Abstract
Various in vitro models have been described that emulate one or more of the processes involved in angiogenesis in vivo. In the present study endothelial cells were cultured in three-dimensional type I collagen lattices in the presence of a mixture of basic fibroblast growth factor, vascular endothelial cell growth factor, and phorbol myristate acetate. Under these conditions, the endothelial cells rapidly assemble into an interconnected network of tube-like structures with a high frequency of intercellular canals or lumens. The formation of the networks and lumens was completely blocked by cycloheximide and by actinomycin D. Monoclonal antibodies directed against CD31 or vascular endothelial cadherin (VE-cadherin) inhibited the formation of endothelial tubes. A subtle difference in the morphology of cells treated with anti-CD31 versus anti-VE-cadherin was noted; namely, cells incubated in the presence of CD31 antibodies were rounded or formed attenuated tube-like structures, both of which were characterized by a single, large intra- or intercellular vacuole. In contrast, tube formation by cells incubated in the presence of VE-cadherin antibodies was also impaired and, most notably, demonstrated a reduction in either vacuole formation or vacuole fusion, depending upon the monoclonal antibody used. We suggest that the two endothelial-junction-associated proteins, CD31 and VE-cadherin, play different roles in the process of tube formation. CD31 appears to be required for cell elongation, migration, and/or invasion in the gels as well as for cell-cell association to form the network structures. VE-cadherin also appears to be required for cell-cell association, but additionally appears to play some role in the process of vacuolization or vacuole fusion leading to intercellular lumen formation.
Angiogenesis in vivo can be distinguished by a variety of sequential steps: cell-mediated proteolytic degradation of the basement membrane, migration of the endothelial cells out of the vessel into the surrounding tissue, proliferation of the endothelial cells, and morphogenesis of the cells into tube-like structures.
Various in vitro systems have been used to model the differentiation of endothelial cells into tubular capillary-like sprouts. The mechanism of tube formation is not clearly understood, and the different in vitro models may yield divergent information due to differences in the matrix components, growth factor requirements, and even the source of the endothelial cells used. A commonly used method of in vitro angiogenesis is the spontaneous or growth-factor-induced formation of cord-like structures when endothelial cells are cultured on an extracellular matrix secreted by Engelbroth-Holm-Swarm sarcoma (EHS) cells, commonly known as Matrigel. This model has been used by many investigators to demonstrate roles for various ligands, receptors, and integrins in the angiogenic process, and many of these findings have been verified by in vivo experiments. However, Matrigel-driven tube formation can occur independent of new protein or mRNA synthesis, 1,2 although there are conflicting reports. 3 Studies by Davis et al suggested that the cords that form in the Matrigel system follow linear distortions, so-called matrix guidance pathways that are generated in the matrix by the generation of mechanical tension between endothelial cells. 4 Analysis of the structure of the cords formed also suggests that lumens are not generally found, and in the rare cases in which they have been described, basement membrane material is present on both apical and basal aspects. 5-8 This probably occurs by enfolding of the supportive scaffold by tractional forces, resulting in the tubular shape. 9 The lack of apical:basal polarity as well as the infrequent generation of patent lumens suggests that the Matrigel model may not be a suitable system to probe the molecular events that lead to lumen formation. Additionally, a variety of cell types will form networks on Matrigel, including certain types of epithelial cells, dermal fibroblasts, smooth muscle cells, osteoblasts, keratinocytes, renal tubular cells, and hepatocytes, 9-15 clearly indicating that the formation of networks on Matrigel is not endothelial specific.
Endothelial cells will also differentiate in three-dimensional gels generated from type I collagen or fibrin. A commonly used method is to sandwich the endothelial cells between two layers of the matrix. Soon after the sandwich is formed, the endothelial cells undergo morphogenesis to form a structure resembling a capillary network that is extensive and well formed within 24 hours 16 with infrequent but appropriately polarized luminal structures. Endothelial cells grown on the surface of a collagen gel can be induced to invade upon addition of phorbol myristate acetate (PMA) 8 or antibodies to α2β1 integrins. 17 Under these conditions, the majority of invading cells have vacuole-like structures and multicellular lumens. A modification of the type I collagen gel model incorporates endothelial cells into the collagen before gelation, and after the gel has formed, culture medium containing PMA, basic fibroblast growth factor (bFGF), and vascular endothelial cell growth factor (VEGF) is added. The endothelial cells rapidly differentiate forming tube-like structures with frequent lumens with appropriate apical:basal polarity. 18
In the present communication, we used the “In Gel” version of the type I collagen gel matrix 18 to begin to dissect the molecules and events that lead to the formation of interconnected tubes and lumens. These studies demonstrate the obligatory role for new mRNA and protein synthesis in this differentiation as well as an important and possibly differential role for two endothelial junction proteins, platelet-endothelial adhesion molecule-1 (PECAM-1, CD31) and vascular-endothelial cadherin (VE-cadherin, CD144).
Materials and Methods
Materials
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA) and maintained in EGM media (Clonetics) supplemented to a final concentration of 10% fetal bovine serum (FBS). Type I rat tail collagen was from Upstate Biotechnology (Lake Success, NY) and recombinant bFGF was purchased from Collaborative Biomedical Products (Becton Dickinson Labware, Bedford, MA). Recombinant VEGF was from Genentech (South San Francisco, CA). 10X medium 199 (M199, M0650), PMA, 4,6′-diamino-2-phenylindole dihydrochloride (DAPI), actinomycin D, cycloheximide, phycoerythrin-conjugated goat anti-mouse IgG, and isotype-matched control (IgG1 and IgG2a) were from Sigma Chemical Co. (St. Louis, MO). Antibodies to CD31 were as follows: clone BV10 (Chemicon, Temecula, CA), clone JC/70A (DAKO, Glostrup, Denmark), clone HEC7 (Endogen, Woburn, MA); antibodies to VE-cadherin: clone RM67 (Chemicon) and TEA1/31 (Immunotech, Marseilles, France). Antibody to ICAM-1 (clone P2A4) was purchased from Chemicon. Rhodamine phalloidin was from Molecular Probes (Eugene, OR). FBS was from Hyclone (Logan, UT). ITS (insulin, transferrin, and selenium-A) and trypsin, and were from Gibco-BRL (Gaithersburg, MD).
Formation of Three-Dimensional Collagen Gels
Collagen gels were formed by mixing together ice-cold gelation solution (10X M199, H2O, 0.53 mol/L NaHCO3, 200 mmol/L l-glutamine, type I collagen, 0.1 mol/L NaOH; 100:27.7:50:10:750:62.5 by volume) and cells in 1X basal medium (see below) at a concentration of 3 × 10 6 cells/ml at a ratio of 4 vol of gelation solution:1 vol of cells. The gels were allowed to form by incubation in a CO2-free incubator at 37°C for 30 minutes to 1 hour. The gels were then overlaid with 1X basal medium consisting of M199 supplemented with 1% FBS, 1X ITS, 2 mmol/L l-glutamine, 50 μg/ml ascorbic acid, 26.5 mmol/L NaHCO3, 100 U/ml penicillin, 100 U/ml streptomycin and, unless otherwise noted, 40 ng/ml bFGF, 40 ng/ml VEGF, and 80 nmol/L PMA. In antibody-blocking and drug studies the substances were added to the 1X basal medium. At the end of the experiment the media were removed by gentle aspiration and the gels routinely fixed in 3.7% formalin. Cells were later stained with rhodamine phalloidin (5 U/ml) and DAPI (4 μmol/L final concentration) to visualize the actin cytoskeletons and nuclei, respectively. In some experiments the lumens were loaded with 6-carboxyfluorescein, single isomer (6-CF), by including the dye solution at a concentration of 0.06 mg/ml with the endothelial cells during the formation of tubes and lumens. Free dye was removed by repeated washing of fixed gels with PBS. In some experiments, fixed cells were stained 30 minutes with toluidine blue in Tris buffer (0.1 mol/L Tris/0.15 mol/L NaCl, pH 9.5). The gels were destained in a bath of Tris buffer (2 to 5 hours depending on size and thickness of gels). Gels were removed from the bath, rinsed once with Tris buffer, and then maintained in a PBS solution. Photographs were taken on a Nikon Eclipse TE300 inverted phase microscope using Hoffmann modulation contrast optics and a Polaroid digital microscope camera. Images were captured using Adobe Photoshop and composite photomicrographs prepared using QuarkExpress software.
Quantification of Tube Formation
Gels were examined on a Nikon TE300 microscope equipped with Hoffman modulation optics and a cooled CCD camera (Optronics, Technical Instruments, San Francisco, CA). Assays were performed using 48-well plates with n=3 wells for each experimental manipulation, and all experiments were repeated at least three times. The optimal optical quality of the 48-well chamber is in the center of each well, and this region was selected for image capture. After focusing on the lowest layer of the gel (ie, the bottom), the stage was moved upward 100 units on the coaxial fine focusing knob and the first image captured. Four additional fields, separated by 100-unit increments were imaged, and the data were imported as TIFF files into NIH Image. After calibration with a stage micrometer, a second observer measured the total length of each tube or the long axis of single cells or groups of adjacent cells that were in clear focus in the image field, and the data were imported into a Microsoft Excel spread sheet. In those instances where several tube-like structures merged together or branched, the total length of the tubes was calculated as the sum of the length of the individual branches. The number of tubes (or cells) containing one or more vacuoles/lumens was also collected for each image. The mean length and percentage of cells/tubes containing vacuoles was determined for each well, followed by determination of the mean data and standard deviation for each experimental group. In some experiments, the number of tube-like structures (defined as those exceeding 100 μm in length) per high-power (20× objective) field was also determined. Data were analyzed by one-way analysis of variance and post hoc Bonferonni’s modified t-test for multiple comparisons. P < 0.05 was accepted as significant.
Results
Tube and Lumen Formation
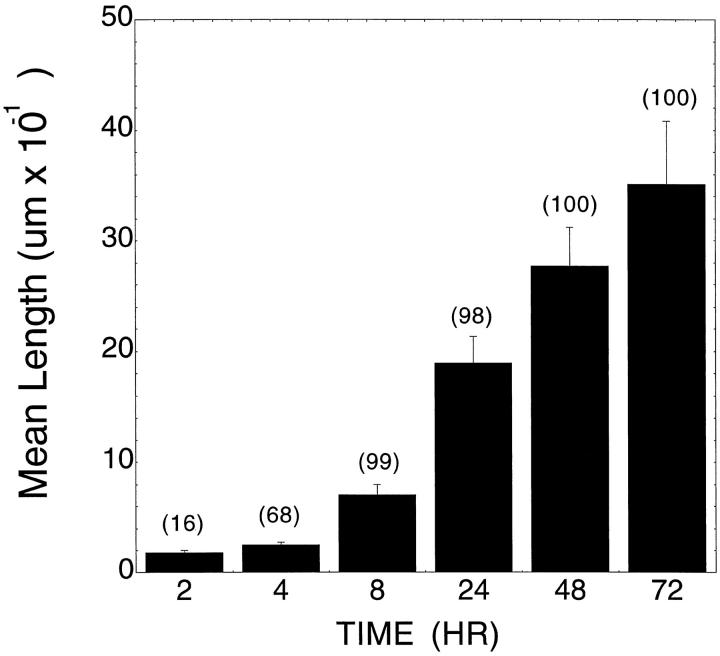
As early as 2 hours, small vacuole-like structures could be observed in select individual endothelial cells (∼16% of the total cell population). The frequency of vacuole formation increased such that at 8 hours, virtually all cells in the image field contained these vacuoles. As early as 4 hours, the smaller vacuoles appeared to fuse to form large vacuole structures. At 48 hours, these structures appeared as interconnecting lumens, formed either by individual cells aligned end to end or, in some cases, by several cells surrounding a single lumen. Although there was some variability from one endothelial cell preparation in the time required to form branching tube-like structures and in the mean length of the tubes at 48 hours, nearly every cell in the gel was associated with a tube-like structure. Those that had not been incorporated into a tube appeared to remain viable and exhibited an elongated morphology and large intracellular vacuoles. The branching structures continued to become more complex over the next 24 hours. However, many preparations of HUVECs appeared to degrade the gels at times approaching or exceeding 72 hours, complicating any longer-term evaluation of tube formation or assessment of possible regression of the tubes. Figure 1, A–I ▶ , shows representative images of the morphology observed at 4, 24, and 48 hours. Figure 2 ▶ provides quantitative analysis of the mean tube length and the percentage of cells or tube-like structures that contained vacuole-like structures.
Figure 1.
Time course of endothelial vacuole and tube formation in collagen gels. Frames A to F: Representative images captured at 4 hours. Note the appearance of small vacuoles. In other cells, smaller vacuoles appear to fuse to form larger vacuoles (A and B), and in other cells, one large intracellular vacuole is observed (C). D: Two endothelial cells with large vacuoles appear to have migrated toward each other and touch. E and F: Identical fields under Hoffmann (E) and DAPI fluorescent optics (F), demonstrating that the structure in E is composed of a single endothelial cell. Arrow, nucleus of endothelial cell. G to J: At 24 hours. G: Apparent fusion of vacuoles from at least three adjacent cells, leading to intercellular canalization, as shown in H. I and J: Identical fields under Hoffman (I) and DAPI fluorescent optics (J), demonstrating that the elongated, vacuolated structure in I is composed a single, elongated endothelial cell. Arrow, nucleus of endothelial cell. K to N: At 48 hours. Long, interconnected tubes are formed with large vacuoles still visible. K and L: Under Hoffman (K) and DAPI fluorescent optics (L), demonstrating that the structure in K is composed of at least four endothelial cells. Arrow, nuclei of endothelial cells.
Figure 2.
Quantification of tube formation versus time. The length of tubes (or, at early time course, the long axis of individual cells) and the frequency (as percentage of total cells or tubes/field; numbers in parentheses over data bars) of vacuoles was determined at 2, 4, 8, 24, 48 and 72 hours of incubation in the gel in the presence of the combination of VEGF, bFGF, and PMA as described in Materials and Methods. Tube length is expressed as the mean ± SD. Data shown are from one representative experiment with n = 3 wells per time point. Five fields/well scored as detailed in Materials and Methods.
The vacuole or lumen-like structures could be loaded with 6-CF if the dye was included in the basal medium (Figure 3, A and B) ▶ . Use of the acetoxy methyl ester forms of 6-CF or other fluorescent dyes did not result in loading of the luminal structures. Acetoxy methyl ester derivatives are membrane permeable and are used to load the cytoplasm of endothelial cells. The acetoxy methyl ester is cleaved by cell esterases and the nonpermeant form of the dye becomes trapped in the cytoplasm. The preferential loading of the free form of the dye is consistent with uptake of the dye by vesicular transport of material from the extracellular space.
Figure 3.
Uptake of 6-carboxyfluorescein (6-CF) by endothelial cells. Endothelial cells were incubated with 0.06 mg/ml 6-CF during the process of tube formation. Excess dye was removed after fixation of the gels with 3.7% formalin by extensive washing with PBS. A: Phase micrograph of endothelial cells cultured in 1X basal media supplemented with VEGF (40 ng/ml), bFGF (40 ng/ml), and PMA (80 nmol/L). B: Same field as A, viewed under fluorescein optics, showing uptake of 6-CF. Note that the dye is concentrated in the large vacuolar structures (arrows).
Tube and Lumen Formation Require Protein and mRNA Synthesis
To determine the role for new protein and mRNA synthesis in the processes of vacuole, tube, and lumen formation, at the time of addition of the basal media supplemented with PMA, bFGF, and VEGF, 1 μg/ml actinomycin D or 10 μg/ml cycloheximide were added to the cultures. Preliminary studies indicated that these concentrations of drugs were sufficient to inhibit >95% of radiolabeled uridine or leucine uptake, respectively. As shown in Figure 4, C and D ▶ , cells co-incubated with either actinomycin D or cycloheximide did not form interconnecting sprouts or observable lumens. Cells treated with actinomycin D or cycloheximide remained round and did not elongate when observed at earlier time points (2, 4, 8, or 24 hrs (not shown)). As shown in Figure 4E ▶ , gels incubated with either actinomycin D or cycloheximide demonstrated a marked reduction in the mean length of the structures (basically the mean long axis of rounded cells), and these effects were statistically significant.
Figure 4.
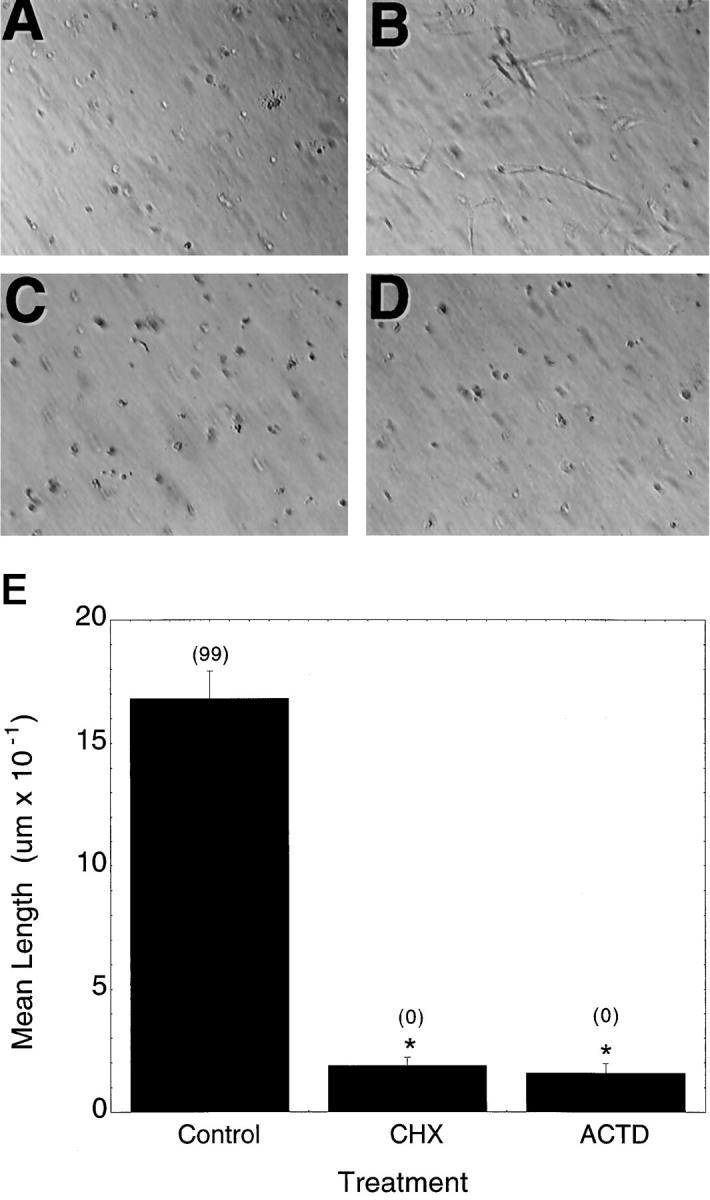
Requirement for new mRNA and protein synthesis. A: Negative control. Endothelial cells were cultured 48 hours in collagen gel in the absence of exogenous growth factors. B: Positive control. Endothelial cells were cultured 48 hours in collagen gel in the presence of 40 ng/ml bFGF, 40 ng/ml VEGF, and 80 nmol/L PMA. C: Cells cultured under conditions as in B in the presence of 1 μg/ml actinomycin D. D: Cells cultured under conditions as in B in the presence of 10 μg/ml cycloheximide. E: Quantitative analysis of the effects of cycloheximide and actinomycin D. The length of tubes (or, at early time points, the long axis of individual cells) and the frequency (as percentage of total cells or tubes/field; numbers in parentheses over data bars) of vacuoles was determined after 48 hours of incubation in the gel in the presence of bFGF, VEGF, or PMA without (control) or with the indicated inhibitor. Tube length is expressed as the mean ± SD. Data shown are from one representative experiment with n = 3 wells per time point. Five fields/well were scored as detailed in Materials and Methods. The frequency of vacuoles or lumens is expressed as the percentage of total cells or tubes per high-power field and is given in parentheses above the data bars. *Significantly different from control, P < 0.05.
Roles for CD31 and VE-Cadherin in Network Formation
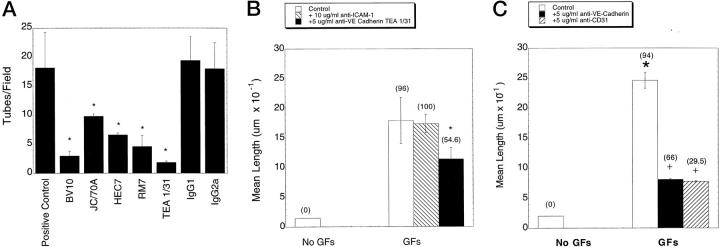
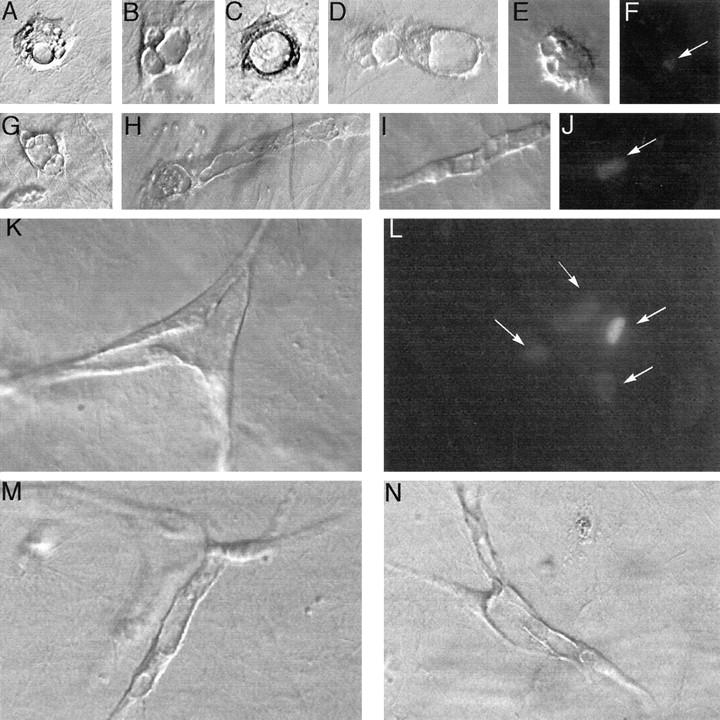
CD31 and VE-cadherin have been implicated in angiogenesis in vitro using several different models. 19-22 We therefore evaluated the respective roles of these matrix and junctional adherence 23 molecules in the three-dimensional collagen gel model. A variety of antibodies for each molecule were acquired from commercial sources as detailed in Materials and Methods. As shown in Figure 5A ▶ , CD31 and VE-cadherin antibodies significantly decreased the number of tube structures (defined as contiguous structures exceeding 100 μm in length) per high-power field in the three-dimensional collagen gel. The corresponding isotype-matched nonimmune control antibodies had no effect on endothelial tube formation. Additionally, an endothelial binding antibody (anti-ICAM-1) also failed to inhibit tube formation (Figure 5B) ▶ . Some interesting morphological differences, in comparison with controls, were found upon high-power examination of the structures formed during incubation with the different antibodies. All of the CD31 antibodies tested attenuated the elongation of the cells into tube-like structures (Figure 5 ▶ and Figure 6, C–F ▶ ). Many of the cells were isolated and had a large intracellular vacuole (Figure 6, E and F) ▶ . Others tended to aggregate and form attenuated (ie, shorter) tube-like structures with a large lumen (Figure 6, C and D) ▶ . Both morphologies were observed with all three anti-CD31 antibodies used, ie, BV10, JC/70A, and HEC7. In contrast, cells incubated with the anti-cadherin antibody TEA 1/31 tended to elongate (although the average length of the tubes formed was significantly shorter than those observed in the absence of antibody, Figure 5B ▶ ) and to not associate with adjacent cells, and furthermore, nearly one-half of these elongated structures were devoid of vacuoles (Figure 6, I and J) ▶ , whereas others had only small attenuated vacuoles usually found at one end of the elongated structure (Figure 6, G and H) ▶ . However, cells incubated in the presence of the anti-cadherin antibody RM67 demonstrated a reduction in mean length and in the number of capillary-like structures (Figure 5, A and C) ▶ , but the morphology was distinct (Figure 6, K–N) ▶ ; ie, cells were somewhat elongated, occasionally associated with adjacent cells, and the vacuoles of these adjacent cells often were not fused (Figure 6, K and L) ▶ . In other instances isolated single cells could be visualized with multiple, nonfused vacuoles (Figure 6, M and N) ▶ .
Figure 5.
Effects of various antibodies on endothelial cell tube formation in three-dimensional collagen gels. HUVECs were resuspended in collagen gels and either left untreated or treated with 5 μg/ml of the indicated antibody. The positive control group was cultured in basal media/VEGF/bFGF/PMA. The negative controls were cultured in 1X basal media without growth factors. A: Antibodies used were clones BV10, JC/70A, and HEC7 directed against CD31 and clones RM67 and TEA 1/31 directed against VE-cadherin. Also shown are results obtained with nonimmune IgG1 and IgG2a isotype-matched control antibodies. Data are expressed as the mean ± SEM number of tube-like structures (exceeding 100 μm in length) per high-power field. *Significantly different from positive control, P < 0.05. B: Morphometric analysis of the effects of anti-ICAM-1 and anti-VE-Cadherin (TEA 1/31) on tube formation. C: Morphometric analysis of the effects of the anti-CD31 antibody (BV10) and anti-VE-cadherin antibody (RM67). In B and C, tube length is expressed as the mean ± SD. Data shown are from one representative experiment with n = 3 wells per time point. Five fields/well were scored as detailed in Materials and Methods. The frequency of vacuoles or lumens is expressed as the percentage of total cells or tubes per high-power field and is given in parentheses above the data bars. *Tube length significantly different from growth factor (VEGF, 40 ng/ml; bFGF, 40 ng/ml; and PMA, 80 nmol/L) treated control group.
Figure 6.
Morphology of endothelial cells in the presence of antibodies to endothelial junctional molecules. HUVECs were resuspended in collagen gels as described in Materials and Methods. Antibodies (at 5 μg/ml final concentration) were added along with 1X basal media containing bFGF, VEGF, and PMA, and the cells were incubated for 48 hours. A: Phase micrograph of positive control cells cultured in 1X basal media supplemented with VEGF (40 ng/ml), bFGF (40 ng/ml), and PMA (80 nmol/L). B: Same field as A, viewed under ultraviolet optics, showing DAPI-stained nuclei. Note the contiguous lumen in A is formed by multiple endothelial cells. C and E: Phase micrograph of endothelial cells cultured in media as in A in the presence of anti-CD31 antibody (BV10; 5 μg/ml). D and F: Same fields as C and E, respectively, viewed under ultraviolet optics, showing DAPI-stained nuclei. Note that the large vacuole observed in C is formed by the non-elongated aggregate of two individual cells, whereas the large vacuole observed in E is formed by a single rounded cell. G and I: Phase micrographs of endothelial cells incubated as in A in the presence of anti-VE-cadherin (TEA 1/31) antibody (5 μg/ml). H and J: Same fields as G and I, viewed under ultraviolet optics, showing DAPI-stained nuclei. Note absence of vacuoles in I, the small attenuated vacuole in G, and that the single elongated structures observed in both G and I are composed of single cells. K and M: Phase micrograph of cells incubated as in A in the presence of anti-VE-cadherin (RM67) antibody (5 μg/ml). L and N: Same fields as K and M viewed under ultraviolet optics. Note the presence of numerous nonfused vacuoles in the attenuated tube formed of adjacent cells (K, arrows) and nonfused, enlarged vacuoles in an isolated endothelial cell (M, arrows). All photographs are shown at the same magnification. Size bar in A, 100 μm.
Discussion
This study uses a modified model of vascular endothelial cell differentiation initially described by Davis and colleagues. 18 In this model, endothelial cells suspended in a three-dimensional gel composed of type I collagen must complete a variety of processes to progress to the final three-dimensional network that is observed. The cells require survival signals to undergo this differentiation. Cells cultured in the absence of PMA, VEGF, and bFGF rapidly exhibited the characteristics of apoptosis, ie, nuclear condensation, blebbing, and disruption of the cytoskeleton. The requirement for survival signals in three-dimensional collagen lattices is in agreement with other reports employing similar in vitro models. 24,25 The survival and downstream differentiation of the cells into tubes and lumens is dependent on mRNA and protein synthesis, indicating that this model provides a useful in vitro system to dissect the molecular events required for this differentiation. Finally, our results suggest that the endothelial-junction-associated molecules, CD31 and VE-cadherin, play important roles in this differentiation process. Curiously, blocking antibodies to CD31 reduced the elongation but did not inhibit the process of vacuole formation, whereas blocking antibodies to VE-cadherin impaired vacuole formation or fusion as well as reduced frequency and mean length of tube-like structures.
Initial observations by time-lapse photography during the first 3 hours after HUVECs were suspended in the three-dimensional gels indicated that the endothelial cells displayed rapid, random extensions of fine pseudopodia that eventually became longer (data not shown). At the same time, the cells were forming numerous vacuoles that were merging to form large intracellular vacuoles. In the presence of CD31 antibodies, cell elongation, and apparently cell migration (as the cells appeared isolated from one another) were blocked, but the vacuolization process did not appear to be impaired. Engagement of CD31 has been reported to activate β1 and β2 integrins in leukocytes and platelets 26-28 and could potentially modulate endothelial integrins. CD31 has been shown to interact with integrin αvβ3, 29 an integrin with a demonstrated role in angiogenesis in vivo. 30 Thus, CD31 may play a role in the invasion, migration, and/or extensions of pseudopodia through the collagen matrix.
Our results differ from an earlier report by Bach et al, 19 which suggested that VE-cadherin, but not CD31, played a role in tube formation in collagen and fibrin gels. However, the model used in the study by Bach and co-workers employed the sandwich methodology as discussed in the introduction. In the sandwich model, endothelial junctions are already formed before addition of the angiogenic stimulus. In contrast, in the three-dimensional suspension model used in the present study, endothelial cells must find each other and link up to form tubes and lumen structures. Thus, CD31/CD31 interactions might be required for the initial formation but not continued maintenance of endothelial cell-cell associations. Consistent with this hypothesis are the observations by Albelda et al 31 and Fawcett et al, 32 which demonstrated that endothelial cells seeded to culture dishes and grown in the presence of CD31 antibodies fail to establish tight cell contacts whereas addition of the CD31 antibodies to intact monolayers did not inhibit cell-cell contacts or disrupt the cobblestone appearance. Importantly, antibodies directed against CD31 block cytokine-induced corneal neovascularization, indicating that inhibitory effects in the three-dimensional collagen gel model may be predictive of in vivo activity. 33
In the presence of VE-cadherin antibodies individual endothelial cells were able to elongate. However, the two VE-cadherin antibodies used resulted in different morphologies, although both morphologies were consistent with an effect on vacuole formation. Cells incubated with the VE-cadherin antibody TEA 1/31 were able to lengthen but remained isolated from adjacent cells. Additionally, nearly one-half of the cells examined did not form visible vacuoles. In contrast, cells incubated the VE-cadherin antibody RM67 also elongated (although shorter than the otherwise untreated PMA plus bFGF plus VEGF group) but contained large vacuoles that apparently had not fused. Additionally, there were also examples of aggregates of three of more cells that exhibited closely apposed, but not fused, vacuoles. These observations support the possibility that the two monoclonal antibodies to VE-cadherin recognize different epitopes (epitope information was not available from the manufacturer) and further suggest that different extracellular domains of VE-cadherin might have different functions in the molecular events involved in tube formation in vitro. Endothelial vacuolization is thought to occur via pinocytosis. 18 Consistent with this hypothesis was our observation that addition of free 6-carboxyfluorescein, but not the acetoxymethyl ester, resulted in dye accumulation in the intracellular vacuoles. It is possible that VE-cadherin is involved in some manner in the process of vacuole fusion to form large vacuoles. Cadherins have been previously reported to occur in endocytic membrane vesicles of bovine endothelial cells undergoing junctional remodeling. 34 To date, there are no reports of in vivo efficacy (or lack thereof) of anti-VE-cadherin antibodies on angiogenesis.
The mechanism of vascular lumen formation is not well defined, and currently there are two different concepts about how vascular lumens form, and one, or both of them, may be correct. Lumens could form by the coordinated migration of several endothelial cells that maintain their polarity and junctional contacts. However, the idea that intracellular vacuolization may generate lumens dates back to early studies by several groups 35,36 that described angiogenic endothelial cells as highly vacuolated. Thus, previous intracellular vacuolization of the endothelial cytoplasm of adjacent endothelial cells could lead to the generation of an intercellular canal by connection of the vacuoles. More recent studies clearly demonstrate a role for VEGF in the formation of vesicular vacuolar organelles, 37,38 suggesting that vacuole formation may be a common feature of VEGF-dependent angiogenesis.
In summary, the present study has further characterized a useful new model of angiogenesis. In this model, endothelial cells undergo tube formation and lumen formation over a short time frame. This process is dependent on new mRNA and protein synthesis, thus offering the opportunity to explore, in a coordinated and time-dependent manner, the molecular events that lead to this differentiation.
Acknowledgments
We are grateful to George Davis (Texas A and M University, College Station, TX) for helpful discussions in establishment of the collagen gel model. We gratefully acknowledge the assistance of Chris Jung and Dr. Franklin Peale (Genentech) for assistance in the staining of the collagen gels.
Footnotes
Address reprint requests to Dr. Mary E. Gerritsen, Department of Cardiovascular Research, MS 42, Genentech Inc., 1 DNA Way, South San Francisco, CA. E-mail: meg@gene.com.
S. Yang and J. Graham contributed equally to this work.
References
- 1.Zimrin AB, Villeponteau B, Maciag T: Models of in vitro angiogenesis: endothelial cell differentiation on fibrin but not matrigel is transcriptionally dependent. Biochem Biophys Res Commun 1995, 213:630-638 [DOI] [PubMed] [Google Scholar]
- 2.Kubota Y, Mizoguchi M: Modulation of morphological differentiation of human endothelial cells in culture by the synthetic peptide YIGSR and cytochalasin B. Clin Exp Dermatol 1993, 18:236-240 [DOI] [PubMed] [Google Scholar]
- 3.Grant DS, Lelkes PI, Fukuda K, Kleinman HK: Intracellular mechanisms involved in basement membrane induced blood vessel differentiation in vitro. In Vitro Cell Dev Biol 1991, 27A:327-336 [DOI] [PubMed] [Google Scholar]
- 4.Davis GE, Camarillo CW: Regulation of endothelial cell morphogenesis by integrins, mechanical forces and matrix guidance pathways. Exp Cell Res 1995, 216:113-123 [DOI] [PubMed] [Google Scholar]
- 5.Bikfalvi A, Cramer EM, Tenza D, Tobelem G: Phenotypic modulations of human umbilical vein endothelial cells and human dermal fibroblasts using two angiogenic assays. Biol Cell 1991, 72:275-278 [DOI] [PubMed] [Google Scholar]
- 6.Nicosia RF, Ottinetti A: Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol 1 26:119–218 [DOI] [PubMed]
- 7.Sage H, Vernon RB: Regulation of angiogenesis by extracellular matrix: the growth and the glue. J Hypertension 1994, 12 (Suppl 10):S145-S152 [PubMed] [Google Scholar]
- 8.Folkman J, Haudenschild C: Angiogenesis in vitro. Nature 1980, 288:551-556 [DOI] [PubMed] [Google Scholar]
- 9.Vernon RB, Sage H: Between molecules and morphology: extracellular matrix and the creation of vascular form. Am J Pathol 1995, 147:873-883 [PMC free article] [PubMed] [Google Scholar]
- 10.Vernon RB, Lane TF, Angello JC, Sage H: Adhesion, shape, proliferation and gene expression of mouse Leydig cells are influenced by extracellular matrix in vitro. Biol Rep 1991, 44:157-170 [DOI] [PubMed] [Google Scholar]
- 11.Kennedy A, Frank RN, Sotolongo LB, Das A, Zhang NL: Proliferative response and macromolecular synthesis by ocular cells cultured on extracellular matrix material. Curr Eye Res 1990, 9:157-170 [DOI] [PubMed] [Google Scholar]
- 12.Vukicevics S, Luyten FP, Kleinman HK, Reddi AH: Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell 1990, 63:437-445 [DOI] [PubMed] [Google Scholar]
- 13.Taub M, Wang Y, Szczesny TM, Kleinman HK: Epidermal growth factor or transforming growth factor α is required for kidney tubulogenesis in matrigel cultures in serum free medium. Proc Natl Acad Sci USA 1990, 87:4002-4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blomme EA, Weckmann MT, Capen CC, Rosol TJ: Influence of extracellular matrix macromolecules on normal human keratinocyte phenotype and parathyroid hormone-related protein secretion and expression in vitro. Exp Cell Res 1998, 238:204-215 [DOI] [PubMed] [Google Scholar]
- 15.Montesano R, Soriano JV, Fialka I, Orci L: Isolation of EpH4 mammary epithelial cell subpopulations which differ in their morphogenetic properties. In Vitro Cell Dev Biol Anim 1998, 34:468-477 [DOI] [PubMed] [Google Scholar]
- 16.Montesano R, Orci L, Vassalli JD: In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol 1983, 1983:1648-1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamble JR, Matthias LJ, Meyer G, Kaur P, Russ G, Faull R, Berndt MC, Vadas MA: Regulation of in vitro capillary tube formation by anti-integrin antibodies. J Cell Biol 1993, 121:931-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis GE, Camarillo CW: An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res 1996, 224:39-51 [DOI] [PubMed] [Google Scholar]
- 19.Bach TL, Barsigian C, Chalupowicz DG, Busler D, Yaen CH, Grant DS, Martinez J: VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res 1998, 238:324-334 [DOI] [PubMed] [Google Scholar]
- 20.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W: Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 1998, 111:1853-1865 [DOI] [PubMed] [Google Scholar]
- 21.Sheibani N, Frazier WA: Down-regulation of platelet endothelial cell adhesion molecule-1 results in thrombospondin-1 expression and concerted regulation of endothelial cell phenotype. Mol Biol Cell 1998, 9:701-713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumura T, Wolff K, Petzelbauer: Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol 1997, 158:3408-3416 [PubMed] [Google Scholar]
- 23.Lampugnani MG, Dejana E: Interendothelial junctions: structure, signalling and functional roles. Curr Opin Cell Biol 1997, 9:674-682 [DOI] [PubMed] [Google Scholar]
- 24.Satake S, Kuziua M, Ramos MA, Kanda S, Igushi A: Angiogenic stimuli are essential for survival of vascular endothelial cells in three dimensional collagen lattice. Biochem Biophys Res Commun 1998, 244:642-646 [DOI] [PubMed] [Google Scholar]
- 25.Ilan N, Mahooti S, Madri JA: District signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J Cell Sci 1998, 111:3621-3631 [DOI] [PubMed] [Google Scholar]
- 26.Piali L, Albelda SM, Baldwin HS, Hammel P, Gisler RH, Imhof BA: Murine platelet endothelial cell adhesion molecule (PECAM-1)/CD31 modulates β2 integrins on lymphokine-activated endothelial cells. Eur J Immunol 1993, 23:2464-2471 [DOI] [PubMed] [Google Scholar]
- 27.Berman ME, Muller WA: Ligation of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) on monocytes and neutrophils increases the binding capacity of leukocyte CR3 (CD11b/CD18). J Immunol 1995, 154:299-307 [PubMed] [Google Scholar]
- 28.Varon D, Jackson DE, Shenkman B, Dardik R, Tamarin I, Savion N, Newman PJ: Platelet/endothelial cell adhesion molecule-1 serves as a costimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood 1998, 91:500-507 [PubMed] [Google Scholar]
- 29.Piali L, Hemmel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA: CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol 1995, 130:451-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 31.Albelda SM, Oliver PD, Romer LH, Buck CA: EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol 1990, 110:1227-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fawcett J, Buckley C, Holness CL, Bird IN, Spragg JH, Saunders J, Harris A, Simmons DL: Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial contacts. J Cell Biol 1995, 128:1229-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM: Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 1997, 151:671-677 [PMC free article] [PubMed] [Google Scholar]
- 34.Kartenbeck J, Schmelz M, Franke WW, Geiger B: Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J Cell Biol 1991, 113:881-892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabin F: Studies on the origin of the blood vessels and of red blood corpuscles as seen in the living blastoderm of chick during the second day of incubation. Contr Embryol 1920, 9:215-262 [Google Scholar]
- 36.Furusato M, Fukunaga M, Kikuchi Y, Yokota S, Joh K, Aizawa S: Two- and three-dimensional ultrastructural observation of angiogenesis in juvenile hemangioma. Virchows Arch Cell Pathol 1984, 46:229-237 [DOI] [PubMed] [Google Scholar]
- 37.Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF: The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol 1996, 59:100-115 [PubMed] [Google Scholar]
- 38.Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM: Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med 1996, 183:1981-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]