Abstract
Besides its proinflammatory properties, interleukin-8 (IL-8) has been suggested as an important promoter for melanoma growth. To study the role of IL-8 in melanoma biology, we determined the in vivo expression of IL-8 mRNA by in situ hybridization in primary melanoma lesions and metastases. High levels of melanoma cell-associated IL-8-specific transcripts were exclusively detected in close vicinity of necrotic/hypoxic areas of melanoma metastases, whereas both in primary melanomas and in non-necrotic metastases IL-8 expression was low or absent. To analyze further the up-regulation of IL-8 mRNA expression in necrotic/hypoxic tumor areas, human melanoma cell lines of different aggressiveness exposed to severe hypoxic stress (anoxia) were used as an in vitro model. Anoxia induced IL-8 mRNA and protein expression in the highly aggressive/metastatic cell lines MV3 and BLM but not in the low aggressive cell lines IF6 and 530. As shown by IL-8 promoter-dependent reporter gene analysis and mRNA stability assays, elevated mRNA levels in melanoma cells were due to both enhanced transcriptional activation and enhanced IL-8 mRNA stability. Interestingly, transcriptional activation was abolished by mutations in the AP-1 and the NF-κB-like binding motifs, indicating that both sites are critical for IL-8 induction. Concomitantly, anoxia induced an enhanced binding activity of AP-1 and NF-κB transcription factors only in the highly aggressive cells. From our in vitro and in vivo data we suggest that anoxia-induced regulation of IL-8 might be a characteristic feature of aggressive tumor cells, thus indicating that IL-8 might play a critical role for tumor progression in human malignant melanoma.
Human malignant melanoma is a highly metastatic tumor of increasing incidence, poor prognosis, and high resistance to treatment, especially when it is metastasized. Factors that influence local growth and metastasis of malignant melanoma as well as of other aggressive tumors are poorly defined. There is considerable evidence that local tumor growth and metastasis are dependent on neovascularization, a process called angiogenesis. 1,2 The induction of new capillary blood vessels promotes local tumor growth and facilitates tumor cell invasion into the circulation and further spreading of the tumor. 3 It has been shown, especially for malignant melanoma, that local tumor progression and prognosis correlate with vessel density. 4-8 Neovascularization is most likely due to the production of angiogenic factors. A local decrease in oxygen tension and nutrient supply due to inadequate vasculature has been considered as a major cause for the induction of these factors. 9,10
Interleukin-8 (IL-8), which belongs to the superfamily of CXC chemokines, has a wide range of proinflammatory effects and was initially described as a neutrophil chemoattractant. 11,12 IL-8 is produced by a wide range of cells, including lymphocytes, monocytes, endothelial cells, fibroblasts, hepatocytes, and keratinocytes. 13 More recently, in vitro data also demonstrated IL-8 production by melanoma cells. 14 In addition, there is accumulating evidence that IL-8 plays a key role in melanoma progression and metastasis as patients with melanoma metastases have elevated serum levels of IL-8. 15 IL-8 production by melanoma cells directly correlated with their metastatic potential in nude mice as well as in humans. 16,17 This has been primarily explained by its properties to act as an autocrine growth factor for melanoma cells 14 and to induce haptotactic migration. 18 More recently, it has been shown that IL-8 also exhibits potent angiogenic activities both in vitro and in vivo. 19-21
As hypoxia is a major stimulus for the expression of angiogenic factors, it is tempting to speculate whether oxygen deficiency may also induce IL-8 expression in malignant melanoma and thereby influence tumor aggressiveness via stimulation of melanoma cell proliferation and stimulation of angiogenesis. In addition, detailed molecular data on the production and transcriptional regulation of IL-8 in melanoma under hypoxia are still missing. Therefore, we applied in situ hybridization for IL-8 mRNA expression and immunohistochemistry for IL-8 receptor expression in primary cutaneous melanomas and melanoma metastases. Furthermore, we studied the regulation of IL-8 production in highly as well as low aggressive human melanoma cell lines under anoxic culture conditions. In the present report we demonstrate that in vivo IL-8 is highly expressed by melanoma cells in close proximity to necrotic/hypoxic tumor areas, whereas the corresponding IL-8-specific receptor A is concomitantly expressed both on melanoma cells and supplying blood vessels. We provide evidence that tumor cell aggressiveness depends on hypoxia-induced IL-8 production which is regulated by the transcription factors AP-1 and NF-κB.
Materials and Methods
In Situ Hybridization of Tissue Specimens
Formalin-fixed sections (5 μm thickness) of 10 primary cutaneous melanomas of intermediate thickness (1 to 3 mm, Clark level III-IV) and 20 cutaneous metastases from different patients were investigated for in situ mRNA expression. We chose 10 metastases with a histopathologically detected central necrotic, and thereby presumably hypoxic, area and 10 metastases with no apparent necrosis.
Labeling of mRNA Sense and Anti-Sense Probe
The IL-8 cDNA probe (bp 137-1350) was kindly provided by C. Weissmann, University of Zürich, Zürich, Switzerland. The specificity of the IL-8 cDNA probe has been previously been shown. 22 Subcloning of the specific cDNA fragment in a plasmid with T3/T7 (Bluescript SK/KS, Stratagene, La Jolla, CA) initiation sites was done according to standard protocols. In vitro transcription of sense and anti-sense probes was performed as described elsewhere. 23 Briefly, plasmid DNA was linearized and 35S-labeled sense and anti-sense probes were obtained by in vitro transcription using T3 and T7 polymerases (Boehringer Mannheim, Mannheim, Germany) together with [35S]uridine triphosphate (Amersham, Braunschweig, Germany) as substrate. The original cDNA template was eliminated with DNase (Pharmacia, Uppsala, Sweden). To facilitate the intracellular accessibility of labeled probes, alkaline hydrolysis was performed to get an average length of 50 to 150 bp. The radioactive IL-8 sense and anti-sense probes were adjusted to a specific activity of 2 × 10 6 cpm/ml in 0.01 mol/L Tris-HCl, pH 7.5, containing 1 mmol/L EDTA.
Hybridization Procedure
Deparaffinized tissue specimens were treated with 10 μg of proteinase K per ml (Boehringer Mannheim) for 30 minutes at 37°C. Afterward, specimens were refixed with 4% paraformaldehyde, acetylated, dehydrated in graded ethanol, and air-dried. Then, slides were overlaid with 20 μl of hybridization solution (50% formamide, 300 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 8.0, 5 mmol/L ethylenediamine tetraacetic acid, 1× Denhardt’s solution, 10% dextran sulfate, 100 mmol/L dithiothreitol, and 2 × 10 5 cpm/μl of heat-denaturated radioactive anti-sense, and for control purposes, sense probe. The slides were coverslipped, sealed, and hybridized at 46°C for 16 hours. Ribonuclease treatment was included as a control and consistently abrogated specific hybridization signals. After removing the coverslips, the nonhybridized RNA probe was removed by incubation with high stringent washing solutions (50% formamide, 2× saline-sodium citrate, and 5 mmol/L ethylenediamine tetraacetic acid at 54–57°C) under constant stirring. To further minimize nonspecific background, noncomplementary unhybridized single-stranded probe was digested with ribonuclease A (20 μl/ml) and ribonuclease T1 (1 unit/ml) (Boehringer Mannheim) for 30 minutes at 37°C. For autoradiography, slides were dipped in NTB-2 Kodak solution (1:2 in 800 mmol/L ammonium acetate) and exposed for 5 weeks at 4°C.
Immunohistochemistry
Formalin-fixed sections (5 μm thickness) of the 10 central necrotic cutaneous metastases were incubated with two monoclonal antibodies raised against CXCR1 and CXCR2 (IL-8 receptors R1 and R2) (PharMingen, Hamburg, Germany; Biozol, München, Germany). Microwaving was used to optimize antigenicity. A biotinylated sheep anti-mouse IgG (Amersham) was used as secondary antibody at a dilution of 1:200. For detection of antibody binding, a streptavidin-peroxidase-complex (streptABC-peroxidase, Dako, Hamburg, Germany) was applied in accordance to the manufacturer’s instructions. Serial sections were stained with monoclonal antibodies against CD68 (expressed on macrophages), against neutrophil elastase (specific for neutrophils), and against the CD3 antigen (T-cell-specific) (Dako, Hamburg, Germany). The reaction was visualized using 0.2 mg/ml 3-amino-9-ethylcarbazole (AEC) as substrate. Sections were counterstained with hematoxylin.
Cell Lines and Culture Conditions
The four human melanoma cell lines MV3, BLM (highly metastatic in nude mice 24 ), IF6, and 530 (low metastatic) were kindly provided by G. N. P. van Muijen, Department of Pathology, University of Nijmegen, The Netherlands, and maintained in RPMI 1640 medium (Linaris, Bettingen, Germany), supplemented with 10% fetal calf serum (FCS, Linaris), 2 mmol/L L-glutamine, 100 units/ml penicillin/streptomycin and 1% nonessential amino acids. Cells were cultured in a humidified incubator (37°C in 5% CO2, 95% air) and passaged when confluent. Before anoxic stimulation cells were kept under low serum conditions (1% FCS) for at least 16 hours. For anoxic treatment cultures were put in an anaerobic culture chamber (Anaerocult A, Merck, Darmstadt, Germany) for different time periods.
Cytokine Enzyme-Linked Immunosorbent Assay
Cell-free supernatants were harvested at the appropriate times and stored at −70°C before analysis. A commercial sandwich enzyme-linked immunosorbent assay (ELISA) kit for human IL-8 (Quantikine, R&D Systems, Bad Nauheim, Germany) was used and assays were performed according to the manufacturer’s specifications.
RNA Extraction from in Vitro Cell Cultures and Northern Blot Analysis
Total cytoplasmic RNA was isolated using the total RNeasy kit (Qiagen, Hilden, Germany). RNA concentration was determined spectrophotometrically at 260 nm. Twenty micrograms of RNA/sample were denatured in 50% formamide in gel running buffer (0.1 mol/L MOPS 3-(N-morpholino)propanesulfonic acid, pH 7.0, 40 mmol/L sodium acetate, 5 mmol/L EDTA, pH 8.0) for 15 minutes at 65°C, fractionated on a 1% agarose gel in formaldehyde buffer and subsequently transferred to a nylon membrane (Hybond N+, Amersham) in 20× SSC. As a probe for IL-8 mRNA, a 402-bp BamHI/SacI IL-8 cDNA fragment was used. For Northern hybridization the purified fragment was labeled to high specific activity with [32P]dATP using a random primer labeling system (Boehringer Mannheim). Membranes were cross-linked by ultraviolet light irradiation and prehybridized in a dextran sulfate buffer (100 g/L dextran sulfate, 0.6 mol/L NaCl, 0.2 mol/L Na2HPO4, 6 mmol/L EDTA, 1.75% lauroylsarcosinate, 50 μg/ml salmon sperm DNA, pH 6.2) for 1 hour at 65°C. Hybridization was carried out in the same prehybridization solution containing 5 × 10 6 cpm/ml of labeled probe. After hybridization for 16 hours at 65°C and 6 hours at 60°C, membranes were washed twice with 2× SSPE/0.1% sodium dodecyl sulfate (SDS) at room temperature, once with 1× SSPE/0.1% SDS at 60°C, and once again with 0.2× SSPE/0.1% SDS. Membranes were then exposed to Hyperfilm (Amersham) with intensifying screens at −80°C for 3 days. IL-8 mRNA loading differences were corrected by the amount of GAPDH mRNA.
mRNA Stability Assay
The half-life of IL-8 mRNA was determined by treating MV3 and BLM melanoma cells with actinomycin D (Sigma, Deisenhofen, Germany) as described by Lindholm et al 25 and Ikeda et al. 26 In short, MV3 and BLM cells were grown under standard normoxic conditions and under anoxia for 15 hours, respectively. After that actinomycin D (10 μg/ml) was added to the culture media to inhibit transcription. Cells were harvested at different time points (O, 1, 2, 4, 6, and 8 hours), total RNA was prepared and Northern analysis performed as described above. The relative amounts of IL-8 mRNA and GAPDH mRNA at each time point were quantified using a phosphoimager (Fujix Bas-2000, Fuji). IL-8 mRNA loading differences were corrected by the amount of GAPDH mRNA.
Transfection and Luciferase Assay
Firefly luciferase expression vectors harboring the 5′ flanking region −133 to +44 bp of the IL-8 gene were the same as described elsewhere. 27 They carry the AP-1 (−126 to −120), NF-IL-6 (−94 to −81) and NF-κB-like (−80 to −71) binding sites. The following constructs were used: wild-type −133-luc, mutant AP-1 (AP-1 mut-luc (TatCTCA)), mutant NF-κB (NF-κB mut-luc (taAcTTTCCTC)). Melanoma cell lines MV3 and 530 were transfected with 2 μg of appropriate plasmid DNA using the DMRIE-C (1,2-dimyristyloxypropyl-3-dimethyl-hydroxyethyl ammonium bromide) reagent (Gibco, Life Technologies, Eggenstein, Germany) according to the manufacturer’s specifications. Briefly, cells were kept under serum-free medium for at least 16 hours. 2 μg of plasmid DNA and DMRIE-C reagent, respectively, were diluted in 500 μl of OptiMEM (Gibco). Both mixtures were put together and incubated at room temperature for 30 minutes. Culture medium was removed and the lipid-DNA complex was overlaid onto cells and incubated overnight. Subsequently, medium was replaced by RPMI medium (10% FCS) and after additional 24 hours changed to low serum conditions (1% FCS) for further 24 hours. Subsequently, cells were exposed to anoxic conditions for 12 hours. As a positive control melanoma cells were stimulated with 10 ng/ml = 12-o-tetradecanoylphorbol-13-acetate (TPA) for 12 hours (Sigma, Deisenhofen, Germany). For luciferase assays, total cell extracts were prepared. Briefly, cells were harvested in 100 μl of lysis buffer (50 mmol/L NaMES, pH 7.8, 50 mmol/L Tris-HCl, pH 7.8, 10 mmol/L dithiothreitol, 2% Triton X-100). The crude cell lysates were cleared by centrifugation and 50 μl of cleared cell extracts were added to 50 μl of luciferase assay buffer (125 mmol/L NaMES, pH 7.8, 25 mmol/L Mg acetate, 2 mg/ml ATP) and activity was measured after injection of 50 μl of 1 mmol/L D-luciferin (AppliChem, Darmstadt, Germany) in a Berthold luminometer (Berthold, Bald Wildbach, Germany). Total protein concentration was measured by the Bradford technique (Biorad, München, Germany). The luciferase activities were normalized on the basis of protein content as well as on β-galactosidase activity of cotransfected RSV-β-gal vector. The β-galactosidase assay was performed with 20 μl of precleared cell lysate according to a standard protocol as mentioned earlier. 28 Mean and standard deviations of four independent experiments, each done in duplicate, are shown in the figures.
Preparation of Nuclear Extracts and Electrophoretic Mobility Shift Assays (EMSA)
Cells were cultured under low serum conditions (1% FCS) for at least 16 hours and then exposed to anoxic conditions for 1, 3 or 24 hours, respectively. Nuclear extracts were prepared according to Dignam’s procedure. 29 Cells were washed with ice-cold phosphate-buffered saline and pelleted. Supernatants were removed and cells resuspended in 500 μl of buffer A (10 mol/L Hepes pH 7.9, 10 mol/L KCl, 0.1 mol/L EDTA, 0.1 mol/L EGTA, 1 mol/L dithiothreitol, 0.5 mol/L phenylmethylsulfonyl fluoride (PMSF)) and allowed to swell for 10 minutes. Cells were pulled 10 to 15 times through a 26G3/8 needle for cell membrane disruption and nuclei were pelleted in a microcentrifuge. Nuclei were washed twice in buffer A and resuspended in 50 μl of buffer C (20 mol/L Hepes, 20% glycerol, 0.4 mol/L NaCl, 0.1 mol/L EDTA, 0.1 mol/L EGTA, 1 mol/L dithiothreitol, 0.2 mol/L PMSF) and incubated on ice for 45 minutes with occasional shaking. After centrifugation, supernatants were harvested, frozen, and stored at −70°C. The following double-stranded oligonucleotides were used: AP-1 binding sequence (derived from IL-8 promoter region): 5′GAAGTGTGATGACTCAGGTTTGCCTGA3′ and NF-κB-like binding sequence (derived from IL-8 promoter region): 5′ GCGATCGTGGAATTTCCTCTGACGCTCGAG 3′. For EMSA, 3 μg of nuclear proteins were incubated at room temperature for 20 minutes in a 20-μl binding reaction mixture containing 20 mol/L Hepes, 50 mol/L KCl, 1 mol/L dithiothreitol, 1 mol/L EDTA, 5% glycerol, 2 μg poly(dI-dC) and 50,000 to 100,000 cpm of 32P-end-labeled probe. The protein DNA complexes were subjected to gel electrophoresis on a 5% non-denaturing polyacrylamide gel in 0.5% TBE buffer. The antibodies α-p65 and α-cRel for supershift analysis of NF-κB complexes were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). The α-p50 antibody was kindly provided by Nancy Ricy, National Cancer Institute, Frederick, MD. Antibodies for AP-1 supershift analyses (α-cJun, α-cFos, α-cJunD) were purchased from Santa Cruz Biotechnology. Gels were dried at 80°C for 1 hour and exposed to Hyperfilm (Amersham) at −70°C.
Nucleotide Sequencing of the Genomic IL-8 Promoter Region
Genomic DNA was isolated from the mentioned cell lines (MV3, BLM, IF6, and 530) using a commercial DNA extraction kit (DNeasy kit, Qiagen) according to the manufacturer’s specifications. 100 ng of genomic DNA were used for polymerase chain reaction (PCR) amplification of an IL-8 promoter region, spanning from the −407 position to the +17 position. The following primers were used: 5′ATCTTCACCATCATGATAGCATCTG3′, 5′GTTTGTGCCTTATGGAGTGCTCG3′. PCR conditions were: an initial 5-minute denaturation step was followed by 35 cycles of 30 seconds of denaturation, 30 seconds of annealing, 1 minute of primer extension, and a terminal primer extension step of 10 minutes. PCR products were electrophoresed on an 1% agarose gel, cut out, and purified using the QiaexII gel extraction kit (Qiagen). Isolated PCR products were cloned into the pCRII vector (Invitrogen, Leek, The Netherlands). Five clones of the PCR product from each cell line were analyzed by sequencing using an automated capillary sequencer (Perkin Elmer, Weiterstadt, Germany).
Statistical Analysis
IL-8 levels in culture supernatants are given as means ± SD. The Student’s t-test was used for statistical analysis and P ≤ 0.05 was regarded as statistically significant.
Results
IL-8 mRNA Is Highly Expressed in Necrotic/Hypoxic Metastases
To determine whether IL-8 is expressed in primary melanomas and melanoma metastases we first studied IL-8 mRNA production by in situ hybridization. Sections of primary melanoma lesions (n = 10), metastases lacking necrotic areas (n = 10), and metastases with clearly defined necrotic areas were hybridized with radioactively labeled 35S-anti-sense, and as a control, sense IL-8 probes. A pronounced IL-8 mRNA expression was detected only in metastases with signs of necrosis represented as a band-like hybridization pattern around necrotic areas (Figure 1) ▶ . More distant to the necrosis only single cells showed a weak IL-8-specific hybridization signal (Figure 1, B and C) ▶ . IL-8 mRNA expression was only occasionally detected in single melanoma cells in primary melanomas and non-necrotic metastases (data not shown). The expression pattern of IL-8 mRNA in necrotic metastases did not correlate with the presence of macrophages and neutrophils, as detected by immunohistochemical staining of serial sections with anti-CD68 monoclonal antibodies and anti-neutrophil elastase, respectively (Figure 1, E and F ▶ ; positively stained cells in the central necrotic area are marked by arrowheads). Thus, these cells seem not to contribute to the rim-like IL-8 mRNA expression. To identify potential target cells for in vivo synthesized IL-8 in necrotic melanoma metastases, we performed immunohistochemical analyses for corresponding IL-8 receptors A and B (CXCR1 and CXCR2). Eight out of 10 metastases displayed strong CXCR1 immunoreactivity of melanoma cells (Figure 2, A and C ▶ ; C, high power view of A). Moreover, endothelial cells of small vessels (Figure 2C ▶ , arrows) in the vicinity of the metastasis were positively stained for CXCR1. Labeling for CXCR2 (Figure 2, B and D ▶ ; D, high power view of B) showed much weaker immunoreactivity of melanoma cells (5 of 10 metastases) whereas endothelial cells were negative (Figure 2D) ▶ . Taken together, our in situ hybridization data demonstrate that IL-8 mRNA is particularly expressed in a band-like pattern around necrotic areas in melanoma metastases and both melanoma cells and endothelial cells may serve as targets for IL-8.
Figure 1.
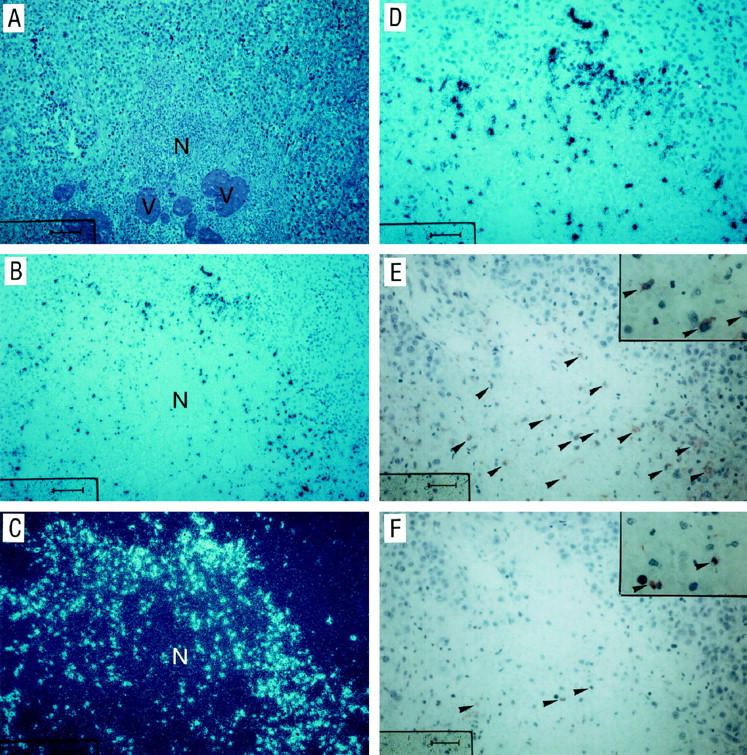
Hematoxylin and eosin stain, in situ hybridization for IL-8 mRNA, and immunohistochemistry for macrophages and neutrophils for a necrotic melanoma metastasis. A cutaneous metastasis (A, hematoxylin and eosin stain) with a necrotic area (N) was stained with a 35S-labeled IL-8 anti-sense probe by in situ hybridization (B, bright field illumination; C, dark field illumination; D, higher magnification of B, bright field illumination). Serial sections of the same tumor were stained immunohistochemically with anti-CD68 (monocytes/macrophages; E) and anti-NE (neutrophil elastase, for detection of neutrophils, F) as described in Materials and Methods. Arrowheads in E and F indicate positively staining cells. V, blood vessel; scale bar in A to C, 100 μm; scale bar in D to F, 50 μm.
Figure 2.
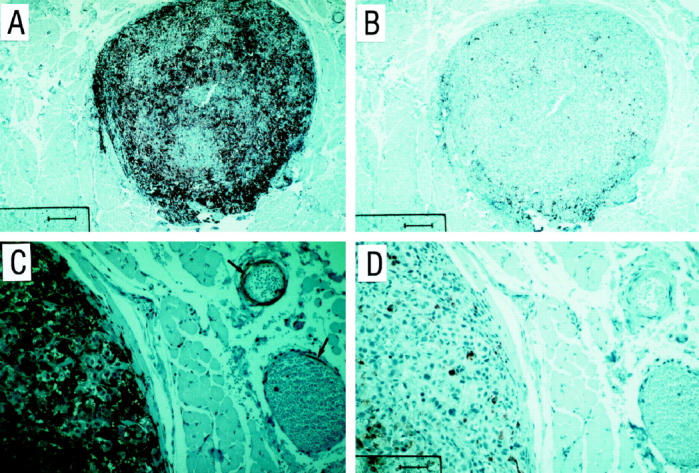
Immunohistochemical staining of a cutaneous melanoma metastasis with antibodies against IL-8 receptors (CXCR1/CXCR2). A three-step peroxidase method was used and 3-amino-9-ethyl-carbazole (AEC) served as substrate as described in Materials and Methods. A: CXCR1 expression; B: CXCR2 expression. C shows a high power view of A; and D shows a high power view of B. Arrows in C indicate positive staining of endothelial cells for CXCR1. Scale bar in A and B, 200 μm; scale bar in C and D, 25 μm.
Anoxia Induces IL-8 mRNA and Protein Synthesis in the Highly Aggressive Melanoma Cell Lines MV3 and BLM but Not in the Low Aggressive Cell Lines IF6 and 530
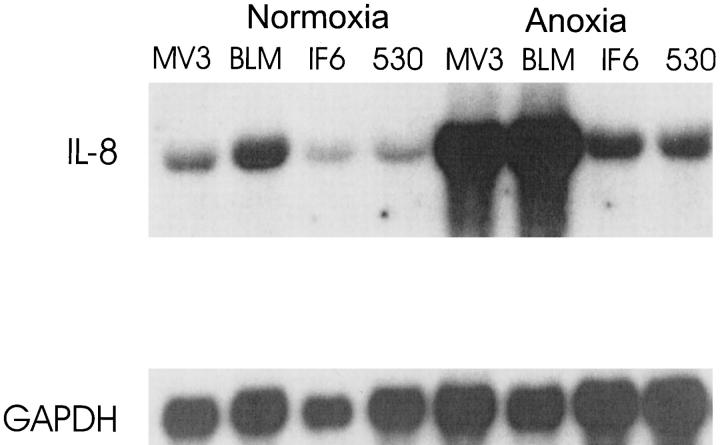
To investigate whether anoxia is a potent stimulus for IL-8 production in melanoma cells we measured IL-8 expression in supernatants from four melanoma cell lines with different aggressiveness. As shown in Figure 3 ▶ , baseline production of IL-8 was similar in all four cell lines. On anoxia there was a sixfold up-regulation of IL-8 production in the highly aggressive cell lines MV3 and BLM (P ≤ 0.05), whereas in the low aggressive cell lines IF6 and 530 IL-8 levels remained unchanged. After 24 hours of reoxygenation IL-8 levels decreased in MV3 and BLM supernatants and returned to baseline levels after an additional 24 hours of incubation (data not shown). The total amount of non-viable cells was estimated by trypan blue staining and neither exceeded 20% nor correlated with cytokine levels, suggesting that IL-8 levels in culture supernatants are not a result of nonspecific release due to cell death. To analyze whether IL-8 production is paralleled by elevated levels of IL-8 mRNA transcripts, we performed Northern blot hybridization after 24 hours of normoxia and anoxia, respectively (Figure 4) ▶ . The two highly aggressive lines (MV3 and BLM) showed a strong up-regulation of IL-8 mRNA (12-fold). In contrast, in the low aggressive cell lines (IF6 and 530) we observed only a slight up-regulation (twofold) of mRNA production under anoxia. To further address the question whether elevated IL-8 levels under anoxia might be due to enhanced mRNA stability we performed Northern blots after inhibition of mRNA transcription by incubation of cells with actinomycin D (Figure 5, A and B) ▶ . These experiments were performed in the highly aggressive cell lines since only these displayed a significant mRNA up-regulation. Before addition of actinomycin D both cell lines expressed low levels IL-8 mRNA under normoxic condition (Figure 5 ▶ , left), but 15 hours of anoxic treatment led to a strong induction of the chemokine (Figure 5 ▶ , right). Addition of actinomycin D decreases IL-8 mRNA levels in a time-dependent fashion (see also diagrams in the lower right corners). Anoxia prolonged mRNA half-life by a factor of 2.3 (MV3) and 2.5 (BLM), respectively, indicating that mRNA stabilization might contribute to the elevated mRNA levels.
Figure 3.
Cytokine ELISAs of culture supernatants from malignant melanoma cell lines. The melanoma cell lines MV3/BLM (highly metastatic) and IF6/530 (low metastatic potential) were exposed to anoxia for 24 hours (solid bars) and parallel cultures were kept under normoxia (open bars). IL-8 production (pg/10 6 cells) was determined by a commercial solid phase ELISA in culture supernatants and data are given as means ± SD of four independent experiments.
Figure 4.
Northern blot analysis for IL-8 mRNA in melanoma cell lines. Melanoma cell lines MV3, BLM, IF6, and 530 were incubated either under normoxia or anoxia for 24 hours. Total RNA (20 μg/lane) was analyzed using a 402-bp BamHI/SacI IL-8 cDNA fragment as a probe (top). GAPDH expression is shown in the bottom. Intensity of the Northern blot bands was quantitated by phosphoimager as described in Materials and Methods.
Figure 5.
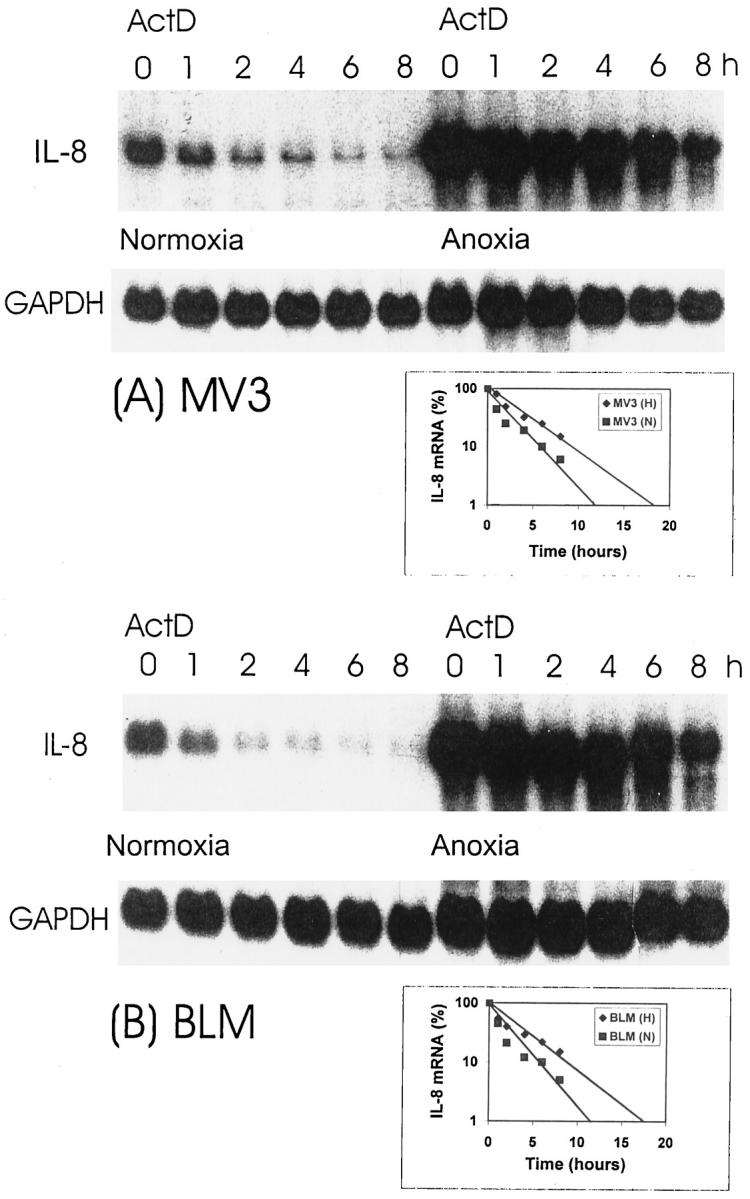
IL-8 mRNA stability under normoxia and anoxia. MV3 (A) and BLM (B) melanoma cells were cultured under normoxia or anoxia. After 15 hours cells were treated with actinomycin D (ActD), 10 μg/ml, to inhibit transcription and cells were harvested at different time points (O, 1, 2, 4, 6, 8 hours) thereafter. Total RNA was prepared and Northern analysis performed for IL-8 mRNA and GAPDH as described. Actinomycin D-dependent decrease of IL-8 mRNA levels in a time-dependent fashion is shown in diagrams in the lower right corners of A and B. Data are given as percentage of total IL-8 mRNA (from time point O h) over a time course of 8 hours.
Anoxia Induces Transcriptional Activation of the IL-8 Promoter
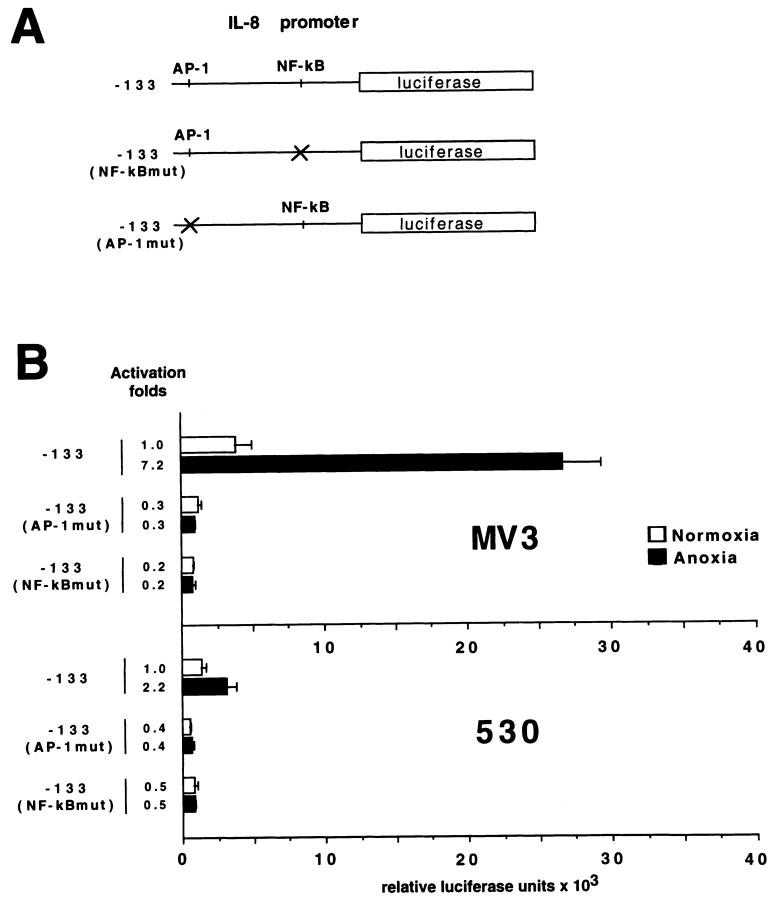
To evaluate whether anoxia induces enhanced transcriptional activation of the IL-8 promoter, we used a firefly luciferase reporter gene linked to wild-type and mutated IL-8 promoters in luciferase assays (Figure 6A) ▶ . On anoxic treatment a 7.2-fold up-regulation of transcriptional activity of the wild-type promoter, harboring AP-1 and NF-κB-like binding sites, was observed in the highly aggressive cell line MV3 (Figure 6B) ▶ . In contrast, the low aggressive cell line 530 showed only a 2.2-fold induction of transcriptional activation. Interestingly, point mutations in the AP-1 or the NF-κB-like binding sites in the IL-8 promoter abolished transcriptional activity, indicating that both factors are important for IL-8 regulation under anoxia. Consistent with previously published data, TPA, a potent inducer of AP-1 and NF-κB activity, was used as a positive control and induced a five- to eightfold stimulation of luciferase activity (data not shown). Taken together, up-regulation of IL-8 mRNA correlates with the enhanced transcriptional activity of the promoter in the highly aggressive melanoma cells. To exclude the fact that different mRNA induction levels, as described above, may be due to genomic mutations within the IL-8 promoter region, genomic DNA was isolated from the four cell lines. The nucleotide position from −407 to nucleotide position +17 was PCR-amplified and sequenced. From position −407 to +17, which carries the binding motifs for AP-1 and NF-κB, no mutations could be detected in the promoter regions of all four cell lines (data not shown).
Figure 6.
Transcriptional activity of IL-8 promoter constructs measured by firefly luciferase assay. A: Firefly luciferase expression vectors harboring the 5′ flanking region −133 to +44 bp of the IL-8 promoter carry the AP-1 (−126 to −120) and NF-κB-like (−80 to −71) binding sites. The following constructs were used: wild-type −133-luc, mutant AP-1 (AP-1mut-luc (TatCTCA)), and mutant NF-κB (NF-κBmut-luc (taAcTTTCCTC)). B: Melanoma cell lines MV3 and 530 were transfected with 2 μg of appropriate plasmid DNA using the DMRIE-C transfection reagent. Subsequently, cells were exposed to for 12 hours to normoxic and anoxic conditions, respectively. The luciferase activities were normalized on the basis of protein content as well as on β-galactosidase activity of cotransfected RSV-β-gal vector. Mean and standard deviations of four independent experiments, each done in duplicate, are shown.
Anoxia Induces Enhanced Binding of AP-1 and NF-κB Transcription Factors in Highly Aggressive Melanoma Cells
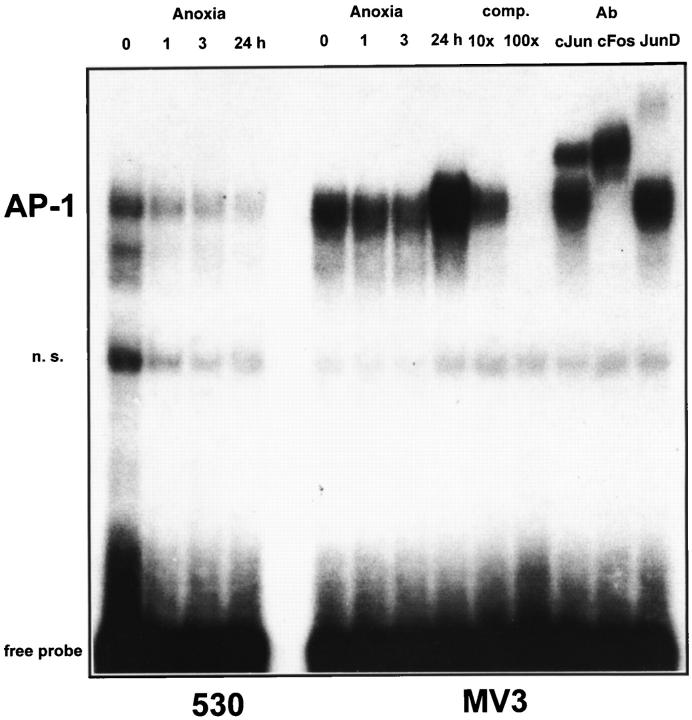
Induction of IL-8 gene expression is dependent on the activation of both transcription factors AP-1 and NF-κB. 30-33 To address the question of whether both transcription factors are stimulated by anoxia in MV3 and 530 cells, we performed EMSA using specific IL-8 promoter-derived sequences as probes. As shown in Figure 7 ▶ , low constitutive AP-1 binding activity was observed in 530 cells under normoxic conditions, which was not inducible and even decreased on anoxic treatment. In contrast, AP-1 complexes were strongly induced in the highly aggressive cell line MV3 after 24 hours of anoxia. TPA was used as a positive control and induced similar AP-1 binding activity in both cell lines (data not shown). To identify specific AP-1 protein complexes, we performed supershift experiments from nuclear extracts of MV3 cells (after 24 hours of anoxia) using AP-1-specific antibodies. As shown in Figure 7 ▶ , anoxia-induced protein complexes were partly supershifted with the anti-cJun antibody and completely supershifted with the anti-cFos antibody. Anti-JunD antibody added a faint band, suggesting that at least a small portion of the AP-1 complex might contain JunD. Moreover, in MV3 we observed a constitutive binding of NF-κB transcription factors, which were slightly induced after 3 hours of anoxia (Figure 8B) ▶ . A further band was induced after 24 hours of anoxia. Both complexes were identified by competition with a 10-fold and 100-fold molar excess, respectively, of cold probe. As demonstrated by supershift analysis these protein complexes contain NF-κB p50 and p65 subunits, respectively. The lower band was supershifted by anti-p50 and the upper band was supershifted by anti-p65 (faint complex, visible only in the highly aggressive MV3 cells). Anti-cRel antibody did not supershift. In the low aggressive cell line 530 (Figure 8A) ▶ a significant induction of NF-κB complexes was not observed and complexes were faint. In summary, these data suggest an important role of both transcription factors, AP-1 and NF-κB, for hypoxic gene regulation in aggressive melanoma cells.
Figure 7.
EMSA for AP-1 of MV3 and 530 cell lines under normoxia and anoxia. Cells were exposed to anoxia for 1, 3, and 24 hours, respectively. Control cultures were kept under normoxia. For EMSA the following 32P-labeled double-stranded oligonucleotide was used: AP-1 binding sequence (derived from IL-8 promoter region); 5′ GAAGTGTGATGACTCAGGTTTGCCTGA 3′. Competition experiments were carried out with a 10- and 100-fold molar excess, respectively, of cold probe. AP-1 complexes of MV3 cells after 24 hours of anoxia were identified by supershift analysis using anti-cJun, anti-cFos, and anti-JunD antibodies, respectively. Free probe is indicated. n. s., nonspecific.
Figure 8.
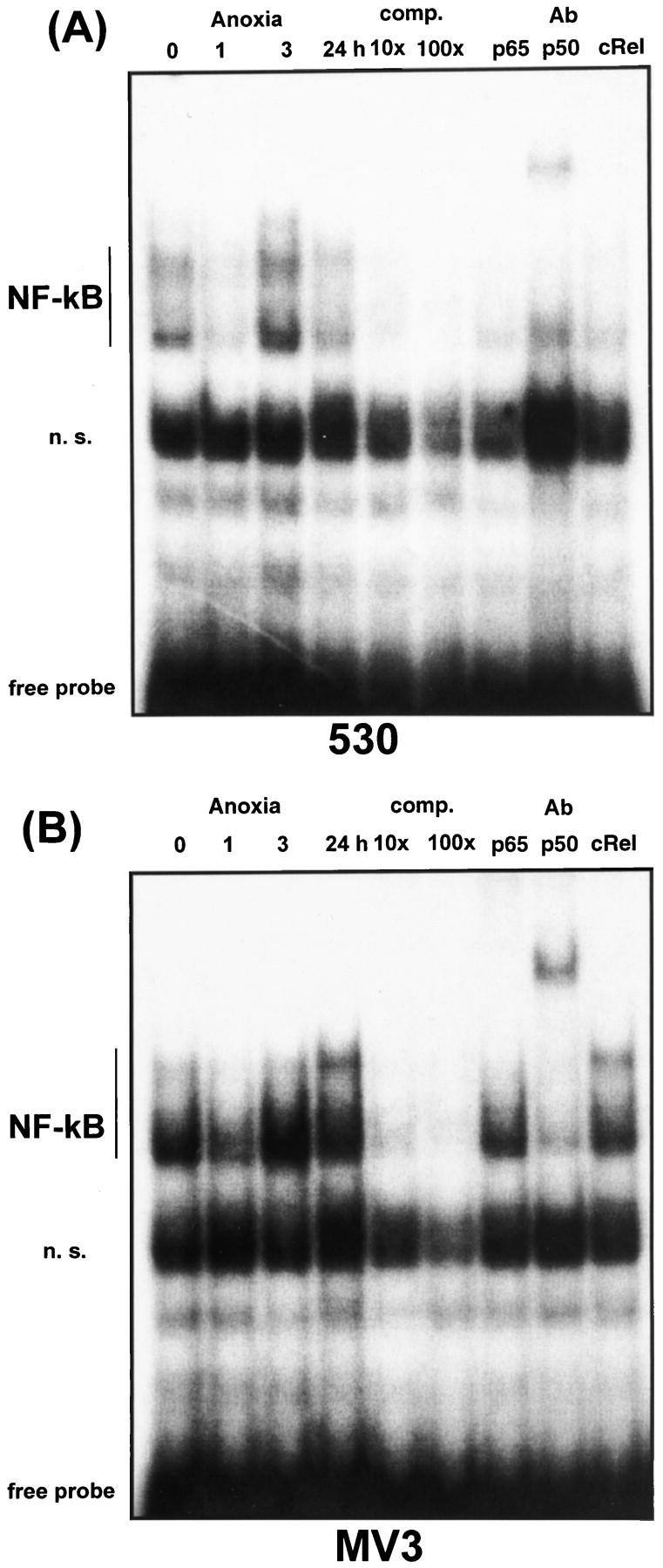
EMSA for NF-κB of MV3 and 530 cell lines under normoxia and anoxia. Cells were exposed to anoxia for 1, 3, and 24 hours, respectively. Control cultures were kept under normoxia. For EMSA the following 32P-labeled double-stranded oligonucleotide was used: NF-κB-like binding sequence (derived from IL-8 promoter region); 5′ GCGATCGTGGAATTTCCTCTGACGCTCGAG 3′. Competition experiments were carried out with a 10- and 100-fold molar excess, respectively, of cold probe. NF-κB complexes of 530 cells (A) and MV3 cells (B) after 24 hours of anoxia were identified by supershift analysis using anti-p65, anti-p50, and anti-cRel antibodies, respectively. Free probe is indicated. n. s., nonspecific.
Discussion
In this report we demonstrate that IL-8 message was highly expressed in perinecrotic/hypoxic areas of melanoma metastases with focal necrotic areas, whereas in primary melanoma lesions and non-necrotic metastases expression was absent or low. Moreover, melanoma cells as well as endothelial cells of supplying vessels which both may serve as targets via autocrine or paracrine stimulation expressed the corresponding IL-8 receptor, CXCR1. The in vivo observations were further substantiated by in vitro studies showing that anoxia strongly induced IL-8 mRNA and protein in highly aggressive human melanoma cell lines, whereas low aggressive melanoma cell lines were non-responsive to the anoxic stimulus. Anoxia induced binding activity of the redox-regulated transcription factors AP-1 and NF-κB and also transcriptional activation of the IL-8 promoter only in the highly aggressive melanoma cells.
High levels of IL-8 were detected exclusively in those areas of cutaneous metastases which were close to focal necrosis and presumably under severe hypoxic/anoxic stress and the IL-8 mRNA expressing cells were identified as melanoma cells. Notably, IL-8 mRNA in primary melanoma lesions and non-necrotic metastasis was absent or low, indicating that IL-8 may not play an important role as a promoter of tumor growth and spreading in primary melanoma. High levels of IL-8 in necrotic areas might even contribute to the high serum levels of IL-8 found in patients with widespread melanoma metastasis. 15 The fact that the local microenvironment might be of importance for IL-8 production in vivo was recently demonstrated by Gutmann et al, 34 who showed that IL-8 production of melanoma metastases after injection into nude mice depended on the organ environment. Interestingly, low vascularized tissue, like skin, induced high IL-8 levels, whereas highly vascularized tissue, especially lung and liver, induced low IL-8 levels. The tumor-promoting features of IL-8 in vivo were demonstrated by recent mouse studies with different melanoma cell lines showing that tumor growth and aggressiveness correlated with IL-8 levels of melanoma cells in vitro. 16,17 Together, all mentioned studies underline the physiological relevance of IL-8 as a potent factor in vivo. Interestingly, similar findings regarding IL-8 production in rapidly growing tumors have recently been reported for glioblastoma, a highly aggressive tumor of the central nervous system. 35,36
Our in vitro studies provide evidence that up-regulation of IL-8 mRNA under anoxia is due to both enhanced transcriptional activation of the IL-8 gene and increased mRNA stability. Similar results had been obtained for VEGF induction under hypoxia in C6 glioma cells and PC12 cells. 26,37,38 Additionally, we could show by EMSA and reporter gene analysis that anoxia-induced IL-8 production in highly aggressive melanoma cells seems to be dependent on the transcription factors AP-1 and NF-κB. Although an interaction of both transcription factors, AP-1 and NF-κB, in IL-8 gene regulation has been shown after a variety of stimuli, eg, mitogens, proinflammatory cytokines, and viral infections, 30-33,39 little is known about the role of both factors in hypoxic gene regulation. 40-41 According to our data we propose that both transcription factors act in a cooperative manner, since both factors are simultaneously activated and mutations in each of the corresponding binding sites totally abrogated transcriptional activity in luciferase assays. Similar data have been recently reported for glioblastoma, showing that anoxia-induced AP-1 activity was paralleled by constitutive NF-κB activity. 36 Interestingly, hypoxia-inducible factor seems to play no role for anoxic induction of IL-8, as no binding motif can be detected in the published IL-8 promoter region. 42,43 However, we cannot rule out that hypoxia-inducible factor binds to and indirectly interacts with AP-1, as has been suggested by recent data. 44 It should also be noted that regulation under hypoxia and anoxia shows differences at least in some regulated genes, eg, VEGF and p53. VEGF mRNA is already up-regulated under physiological hypoxia. In contrast, p53 requires pathological anoxia. 45 This might provide an explanation for the different mRNA kinetics for VEGF and IL-8 under anoxia and the different distribution pattern for both cytokines observed in glioblastoma. 36 Since rapidly growing tumors display both hypoxia and anoxia, 46 both factors might be of importance for tumor biology.
Our in vitro data showed that the high aggressiveness of our melanoma cell lines correlated with elevated IL-8 production and activation of the transcription factors AP-1 and NF-κB under anoxic stress. The underlying mechanisms which contribute to the different responsiveness of our cell lines are not yet clear, although it seems likely that upstream activators might be differently regulated under anoxic conditions. Recent data suggest that the family of mitogen-activated protein kinases is involved in hypoxic/anoxic gene regulation. 47-48 Further upstream activators of mitogen-activated kinases remain to be defined; eg, the existence of a membrane-bound oxygen sensor is speculative up to now. However, the different responsiveness of such a sensor might be a further explanation for our findings.
It has been shown that tissue hypoxia/anoxia indeed renders tumors highly aggressive. 49 In those experiments tumor aggressiveness had been attributed to subsequent DNA over-replication and the induction of mutations after hypoxia/reoxygenation. Although this in fact might be the case, our data provide an alternative explanation for anoxia-induced tumor aggressiveness. Anoxia-induced tumor aggressiveness via IL-8 up-regulation might be explained by at least two different mechanisms, induction of angiogenesis and induction of tumor growth as an autocrine growth factor for melanoma cells. It is well established in in vivo models that IL-8 is a potent angiogenic factor. 19-21,50,51 Although the corresponding receptors CXCR1 and CXCR2 could not be detected on cultured endothelial cells in vitro, 52 in vivo angiogenesis was blocked by IL-8-specific antibodies, 20 suggesting that the lacking of IL-8 receptors in vitro is a culture artifact. The pivotal role of IL-8 in angiogenesis was emphasized by Yoshida et al, 51 who could demonstrate that tumor necrosis factor-α-induced tubulus formation of endothelial cells in collagen gels is attributable to IL-8. In accordance with these data, we could clearly detect CXCR1 on endothelial cells of small vessels in the melanoma metastases. Therefore, we conclude that IL-8 might act as an angiogenic factor in vivo and transduces its angiogenic effects through CXCR1 which in contrast to CXCR2 only binds IL-8 as the specific ligand. Moreover, it has been recently shown that melanoma cells express the CXCR1 and CXCR2 and IL-8 binds to both receptors and acts as an autocrine growth factor, inducing proliferation. 14,53 The CXCR2 also binds another member of the CXC chemokine superfamily, the chemokine growth- related oncogene α (synonymous with melanoma growth-stimulating activity) Gro-α, which is also known to act as an autocrine growth factor for melanoma. 53,54 The signaling mechanisms are not yet clear but recent data implicate that activated CXCR1 and CXCR2 are able to further activate extracellular signal regulated kinase 1/2 (ERK1/2) and in this way induce proliferation. 55-57 We found a strong positive reaction for CXCR1 on melanoma cells in metastases, and considerably weaker staining for CXCR2. This implicates that in vivo melanoma cells might serve as autocrine target cells for IL-8 mainly via CXCR1.
In summary, our data provide evidence that the differential responsiveness to local tissue factors such as anoxia might be an explanation for the different aggressiveness of tumor cells. Tumor cells that are able to overcome the adverse conditions of the microenvironment have a higher potential to survive and induce further metastasis. Further, anoxia-regulated genes such as IL-8 are involved in melanoma progression and metastasis and the described regulatory mechanisms for anoxia-induced IL-8 production might serve as a target for further therapeutic strategies.
Acknowledgments
We thank Dr. G. N. P. van Muijen and Dr. D. J. Ruiter (Institute of Pathology, University of Nijmegen, The Netherlands) for providing the cell lines MV3, BLM, IF6, and 530. We thank S. Schmid and C. Siedel for excellent technical assistance and Dr. A. McLellan for critically reading the manuscript.
Footnotes
Address reprint requests to Dr. M. Kunz, Department of Dermatology, University of Rostock, Augusten Strasse 80, 18055 Rostock, Germany.
Supported by Wilhelm Sander-Stiftung, Contract Grant 95:064 and IZKF (Interdisciplinary Clinical Research Center), Würzburg, Project B 5.
References
- 1.Folkman J, Klagsbrunn M: Angiogenic factors. Science 1987, 235:442-445 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J: What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990, 82:4-6 [DOI] [PubMed] [Google Scholar]
- 3.Liotta L, Kleinerman J, Saidel G: Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastasis following tumor implantation. Cancer Res 1974, 34:997-1004 [PubMed] [Google Scholar]
- 4.Graham CH, Rivers J, Kerbel RS, Stankiewicz KS, White WL: Extent of vascularization as a prognostic indicator in thin (<0.76 mm) malignant melanomas. Am J Pathol 1994, 145:510-514 [PMC free article] [PubMed] [Google Scholar]
- 5.Barnhill RL, Fandrey K, Levy MA, Mihm MC Jr, Hyman B: Angiogenesis and tumor progression of melanoma. Quantification of vascularity in melanocytic nevi and cutaneous malignant melanoma. Lab Invest 1992, 67:331–337 [PubMed]
- 6.Srivastava A, Laidler P, Hughes LE, Woodcock J, Shedden EJ: Neovascularization in human cutaneous melanoma: a quantitative morphological and Doppler ultrasound study. Eur J Cancer Clin Oncol 1986, 22:1205-1209 [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Laidler P, Davies RP, Horgan K, Hughes LE: The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma: a quantitative histologic study. Am J Pathol 1988, 133:419-423 [PMC free article] [PubMed] [Google Scholar]
- 8.Herlyn M, Clark WH, Rodeck U, Mancianti ML, Jambrosic J, Koprowski H: Biology of tumor progression in human melanocytes. Lab Invest 1987, 56:461-474 [PubMed] [Google Scholar]
- 9.Bouck N, Stellmach V, Hsu SC: How tumors become angiogenic. Adv Cancer Res 1996, 69:135-174 [DOI] [PubMed] [Google Scholar]
- 10.Ladoux A, Frelin C: Hypoxia is a strong inducer of vascular endothelial growth factor mRNA expression in the heart. Biochem Biophys Res Commun 1993, 195:1005-1010 [DOI] [PubMed] [Google Scholar]
- 11.Schröder JM, Christophers E: Identification of C5adesarg and an anionic neutrophil activating peptide (ANAP) in psoriatic scales. J Invest Dermatol 1986, 87:53-58 [DOI] [PubMed] [Google Scholar]
- 12.Walz A, Peveri P, Aschauer AO, Baggiolini M: Purification and amino acid sequencing of NAF, a novel neutrophil activating factor produced by monocytes. Biochem Biophys Res Commun 1987, 149:755-761 [DOI] [PubMed] [Google Scholar]
- 13.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K: Properties of the novel proinflammatory supergene intercrine cytokine family. Annu Rev Immunol 1991, 9:617-648 [DOI] [PubMed] [Google Scholar]
- 14.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki B: IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol 1993, 151:2667-2675 [PubMed] [Google Scholar]
- 15.Scheibenbogen C, Mohler T, Haefele J, Hunstein W, Keilholz U: Serum interleukin-8 is elevated in patients with metastatic melanoma and correlates with tumor load. Melanoma Res 1995, 5:179-181 [DOI] [PubMed] [Google Scholar]
- 16.Singh RK, Gutman M, Radinsky R, Bucana CD, Fiedler IJ: Expression of interleukin-8 correlates with metastatic potential of human melanoma cells in nude mice. Cancer Res 1994, 54:3242-3247 [PubMed] [Google Scholar]
- 17.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M: Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 1997, 151:1105-1113 [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JM, Taroboletti G, Matsushima K, Damme JV, Mantovani A: Induction of haptotactic migration of melanoma cells by neutrophil activating protein/IL-8. Biochem Biophys Res Commun 1990, 169:165-170 [DOI] [PubMed] [Google Scholar]
- 19.Keane MP, Arenberg DA, Lynch III JP, White RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM: The CXC chemokines: IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 1997, 159:1437–1443 [PubMed]
- 20.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM: Interleukin-8 as a macrophage derived mediator of angiogenesis. Science 1992, 258:1798-1801 [DOI] [PubMed] [Google Scholar]
- 21.Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG: Interleukin-8: a corneal factor that induces neovascularization. Am J Pathol 1992, 141:1279-1284 [PMC free article] [PubMed] [Google Scholar]
- 22.Gillitzer R, Berger R, Mielke V, Müller C, Wolff K, Stingl G: Upper level keratinocytes of psoriatic lesions express high levels of NAP-1/IL-8 mRNA in situ. J Invest Dermatol 1991, 97:73-79 [DOI] [PubMed] [Google Scholar]
- 23.Ritter U, Moll H, Laskay T, Bröcker E-B, Velazco O, Becker J, Gillitzer R: Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J Infect Dis 1996, 173:699-709 [DOI] [PubMed] [Google Scholar]
- 24.Muijen van GNP, Jansen CFJ, Cornelissen LMHA, Beck JLM, Ruiter DJ: Establishment and characterization of a human melanoma cell line (MV3) which is highly metastatic in nude mice. Int J Cancer 1991, 48:85-91 [DOI] [PubMed] [Google Scholar]
- 25.Lindholm D, Heumann R, Hengerer B, Thoenen H: Interleukin 1 increases stability, and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J Biol Chem 1988, 263:16348-16351 [PubMed] [Google Scholar]
- 26.Ikeda E, Achen MG, Breier G, Risau W: Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem 1995, 270:19761-19766 [DOI] [PubMed] [Google Scholar]
- 27.Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K: Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-κB is a target for glucocorticoid-mediated interleukin-8 gene repression. J Biol Chem 1994, 269:13289-13295 [PubMed] [Google Scholar]
- 28.Hoffmeyer A, Grosse-Wilde A, Flory E, Neufeld B, Kunz M, Rapp UR, Ludwig S: Different mitogen-activated protein kinase signaling pathways cooperate to regulate tumor necrosis factor α gene expression in T lymphocytes. J Biol Chem 1999, 274:4319-4327 [DOI] [PubMed] [Google Scholar]
- 29.Dignam JD, Lebovitz RM, Roeder RG: Accurate transcription regulation initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 1983, 11:1475-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LF, Haskill S, Mukaida N, Matsushima K, Ting JPY: Identification of tumor-specific paclitaxel (Taxol)-responsive regulatory elements in the interleukin-8 promoter. Mol Cell Biol 1997, 17:5097-5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukaida N, Mahe Y, Matsushima K: Cooperative interaction of nuclear factor-κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by proinflammatory cytokines. J Biol Chem 1990, 265:21128-21133 [PubMed] [Google Scholar]
- 32.Murayama T, Ohara Y, Obuchi M, Khabar KSA, Higashi H, Mukaida N, Matsushima K: Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1 and NF-κB-binding sites of the interleukin-8 gene. J Virol 1997, 71:5692-5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K: Tumor necrosis factor α and interferon γ synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem 1992, 267:22506-22511 [PubMed] [Google Scholar]
- 34.Gutman M, Singh RK, Xie K, Bucana CD, Fiedler IJ: Regulation of interleukin-8 expression in human melanoma cells by the organ environment. Cancer Res 1995, 55:2470-2475 [PubMed] [Google Scholar]
- 35.Desbaillets I, Diserens AC, de Tribolet N, Hamou MF, van Meir EG: Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med 1997, 8:1201-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desbaillets I, Diserens AC, de Tribolet N, Hamou MF, van Meir EG: Regulation of interleukin-8 expression by reduced oxygen pressure in human glioblastoma. Oncogene 1999, 18:1447-1456 [DOI] [PubMed] [Google Scholar]
- 37.Stein I, Neeman M, Shweiki D, Hin H, Keshet E: Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol 1995, 15:5363-5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy AP, Levy NS, Goldberg MA: Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem 1996, 271:2746-2753 [DOI] [PubMed] [Google Scholar]
- 39.Mahe Y, Mukaida N, Kuno K, Akiyama M, Ikeda N, Matsushima K, Murakami S: Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor κB, and CCAAT/enhancer-binding protein-like cis-elements. J Biol Chem 1991, 266:13759-13763 [PubMed] [Google Scholar]
- 40.Rupec RA, Baeuerle PA: The genomic response of tumor cells to hypoxia and reoxygenation: differential activation of transcription factors AP-1 and NF-κB. Eur J Biochem 1995, 234:632-640 [DOI] [PubMed] [Google Scholar]
- 41.Bandyopadhyay RS, Phelan M, Faller DV: Hypoxia induces AP-1-regulated genes and AP-1 transcription factor binding in human endothelial and other cell types. Biochim Biophys Acta 1995, 1264:72-78 [DOI] [PubMed] [Google Scholar]
- 42.Mukaida N, Shiroo M, Matsushima K: Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol 1989, 143:1366-1371 [PubMed] [Google Scholar]
- 43.Semenza GL, Jiang BH, Leung SW, Passatino R, Concordet JP, Maire P, Giallongo A: Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sides for hypoxia-inducible factor 1. J Biol Chem 1996, 271:32529-32537 [DOI] [PubMed] [Google Scholar]
- 44.Damert A, Ikeda E, Risau W: Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J 1997, 327:419-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wenger RH, Gassmann M: Little difference. Nature 1996, 380:100. [DOI] [PubMed] [Google Scholar]
- 46.Helmlinger G, Yuan F, Dellian M, Jain RK: Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 1997, 3:177-182 [DOI] [PubMed] [Google Scholar]
- 47.Karin M: The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 1995, 270:16483-16486 [DOI] [PubMed] [Google Scholar]
- 48.Karin M, Zheng-gang L, Zandi E: AP-1 function and regulation. Curr Opin Cell Biol 1997, 9:240-246 [DOI] [PubMed] [Google Scholar]
- 49.Young SD, Marshall RS, Hill RP: Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci USA 1993, 85:9533-9537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norrby K: Interleukin-8 and de novo mammalian angiogenesis. Cell Prolif 1996, 29:315-323 [DOI] [PubMed] [Google Scholar]
- 51.Yoshida S, Mayumi O, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M: Involvement of Interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor α-dependent angiogenesis. Mol Cell Biol 1997, 17:4015-4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petzelbauer P: IL-8 and angiogenesis. Evidence that human endothelial cells lack receptors and do not respond to IL-8 in vitro. Cytokine 1995, 7:267–272 [DOI] [PubMed]
- 53.Norgauer J, Metzner B, Schraufstetter I: Expression and growth-promoting function of the IL-8 receptor β in human melanoma cells. J Immunol 1996, 156:1132-1137 [PubMed] [Google Scholar]
- 54.Bordoni R, Fine R, Murray D, Richmond A: Characterization of the role of melanoma growth stimulatory activity (MGSA) in the growth of normal melanocytes, nevocytes, and malignant melanocytes. J Cell Biochem 1990, 44:207-219 [DOI] [PubMed] [Google Scholar]
- 55.Jones SA, Moser B, Thelen M: A comparison of post-receptor signal transduction events in Jurkat cells transfected with either IL-8R1 or IL-8R2: chemokine mediated activation of p42/p44 MAP-kinase (ERK-2). FEBS Lett 1995, 364:211-214 [DOI] [PubMed] [Google Scholar]
- 56.Robinson MJ, Copp MH: Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 1997, 9:180-186 [DOI] [PubMed] [Google Scholar]
- 57.Horuk R: The interleukin-8 receptor family: from chemokines to malaria. Immunol Today 1994, 15:169-174 [DOI] [PubMed] [Google Scholar]