Abstract
Despite of the increased availability of genetically modified mouse strains, the experimental models in the rat have provided the most widely employed and versatile models for the study of renal pathophysiology and functional genetics. The identification of the human gene mutated in the congenital nephrotic syndrome of the Finnish type (NPHS1) has recently been reported, and its protein product has been termed nephrin. Here we report the molecular cloning and characterization of rat nephrin cDNA. Rat nephrin cDNA has an open reading frame of 3705 bp, shows 82% sequence identity with human nephrin cDNA, and shows characteristic rat-specific splicing variants. The translated nucleotide sequence has 89% sequence identity at the amino acid level. The signal sequence, glycosylation, and cysteine localization patterns are nearly identical to those of human nephrin. As in the human, the rat nephrin transcript is expressed in a tissue-restricted pattern. Antipeptide antibodies raised to the intracellular nephrin-specific domain identified immunoreactivity exclusively within the rat kidney glomerulus by indirect immunofluorescence. Initial results with semiquantitative reverse transcriptase-polymerase chain reaction analysis showed a remarkable down-regulation of nephrin-specific mRNA in the puromycin nephrosis of the rat.
The molecular pathogenesis of diseases that result in abnormalities of glomerular filtration has remained poorly understood. Recent results indicate that podocytes play an important role in regulating the passage of macromolecules and have several structural properties suitable for allowing the rapid modulation of the permeability barrier. 1-4 Detailed studies of the recently discovered podocyte-specific proteins, including podocalyxin, 5 GLEPP-1, 6 synaptopodin, 7 and lipids, including 0-acetylGD3 ganglioside, 8,9 should shed new light on the glomerular filter. However, the functional characterization of many of these is still under way.
Experimental models of glomerular disease are important tools for investigating the functional significance of the novel molecules by providing a means to effectively modulate the basic glomerular functions. Thus, even if transgenic and knockout mouse models are extremely useful in the targeted analysis of molecules, the well-established experimental models in the rat remain best characterized and most widely used for glomerular pathophysiology. In particular, the classic models of Heyman nephritis, which mimicks membranous nephropathy, and anti-GBM nephritis and puromycin aminonucleoside (PAN) nephrosis of the rat, which models minimal change nephropathy, are still widely used. 10-13 Similar modeling is often impossible in the mouse.
Kestilä et al 14 recently cloned a new gene, NPHS1, which is mutated in the congenital nephrotic syndrome of the Finnish type (CNF). The current treatment of CNF with early nephrectomy and final renal transplantation appears to cure all symptoms. 15 After transplantation, no new symptoms are reported after several years of follow-up. The results of Kestilä et al 14 showed that NPHS1 is only expressed in the kidney glomerulus. However, little is known thus far of the regulation of this gene of apparently great functional significance in various proteinuria-associated diseases.
Here we report the molecular cloning and characterization of rat nephrin cDNA.
Materials and Methods
Animals and Induction of Nephritis
Male Sprague-Dawley rats weighing 175–190 g, with free access to tapwater and standard rat chow throughout the study, were used.
To induce PAN nephrosis, puromycin aminonucleoside (20 mg/100 g; Sigma Chemical Company, St Louis, MO) in phosphate-buffered saline (PBS) was given by a single intraperitoneal injection to 10 rats, as described earlier. 16 All injected animals developed significant albuminuria (>30 mg/24 hours) by day 5 after injection, as measured by nephelometry (Behring Nephelometer 100 Analyzer; Behringwerke, Marburg, Germany). Five animals were sacrificed at day 3 before the onset of albuminuria (<10 mg of urinary albumin/24 hours), and five at day 10 at the peak of albuminuria (>100 mg/24 hours). One kidney from each animal was used for immediate isolation of cortical RNA. For immunofluorescence studies the remaining kidney was frozen in isopentane and stored appropriately at −70°C until used.
Cloning of Rat Nephrin cDNA
For the cloning of the rat nephrin cDNA, a rat glomerular cDNA library was prepared and used as previously described. 17 Briefly, the library was constructed using total RNA isolated from glomeruli of 1-month-old rats by the isothiocyanate/CsCl− method. 18 Furthermore, poly(A+) mRNA was isolated using a PolyATrac mRNA isolation System III kit (Promega, Madison, WI). mRNA was used to construct a poly(T)-primed directional glomerular library, using a ZAP Express cDNA Synthesis kit and a ZAP Express cDNA Gigapack II Cloning kit (Stratagene, La Jolla, CA).
For the library screening 50 × 10 3 plaque-forming units of the library per 13-cm plate was transferred to a nylon membrane (Hybond N+; Amersham Life Sciences, Buckinghamshire, England), according to the instructions of the manufacturer. The prehybridization and further hybridization were performed as described previously. 17 Briefly, the filter was prehybridized at 42°C and further hybridized in the hybridization solution containing the 32P probe (10 7 cpm; see below) labeled with [α-32P]CTP (3000 Ci/mmol, 10 mCi/ml; Amersham), using a Random priming kit (Boehringer Mannheim, Germany). After an overnight hybridization the filter was washed twice with low-stringency buffer at 42°C, twice with high-stringency buffer at 50°C, and exposed to the film (Fuji Photo Film, Japan) with intensifying screens at −70°C. The positive plaques were picked and used for successive rounds of screening. After single-clone excision protocol (ZAP Express cDNA Gigapack II Cloning kit; Stratagene), the DNA of the pBK-CMV phagemid clone was isolated (Quiagen plasmid Mini Kit; Quiagen, Hilden, Germany) and sequenced from three clones along both strands (ABIPrism 310; Perkin-Elmer Applied Biosystems, Foster City, CA), and the sequences were screened for homology with database sequences, using the BLAST search algorithm at the National Center for Biotechnology (Washington, DC) via the Internet as described earlier. 19
Probes
For screening of the rat glomerular cDNA library the probe (HN3′) was constructed by polymerase chain reaction (PCR) amplification of normal human kidney cortex cDNA, using primer pair (numbering according to NPHS1; Gene Bank accession AF035835) NPHS-2606U (5′-cca aca tcg ttt tca ctt gg) and NPHS-3515L (5′-ggg aag gcc ata tcc tca t), producing a 909-bp-long PCR product, which was gel-purified after cloning to pGEM-T vector (Promega). The clone was digested by restriction enzymes ApaI and SacI, yielding a 777-bp-long fragment that was further used as a probe.
For screening the long transcripts from the library, a probe for the 5′ end of rat nephrin (RN5′) was constructed by PCR amplification of rat kidney cortex cDNA. The primers were designed according to the sequence of mouse nephrin (Holzman et al., manuscript in preparation) for the regions conserved in human and mouse. PCR reaction with the primers RN-S1 (5′-cca cct cag cac ctc gag) and RN-AS1 (5′-gag aca cga gct cgg gac c) produced a PCR product of 293 bp, which was cloned to pGEM-T vector as above. The isolated insert was further used as a probe.
Screening of the Splicing Variants
Screening of the splicing variants of rat nephrin gene by PCR was performed by amplification of normal rat kidney cortex cDNA, using sense primer RN-S4 (5′-ctgctgcctgtgctctttgc) and antisense primer RN-AS2 (5′-ggccataggctctctccact), corresponding to nucleotides 3172–3541 (all numberings according to rat nephrin gene; Gen Bank accession number AF125521).
PCR screening was also done by nested PCR, using primers (sense primer: RN-S3 5′-agcctcttgaccatcgctaa; antisense primer: RN-AS2) flanking the transmembrane region corresponding to nucleotides 2692–3541. The nested sense primer was RN-S2 (5′-tccaggtctccgtcactacc), and the antisense primer was RN-AS2, as in the first reaction.
All PCR reactions were performed as before, 19 using AmpliTaq DNA polymerase (Perkin Elmer) and the following amplification program: after the initial denaturation at 94°C for 5 minutes, DNA was amplified using 30 cycles (94°C 1 minute, 55°C 1 minute, and 72°C 1 minute), followed by final elongation at 72°C for 10 minutes. In the nested PCR reactions, denaturation, annealing, and extension times were reduced to 30 seconds with 20 cycles.
Tissue Distribution of Nephrin by Northern Blotting and RT-PCR
Twenty-five micrograms of total RNAs from rat tissues (kidney cortex, liver, heart, muscle, testis, brain, lung, and spleen) were used for Northern blottings as previously described. 20 To reveal the transcript levels of rat nephrin, the respective cDNA probes as described above were used. To control the total RNA content and lack of degradation in the preparations, blots were hybridized with a β-actin probe. 19 For autoradiography of Northern blots the filters were exposed on Fuji Bas IIIS Imaging Plates, and the expression was recorded using a Fuji phosphoimager and accompanying MacBAS software (Fuji Photo Film Co, Tokyo, Japan).
Because Northern blotting was not sensitive enough for detection of nephrin mRNA in renal cortex and other tissues mentioned above, we determined its transcript levels by reverse transcriptase-polymerase chain reaction (RT-PCR) with controls as previously used. 19
Semiquantitative RT-PCR
RNA-samples from isolated kidney cortex of the PAN rats were used as a starting material in this analysis. 19,20 These analyses were done in triplicate. Sequence-specific primers RN-S3 (see above) and RN-AS3 (5′-ccc agt cag cgt gaa ggt ag) yielded a 302-bp product. The semiquantitation of nephrin expression was done as previously described, 19 using serial dilutions of sample cDNAs in the linear range of amplification and normalization to the amount of β-actin amplification product.
Design of Synthetic Peptides
Sequence-specific polypeptides against intracellular and extracellular nephrin domains were selected over the human nephrin sequence, using the MacDNAsis and DNAStar programs as described. The sequence from amino acid 1101 to amino acid 1126 for the intracellular domain (Figure 1) ▶ was selected. This sequence was identical with the human sequence, whereas no homology to other known protein sequences could be found as screened by the PredictProtein program (European Molecular Biology Laboratory, Heidelberg, Germany). The peptides, coupled to a high-density multiple antigenic peptide-polylysine matrix, 21 were synthesized and purified at a local peptide synthesis unit (Haartman Institute, University of Helsinki).
Figure 1.
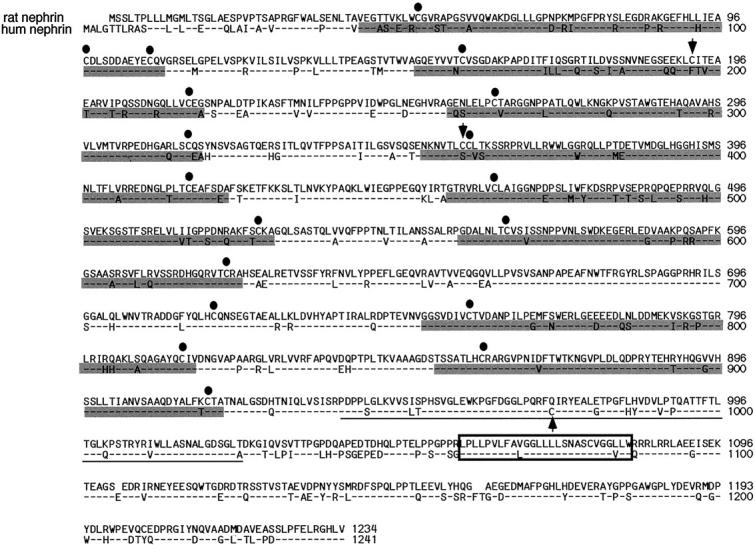
Alignment of nucleotide-derived amino acid sequences of rat and human nephrin. The putative transmembrane domain appears in the box, Ig-like modules are marked by shading, and fibronectin type III-like module is underlined. Cysteine residues identical in human and rat are shown by solid circles, and the two additional cysteines of rat are indicated by arrows (an additional cysteine is found in human locus 974). The cleavage site for predicted N-terminal signal sequence is identical for human and rat, as are the putative N-glycosylation sites (not marked).
Antipeptide Antibodies
The polyclonal antipeptide antibodies were produced in the rabbit. Briefly, the synthetic peptides in Freund’s complete adjuvant were injected into two rabbits. After three booster immunizations 4 weeks apart, peptide-specific IgG fractions were further immunoaffinity purified on CNBr-Sepharose coupled to the corresponding peptides. The specificity of the antisera was tested by immunofluorescence (IF) on kidney sections and by Western blotting of rat glomerular extracts. 22
Immunofluorescence Microscopy
To study the presence and tissue distribution of nephrin, frozen sections of the rat kidneys were cut at 5 μm, fixed in acetone at −20°C for 5 minutes, and washed in PBS. 9 Thereafter, the rabbit antibodies were flooded over sections for 1 hour. After washing the tissue sections were further incubated with rat anti-rabbit IgG (Boehringer Mannheim, Mannheim, Germany) coupled with fluorescein isothiocyanate (FITC). An Olympus OX50 microscope equipped with an epiilluminator and a filter system for FITC fluorescence was used for microscopy.
Results
Isolation of Rat Nephrin cDNA
Molecular cloning of the rat nephrin cDNA was undertaken to generate reagents necessary for studying nephrin in rat model systems. A human nephrin cDNA probe was amplified by PCR and was used to screen the rat glomerular cDNA library. Additional library screening was necessary to obtain the entire open reading frame of the rat nephrin sequence. Using the mouse nephrin cDNA sequence (Holzman, manuscript in preparation), we designed primers that allowed the amplification of 5′ rat nephrin cDNA probe. This cDNA fragment showed 93% sequence identity with mouse nephrin and was successfully employed to assemble the full-length cDNA sequence.
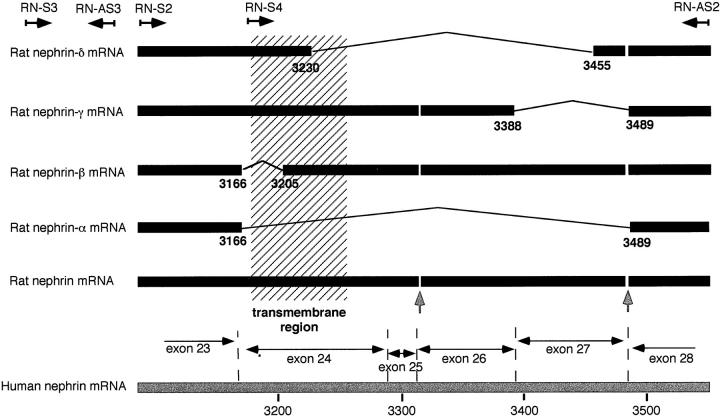
The rat nephrin cDNA coding region of 1234 nucleotides revealed 82% sequence identity with the open reading frame of the human nephrin cDNA sequence. The predicted amino acid sequence, with a calculated molecular mass of 134 kd was 89% identical to the human sequence (see Figure 1 ▶ ) and the extracellular, transmembrane, and intracellular domains showed 90%, 99%, and 83% sequence identity with human nephrin. A highly conserved general structure of the extracellular region with the conserved Ig-like and fibronectin type III-like modules and signal sequence were found as in human nephrin. Putative N-glycosylation sites were also identically located in human and rat nephrin. Cysteine residues were also similarly conserved, showing similarity in location, and two additional cysteine residues in rat (positions 192 and 356) could be found. In addition to the whole coding sequence, two splicing variants (rat nephrin-α and nephrin-β; see below) could be detected with library screening (see Figure 2 ▶ ).
Figure 2.
Splicing of rat nephrin mRNA at the transmembrane region. Full-length rat nephrin and rat nephrin-α and -β were found by screening of rat glomerular cDNA library, and rat nephrin-γ was detected by PCR and rat nephrin-δ by nested PCR. Locations of primers used are indicated at the top of the figure by angled arrows (for details, see Materials and Methods). Exons of human nephrin cDNA of the region studied are shown at the lower part. The vertical arrows indicate nucleotides 3315–3317 (gaa) and 3483–3488 (ttc aca) missing in rat nephrin cDNA.
Splicing Variants by PCR Screening
Our previous data suggested the presence of splice isoforms of human nephrin. Evidence of alternatively spliced rat nephrin cDNA clones prompted further examination for alternatively spliced forms. Primers RN-S2, RN-AS2, RN-S3, and RN-S4 (see Figure 2 ▶ ) were particularly designed to reveal possible additional splicing of the transmembrane coding sequence in the rat.
PCR with primers RN-S4 and RN-AS2 amplified the expected full-size product (370 bp) as well as a product that was about 100 bp smaller. After isolation and direct sequencing, the smaller PCR product was identified as a splicing variant rat nephrin-γ (Figure 2) ▶ .
Nested amplification using primers RN-S2 and RN-AS2 (first PCR reaction with primers RN-S3 and RN-AS2) showed five reaction products. In addition to the expected full-size product (460 bp) and the already identified variants nephrin-α, -β and -γ, a fourth splicing variant giving a 230-bp product (nephrin-δ) was seen and verified by direct sequencing of the PCR product. The sequences of all splicing variants translated the COOH-terminal rat nephrin in the correct reading frame.
Tissue Distribution of Rat Nephrin Specific mRNA
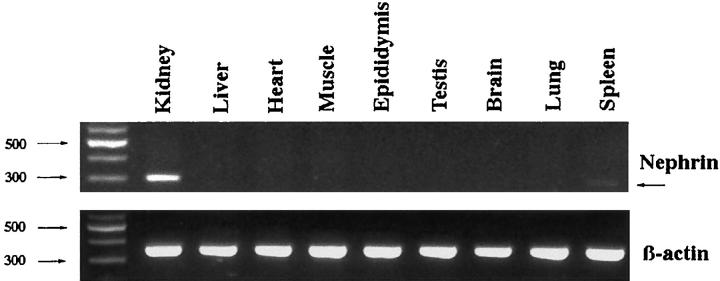
The tissue distribution of rat nephrin mRNA was further studied using Northern blotting and RT-PCR of the following tissues: liver, skeletal muscle, cardiac muscle, spleen, kidney cortex, and brain. Whereas the sensitivity of Northern blotting was found to be too weak for clear detection of the nephrin specific RNA (data not shown), the RT-PCR analysis could verify expression in kidney cortex and in the spleen (Figure 3) ▶ .
Figure 3.
Tissue expression of rat nephrin mRNA as detected by RT-PCR. In the kidney strong expression is seen, whereas faint expression was found in the spleen, and no expression was found in the other tissues studied. Signal intensity is compared to the level of housekeeping β-actin.
Nephrin in PAN
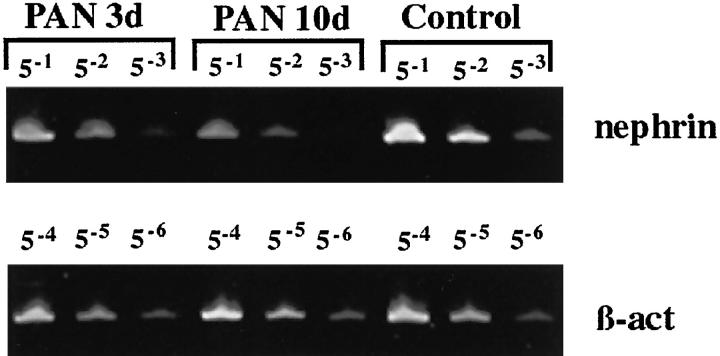
In semiquantitative RT-PCR analysis of rat cortical kidney in the PAN nephrosis model, a distinct down-regulation of the rat nephrin homolog was constantly seen (see Figure 4 ▶ ). Interestingly, this down-regulation up to 60% was observed already at 3 days after the single injection of puromycin amino nucleoside and further decreased down to 20% of the level of controls at day 10 in this model.
Figure 4.
Semiquantitative RT-PCR analysis of nephrin in glomeruli in puromycin nephrosis of the rat (PAN). Dilution of cDNAs was normalized to amounts of β-actin RT-PCR products. The nephrin-specific mRNA level decreases to 60% and 20% of the control level at 3 and 10 days, respectively (data from three independent quantitations).
Immunohistochemistry of Nephrin in Rat Kidney
Immunohistochemical staining of cortical kidney with antipeptide antibodies to the intracellular nephrin domain showed distinct reactivity exclusively with glomeruli (Figure 5) ▶ . Notably, finely granular dotted lines of reactivity resembling a podocyte-like reactivity at the elements facing the urinary space could be seen (Figure 5) ▶ . In the kidneys of PAN rats a systematic decrease (from intense +++ to faint/moderate +/++) in glomerular nephrin reactivity could be seen (data not shown).
Figure 5.
A frozen section of normal rat kidney stained with antibodies to the intracellular nephrin-specific oligopeptide shows reactivity only in the glomerulus (A). At higher magnification (B), a finely granular linear staining is seen (arrows). A, ×160; B, ×450.
In Western blotting of isolated rat glomeruli, the antipeptide antibodies gave a double band at 200 kd (Figure 6) ▶ .
Figure 6.

Immunoblotting of rat glomerular lysate. The lysate was immunoblotted with antibodies to the intracellular nephrin-specific oligopeptide. A double band at 200 kd can be seen. Molecular mass markers appear on the left.
Discussion
Here we report the cloning and characterization of the rat nephrin cDNA and the characteristic splicing of the respective mRNA. Distinct reactivity exclusively in the kidney glomerulus was observed with antipeptide antibodies to the intracellular nephrin-specific domain. Initial results showed down-regulation of its glomerular expression in the puromycin amino nucleoside nephrosis model.
The characteristic massive proteinuria of the congenital nephrotic syndrome of the Finnish type has long been considered a unique model disease of the perturbed glomerular filtration function. 15,19,20,23 Thus, the recent identification of the NPHS1 gene 14 responsible for this disease phenotype has suggested that this gene and the respective protein product nephrin play some key roles in the maintenance of the glomerular filtration barrier. Kestilä et al 14 showed that NPHS1 encodes a putative transmembrane protein with sequence similarity to that of cell adhesion molecules of the immunoglobulin superfamily and with expression in the kidney but not in other tissues. Here we show that in addition to the kidney, nephrin can be found in the spleen of the rat. The significance of this additional expression remains to be studied in detail.
The cDNA sequence cloned from the rat glomerular cDNA library shows 82% identity with the respective human cDNA sequence. No other related sequences were obtained in similarity searches. Furthermore, the rat nephrin cDNA bears high sequence similarity to human 14 and mouse cDNA (Holzman et al, manuscript in preparation) nephrin sequences. Similarly, the rat nephrin cDNA sequence translates into a closely related amino acid sequence of 89% homology to human nephrin. Indeed, the translated open reading frame includes conserved extra-, intra-, and transmembrane domains, identically located cysteines, signal sequence, and glycosylation sites. Together this suggests that our rat nephrin cDNA is a true homolog of the respective human nephrin cDNA.
Our results have indicated (Holthöfer et al, manuscript submitted for publication) that NPHS1-specific mRNA is spliced in the human kidney glomeruli with a variant lacking exon 24 covering the whole transmembrane domain. Interestingly, the splicing at this apparently important functional domain appears to be more complex in the rat with all of the splicing variants identified here. The unspliced form appears as the dominant one, whereas the less dominant forms lacking human exons 24–27 are found in the order of nephrin-α > β > γ > δ. Kendall and Thomas have shown suppression of endothelial cell growth factor activity by a secreted splice variant of vascular endothelial cell growth factor receptor lacking the transmembrane domain. 24 In addition, similar transmembrane-negative modifications generated by alternative splicing have been reported, e.g., for the T-cell receptor, 25 angiotensin-converting enzyme, 26 and interleukin-6 receptor. 27 Furthermore, the distinct functional role of such splicing variants was recently shown for the soluble receptor of advanced glycosylation, 28 which appears to be an important physiological mechanism regulating atherogenesis in hyperglycemia. 28 What is the significance of the splicing variants of nephrin for glomerular functions and whether their mutual ratios are changing in experimental glomerular diseases is currently being studied in detail.
PAN nephrosis in rats is a model for minimal change nephropathy in humans. Interestingly, our initial results on PAN nephrosis show distinct down-regulation of rat nephrin gene-specific mRNA to 60% of the level of controls at day 3 before peak proteinuria, and an additional drop to only 20% at day 10 of the controls at the highest proteinuria. This was supported by findings from immunofluorescence microscopy in which weaker fluorescence was observed in the PAN rat kidney glomeruli, especially at day 10, although immunofluorescence can be considered semiquantitative at best. Currently nothing is known of the metabolic rates of nephrin, but our results suggest a relatively slow degradation. Further studies are in progress to accurately study the decrease in the specific mRNA and the respective nephrin protein in various experimental models of glomerular disease in the rat.
A double band in immunoblotting at 200 kd, the molecular mass also found for human nephrin (Holthöfer et al, manuscript submitted for publication), suggests that two protein transcripts of nephrin are actively produced in the normal rat kidney. The calculated molecular mass of nephrin is 134 kd; the difference from the observed molecular mass is most likely explained by glycosylation at the multiple glycosylation sites of the nephrin sequence.
Taken together, the data presented here show the close identity of rat nephrin cDNA with the respective human cDNA. The observed characteristic alternative splicing suggests that the splicing variants may be important for nephrin functions. The widely used experimental models of glomerular damage in the rat may now be fully exploited to study the functions of nephrin. (Review started on January 22, 1999).
Acknowledgments
The expert technical assistance of Ms Riitta Väisänen and Ms Liisa Pirinen is gratefully acknowledged.
Footnotes
Address reprint requests to Dr. Harry Holthöfer, Division of Bacteriology and Immunology, The Haartman Institute, PB 21 (Haartmaninkatu 3), University of Helsinki, FIN-00014 Helsinki, Finland. E-mail: harry.holthofer@helsinki.fi.
Supported by the Academy of Finland, a research grant from Helsinki University Hospital, the Finnish Foundation of Heart Disease, and the Sigrid Juselius Foundation.
References
- 1.Rennke HG: How does glomerular epithelial cell injury contribute to progressive glomerular damage? Kidney Int 1994, 45:S58-S63 [PubMed] [Google Scholar]
- 2.Kerjaschki D: Dysfunctions of cell biological mechanisms of visceral epithelial cells (podocytes) in glomerular diseases. Kidney Int 1994, 45:300-313 [DOI] [PubMed] [Google Scholar]
- 3.Mundel P, Kriz W: Structure and function of podocytes: an update. Anat Embryol 1997, 192:385-397 [DOI] [PubMed] [Google Scholar]
- 4.Smoyer WE, Mundel P: Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med 1998, 76:172-183 [DOI] [PubMed] [Google Scholar]
- 5.Kerjaschki D, Sharkey DJ, Farquhar MG: Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 1984, 98:1591-1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas PE, Wharram BL, Goyal M, Wiggins JE, Holzman LB, Wiggins RC: GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. Identification, molecular cloning, and characterization in rabbit. J Biol Chem 1994, 269:19953-19962 [PubMed] [Google Scholar]
- 7.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W: Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997, 139:193-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reivinen J, Holthöfer H, Miettinen A: A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int 1992, 42:624-631 [DOI] [PubMed] [Google Scholar]
- 9.Holthöfer H, Reivinen J, Miettinen A: Nephron segment and cell-type specific expression of gangliosides in the developing and adult kidney. Kidney Int 1994, 45:123-130 [DOI] [PubMed] [Google Scholar]
- 10.Heymann W, Hackel DB, Harwood S, Wilson SGF, Hunter JLP: Production of nephritic syndrome in rats by Freunds adjuvant and rat kidney suspension. Proc Soc Exp Biol Med 1959, 100:660-664 [DOI] [PubMed] [Google Scholar]
- 11.Krakower CA, Greenspon SA: Localization of the nephrotoxic antigen within the isolated glomerulus. Arch Pathol Lab Med 1951, 51:629-639 [PubMed] [Google Scholar]
- 12.Michael AF, Blau E, Vernier RL: Glomerular polyanion: alteration in aminonucleoside nephrosis. Lab Invest 1970, 23:649-657 [PubMed] [Google Scholar]
- 13.Groggel GC, Hovingh P, Border WA, Linker A: Changes in glomerular heparan sulphate in puromycin aminonucleoside nephrosis. Am J Pathol 1987, 128:521-527 [PMC free article] [PubMed] [Google Scholar]
- 14.Kestilä M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1998, 1:575-582 [DOI] [PubMed] [Google Scholar]
- 15.Holmberg C, Antikainen M, Rönnholm K, Ala-Houhala M, Jalanko H: Management of congenital nephrotic syndrome of the Finnish type. Ped Nephrol 1995, 9:87-93 [DOI] [PubMed] [Google Scholar]
- 16.Holthöfer H, Reivinen J, Miettinen A: Decrease of glomerular disialogangliosides in puromycin nephrosis of the rat. Am J Pathol 1996, 149:1009-1015 [PMC free article] [PubMed] [Google Scholar]
- 17.Miettinen A, Solin M-L, Reivinen J, Juvonen E, Väisänen R, Holthöfer H: Podocalyxin in rat platelets and megakaryocytes. Am J Pathol 1999, 154:813-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirgwin JM, Przybyla AC, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 8:5294-5295 [DOI] [PubMed] [Google Scholar]
- 19.Holthöfer H, Kretzler M, Haltia A, Solin M-L, Taanman J-W, Schägger H, Kriz W, Kerjaschki D, Schlöndorff D: Altered gene expression and functions of mitochondria in human nephrotic syndrome. FASEB J 1999, 13:523-532 [DOI] [PubMed] [Google Scholar]
- 20.Haltia A, Solin M-L, Jalanko H, Holmberg C, Miettinen A, Holthöfer H: Mechanisms of proteinuria: vascular permeability factor in congenital nephrotic syndrome of the Finnish type. Pediatr Res 1996, 40:652-657 [DOI] [PubMed] [Google Scholar]
- 21.Tam JP: Synthetic peptide vaccine design. Synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA 1988, 85:5409-5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ljungberg P, Haltia A, Kuusela P, Jalanko H, Holmberg C, Holthöfer H: Noncollagenous matrix components of glomeruli in congenital nephrotic syndrome of the Finnish type: evidence of abnormal splitting of nidogen. Exp Nephrol 1996, 4:286-294 [PubMed] [Google Scholar]
- 23.Rapola J: Congenital nephrotic syndrome. Ped Nephrol 1987, 1:441-446 [DOI] [PubMed] [Google Scholar]
- 24.Kendall RL, Thomas KA: Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993, 90:10705-10709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takase K, Okazaki Y, Wakizaka K, Shevchenko A, Mann M, Saito T: Molecular cloning of pTAC12 an alternative splicing product of the CD3uchain as a component of the pre-T cell antigen receptor complex. J Biol Chem 1998, 273:30675-30679 [DOI] [PubMed] [Google Scholar]
- 26.Sugimura K, Tian X-l, Hoffmann S, Ganten D, Bader M: Alternative splicing of the mRNA coding for the human endothelial angiotensin converting enzyme: a new mechanism for solubilization. Biochem Biophys Res Commun 1998, 247:466-472 [DOI] [PubMed] [Google Scholar]
- 27.Säily M, Koistinen P, Pulkki K, Zheng A, Savolainen E-R: Acute myeloblastic leukemia cells produce soluble interleukin 6 receptor by a mechanism of alternative splicing. Cytokine 1998, 10:860-867 [DOI] [PubMed] [Google Scholar]
- 28.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Chow WS, Stern D, Schmidt AM: Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nature Med 1998, 4:1025-1031 [DOI] [PubMed] [Google Scholar]