnant or pregnant myometrium despite the presence of protein. The authors suggested this inconsistency might have been due to a reduction in HO mRNA following incubation of tissues for 48 hours in medium devoid of steroids. Our consistent results do not suggest that HO-2 is up-regulated in pregnancy to maintain uterine quiescence.
HO-1 protein was not expressed in myometrium. In contrast, the study by Acevedo 34 reported a 16- to 17-fold up-regulation of HO-1 in pregnant nonlaboring myometrium from almost undetectable levels in nonpregnant myometrium. No differences in HO-1 protein expression was reported between pregnant nonlaboring and laboring myometrium. These authors also reported a similar immunohistochemical staining pattern for HO-1 in all of their groups, a finding which differs from our in this study. Our data are also supported by an abstract published by Bartlett et al, 39 which showed that HO-1 protein was undetectable in human myometrial cells cultured in normal growth conditions, and by our own findings showing that although HO-1 is undetectable in cultured myometrial cells, it can be induced in response to stimulation with known inducers of HO-1. These results also show that when HO-1 is present in cells it can be detected.
The absence of eNOS and iNOS protein strongly suggests that NO is not involved in human uterine quiescence. These findings confirm those of Campa et al, who reported that eNOS expression in term pregnant myometrium was localized to endothelium. 40 Nonpregnant myometrium was not included in this study. The same study also found no iNOS protein in pregnant myometrium (Campa, personal communication). In the discussion section of their paper Acevedo and Ahmed reported that HO expression in myometrium was similar to that which they had previously reported for eNOS; ie, there is a marked increase in immunoreactive NOS in pregnant compared to nonpregnant myometrium. 34 However, the study was in fact a comparison of pregnant laboring and nonlaboring myometrium. 41 We did not confirm this observation. Rather, our findings are in agreement with those on rat myometrium, where HO-2 expression is widespread compared with eNOS. 42
The role of NO synthesis in contractility of term and preterm human myometrium has been investigated recently. 32 Neither addition of L-arginine, the substrate for NOS, nor NG-nitro-L-arginine methyl ester, an inhibitor of NOS, led to any specific changes in spontaneous myometrial contraction. These results suggest that NO production does not play an important role in human term or preterm nonlaboring myometrial contractility.
With the increased uterine stretch as the fetus grows, additional mechanisms are required to maintain uterine quiescence. Previous reports have shown that expression of the human myometrial adenylate cyclase stimulatory G-protein (Gαs) increases during pregnancy, then decreases at the onset of labor. 43 The increase in Gαs is also accompanied by an increase in coupled adenylate cyclase activity, which also decreases to nonpregnant levels at the onset of labor. 44 The increased Gαs levels leads to increased cAMP levels; this will favor myometrial relaxation. The onset of labor is thought to be due to the gradual coordination of several mechanisms to produce myometrial contractions. 45 Thus, it is likely that other mechanisms in addition to G-protein-cAMP signaling will operate to maintain uterine quiescence.
Although NO and HO do not appear to be important in myometrial quiescence during pregnancy, this does not rule out a potential role for the cGMP signaling pathway in the control of human uterine quiescence or labor. Other factors which operate by stimulating guanylate cyclase may be involved. Indeed, in support of this, myometrial cGMP has been reported to increase during guinea pig pregnancy independent of changes in NO synthesis. 33 The possibility that such mechanisms may occur in human pregnancy requires investigation. Possible candidates for increasing cGMP in myometrium include the natriuretic peptides, such as atrial natriuretic peptide, BNP, and renal synthesized C-type natriuretic peptide, which activate particulate guanylate cyclase by binding to their specific receptors, GCA and GCB. It has recently been reported that in the guinea pig, pregnancy decreased the myometrial expression of mRNA for GCB receptors but not GCA receptors in myometrium. 46 Because atrial natriuretic peptide has been previously been reported to down-regulate the GCA receptor, the authors speculated that down-regulation of GCB receptors may reflect an increased presence of endogenous CNP, which may contribute to uterine quiescence by stimulating cGMP. In the sheep, pregnancy has been shown to be associated with increased soluble and particulate guanylate cyclases and to decrease the clearance receptor of natriuretic peptides in uterine arteries. 47
In conclusion, our results do not suggest that the expression of HO or NOS proteins are increased in pregnancy. They do suggest that the CO/NO pathways are not likely to be involved in uterine quiescence or contractility in labor.
Acknowledgments
The authors are grateful to Dr. Helena Kelly, Mrs. Alison Condie, Dr. Colette Sparey, and Dr. Helen Simpson for help in collection of myometrial tissue and to the British Heart Foundation for financial support.
Footnotes
Address reprint requests to Dr Fiona Lyall, Maternal and Fetal Medicine Section, Institute of Medical Genetics, Yorkhill, Glasgow, G3 8SJ, United Kingdom. E-mail: f.lyall@udcf.gla.ac.uk.
References
- 1.Berkowitz C, Papiernik E: Epidemiology of preterm birth. Epidemiol Rev 1993, 13:414-443 [DOI] [PubMed] [Google Scholar]
- 2.Rush RW, Keirse MLNC, Howart P, Baum JD, Anderson ABM, Turnbull AC: Contribution of preterm delivery to perinatal mortality. Br Med J 1976, 3:965-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J, Ezcurra EJ: Gurtner de la Fuente V, Campodonico L: Preterm delivery syndrome: the unmet need. Res Clin Forums 1994, 16:9-38 [Google Scholar]
- 4.Besinger RE: A systematic review of adverse events documented in the use of currently available treatment of pre-term labour. Res Clin Forums 1994, 16:89-126 [Google Scholar]
- 5.Schneider H: Pharmacological intervention in pre-term labour. Res Clin Forums 1994, 16:59-87 [Google Scholar]
- 6.Sibai BM, Romero R, Sanchez-Ramos L, Valenzuela G, Veille JC, Tabor B, Morrison J, Varner M, Goodwin T, Menard K, Lane R, Smith J, Creasy G: A double-blinded placebo-controlled trial of an oxytocin-receptor antagonist (Antocin) in the treatment of preterm labor. Am J Obstet Gynecol 1997, 176:S2. [DOI] [PubMed] [Google Scholar]
- 7.Lees C, Campbell S, Jauniaux E, Brown R, Ramsay B, Gibb D, Moncada S, Martin JF: Arrest of preterm labour and prolongation of gestation with glycerol trinitrate, a nitric oxide donor. Lancet 1994, 343:1325-1326 [DOI] [PubMed] [Google Scholar]
- 8.Challis JRG, Mitchell MD: The mechanisms of preterm labour. Res Clin Forums 1994, 16:39-58 [Google Scholar]
- 9.Lye SJ: Inititation of parturition. Animal Reprod Sci 1996, 42:495-503 [Google Scholar]
- 10.Maines MD: Carbon monoxide: An emerging regulator of cGMP in the brain. Mol Cell Neurosci 1993, 4:389-397 [DOI] [PubMed] [Google Scholar]
- 11.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH: Carbon monoxide: a putative neural messenger. Science 1993, 259:381-381 [DOI] [PubMed] [Google Scholar]
- 12.Maines MD: Heme oxygenase: function, multiplicity, regulatory mechanisms and clinical applications. FASEB J 1988, 2:2257-2568 [PubMed] [Google Scholar]
- 13.Trakshel GM, Maines MD: Multiplicity of heme oxygenase isoenzymes. HO-1 and HO-2 are different molecular species in rat and rabbit. J Biol Chem 1989, 264:1323-1328 [PubMed] [Google Scholar]
- 14.Cruse I, Maines MD: Evidence suggesting that the two forms of heme oxygenase are the products of different genes. J Biol Chem 1988, 263:2348-3353 [PubMed] [Google Scholar]
- 15.Keyse SM, Tyrell RM: Heme oxygenase is the major 32-kd stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide and sodium arsenite. Proc Natl Acad Sci USA 1989, 86:99-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maines MD, Mayer RD, Ewing JF, McCoubrey WKJ: Induction of kidney heme-oxygenase-1 (HP32) mRNA and protein by ischaemia: possible role of heme as both promoter of tissue damage and regulator of HSP32. J Pharmacol Exp Ther 1993, 264:457-462 [PubMed] [Google Scholar]
- 17.Levere RD, Staudinger R, Loewy G, Kappas A, Shibahara S, Abraham NG: Elevated levels of haem oxygenase-1 activity and mRNA in peripheral blood adherent cells of acquired immunodeficiency syndrome patients. Am J Hematol 1993, 43:19-23 [DOI] [PubMed] [Google Scholar]
- 18.Kutty RK, Maines MD: Selective induction of heme oxygenase-1 isoenzyme in rat testis by human chorioin gonadotrophin. Arch Biochem Biophys 1989, 268:100-107 [DOI] [PubMed] [Google Scholar]
- 19.Shibahara S, Müller RM, Taguchi H: Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem 1987, 262:12889-12892 [PubMed] [Google Scholar]
- 20.Morita T, Perrella MA, Lee ME, Kourembannas S: Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 1995, 92:1475-1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewing JF, Maines MD: Glutathione depletion induces heme-oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem 1993, 60:1512-1519 [DOI] [PubMed] [Google Scholar]
- 22.Mansouri A, Perry CA: Alteration of platelet aggregation by cigarette smoke and carbon monoxide. Thromb-Haemostas (Stuttgart) 1982, 48:286-288 [PubMed] [Google Scholar]
- 23.McFaul SJ, McGrath JJ: Studies on the mechanism of carbon monoxide-induced vasodilatation in the isolated perfused rat heart. Toxicol Appl Pharmacol 1987, 87:464-473 [DOI] [PubMed] [Google Scholar]
- 24.Farrugia G, Irons WA, Rae JL, Sarr MG, Szurszewski JH: Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am J Physiol 1993, 264:G1184-G1189 [DOI] [PubMed] [Google Scholar]
- 25.Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH: Heme-oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci USA 1996, 93:795-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sladek SM, Magness RR, Conrad KP: Nitric oxide and pregnancy. Am J Physiol 1997, 41:R441-R463 [DOI] [PubMed] [Google Scholar]
- 27.Yallampalli C, Izumi H, Byam-Smith M, Garfield RE: An L-arginine-nitric oxide-cyclic guanosine monophospahte system exists in the uterus, and inhibits contractility during pregnancy. Am J Obstet Gynecol 1993, 170:175-185 [DOI] [PubMed] [Google Scholar]
- 28.Yallampalli C, Garfield RE, Byam-Smith M: Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology 1994, 133:1899-1902 [DOI] [PubMed] [Google Scholar]
- 29.Izumi H, Garfield RE: Relaxant effects of nitric oxide and cyclic GMP on pregnant rat uterine longitudinal smooth muscle. Eur J Obstet Gynecol Reprod Biol 1995, 60:171-180 [DOI] [PubMed] [Google Scholar]
- 30.Buhimschi IM, Ali M, Jain V, Chwalisz K, Garfield RE: Differential regulation of nitric oxide in rat uterus and cervix during pregnancy and labour. Hum Reprod 1996, 11:1755-1766 [DOI] [PubMed] [Google Scholar]
- 31.Buhimschi I, Yallampalli C, Dong Y-L, Garfield RE: Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol 1995, 172:1577-1584 [DOI] [PubMed] [Google Scholar]
- 32.Jones GD, Poston L: The role of endogenous nitric oxide synthesis in contractility of term or preterm human myometrium. Br J Obstet Gynaecol 1997, 104:241-345 [DOI] [PubMed] [Google Scholar]
- 33.Weiner CP, Knowles RG, Nelson SE, Stegink LD: Pregnancy increases guanosine 3′,5′-monophosphate in the myometrium independent of nitric oxide synthesis. Endocrinology 1994, 135:2473-2478 [DOI] [PubMed] [Google Scholar]
- 34.Acevedo CH, Ahmed A: Hemoxygenase-1 inhibits human myometrial contractility via carbon monoxide and is upregulated by progesterone during pregnancy. J Clin Invest 1998, 101:949-955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 36.Lyall F, Jablonka-Shariff A, Johnson RD, Olson LM, Nelson DM: Gene expression of nitric oxide synthase in cultured human term placental trophoblast during in vitro differentiation. Placenta 1998, 17:253-260 [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T, Biro P, Cohen T, Muller R, Shibahara S: Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem 1988, 171:457-461 [DOI] [PubMed] [Google Scholar]
- 38.McCoubrey WK, Ewing JF, Maines MD: Human Heme Oxygenase-2: characterization and expression of a full-length cDNA and evidence suggesting that the two HO-2 transcripts may differ by choice of polyadenylation signal. Arch Biochem Biophys 1992, 295:13-20 [DOI] [PubMed] [Google Scholar]
- 39.Bartlett SR, Siow RCM, Mann GE: Induction of heme oxygenase-1 in human myometrial smooth muscle cells exposed to oxidative stress. 1997. SFRR Meeting Padua, Italy
- 40.Campa JS, Poston R, Poston L: Nitirc oxide synthase (eNOS) is localized to the vascular endothelium of myometrium from pregnant at term. J Physiol 1998, 507:69P [Google Scholar]
- 41.Howe DH, Sangha R, Kilby MD, Whittle MJ, Ahmed A: Identification and expression of nitric oxide synthase isoforms in human myometrium before and after the onset of labour. J Soc Gynecol Invest 1996, 3:A326 [Google Scholar]
- 42.Grozdanovic Z, Gossrau R: Expression of heme oxygenase-2 (HO-2) like immunoreactivity in rat tissues. Acta Histochem 1996, 98:203-214 [DOI] [PubMed] [Google Scholar]
- 43.Europe-Finner GN, Phaneuf S, Watson SP, López Bernal A: Identification and expression of G-proteins in human myometrium: upregualation of Gαs in pregnancy. Endocrinology 1993, 132:2484-2490 [DOI] [PubMed] [Google Scholar]
- 44.Europe-Finner GN, Phaneuf S, Tolkovsky AM, Watson SP, López Bernal A: Down-regulation of Gas in human myometrium in term and pre-term labour: a mechanism for parturition. J Clin Endocrinol Metab 1994, 79:1835-1839 [DOI] [PubMed] [Google Scholar]
- 45.Lye SJ, Ou C-W, Teoh T-G, Erb G, Stevens Y, Casper R, Patel FA, Challis JRG: The molecular basis of labour and tocolysis. Fetal Maternal Med Rev 1998, 121–136
- 46.Aguan K, Mozumdar J, Thompson LP, Weiner CP: Pregnancy selectively downregulates GCB receptor but not GCA receptor mRNA in guinea pig myometrium. J Soc Gynecol Invest 1998, 5:186A [Google Scholar]
- 47.Itoh H, Bird IM, Nakao K, Magness RR: Pregnancy increases soluble and particulate gunaylate cyclases and decreases the clearance receptor of the natriuretic peptides in ovine arteries but not the systemic arteries. Endocrinology 1998, 139:3329-3341 [DOI] [PubMed] [Google Scholar]