Abstract
Deficiency of CD95 (Apo-1/Fas)-mediated apoptosis has recently been found in some autoimmune lymphoproliferative disorders due to inherited mutations of the CD95 gene. In this study, impairment of CD95 ligand-mediated killing of lymphocytes and eosinophils in Churg-Strauss Syndrome (CSS), which was a result of variation of CD95 receptor isoform expression, is demonstrated. Compared to those from healthy individuals, peripheral blood lymphocytes from eight CSS patients exhibit a switch from the membrane-bound CD95 receptor expression to its soluble splice variant, which protects from CD95L-mediated apoptosis. In five out of seven CSS patients recurrent oligoclonal T cell expansions were found, all using a Vβ-gene from the Vβ21 family associated with similar CDR3 motifs, indicating the predominance of T cell clones of a similar specificity in the CSS patients. In two of them, the effect of immunosuppressive therapy was studied. In both cases aberrant overexpression of the soluble CD95 receptor isoform and deviations from normal TCR Vβ-gene usage normalized in parallel with the clinical improvement. Furthermore, soluble CD95 was identified as a survival factor for eosinophils rescuing eosinophils from apoptosis in the absence of growth factors in vitro. Given the role of eosinophils as effector cells in CSS, these findings suggest that soluble CD95 may be mechanistically involved in the disease.
Churg-Strauss Syndrome (CSS) was for the first time described by Lotte Strauss and Jacob Churg in 1951 in The American Journal of Pathology, 1 defining a new entity with systemic vasculitis, blood and tissue eosinophilia, and a long-term history of asthma. Systemic vasculitis in this rare syndrome mainly affects the lower respiratory tract, kidneys, skin, and nervous system. 2 The gastrointestinal tract is involved in about 35% of the CSS cases. 2 Infiltrating eosinophils are frequently found in granulomatous lesions 3 and the fraction of eosinophils in the peripheral blood correlates with the course of the disease, 3,4 suggestive of a role of eosinophils in the pathogenesis of CSS. 5 On the other hand, T cells were frequently implicated in autoreactivity and inflammatory lesions. 6 However, a potential role of T lymphocytes in CSS and the molecular mechanisms underlying this disease have not yet been studied.
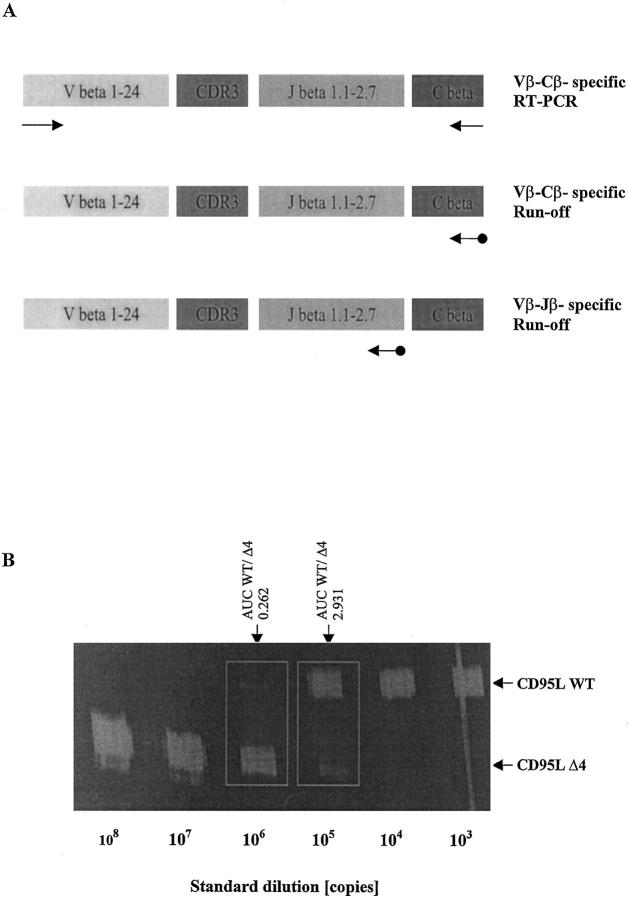
One tool to analyze T cell populations in clinical samples at the molecular level is the Immunoscope technique. 5 This approach is based on the hypothesis that the expression of unique, rearranged T cell receptor (TCR) genes reflects the specificity of a given T cell. 7 Each TCR-β chain consists of a variable (V), diversity (D), joining (J), and a constant region, the first three determining the antigen specificity in their VDJ-junction, including complementarity-determining region III (CDR3). During T cell differentiation, unique variable region genes are created by recombination of V, D and J segments for the TCR-β locus. More than 70 Vβ gene segments have been characterized and are classified into 24 gene families 8 (Figure 1A) ▶ .
Figure 1.
A: Immunoscope-based analysis of the clonality of TCRβ VDJ-transcripts. T cell receptor β (TCRβ) transcripts are reverse transcribed and amplified using a panel of 24 Vβ- and Cβ-specific primers. Thus, the first step of the Immunoscope technique involves 24 RT-PCR reactions run to saturation (top). In an initial low-resolution analysis, a dye labeled Cβ-specific primer is used to visualize the amplified products in run-off reactions (middle). If higher resolution is required, run-off experiments are carried out using 13 dye-labeled Jβ-specific primers in theoretically 24 × 13 = 312 run-off reactions (bottom). After electrophoresis on an automated sequencer and subsequent analysis, the different size peaks are separated and their CDR3 size in amino acids (aa) and area are calculated. B: Quantification of CD95 ligand and receptor isoform mRNA levels by quantitative competitive RT-PCR. The cDNA to be assayed (WT) was co-amplified with known amounts (108, 107, … 10 3 copies) of an internal DNA standard (Δ4), which was apart from a deletion of four nucleotides identical to the corresponding fragment of the assayed cDNA. PCR products were specifically labeled in run-off reactions, loaded on an acrylamide gel, and analyzed by an automated sequencer. The fluorescent profiles were recorded and the profile areas were analyzed. For co-amplifications with 10 6 and 10 5 copies of the CD95L standard, respectively, the peak area ratios for CD95L wild-type (CD95L WT) and standard (CD95L Δ4) were calculated. The number of CD95L WT copies in the cDNA sample was calculated as the mean of CD95L WT/CD95L Δ4 peak area ratios at two standard dilutions (eg, for the sample shown here: (0.262 × 106 + 2.931 × 105)/2, or 277,550 copies).
In T cells the CD95/CD95 ligand system is a major pathway of apoptotic cell death and thus essential for prevention of lymphoproliferative disorders and for autoimmunity. 9 The CD95 system, which consists of membrane-bound (CD95Tm) and soluble (CD95Sol) receptor isoforms generated by alternative splicing and their natural ligand, CD95L, holds a key position in the regulation of the immune response. 9 CD95Tm transduces the apoptotic signal after CD95L binding. 10 In contrast, soluble CD95 can prevent target cells from undergoing apoptosis by neutralizing CD95L. 11,12
Some lymphoproliferative diseases and autoimmune disorders have been explained by distinct mutations of the CD95 gene. 13-15 But somatic variations in CD95 expression have also recently been shown in two adult individuals, one with idiopathic eosinophilia and the second with HIV infection. 16 Recently, we described a deranged expression pattern of CD95 isoforms in a single case of Churg-Strauss vasculitis. 17 In the present study we demonstrate somatic deficiency of CD95-function with partial resistance of T cells toward CD95L-mediated apoptosis in peripheral blood T lymphocytes (PBL) from all eight CSS patients. Defective CD95L-mediated apoptosis was associated in five out of seven CSS cases with recurrent clonal T cell expansions all using the same V-gene with similar amino acid motifs in their CDR3.
Materials and Methods
Patients
Characteristics of eight CSS patients are summarized in Table 1 ▶ . The CSS patients studied here share the main criteria for CSS 3 with a history of asthma, eosinophilia, and systemic vasculitis. In patients LM and WI, the effect of immunosuppressive therapy on the clinical course of the disease, the expression pattern of CD95 receptor isoforms, and oligoclonal T-cell expansions in the peripheral blood were also studied.
Table 1.
Characteristics of CSS Patients
| Patient | Age | Sex | Organs involved | Eosinophilic counts (%) | ANCA titer |
|---|---|---|---|---|---|
| GG | 69 | male | lung, polyneuropathy | 19 | p-ANCA (1:80) |
| GM* | 23 | male | lung, kidney | 23 | c-ANCA (1:160) |
| LM* | 57 | female | lung, polyneuropathy | 36 | p-ANCA (1:80) |
| NB | 57 | female | lung, paranasal sinus | 13 | negative |
| PF* | 43 | male | lung, skin, gastrointestinal | 32 | c-ANCA (1:160) |
| SJ* | 56 | male | lung | 10 | negative |
| VN* | 49 | female | lung | 6 | negative |
| WI* | 48 | female | gastrointestinal, lung, skin | 35 | x-ANCA (1:80) |
*PBL from these patients were also used for functional apoptosis tests and for enzyme-linked immunosorbent assay for soluble CD95 of culture supernatants.
Patient LM is a 57-year-old woman in whom CSS was diagnosed in 1998 by peripheral blood and tissue eosinophilia, long-term history of asthma, pulmonary vasculitis, and the histopathological finding of eosinophilic granulomatous necrotizing vasculitis in a biopsy of N. suralis associated with peripheral polyneuropathy. Consecutive immunosuppressive treatments led to clinical improvement within 1 month.
Patient WI was recently described 16 and is a 48-year-old woman with a 22-year history of asthma. She presented in 1992 for the first time with severe alveolar hemorrhage. During the following two years there were three recurrences of pulmonary hemorrhage due to lung infarctions. In 1995, multiple cutaneous ulcerations appeared together with episodes of severe gastrointestinal bleeding. In 1997, twelve ulcerations of the upper gastrointestinal tract and four ulcerations of the colon were found. The ulcerations showed extensive eosinophilic infiltrates and granulomatous lesions. In parallel to the eosinophilic vasculitis and eosinophilic lung infiltrates, the counts of eosinophils in the peripheral blood were significantly increased (>10%). Because of severe gastric hemorrhage due to multiple ulcerations, gastrectomy and Y-Roux reconstruction were performed. The patient was treated with corticosteroids (prednisone 1 mg/kg/day) for 14 days, followed by significant clinical improvement. After discharge, immunosuppressive treatment with azathioprine (5 mg/kg/day) was continued for about 6 months. In 1998 the patient relapsed with ulcerations of the skin and an esophageal residue of gastric mucosa.
Tissue and Blood Samples
From patient WI, five independent tissue samples were obtained from normal gastric mucosa and from ulcerative gastric mucosa at the time of gastrectomy. After recurrence of the disease 1 year later, a biopsy from an ulcerative esophageal residue of gastric mucosa was obtained.
In peripheral blood from eight CSS patients and seven healthy volunteers, erythrocytes were selectively lysed using an erythrocyte lysis buffer (Qiagen, Hilden, Germany) and lymphocytes were recovered by centrifugation. From patients WI and LM blood samples were also available during and after immunosuppressive treatment. From patient PF peripheral blood eosinophils were isolated and purified as described below. In addition, peripheral blood eosinophils were purified from one 24-year-old male patient (DA) with infectious eosinophilia (25% eosinophilic counts) as a control and from three healthy donors.
Anti-neutrophil cytoplasmic antibody (ANCA) titers were determined by using immunofluorescence test.
Purification of Peripheral Blood Eosinophils
Eosinophils were purified from peripheral blood from non-allergic healthy donors (n = 3), one patient with infectious eosinophilia (patient DA, 25% eosinophilic counts) and one CSS-patient (patient PF, 32% eosinophilic counts) using Percoll (Pharmacia, Uppsala, Sweden) density gradient centrifugation and negative selection with an anti-CD16 monoclonal antibody and immunomagnetic beads coated with goat anti-mouse IgG (Miltenyi Biotec, Bergisch Gladbach, Germany) according to Hansel et al. 18 The purity of eosinophils based on light microscopic examination of the purified cells after staining with Diff-Quick (American Scientific Products, McGraw Park, IL) was >95% and viability was >98% as assessed by trypan blue (Sigma, Deisenhofen, Germany) exclusion. After purification, eosinophils were suspended in RPMI-1640 medium and supplemented with 10% fetal bovine serum (FBS).
Reagents and Antibodies
RPMI 1640 medium and FBS were purchased from Biochrom (Berlin, Germany), phytohemagglutinin (PHA) from Seromed (Berlin, Germany), and all other chemicals from Sigma (Deisenhofen, Germany). Oligonucleotides were synthesized by MWG-Biotech (Ebersberg, Germany). A rabbit polyclonal antibody raised against human CD95L and a mouse monoclonal antibody against human CD3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A rabbit polyclonal antibody (Santa Cruz Biotechnology) that binds to CD95 of human origin without induction of apoptosis was used to neutralize the anti-apoptotic activity of soluble CD95 in culture supernatants. A monoclonal agonistic mouse anti-human CD95 antibody (clone CH-11; Immunotech, Marseille, France) was used to induce apoptosis in CD95-bearing lymphocytes. Human recombinant CD95 ligand protein corresponding to soluble CD95 ligand (amino acids 103–261) and CD95:Fc (Ig) fusion molecule were from Alexis (San Diego, CA). Anti-CD16 microbeads were purchased from Miltenyi Biotec.
Immunoscope-Based Analysis of T Cell Receptor Repertoires
The Immunoscope approach to characterize the clonality of a given T-cell population is described in detail by Pannetier et al. 6 Briefly, T cell receptor β (TCRβ) transcripts are reverse transcribed and amplified using a panel of 24 Vβ-family and Cβ-specific primers 8 (Figure 1A ▶ , top). In an initial low-resolution analysis, a dye-labeled Cβ-specific primer is used to visualize the amplified products in run-off reactions (Figure 1A ▶ , middle). Because the amplified sequences are identical except for their CDR3 segment, PCR reactions run to saturation are quantitative. Accordingly, the relative intensity of a given size peak is proportional to the number of cDNA molecules sharing this CDR3 size. Thus, an increase in the height and area of a size peak signals a clonal expansion over the polyclonal background.
If higher resolution is required, run-off experiments are carried out using 13 dye-labeled Jβ-specific primers in theoretically 24 × 13 = 312 run-off reactions (Figure 1A ▶ , bottom). In practice, one may focus only on those Vβ segments that yielded potentially useful information in the first step. After electrophoresis on an automated sequencer and subsequent analysis, the different size peaks are separated and their CDR3 size in amino acids (aa) and area are calculated.
A normal transcript size distribution reflecting polyclonal cDNAs is bell-shaped and contains 6 to 8 peaks with a mean CDR3 size of 27 to 33 nucleotides or nine to 11 amino acids (aa). On the other hand, emergence of one or more dominant peaks reveals the presence of one or more cDNAs with a similar or identical in frame junctional region.
Quantitative Competitive Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA from snap-frozen tissue sections and freshly isolated T lymphocytes was preparated using a total RNA extraction kit (Qiagen, Hilden, Germany) and reverse transcribed using a first-strand cDNA synthesis kit (Boehringer Mannheim, Mannheim, Germany). The mRNA levels of CD95 ligand (CD95L), CD95 isoforms, CD3δ chain and hypoxanthine guanine phosphoribosyltransferase (HPRT), which was used for standardization, were determined using quantitative competitive RT-PCR. As shown in Figure 1B ▶ , the cDNA to be assayed was co-amplified with known amounts of an internal DNA standard (Δ4), which was, apart from a deletion of four nucleotides, identical with the corresponding fragment of the assayed cDNA. DNA standards were essentially constructed as previously described. 19 For construction of CD95L, CD95tm, CD95sol, CD3δ chain, and HPRT DNA standards, the respective 5′ and 3′ PCR primers (see below) were used to amplify a specific fragment in a human peripheral blood lymphocyte-derived cDNA. A 1000-fold dilution of this product was reamplified using the respective 3′ PCR primer (see below) and an additional construct primer containing a four-nucleotide deletion compared with the wild-type sequence. For quantification of the transcripts for CD95L, CD95Tm, CD95Sol, CD3δ chain, and HPRT, respectively, a constant amount of cDNA, corresponding to 50 ng reverse transcribed total RNA, was mixed with 108, 10 7 … 10 3 or 0 copies of the respective standard (Δ4) and then amplified to saturation (40 cycles of 94°C for 20 seconds, 58°C for 45 seconds, and 72°C for 45 seconds with 10 minutes’ extension time at 72°C on cycle 40). The primers used for PCR were 5′-GGCCACCCCAGTCCACC and 5′-CCGAAAAACGTCTGAGATTCC for CD95L, 5′-GGACATGGCTTAGAAGTGG and 5′-GGTTGGAGATTCATGAGAACC for both CD95 receptor isoforms, 5′-CCAGGCTGATAGTTCGGTGACC and 5′-TGTCTGAGAGCAGTGTTCCCAC for CD3δ chain and 5′-CCTGCTGGATTACATCAAAGCACTG and 5′-CACCAGCAAGCTTGCGACC for HPRT.
The read-out of the amplification involved one additional fluorescent dye-labeled oligonucleotide, which allows to discriminate between wild-type (WT) and standard (Δ4) DNA species (Figure 1B) ▶ . PCR amplification products were specifically labeled in run-off reactions, loaded on an acrylamide gel, and analyzed by an automated sequencer (ABI 373A, Applied Biosystems, Foster City, CA). The fluorescent dye-labeled (FAM) oligonucleotides used in run-off reactions were 5′-CATTGATCACAAGGCCACCC for CD95L, 5′-TCACCAGCAACACCAAGTGCAA for both CD95 isoforms, 5′-TCTATAGGTATCTTGAAGGGGCTC for CD3δ chain, or 5′-CCCCTGTTGACTGGTCATTACAATAG for HPRT. For CD95 receptor, both splice variants were simultaneously detected. In comparison to CD95Tm, the mRNA encoding for the soluble CD95 isoform (CD95Sol) lacking the transmembrane domain was shorter by 62 base pairs. The fluorescent profiles were recorded and the profile areas were analyzed using the software Immunoscope, which was kindly provided by Dr. C. Pannetier (Unité de Biologie Moléculaire du Gène, INSERM U277, Institut Pasteur, Paris, France).
Sequence Analysis of Rearranged TCRβ Genes
cDNAs derived from PBL of eight CSS patients and one patient with infectious eosinophilia were amplified with a primer for all members of the Vβ21 gene family (5′-AGGCAGAGTGTGGCTTTTTGG-3′) and a primer for the Jβ1.2 segment (5′-GGCTCGGGGACCAGGTTAACC-3′) yielding PCR products varying between 294 and 306 bp in length, depending on the number of N-nucleotides in the CDR3. After gel electrophoresis, PCR products were excised from the gels, the cDNA extracted with the QiaExII gel extraction kit (Qiagen) and directly sequenced using the BigDye Terminator cycle sequencing kit and an automated sequencer (ABI 377, Applied Biosystems, Weiterstadt, Germany). TCRβ sequences were compared with the EMBL IMGT database (http://genetik.uni-koeln.de). Sequence data are available under Genbank EMBL accession number AJ243648.
Detection of CD95-Specific Apoptosis in Lymphocytes and Eosinophils Incubated with an Agonistic Anti-CD95 Antibody
T lymphocytes freshly isolated from peripheral blood were activated by PHA (2.4 μg/ml). T lymphocytes or untreated eosinophils were incubated in the presence or absence of an agonistic anti-CD95 antibody at various concentrations. In control experiments the CD95:Fc (Ig) fusion molecule was used to neutralize CD95 ligand. After 24 hours, apoptosis in the lymphocytes was determined by the TdT-mediated fluorescein-dUTP nick end labeling (TUNEL) method as described recently. 20,21 Nuclei staining positive for TUNEL were counted and percentages of TUNEL-positive cells were calculated. The anti-apoptotic activity of the soluble CD95-splice variant in culture supernatants was blocked by a neutralizing rabbit anti-human CD95 IgG1.
Enzyme-Linked Immunosorbent Assay (ELISA) for Soluble CD95
For quantitative assessment of soluble CD95 levels in sera from seven CSS patients and 15 healthy donors (8 men and 7 women, age range, 19–36 years) an ELISA for soluble CD95 from Alexis was used. For each blood sample, 10 μl serum were collected and the ELISA was performed following the manufacturer’s protocol. In addition, the content of soluble CD95 in supernatants derived from cultured T lymphocytes was determined. From four healthy volunteers (3 men, 1 woman, aged 24–35 yrs) and five CSS patients (Table 1 ▶ , marked by asterisks) lymphocytes were isolated from the peripheral blood and 5 × 10 6 lymphocytes were incubated in 1 ml of RPMI medium supplemented with 10% fetal bovine serum for 24 hours. Lymphocytes were removed by centrifugation and the supernatants were collected. Determination of soluble CD95 was carried out with supernatants derived from the same lymphocyte preparations which were also used for the TUNEL assay (see above). Each determination (serum and supernatant) was carried out in duplicate. Contents of soluble CD95 were calculated using an automated ELISA reader (Anthos, Cologne, Germany) at a wavelength of 450 nm, based on the results of a standard dilution curve.
Immunohistochemical Procedures
From patient WI cryosections (5 μm) of ulcerative and nonulcerative gastric tissue (mucosa and muscularis propria) were double-stained with mouse anti-human CD3 IgG1 and rabbit anti-human CD95L IgG1 antibodies as previously described. 20,21 Goat anti-mouse IgG1 (FITC-labeled) and goat anti-rabbit IgG1 (CY3-labeled) were used as secondary antibodies. No cross-reactivity between the secondary antibodies was found. Nonspecific binding of primary antibodies was excluded by parallel stainings with normal mouse IgG1 and normal rabbit IgG1 instead of the primary antibodies.
Statistics
Data are expressed as means ± SE (n = number of independent experiments). Statistical analysis was performed using Student’s t-test. P < 0.05 was considered to be statistically significant.
Results
The clinical and biochemical features of the eight patients with CSS are summarized in Table 1 ▶ . The patients showed a history of asthma, predominant lung involvement in six of seven cases and elevated peripheral blood eosinophilic counts ranging between 6 and 36% (normal eosinophilic counts range between 1 and 3%). Anti-neutrophil cytoplasmic antibodies (ANCAs) have been detected in five of eight cases.
Deviations from Normal TCR Vβ Chain Repertoire of T Lymphocytes in CSS Patients
In order to characterize the clonality of T lymphocytes in the CSS patients, T cell receptor (TCR) β chain transcripts were analyzed in peripheral blood lymphocytes (PBL) from seven CSS patients for all 24 Vβ gene families. In all cases deviations from normal diversity possibly corresponding to clonal expansions were found in several Vβ subfamilies. The Vβ21 segment was involved in all, the Vβ11 segment in three, and the Vβ12 subfamily in two out of seven CSS patients. There were five clonal expansions that shared the same CDR3 length using a V-gene from the Vβ21 gene family and two in Vβ11 using T cells, whereas expansions involving the Vβ12 family had different CDR3 sizes. The clonality of these Vβ11- and Vβ21-specific transcripts in T lymphocytes from the CSS patients was further analyzed using Jβ-specific primers. Interestingly, in five out of seven CSS cases clonal expansions were detected, all using a V-gene from the Vβ21 family and the Jβ1.2 segment with a dominant peak corresponding to a translated CDR3 size of 11 aa in length (Figure 2) ▶ . In one of the two remaining cases, oligoclonal expansions using a V-gene from the Vβ21 family were also detectable, albeit with CDR3 size peaks corresponding to CDR3 sizes of 8 or 9 aa in length (patient NB). In one case (patient GG), RT-PCR amplification of Vβ21 family specific transcripts was not sufficient (not shown).
Figure 2.
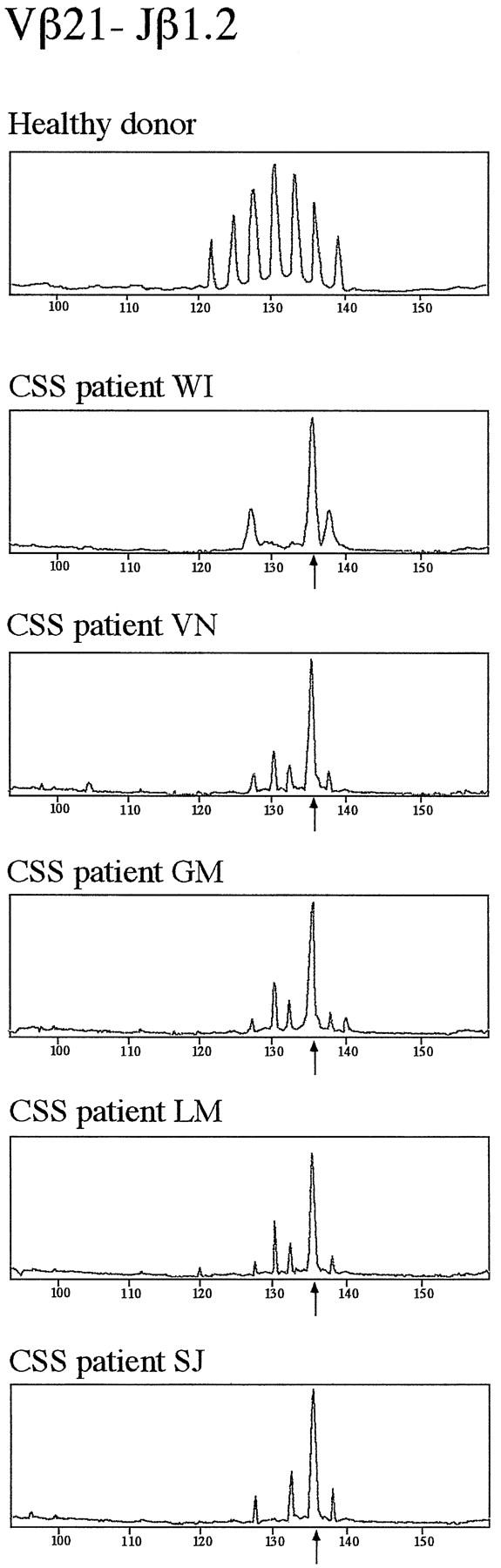
Preferential usage of distinct TCR-Vβ and Jβ genes in T cells from five CSS patients. Fluorescent profiles of the Vβ21-Jβ1.2 rearrangements are shown in samples from the peripheral blood of one healthy donor and five CSS patients (WI, VN, GM, LM, and SJ). The peripheral blood samples of these five CSS patients contained clonally expanded T cells carrying a gene from the Vβ21 gene family rearranged to the Jβ1.2 gene with a dominant CDR3-size peak corresponding to a CDR3 of 11 aa in length (arrows).
PBL-derived cDNAs from all eight cases of CSS were amplified with a set of Vβ21- and Jβ1.2-specific primers that was distinct from the primers used for Immunoscope. As for Immunoscope analysis (Figure 2) ▶ , Vβ21- and Jβ1.2-specific PCR yielded one predominant product, which was directly sequenced. In Table 2 ▶ the protein sequences of TCRβ VDJ junctions, translated from DNA sequence, are aligned. In patients GG and NB, no readable junctional sequence could be obtained. This is consistent with the absence of a dominant CDR3-size peak in the Immunoscope analysis, which is obviously due to polyclonality of Vβ21-positive T cells in these patients. From the six remaining CSS patients, similar CDR3 motifs corresponding to a CDR3 length of 11 aa could be obtained. In all six patients the Vβ21.3 gene was used. Vβ21.3- and Jβ1.2 transcripts were unmutated in all patients and their VDJ junction yielded a potentially functional gene product. Within the junctional region, the 3′ end of the Vβ21.3 segment as well as the 5′ end of the Jβ1.2 segment were conserved in all CSS patients. Regarding the N-regions, four out of six patients share a glycine-threonine (GT) motif at positions two and three. The two remaining patients share an alanine (A) at position two. From three patients (PF, SJ, and WI), a second Vβ21.3-Jβ1.2 rearrangement could be amplified with a CDR3 length corresponding to 12 aa in length (Table 2 ▶ , asterisk). Whereas patients PF and SJ have the first three amino acids in common for these junctions (leucine-glutamine-alanine, LQA), there was no sequence homology with the 12aa CDR3 motif from patient WI. However, the 11-aa CDR3-junction from patient LM shares all three positions of its N-region with the 12-aa CDR3 from patient WI (serine-alanine-threonine, SAT) but only one with the 11-aa CDR3 junction from the same patient (threonine, T, at position three; Table 2 ▶ ). Taken together, the sequence data suggest the emergence of two motifs of significant homology in the TCRβ N-regions of the six CSS-patients, S〈A/G〉T (serine-alanine/glycine-threonine) and LQA (leucine-glutamine-alanine) and thus similar specificities of the TCRs of the clonally expanded T cells in these patients.
Table 2.
Sequence Analysis of TCRβ-VDJ Junctions in CSS Patients
| Patient | Junctional Sequence | ||
|---|---|---|---|
| Vβ21 | D, N | Jβ 1.2 | |
| VN | CASS | FAF | YGYT |
| WI | CASS | FGT | YGYT |
| GM | CASS | AGT | YGYT |
| PF | CASS | SGT | YGYT |
| LM | CASS | SAT | YGYT |
| WI* | CASS | FSAT | YGYT |
| SJ | CASS | LGT | YGYT |
| PF* | CASS | LQAGS | GYT |
| SJ* | CASS | LQAA | YGYT |
Transcripts from rearranged TCRβ VDJ genes were amplified from cDNAs derived from PBL of eight CSS patients using Vβ21- and Jβ1.2-specific primers. With the exception of cDNAs from patients GG and NB, the sequence of the TCRβ VDJ-junctions were readable in six patients with a CDR3 of 11 aa in length. Protein sequence information is given as single letter amino acids derived from the 3′ end of the rearranged Vβ21.3 gene (Vβ21), the germline encoded D-region, the N-region generated during VDJ-recombination (D,N), and the 5′ end of the rearranged Jβ1.2 gene (Jβ1.2). Amino acids matching the S〈G/A〉T motif are underlined. Those fitting the LQA motif are in bold type.
*Sequences that correspond to additional TCRβ VDJ-rearrangements with a CDR3 length of 12aa amplified from patients PF, SJ and WI.
Diversity of TCR Vβ Chain Repertoire in Gastric IEL from Patient WI
In patient WI systemic vasculitis due to CSS mainly involved the gastrointestinal tract with multiple highly infiltrated gastric ulcerations. Intraepithelial lymphocytes (IEL) from five ulcerative and non-ulcerative gastric tissue samples of this patient were studied for their TCRβ chain repertoire and oligoclonal expansions were found. Expansions that were also found in non-ulcerative gastric mucosa were excluded from further analysis. Four clonal IEL expansions were detected in all biopsies from different ulcerative sites but not in any of five non-ulcerative sites, suggesting that these expansions are specific for ulcerative gastric mucosa from this patient. Analysis of TCR Vβ gene usage focused on these expansions. Among them, clonally expanded T cells using the Vβ11 and the Vβ21 segment from ulcerative gastric mucosa were also found in the peripheral blood (Figure 3) ▶ . However, Vβ11-Cβ and Vβ21-Cβ profiles were clonal for gastric IEL but polyclonal for PBL. Whereas clonal expansions in IEL from ulcerative gastric mucosa represent the most part of the peak area of the Vβ-Cβ profiles, this is not the case in PBL, indicating that cells with expansion in the Vβ11 and the Vβ21 segment are concentrated in the ulcerative gastric mucosa when compared to the peripheral blood (Figure 3) ▶ . The clonality of the Vβ11-Cβ and Vβ21-Cβ transcripts was further confirmed by the results of run-off reactions using Jβ-specific primers (Figure 3) ▶ .
Figure 3.
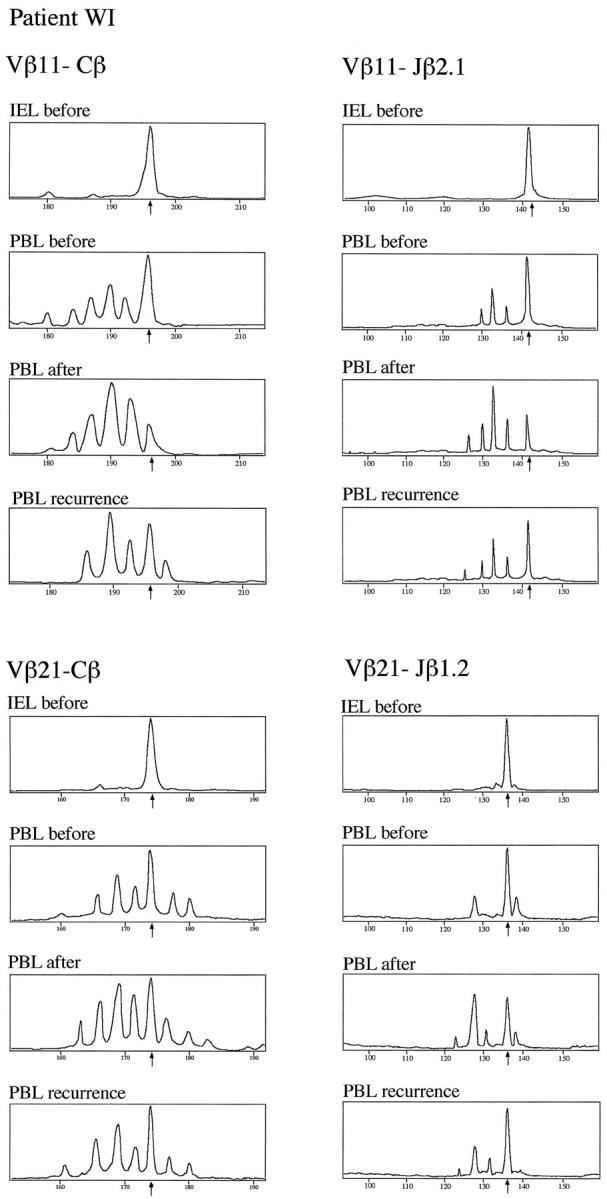
T cell repertoire diversity and clonal expansions in gastric IEL and PBL from Churg-Strauss Syndrome patient WI. Using the Immunoscope RT-PCR technique, TCRVβ chain segments in IEL and PBL from patient WI were amplified using a panel of 24 Vβ family-specific PCR primers. Clonal expansions were detected in several Vβ-Cβ profiles. Clonality of Vβ11- and Vβ21-specific transcripts is shown here. Samples were analyzed from infiltrated ulcerative gastric mucosa (IEL before) and the peripheral blood before (PBL before) and after immunosuppressive treatment (PBL after) as well as after recurrence of CSS (PBL recurrence). The peak sizes of clonal expansions are indicated by arrows. Clonality of the Vβ-Cβ profiles was further analyzed using Jβ-specific primers. For both Vβ-Cβ profiles, each one of 13 Vβ-Jβ recombinations was selected to demonstrate the strongest deviation from normal polyclonal gaussian-like peak size distribution, ie, Vβ11-Jβ2.1 for the Vβ11 gene family and Vβ21-Jβ1.2 for the Vβ21 gene family.
Effect of Immunosuppressive Treatment on Clonal T Cell Expansions in Patients LM and WI
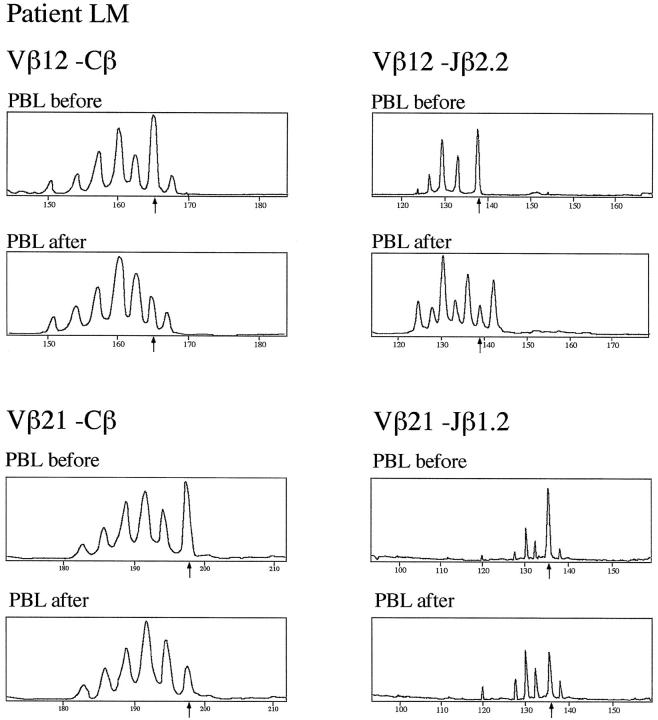
In patients LM and WI, the effect of immunosuppressive therapy on the clonality of T cells in the peripheral blood was studied. In patient LM, preexisting clonal expansions using the Vβ12 and the Vβ21 family were diminished after 1 month of immunosuppressive treatment (Figure 4) ▶ . The Vβ-Cβ profiles of both Vβ subfamilies regained almost normal gaussian-like distribution, which was confirmed using Jβ-specific run-off primers. Similarly, in patient WI, immunosuppressive treatment led within 14 days to a marked reduction of the clonal deviations from normal diversity that were found in the Vβ11-Cβ and the Vβ21-Cβ profiles (Figure 3) ▶ . Interestingly, after cessation of immunosuppressive treatment after 6 months, patient WI sustained a recurrence of CSS and the deviating peaks in the Vβ11-Cβ and the Vβ21-Cβ profiles (both corresponding to a CDR3 size of 11 aa) were again markedly increased (Figure 3) ▶ .
Figure 4.
T-cell repertoire diversity and clonal expansions in PBL from Churg-Strauss syndrome patient LM. Using the Immunoscope RT-PCR technique, TCRVβ chain segments in PBL from patient LM was amplified using a panel of 24 Vβ subfamily-specific PCR primers. Clonal expansions was detected in several Vβ-Cβ profiles. Clonality of Vβ12- and Vβ21-specific transcripts is shown here. Samples were analyzed from the peripheral blood prior to (PBL before) and after immunosuppressive treatment (PBL after). Clonal expansions are indicated by arrows. Clonality of the Vβ-Cβ profiles was confirmed using Jβ-specific primers. For both Vβ-Cβ profiles, each of 13 Vβ-Jβ recombinations was selected to demonstrate the strongest deviation from normal polyclonal gaussian-like peak size distribution, ie, Vβ12-Jβ2.2 for the Vβ12 family and Vβ21-Jβ1.2 for the Vβ21 family.
The association of the occurrence and disappearance of clonal T cell expansions with the clinical course of CSS in these two patients may suggest a role for specific T cell expansions in these cases of CSS.
CD95 Ligand and Receptor mRNA Expression by PBL and Gastric IEL
In order to elucidate the potential role of T cells in CSS, the regulation of the CD95 system, a key regulator of lymphocyte maintenance, 22 was studied in T cells. The mRNA expression for CD95 ligand (CD95L), the membrane-bound CD95 receptor (CD95Tm), and its soluble splice variant (CD95Sol) were studied by means of quantitative RT-PCR in PBL from five healthy volunteers, in PBL from seven CSS patients, and in gastric IEL from ulcerative and non-ulcerative gastric mucosa of CSS patient WI.
In PBL from the CSS patients CD95L mRNA expression was about eightfold higher when compared to healthy volunteers (Table 3) ▶ . CD95Tm mRNA levels were reduced to about 50% in the CSS patients, whereas mRNA levels for its soluble splice variant, which neutralizes CD95L, were about fivefold higher than in normal PBL (Table 3) ▶ .
Table 3.
CD95 Ligand and Receptor Isoform mRNA Expression in CSS Patients
| CD95L | CD95Tm [copies/HPRT copy] | CD95Sol | |
|---|---|---|---|
| CSS patients (n = 7) | 1.79 ± 0.44* | 0.14 ± 0.02* | 0.24 ± 0.04* |
| Healthy donors (n = 7) | 0.21 ± 0.02 | 0.25 ± 0.02 | 0.05 ± 0.01 |
| Patient LM | |||
| before treatment | 2.31 | 0.15 | 0.19 |
| after treatment | 0.32 | 0.21 | 0.08 |
| Patient WI | |||
| before treatment | 1.17 | 0.12 | 0.42 |
| after treatment | 0.46 | 0.19 | 0.06 |
| recurrence | 1.31 | 0.13 | 0.28 |
T lymphocytes were isolated from the peripheral blood, mRNA was extracted and reverse transcribed as described in Materials and Methods. Quantitative competitive RT-PCR was performed on the mRNA samples. mRNA levels are given as copies/copy HPRT.
*Significantly different from the healthy donors (P < 0.05).
As assessed by an ELISA technique, serum-levels of soluble CD95 were increased fivefold in the CSS patients when compared to 15 healthy donors (Table 4) ▶ . Moreover, levels of soluble CD95 in supernatants derived from lymphocyte cultures from CSS patients were also fivefold higher when compared to supernatants derived from cultured lymphocytes from healthy donors. Thus, increased serum levels of soluble CD95 originate mainly from PBL.
Table 4.
Levels of Soluble CD95 in Serum and in Supernatants from Cultured Lymphocytes and Sensitivity to CD95-Mediated Apoptosis
| CD95Sol [ng/ml] | Apoptotic lymphocytes [%] | |
|---|---|---|
| Serum | ||
| CSS patients (n = 7) | 71.9 ± 13.7 | n.a. |
| healthy donors (n = 15) | 13.9 ± 1.5 | n.a. |
| Supernatants | ||
| cultured PBL, CSS (n = 5) | 321.6 ± 14.8 | 41 ± 6 |
| cultured PBL, HD (n = 4) | 68.3 ± 16.8 | 90 ± 2 |
| Supernatants | ||
| cultured PBL, patient WI | ||
| before treatment | 361.9 | 37 |
| after treatment | 99.5 | 81 |
| Supernatants | ||
| cultured PBL, patient LM | ||
| before treatment | 350.6 | 29 |
| after treatment | 48.1 | 84 |
Sera from blood samples of seven CSS patients and 15 healthy volunteers were collected and analyzed for their content of soluble CD95 by an ELISA technique as described in Materials and Methods. T lymphocytes were isolated from the peripheral blood of five CSS patients (PBL, CSS) and four healthy donors (PBL, HD) and were incubated in the presence of PHA (2.4 μg/ml) for 24 hours. Half of the lymphocytes were treated with 100 ng/ml of an agonistic anti-CD95 antibody for 24 hours. Percentages of apoptotic lymphocytes were assessed by the TUNEL method. From the remaining cultures lymphocytes were removed, supernatants were collected, and their content of soluble CD95 was determined by ELISA. For patients LM and WI the effect of immunosuppressive therapy is shown. Data are expressed as means ± SE, n.a., not applicable.
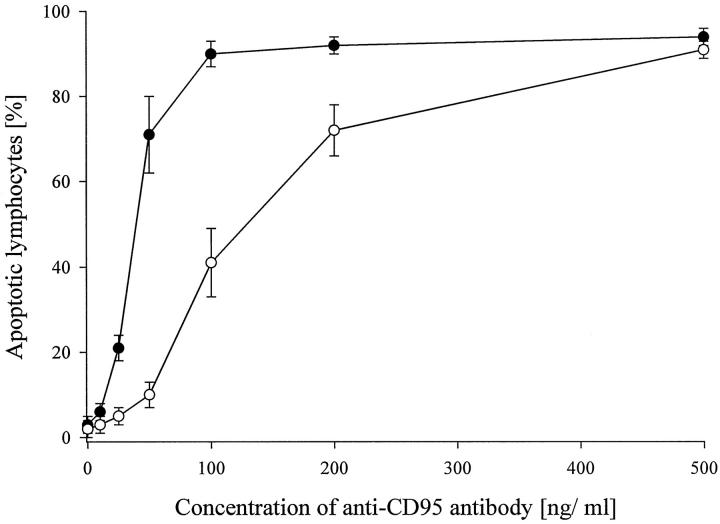
The functional significance of the aberrant distribution of the mRNA expression for the two CD95 receptor isoforms was assessed using an agonistic anti-CD95 antibody, which induces apoptosis in CD95L-sensitive cells. Only about 40% of the PBL of the CSS patients underwent apoptosis upon exposure to an agonistic anti-CD95 antibody (100 ng/ml), whereas 90% of the PBLs from five healthy donors became apoptotic under these conditions (Figure 5) ▶ . CD95 was functional in all CSS patients except that, for a yield of more than 90% apoptotic PBLs, about fivefold higher agonistic anti-CD95 antibody concentrations were required (Figure 5) ▶ . PBL from healthy donors acquired secondary resistance to CD95-mediated apoptosis when they were incubated with supernatants derived from PBL of the CSS patients (Table 5) ▶ , indicating that soluble CD95 in the supernatants also may rescue lymphocytes of healthy individuals from CD95L-mediated apoptosis. Supernatants derived from cultured CSS lymphocytes were less protective when they were pretreated with an anti-CD95 antibody that was raised against the binding site for CD95L (Table 5) ▶ .
Figure 5.
Sensitivity of PBL to CD95-mediated apoptosis. Lymphocytes were isolated from the peripheral blood from four healthy donors (filled circles) and from five CSS-patients (open circles). After treatment with PHA (2.4 μg/ml) for 24 hours, the lymphocytes were incubated with an agonistic anti-CD95 antibody at various concentrations. After another 24 hours, the lymphocytes were subjected to TUNEL analysis as described in Materials and Methods and percentages of apoptotic lymphocytes were counted. Data are given as means ± SE.
Table 5.
PBL from Healthy Donors Are Rescued from CD95-Mediated Apoptosis by Supernatants from Cultured CSS-PBL
| Apoptotic lymphocytes [%] | |
|---|---|
| Control | 89 ± 4 |
| CSS-Sn | 66 ± 7* |
| CSS-Sn+ neutralizing anti-CD95 | 90 ± 6 |
T lymphocytes from three healthy volunteers were isolated from the peripheral blood and incubated either in RPMI medium (control) or with a mixture of supernatants which have been derived from cultured lymphocytes from three CSS-patients (CSS-Sn). After 24 hours, lymphocytes were treated with 100 ng/ml of an agonistic anti-CD95 antibody for another 24 hours and subjected to TUNEL analysis. In some experiments, the supernatant mixture was pretreated with a neutralizing anti-CD95 antibody at 1 μg/ml (neutralizing anti-CD95). Data are from three independent experiments and given as means ± SE.
*Significantly different from the control (P < 0.05).
Taken together, these results identify soluble CD95 originating from PBL as an important inhibitor of CD95L-mediated apoptosis in CSS, which may protect lymphocytes themselves, as well as other CD95-bearing cells, from CD95L-dependent apoptosis.
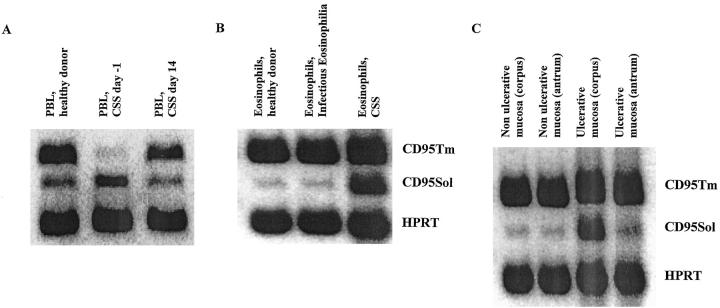
Regarding intraepithelial lymphocytes (IEL) infiltrating the gastric mucosa in patient WI, mRNA levels for CD95L were about 20-fold higher in ulcerative mucosa when compared to non-ulcerative gastric mucosa (ulcerative: 3.16 ± 0.71 CD95L copies/HPRT copy; non-ulcerative 0.14 ± 0.06 CD95L copies/HPRT copy; samples from five different areas). Comparing mRNA levels for CD95 isoforms in normal and ulcerative gastric mucosa, the soluble splice variant was overexpressed in ulcerative tissue from various ulcerative sites (Figure 6C) ▶ .
Figure 6.
Distribution of CD95 receptor isoform mRNA expression in peripheral blood T cells (PBL), gastric intraepithelial T cells (IEL) and eosinophils. A: cDNAs derived from PBL of one healthy donor, patient WI 1 day prior to (CSS, day −1) and 14 days after gastrectomy and immunosuppressive treatment (CSS, day 14) were amplified using CD95 isoform- and HPRT-specific primers as described in Materials and Methods in 30 PCR cycles. Amplification products for membrane-bound CD95 (CD95Tm, 392 bp), soluble CD95 (CD95Sol, 330 bp), and HPRT (237 bp) were separated on a 2% agarose gel. B: CD95 membrane-bound and soluble isoform mRNA expression was analyzed in eosinophils from healthy donors (representative for three donors), one patient with infectious eosinophilia (patient DA, 25% eosinophilic counts), and one CSS patient (patient PF, 32% eosinophilic counts). C: CD95 isoform mRNA expression in ulcerative and non-ulcerative gastric mucosa was studied in samples from gastric antrum and corpus of patient WI at the time of gastrectomy.
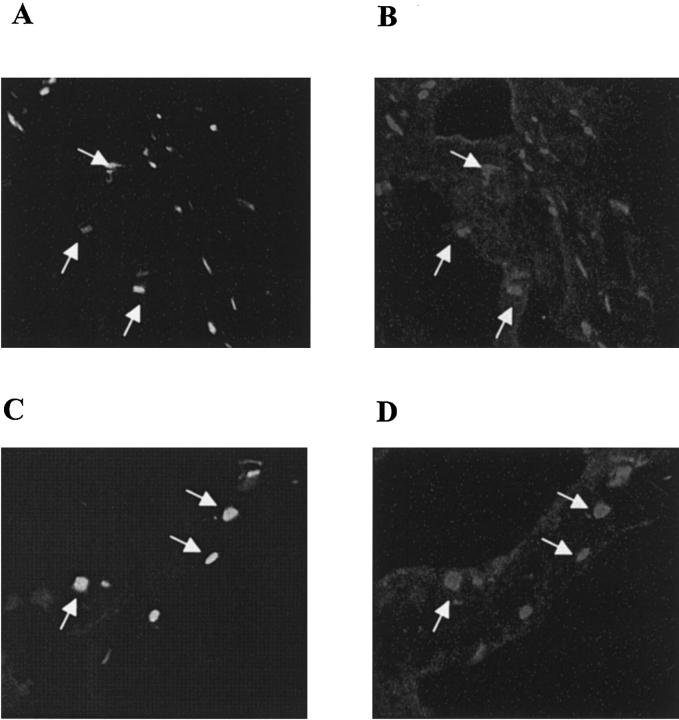
Because normal gastric epithelia are known to express CD95 but not CD95L, 23 extensive CD95L expression in the area of affected gastric mucosa could account for the ulcerative tissue damage observed in this case. The contribution of IEL to CD95L expression in ulcerative gastric mucosa was estimated by quantitative RT-PCR using CD3δ chain-specific primers. In five non-ulcerative gastric tissue samples, both CD95L and CD3δ chain mRNA levels were low and tightly correlated (CD3δ copies/HPRT copy: 0.43 ± 0.1; CD95L copies/HPRT copy: 0.14 ± 0.03; r = 0.94; P < 0.01). In contrast, in five ulcerative tissue samples high copy numbers for both CD95L and CD3δ chain transcripts were present with a strong correlation (CD3δ copies/HPRT copy: 2.67 ± 0.46; CD95L copies/HPRT copy: 3.16 ± 0.32; r = 0.96; P < 0.01). Because gastric epithelia lack CD95L expression, 23 gastric IEL are a likely source of CD95L expression in the ulcerative lesions. In cryosections from ulcerative gastric mucosa of patient WI CD3-expressing cells (ie, T lymphocytes) and CD95L expression were colocalized, identifying T lymphocytes as CD95L-expressing cells in gastric ulcerations (Figure 7) ▶ .
Figure 7.
Identification of T cells as CD95L-expressing cells in ulcerative gastric mucosa in patient WI. Cryosections from ulcerative gastric mucosa from patient WI were double-stained using CD3-specific and CD95L-specific antibodies as described in Materials and Methods. In A and B, one cryosection from infiltrated muscularis propria and in C and D one from infiltrated mucosa is shown. Double fluorescence staining for CD3 and CD95L was performed on the same cryosection. In A and C, signals are specific for CD3 (FITC-labeled), in B and D, for CD95L expression (CY3-labeled). CD3-expressing cells (ie, T lymphocytes) and CD95L expression were colocalized (arrows).
In patients LM and WI the effect of immunosuppressive therapy on the expression of CD95L and CD95 receptor isoforms was studied. Interestingly, the aberrantly increased mRNA levels for CD95L and soluble CD95 were largely reduced in both patients in response to immunosuppressive treatment (Table 3) ▶ . Normalization of the CD95 system was reached in parallel with clinical improvement. Recovery of patient WI after 14 days of treatment and patient LM after 1 month involved resistance to CD95-mediated apoptosis in cultured lymphocytes, elevated serum levels of soluble CD95, increased mRNA levels for soluble CD95, and oligoclonal T cell expansions (see above). Similarly, CD95L mRNA levels in PBL from patients LM and WI were reduced to mRNA levels observed in healthy individuals (Table 3) ▶ . However, a recurrence of CSS in patient WI after 1 year was associated with newly increased mRNA levels for both soluble CD95 (sixfold) and CD95L (fourfold) in PBL as well as increased serum levels for soluble CD95.
Soluble CD95 as an Eosinophil Survival Factor
Given the role for eosinophils as specific effector cells in CSS, 5 the expression of CD95 isoforms was analyzed in eosinophils as well as the effect of soluble CD95 on eosinophil survival under cell culture conditions. For this analysis, highly purified eosinophils were isolated from the peripheral blood of one untreated CSS patient (PF), one patient with infectious eosinophilia (DA), and three nonallergic healthy donors. As shown in Figure 6B ▶ , eosinophils purified from CSS patient PF exhibit high mRNA levels for soluble CD95, whereas eosinophils from healthy donors, as well as from one patient with infectious eosinophilia, expressed mRNA for soluble CD95 at low levels.
After incubation for 48 hours under cell culture conditions, 42 ± 7% (n = 3) of freshly isolated eosinophils underwent spontaneous apoptosis in the absence of exogenous survival factors (eg, interleukin-5). In comparison, only 13% of the eosinophils isolated from the CSS patient (PF) became apoptotic under the same conditions. When 100 μg/ml of an artificial soluble CD95 molecule, which blocks engagement of CD95 by CD95L, was added to the eosinophil cultures from healthy donors, susceptibility of eosinophils to CD95L-mediated apoptosis was significantly reduced, as only 16 ± 5% (n = 3) of the eosinophils underwent apoptosis in the presence of the CD95:Fc (Ig) fusion molecule. When 100 ng/ml of an agonistic anti-CD95 antibody was supplemented to eosinophil cultures from the CSS patient and healthy donors after 24 hours of cell culture for another 24 hours, the fraction of apoptotic eosinophils increased for the CSS patient up to 62% and, for eosinophils from healthy donors, above 90% (n = 3).
In conclusion, the CD95/CD95L system interferes with eosinophil survival in vitro and in particular soluble CD95 favors eosinophil survival, delivering partial resistance of eosinophils towards elimination by apoptosis.
Discussion
Impairment of CD95 Function in CSS
In some cases autoimmune disorders have been explained by mutations of the CD95 gene. 13-15 Likewise, lpr/lpr-mice, lacking functional CD95, show several features of lymphoproliferation 9 and autoimmune disorder 24 such as systemic vasculitis and glomerulonephritis. In humans, CD95 mutations have been found in Canale-Smith Syndrome 13 and autoimmune lymphoproliferative syndrome (ALPS). 14,15
Because almost normal CD95 function could be demonstrated after immunosuppressive treatment in PBL of CSS patients LM and WI (Tables 4 and 5) ▶ ▶ , deficiency of CD95-mediated lymphocyte killing in the CSS cases studied here cannot be explained by inherited mutations of the CD95 gene. Moreover, PBL from all seven CSS patients were sensitive towards CD95L-mediated apoptosis, except that, for a yield of more than 90% apoptotic PBL, about fivefold higher concentrations of the agonistic anti-CD95 antibody were required (Figure 5) ▶ .
The data are suggestive of an intervening factor competing with CD95 for binding to its natural ligand, CD95L. The soluble isoform of CD95 that is generated by alternative splicing was identified as the competitive agent. The soluble isoform of CD95 has been demonstrated to neutralize CD95L and thus to protect CD95-bearing cells from CD95 ligation and consecutive induction of apoptosis. 11,12 Blocking CD95L by the soluble CD95 isoform may be implicated in the failure of termination of the immune response and autoimmunity.
Given the high eosinophilic counts typically found in CSS patients and the fact that eosinophils are efficiently eliminated by CD95-mediated apoptosis, 25 it appears conceivable that soluble CD95 could act as a survival factor for eosinophils in CSS. In the present study, this hypothesis is supported by the findings that eosinophils from one CSS patient overexpress soluble CD95, whereas eosinophils from healthy donors do not, and that healthy eosinophils can be rescued by an artificial soluble CD95 molecule (CD95:Fc) from the spontaneous apoptosis they otherwise would undergo in the absence of other survival factors such as IL-5. 25 Because eosinophils are known for their role as specific effector cells in CSS, 5 the effect of soluble CD95 on eosinophil survival suggests that soluble CD95 may be mechanistically involved in the disease. In the presence of soluble CD95, eosinophils may bypass negative selection and escape removal by induction of apoptosis even after withdrawal of essential survival factors as, eg, IL-5.
As shown at the mRNA and protein levels, soluble CD95 is also strongly expressed by PBL in the CSS patients studied here. In addition, T cells and eosinophils overexpressing soluble CD95 were similarly protected against CD95L-mediated apoptosis. Moreover, T cells from healthy volunteers escaped CD95L-mediated apoptosis when they were incubated in medium conditioned by CSS lymphocytes that released soluble CD95.
Soluble CD95: A Survival Factor for Autoaggressive T Cells?
Because the CD95 system is a key regulator of T cell homoeostasis, 22 imbalance of its constituents may cause autoimmunity or immunodeficiency. In all eight cases of CSS studied here, overexpression of soluble CD95, which protects T cells from CD95-mediated apoptosis, was associated with clonal T cell expansions. In two cases the effect of immunosuppressive therapy could be studied and in both, expression of soluble CD95 and clonal T cell expansions were markedly reduced. In view of previously published data, 16,22 one might speculate that the clonal T cell expansions might represent autoaggressive T cell populations and that high levels of soluble CD95 may protect them from apoptotic removal. Overexpression of soluble CD95 might then equally favor survival and proliferation of eosinophils and self-reactive T cells. Clonally expanded T cells in the CSS patients studied here show preferential V-gene usage for a gene from the Vβ21 family. Furthermore, sequence analysis of the dominant T cell clones revealed two recurrent motifs of their TCRβ-VDJ junction, reflecting similar TCR specificities. The N-region homologies among the six CSS patients cannot be explained as random events, and, together with the preferential use of one individual gene in the Vβ21 family, this strongly argues for the recognition of one or a limited number of common antigens in six of the CSS patients by consecutively expanding T cell clones. In fact, based on clinical observations, inhaled antigen was proposed to be implicated in triggering CSS. 26 For instance, one of these antigens could originate from Actinomycetes thermophilus, which in some cases has been shown to precipitate the onset of active CSS. 26
Acknowledgments
We thank Prof. Dr. Philippe Kourilsky (Institut Pasteur, Paris) for encouraging discussions, Dr. Christophe Pannetier (Institut Pasteur) for providing the Immunoscope software, and Helga Landmann-Crijns for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Ulrich Warskulat, Medizinische Universitätsklinik, Heinrich-Heine Universität Düsseldorf, Moorenstrasse 5, D-40225 Düsseldorf, Germany. E-mail: warskula@uni-duesseldorf.de.
M. M. and U. W. contributed equally to this work.
References
- 1.Churg J, Strauss L: Allergic granulomatosis. Allergic angiitis, and periarteriitis nodosa. Am J Pathol 1951, 27:277-301 [PMC free article] [PubMed] [Google Scholar]
- 2.Modigliani R, Muschart JM, Galian A, Clauvel JP, Desruisseaux JL: Allergic granulomatous vasculitis: report of a case with digestive involvement. Dig Dis Sci 1981, 26:264-270 [DOI] [PubMed] [Google Scholar]
- 3.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY: The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome. Arthritis Rheum 1990, 33:1094-1102 [DOI] [PubMed] [Google Scholar]
- 4.Lanham GJ, Elkon KB, Pusey CD, Hughes GR: System vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine 1984, 63:65-81 [DOI] [PubMed] [Google Scholar]
- 5.Schmitt WH, Csernok E, Kobayashi S, Klinkenborg A, Reinhold-Keller E, Gross WL: Churg-Strauss syndrome: serum markers of lymphocyte activation and endothelial damage. Arthritis Rheum 1998, 41:445-452 [DOI] [PubMed] [Google Scholar]
- 6.Pannetier C, Even J, Kourilsky P: T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today 1995, 16:176-181 [DOI] [PubMed] [Google Scholar]
- 7.Davis MM, Bjorkmann PJ: T-cell antigen receptor genes and T-cell recognition. Nature 1988, 334:395-402 [DOI] [PubMed] [Google Scholar]
- 8.Génevée C, Diu A, Nierat J, Caignard A, Dietrich PY, Ferradini L, Roman-Roman S, Triebel F, Hercend T: An experimentally validated panel of subfamily-specific oligonucleotide primers (Vα1-W29/Vβ1-W24) for the study of human T-cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol 1992, 22:1261-1269 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S: Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992, 356:314-317 [DOI] [PubMed] [Google Scholar]
- 10.Trauth BC, Klas C, Peters AMJ, Matzku S, Möller P, Falk W, Debatin KM, Krammer PH: Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989, 245:301-305 [DOI] [PubMed] [Google Scholar]
- 11.Hughes DPM, Crispe NI: A naturally occurring soluble isoform of murine Fas generated by alternative splicing. J Exp Med 1995, 182:1395-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD: Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 1994, 263:1759-1762 [DOI] [PubMed] [Google Scholar]
- 13.Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB: Fas gene mutations in the Canale-Smith Syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med 1996, 335:1643-1645 [DOI] [PubMed] [Google Scholar]
- 14.Fisher GH, Rosenberg FJ, Strauss SE, Dale JK, Middelton LA, Lin AY, Strober W, Lenardo MJ, Puck JM: Dominant interfering Fas gene mutations impair apoptosis in human autoimmune lymphoproliferative disorders. Cell 1995, 81:935-946 [DOI] [PubMed] [Google Scholar]
- 15.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IAG, Debatin KM, Fischer A, deVillartay JP: Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995, 268:1347-1350 [DOI] [PubMed] [Google Scholar]
- 16.Simon HU, Yousefi S, Dommann-Scherrer C, Zimmermann DR, Bauer S, Barandun, Blaser K: Expansion of cytokine-producing CD4−CD8− T cells associated with abnormal Fas expression and hypereosinophilia. J Exp Med 1996, 183:1071–1082 [DOI] [PMC free article] [PubMed]
- 17.Müschen M, Warskulat U, Häussinger D, Moers C, Simon D, Even J: Derangement of CD95 system in a case of Churg-Strauss vasculitis. Gastroenterology 1998, 114:1351-1352 [DOI] [PubMed] [Google Scholar]
- 18.Hansel TT, Pound JD, Pilling D, Kitas GD, Salmon M, Gentle TA, Lee SS, Thompson RA: Purification of human blood eosinophils by negative selection using immunomagnetic beads. J Immunol Methods 1989, 122:97-103 [DOI] [PubMed] [Google Scholar]
- 19.Pannetier C, Delassus S, Darche S, Saucier C, Kourilsky P: Quantitative titration of nucleic acids by enzymatic amplification reactions run to saturation. Nucleic Acids Res 1993, 21:577-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müschen M, Warskulat U, Douillard P, Gilbert E, Häussinger D: Regulation of CD95 (Apo-1/Fas) receptor and ligand expression by lipopolysaccharide and dexamethasone in parenchymal and nonparenchymal rat liver cells. Hepatology 1998, 27:200-208 [DOI] [PubMed] [Google Scholar]
- 21.Müschen M, Warskulat U, Peters-Regehr T, Bode JG, Kubitz R, Häussinger D: Involvement of CD95 (Apo-1/Fas) ligand expressed by rat Kupffer cells in hepatic immunoregulation. Gastroenterology 1999, 116:666-677 [DOI] [PubMed] [Google Scholar]
- 22.Lenardo MJ: Fas and the art of lymphocyte maintenance. J Exp Med 1996, 183:721-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xerri L, Devilard E, Hassoun J, Mawas C, Birg F: Fas ligand is not only expressed in immune privileged human organs but is also coexpressed with Fas in various epithelial tissues. J Clin Pathol 1997, 50:87-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell JH, Rush B, Weaver C, Wang R: Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA 1993, 90:4409-4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner B: Induction of apoptosis in human eosinophils by anti-Fas antibody treatment in vitro. Blood 1995, 86:1437-1443 [PubMed] [Google Scholar]
- 26.Guillevin L, Amouroux J, Arbeille B, Boura R: Churg-Strauss angiitis: arguments favoring the responsibility of inhaled antigens. Chest 1991, 100:1472-1473 [DOI] [PubMed] [Google Scholar]