Abstract
To demonstrate that aluminum III (Al) interacts with PHFτ in neurofibrillary degeneration (NFD) of Alzheimer’s disease (AD) brain, we developed a “chelating autoclave method” that allows Al chelation by using trivalent-cationic chelator desferrioxamine. Its application to AD brain sections before Morin histochemistry for Al attenuated the positive fluorescence of neurofibrillary tangles, indicating Al removal from them. This method, applied for immunostaining with phosphorylation-dependent anti-τ antibodies, significantly enhanced the PHFτ immunoreactivity of the NFD. These results suggest that each of the phosphorylated epitopes in PHFτ are partially masked by Al binding. Incubation of AD sections with AlCl3 before Morin staining revealed Al accumulation with association to neurofibrillary tangles. Such incubation before immunostaining with the phosphorylation-dependent anti-τ antibodies abolished the immunolabeling of the NFD and this abolition was reversed by the Al chelation. These findings indicate cumulative Al binding to and thereby antigenic masking of the phosphorylated epitopes of PHFτ. Al binding was further documented for electrophoretically-resolved PHFτ on immunoblots, indicating direct Al binding to PHFτ. In vitro aggregation by AlCl3 was observed for PHFτ but was lost on dephosphorylation of PHFτ. Taken together, phosphorylation-dependent and direct PHFτ-Al interaction occurs in the NFD of the AD brain.
Alzheimer’s disease (AD) is a heterogeneous group of neurodegenerative disorders which clinically manifest with progressive dementia. The major histopathological abnormalities that characterize the brains affected with AD include intracellular neurofibrillary degeneration (NFD) and extracellular senile plaques (SPs). The NFD includes neurofibrillary tangles (NFTs), dystrophic neurites associated with SPs, and neuropil threads. Ultrastructurally these lesions contain abnormal filamentous structures called paired helical filaments (PHFs) and straight filaments as well as amorphous nonfilamentous aggregates. These structures are formed from hyperphosphorylated adult central nervous system (CNS) τ protein known as PHFτ. 1-3 This form of τ protein generated in AD differs in several biochemical properties from that of the normal adult brain. The most prominent feature distinguishing τ in normal adult brain from that in AD brains derives from their phosphorylation state. In the living normal adult brain, τ is phosphorylated at many of the same sites as PHFτ, but the possession of phosphate groups is given only to a small fraction of τ (biopsy-derived τ). In the postmortem normal adult brain, however, τ is subject to rapid dephosphorylation during the postmortem period, yielding far less phosphorylated form of τ (autopsy-derived τ). 4 In contrast, PHFτ remains highly phosphorylated in the AD brain even after a long postmortem interval. There are thus apparently quantitative and dynamic differences in phosphorylation between normal adult τ and PHFτ. In AD brain PHFτ is accumulated as nonfilamentous aggregates in a subpopulation of neuronal cells at the initial pretangle stage, 5-7 followed by progressive accumulation as PHFs forming NFD. 6,8-10 At the pretangle stage it appears that additional factors should be implicated in the aggregation of freshly generated PHFτ, because the mere phosphorylation of τ does not fully account for its aggregation.
Aluminum III (Al) has long been a target of research concerning its role as an environmental risk factor in the etiology of AD. 11-13 Our previous study suggested the role of Al as a cofactor in the formation of the NFD. 14 Specifically, Al induces PHFτ to aggregate in vitro and to resist proteolysis in vivo. Recent in vivo study showed additional evidence supporting the aggregation of phosphorylated τ by Al. 15 These data explain the mechanism whereby the hyperphosphorylated τ undergoes aggregation and deposition and strengthen the idea that Al plays a role in the pathogenesis of AD. In our continuing efforts to elucidate the pathobiological involvement of Al in the formation of the NFD, we herein provide evidence that phosphorylation-dependent and direct interaction between PHFτ and Al occurs in the NFD of the AD brain.
Materials and Methods
Search for a Desferrioxamine-Assisted Procedure Aimed at Enhancing PHFτ Immunoreactivity of AD Brain Sections
Demonstration that Al interacts with PHFτ, which constitutes the NFD in the AD brain, was executed by testing whether the immunoreactivity of PHFτ in these lesions is altered when Al is chelated from AD brain sections. To develop a method to accomplish this purpose, we examined procedures which employ desferrioxamine mesylate (DFO) (Sigma, St. Louis, MO), a chelator of trivalent cation, under the following conditions: i) incubation of brain sections at 37°C with solution of 10 mmol/L DFO dissolved in deionized water, which resulted in pH 4.7 (10 mmol/L DFO, pH 4.7) or in 50 mmol/L Tris buffer which was adjusted to pH 7.0 (10 mmol/L DFO, pH 7.0), and ii) autoclaving of brain sections immersed into the solution of 10 mmol/L DFO pH 4.7 or 10 mmol/L DFO pH 7.0. In some experiments di- and trivalent cationic chelator ethylenediaminetetraacetic acid (EDTA) was used instead of DFO. Autoclaving was done at 121°C with a set time of 10 minutes. This procedure of autoclaving with a cationic chelator is referred to here as the chelating autoclave method. Experiments using these procedures were performed in parallel with control experiments in which the DFO solution was replaced by deionized water or 50 mmol/L Tris buffer, pH 7.0, in the incubation at 37°C or in the autoclaving (namely, the hydrated autoclave method). 10,16
Each of these procedures was applied to paraffin sections prepared from pathologically confirmed AD brains (n = 5) fixed with 10% neutral buffered-formalin or 70% ethanol/0.15 mol/L NaCl, followed by immunohistochemical analysis for PHFτ using a panel of antibodies to τ listed in Table 1 ▶ . The antibodies are classified into three groups: phosphorylation-dependent antibodies AT8 (Innogenetics, Belgium), 17,18 PHF1, 19,20 and AP422; 21 phosphorylation-independent antibody T14 and T46; 22,23 and dephosphorylation-dependent antibody Tau1 (Cedar Lane, Hornby, Ontario). 24 For immunostaining with Tau1, the sections were dephosphorylated with 12 units/ml of Escherichia coli alkaline phosphatase Type III (Sigma) at 67°C 25 for 3 hours to reveal the nonphosphorylated epitopes of this antibody. By comparison among the immunohistochemical analyses combined with pretreatments by the procedures described above, we searched for a procedure which enhances PHFτ immunoreactivity of the NFD. As control experiments we analyzed other protein components constituting NFD and SPs using antibodies to ubiquitin (Mab 1510, Chemicon, Temecula, CA) at a dilution of 1:10,000, heparan sulfate (10E4, Seikagaku, Japan) at 1:500, Aβ (4G8, Senetek, Maryland Heights, MO) at 1:10,000, and ApoE (Chemicon) at 1:1000 under the same conditions as used for the anti-τ antibodies. In addition we analyzed neurofilament proteins in the AD and rat brains using phosphorylation-dependent antibodies to the high molecular neurofilament subunit (NF-H) (TA51 and RMO24) 26 at 1:100 and 1:10, respectively, and dephosphorylation-dependent antibody to NF-H (RMdO20) 26 at 1:10. As the secondary antibody was used the goat anti-mouse or anti-rabbit immunoglobulins peroxidase-labeled dextran polymer conjugate Envision (Dako, Glostrup, Denmark).
Table 1.
Summary of Antibodies to τ/PHFτ
| Antibody | Specificity | Type | Dilution |
|---|---|---|---|
| AT8 | phos Ser202, Thr205 | M | 1:500 |
| PHF1 | phos Ser396, Ser404 | M | 1:2000 |
| AP422 | phos Ser422 | P | 1:1000 |
| T14 | p-ind τ 141–178 | M | 1:1000 |
| T46 | p-ind τ 404–441 | M | 1:1000 |
| Tau1 | dephos Ser202 | M | 1:2000 |
AT8, PHF1 and AP422 are phosphorylation-dependent antibodies to τ that recognize PHFτ, but not enzymatically dephosphorylated PHFτ as well as autopsy-derived adult human CNS τ. T14 and T46 are phosphorylation-independent antibodies to τ that recognize adult human CNS τ as well as PHFτ and its dephosphorylated preparation. Tau1 is a dephosphorylation-dependent antibody to τ that recognizes normal CNS τ, and it only binds to PHFτ after enzymatic dephosphorylation. The numbering system for the amino acid sequences of the epitopes in τ recognized by the anti-τ antibodies listed here is based on the largest human CNS τ isoform. 51
phos, phosphorylated; Ser, serine; Thr, threonine; p-ind, phosphorylation-independent; dephos, dephosphorylated; M, monoclonal antibody; P, polyclonal antibody.
Morin Histochemical Stain for Al
Among the procedures described above, the chelating autoclave method proved to effectively elicit immunoreactive enhancement of PHFτ (see Results). To demonstrate that the chelating autoclave method is indeed capable of chelating Al from the NFD of the AD brain sections, we used fluorescent Morin stain for Al and thereby monitored Al in the sections. Following pretreatment by either the chelating or the hydrated autoclave method, the sections were stained with Morin as reported, 27-29 and viewed under the fluorescence microscope (Olympus AX70, Japan).
Assessment of Binding of Exogenous Al to PHFτ in NFD in Situ
To assess the ability of Al salts to bind to PHFτ in the NFD, AD brain sections were incubated in 0.1 mol/L Tris, pH 6.5, with and without 10 mmol/L AlCl3 as reported, 14 followed by Morin histochemical or immunohistochemical analysis. Before Al incubation for the Morin staining, AD brain sections were subjected to Al removal and then were incubated with or without alkaline phosphatase as described above. To determine whether the chelating autoclave method is also useful to chelate exogenously added Al, the sections were treated by the chelating autoclave method with DFO or EDTA in concentrations ranging from 10 to 50 mmol/L after the Al incubation and then subjected to Morin or immunohistochemical analysis. Effects of other metal salts including FeCl3, CaCl2, and ZnSO4 on the PHFτ immunoreactivity of the NFD were examined under the same conditions as used for AlCl3 in experiments consisting of τ immunostaining. It should be pointed out that the solution of AlCl3 contains free Al3+ at concentrations much lower than the indicated concentration, because AlCl3 forms insoluble hydroxyl complexes at pH 6.5. 30 The same situation also held in all of the following experiments in which AlCl3 was used.
Isolation of PHFτ from AD Brain and That of τ from Autopsy Normal Adult Human Brain
The aqueous-soluble fraction of PHFτ was prepared from the postmortem brain affected with AD as reported, 14 with modifications. The tangle-rich regions of the brain tissue were glass-homogenized in 1:1 vol of ice-cold reassembly buffer (0.1 mol/L MES, pH 6.8, 0.5 mmol/L MgSO4, 1 mmol/L EGTA, 2 mmol/L dithiothreitol) containing 0.75 mol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride, and a cocktail of protease inhibitors (Boehringer Mannheim, Mannheim, Germany) and then sonicated (Sonifier 250, Branson, Danbury, CT) at the lowest output in the Eppendorf tube for 5 minutes before centrifugation at 100,000 × g for 30 minutes. The resulting supernatant was boiled for 5 minutes and centrifuged at 100,000 × g for 30 minutes. The final 100,000 × g supernatant contained the aqueous-soluble fraction of PHFτ (buffer-PHFτ) as well as normal adult human τ. The procedure of sonication used was efficient in enrichment of the aqueous-soluble fraction of PHFτ. Aqueous-soluble samples containing PHFτ that was separated from normal adult human τ were prepared from the AD brain as described previously, 31-33 with modifications. The pellet obtained from the AD brain homogenate as described above was re-homogenized in 10 volumes (per gram starting brain tissue) of extraction buffer (10 mmol/L Tris, pH 7.6, 10% sucrose, 1 mmol/L EGTA, 0.75 mol/L NaCl) containing protease inhibitors cocktail, and centrifuged at 12,000 × g for 30 minutes. To the resulting supernatant was added sodium N-lauroylsarcosinate (Sarkosyl) (Fluka, Switzerland) to yield 1% solution, which was incubated stirring at room temperature for 1 hour, and centrifuged at 100,000 × g for 30 minutes. The resultant pellet was resuspended in the reassembly buffer by sonication for 5 minutes, and boiled for 5 minutes prior to centrifugation at 12,000 × g for 5 minutes. The resulting supernatant was sonicated again and centrifuged at 100,000 × g for 30 minutes. The final supernatant contained 100,000 × g aqueous-soluble fraction of PHFτ (Sarkosyl-PHFτ) but not normal adult τ. Aliquots of this sample were dephospho-rylated by reacting with alkaline phosphatase at 12 U/ml at 67°C for 3 hours. After the reaction the sample was boiled for 3 minutes and cleared by centrifugation at 100,000 × g for 10 minutes to reduce the enzyme. Samples containing normal adult human τ but not PHFτ were prepared from the postmortem normal adult human brain by the same method used to isolate buffer-PHFτ, except that the procedure of sonication was omitted.
Assessment of Binding of Exogenous Al to PHFτ on Immunoblots
The protein samples containing each of PHFτ, dephosphorylated PHFτ, and autopsy-derived normal adult human τ were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to polyvinylidene difluoride (PVDF) membranes (Amersham, Poole, UK). A series of the membrane strips including each of the protein samples were preincubated for 1 hour at room temperature in 0.1 mol/L Tris buffer, pH 6.5, with or without 10 mmol/L AlCl3. After washing with TTBS, the membrane strips were probed using the anti-τ antibodies. The effect of Al on the access of the primary antibodies to their antigens on the immunoblots was visualized by alkaline phosphatase-linked anti-mouse or anti-rabbit immunoglobulins and nitro blue tetrazolium/5-bromo-4-chloro-3-indolylphosphate p-toluidine salt as a color development substrate (Promega, Madison, WI). To determine the effect by the chelating autoclave method on exogenous Al, which presumably was bound to PHFτ on immunoblots, the membrane strips were treated by this method with concentrations of DFO or EDTA ranging from 0 to 100 mmol/L after the incubation with Al and then were subjected to immunoblot analysis using the anti-τ antibodies.
Assessment of in Vitro Interaction between PHFτ and Al
The in vitro effect of AlCl3 on PHFτ before and after dephosphorylation was assayed as described, 14 with modifications. The protein sample including PHFτ or dephosphorylated PHFτ was incubated at 37°C for 1 hour with various concentrations of AlCl3 ranging from 0 to 1 mmol/L and then centrifuged at 100,000 × g for 30 minutes. The resulting supernatants and pellets were subjected to 10% SDS-PAGE, followed by immunoblot analysis using PHF1 or Tau1. To determine the effect of DFO on the observed Al-induced aggregation of PHFτ, the aqueous-soluble samples containing the buffer-PHFτ was mixed with 1 mmol/L AlCl3 plus various concentrations of DFO ranging from 0.1 to 2 mmol/L and incubated at 37°C for 1 hour. After centrifugation at 100,000 × g for 30 minutes, the resulting supernatants and pellets were analyzed by Western blot analysis with PHF1.
Results
Chelating Autoclave Method Produces Immunoreactive Enhancement in Phosphorylated Epitopes of PHFτ in NFD
We examined and compared AD brain sections which were pretreated as described in Materials and Methods and then immunostained with AT8 or PHF1. Incubation of the sections at 37°C for 1 hour to overnight with 10 mmol/L DFO, pH 4.7, or 10 mmol/L DFO, pH 7.0, before applying the antibodies failed to produce any immunoreactive alterations in PHFτ of the NFD compared with standard immunostaining without application of any pretreatments (not shown). After the hydrated autoclave pretreatment, immunostaining with AT8 or PHF1 induced immunoreactive enhancement in PHFτ of the NFD (Figure 1, A and C) ▶ compared with the standard immunostaining, a finding known as the antigen retrieval. Employment of 10 mmol/L DFO, pH 4.7, or 10 mmol/L DFO, pH 7.0, for the chelating autoclave method produced more intense immunoreactivity of PHFτ in the NFD (Figure 1, B and D) ▶ than the hydrated autoclaving. The enhancement of the PHFτ immunoreactivity appeared especially prominent in neuropil threads and degenerative neurites of SPs. There was no appreciable difference in the immunoreactivity of PHFτ between the chelating autoclave method using 10 mmol/L DFO, pH 4.7, and that using 10 mmol/L DFO, pH 7.0. Pretreatment by the chelating autoclave method using 10 mmol/L EDTA, pH 4.7, or 10 mmol/L EDTA, pH 7.0, markedly enhanced PHFτ immunoreactivity of the NFD as did that using 10 mmol/L DFO. Higher DFO concentrations (up to 50 mmol/L) used for the chelating autoclave method gave similar to only slightly increased enhancement compared with 10 mmol/L DFO. We examined other antibodies to τ listed in Table 1 ▶ after pretreatment by the chelating autoclave method with 10 mmol/L DFO, pH 4.7 (which was used as the standard chelating autoclave method unless otherwise stated hereafter). AP422 produced marked enhancement as similarly as AT8 and PHF1 (not shown), whereas T14, T46, and Tau1 did not show appreciable immunoreactive enhancement (Figure 1, E and F) ▶ . The chelating autoclave pretreatment for the immunostaining with the phosphorylation-dependent anti-τ antibodies worked efficiently in both the formalin- and ethanol-fixed brain sections. Immunostaining with the antibodies to ubiquitin, heparan sulfate, Aβ, or ApoE (see Materials and Methods) for the sections pretreated with the chelating autoclave method did not enhance the immunolabeling of the NFD and SPs compared with that using the hydrated autoclave pretreatment or without pretreatments. Immunostaining of AD and rat brain sections with TA51, RMO24, and RMdO20 revealed NF-H distributed in the neuronal compartment as reported. 26 The immunoreactive intensity of NF-H was altered only negligibly between following the hydrated and chelating autoclave pretreatments (not shown). Thus the immunoreactive enhancement following the chelating autoclave method was selectively seen for PHFτ, notably its phosphorylated epitopes such as Ser202, Thr205, Ser396, Ser404, and Ser422, but not for other protein components of the AD brain lesions or for phosphorylated and nonphosphorylated NF-H in the AD and rat brains. These findings might suggest that the phosphorylated epitopes of PHFτ studied here are partially masked by the association of trivalent cations to them and these masked epitopes are exposed by the chelating autoclave pretreatment. In light of the high level of Al in the NFD, Al is likely to be the candidate element that binds to and masks the epitopes of PHFτ in these lesions.
Figure 1.
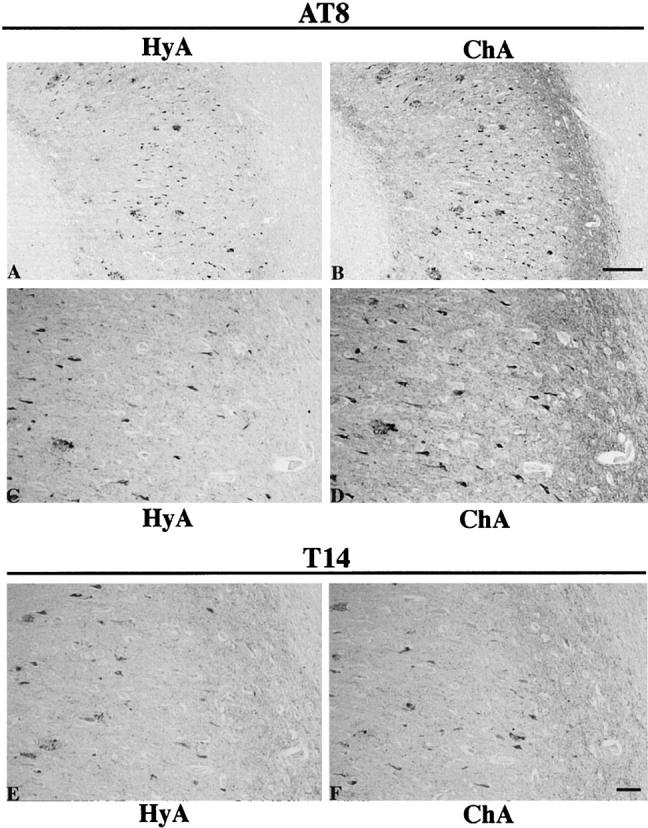
The immunoreactive enhancement in the phosphorylated epitope of PHFτ which constitutes neurofibrillary degeneration (NFD) of the AD brain sections pretreated by the chelating autoclave method. Closely adjacent sections of the hippocampus from the AD brain were pretreated by the control hydrated autoclave method (HyA) (A, C, E) or by the chelating autoclave method with 10 mmol/L DFO pH4.7 (ChA) (B, D, F), followed by immunostaining with AT8 (A-D) or T14 (E and F). Note that, compared with the hydrated autoclaving, the chelating autoclave method produced enhanced immunolabeling of the NFD as recognized by AT8. In contrast, no appreciable immunoreactive alteration was noted for the immunolabeling of the NFD revealed by T14. C and D are higher magnifications of A and B, respectively. A and B are at the same magnification, as are panels C-F. Scale bars, 100 μm (A and B) and 50 μm (C-F).
The Chelating Autoclave Method Chelates Al from Neurofibrillary Tangles
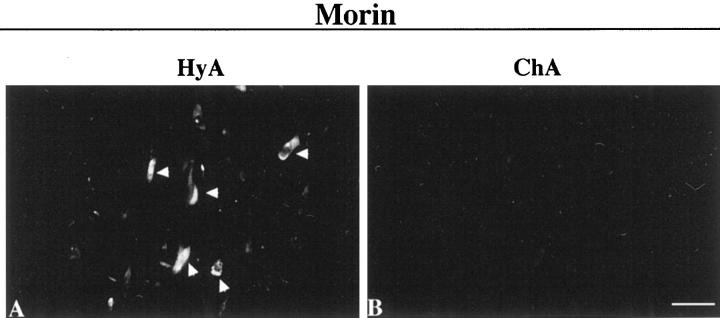
The fluorescent Morin stain for Al was used to monitor the removal of Al attainable by the chelating autoclave method in the AD brains. In the Morin-stained sections without pretreatments or pretreated with the hydrated autoclaving, there were positively illuminating NFTs, indicating that high level of Al exists in the NFTs. In the adjacent sections pretreated with the chelating autoclave method, fewer NFTs were only faintly stained, indicating the chelation of Al. To further confirm the Al removal from NFTs, individual NFTs were analyzed for Morin-positive staining by comparing the same sections before and after the chelating autoclave method as follows: first, sections were pretreated by the control hydrated autoclaving and stained with Morin. Positively stained NFTs were identified under the fluorescence microscope (Figure 2A) ▶ . The sections were bleached with ethanol and then deionized water to eliminate the Morin fluorescence in the NFTs. Then the same sections were pretreated by the chelating autoclave method and stained again with Morin. In the same areas, those NFTs which had been Morin-positive failed to reproduce their Morin fluorescence (Figure 2B) ▶ , indicating Al chelation. In contrast, control experiments, in which sections were pretreated again by the hydrated autoclaving for the second pretreatment reproduced the Morin fluorescence, indicating failure of Al chelation. Thus the pretreatment by the chelating autoclave method but not by the nonchelating autoclave method proved capable of chelating Al from the NFTs. The Al chelation from other lesions, including the neuropil threads and SP neurites, however, remained unidentified due to the limited sensitivity of the Morin staining. The Al chelation from NFTs and perhaps from other neurofibrillary lesions could reasonably be related to the immunoreactive enhancement of PHFτ because these two phenomena were induced at the same brain sites by the same procedure. Thus, Al existing in the NFD is highly likely to be in a condition to interact intimately with PHFτ in these lesions.
Figure 2.
Chelation of Al from the AD brain section following the chelating autoclave method. The section of the hippocampus from the AD brain was pretreated by the control hydrated autoclaving and then stained with Morin. An area including some illuminating NFTs was photomicrographed under the fluorescence microscope (A). Following bleaching the Morin fluorescence, the same section was then pretreated by the chelating autoclave method and stained again with Morin. The same area was photomicrographed (B) for comparison of the Morin fluorescence in those NFTs between before and after the chelating autoclave method. Note that the Morin positive staining that was observed in NFTs (arrowheads in A) was significantly attenuated after the chelating autoclave method (B). Scale bar, 50 μm.
Binding of Exogenous Al to the Phosphorylated Epitopes of PHFτ in NFD
Results described above further indicate that each of the phosphorylated epitopes of PHFτ is partially masked by Al and the rest of it, therefore, remains unassociated with Al. To confirm that exogenous Al can bind specifically to the phosphorylated epitopes in PHFτ, we performed experiments consisting of Al incubation as described in Materials and Methods. Morin staining of the Al-treated sections revealed Al accumulation with association to NFTs (Figure 3, A and B) ▶ , and this Morin-positive illumination was attenuated after subsequent treatment by the chelating autoclave method (not shown). The ability of exogenous Al to bind to NFTs was lost upon dephosphorylation of the AD brain sections (not shown). Immunostaining with AT8, PHF1, and AP422 after the Al incubation showed total abolition of the immunolabeling of the NFD (Figure 3, C and D) ▶ . This immunoreactive abolition was reversed by the chelating autoclave method (Figure 3F) ▶ , but remained unchanged by the nonchelating autoclave method (Figure 3E) ▶ or by incubation at 37°C with DFO overnight (not shown). The reversing effect by the chelating autoclave method was dependent on DFO, because the reversed immunoreactivity increased in intensity with the concentration of DFO, reaching a maximum at 30 mmol/L of DFO. Similar reversing effects were obtained using EDTA under the same conditions used for DFO. In contrast, the immunolabeling of the NFD with T14, T46, or Tau1 produced no discernable alterations with or without preincubation with AlCl3 (not shown). These results indicate that i) in each of the phosphorylated epitopes of PHFτ in the NFD, there is Al-free fraction which retains an ability to bind with exogenous Al, and ii) the Al binding is reflected by epitopic masking at the Al binding sites of its ligand PHFτ and, conversely, Al removal unmasks the epitopes. No effect by the Al incubation was observed for the immunolabeling of the NFD and SPs revealed by the antibodies to ubiquitin, heparan sulfate, Aβ, or ApoE. FeCl3, CaCl2, or ZnSO4 examined under the same conditions as used for AlCl3 did not affect significantly the immunolabeling of the NFD revealed by the anti-τ antibodies.
Figure 3.
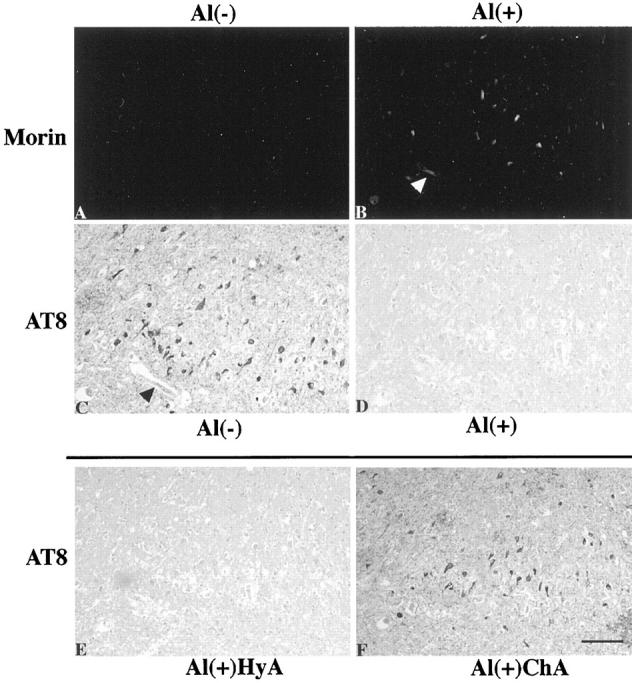
The Al accumulation with association to NFTs and the Al-induced immunoreactive abolishment of the phosphorylated epitope in PHFτ of the NFD. Closely adjacent sections of the AD hippocampus were pretreated by different sets of procedures before Morin staining (A and B) or immunostaining with AT8 (C-F). A and B: The sections were subjected to prior Al removal by the chelating autoclave method to differentiate binding of exogenous Al. They were then incubated with control buffer without Al (A) or with buffer containing 10 mmol/L AlCl3 pH 6.5 (B). C-F: The sections were initially treated by the hydrated autoclave method as the routine antigen retrieval. They were then incubated with control buffer without Al (C) or with buffer containing 10 mmol/L AlCl3, pH 6.5 (D-F), followed by incubation in control buffer without DFO (C and D), by the hydrated autoclave method (HyA) (E) or by the chelating autoclave method with 30 mmol/L DFO, pH 4.7 (ChA) (F). Incubation with AlCl3 produced Al accumulation with association to NFTs (B) and abolished the immunolabeling of the NFD revealed by AT8 (D). This immunoreactive abolishment was retrieved by the chelating autoclave method (F), but not by the hydrated autoclave method (E). Arrowheads in B and C indicates the same blood vessel. All panels are at the same magnification. Scale bar, 50 μm.
Binding of Exogenous Al to the Phosphorylated Epitopes of PHFτ on Immunoblots
Preincubation of the immunoblot membranes including PHFτ with AlCl3 abolished the immunoreactive bands and smear corresponding to those of PHFτ when probed with AT8, PHF1 (Figure 4A) ▶ , and AP422 but not with T14 (Figure 4B) ▶ or T46. In contrast, no immunoreactive alteration was observed for dephosphorylated PHFτ and autopsy-derived normal adult human τ as recognized by T14 (Figure 4B) ▶ , T46, or Tau1 (Figure 4C) ▶ . The Al-induced immunoreactive abolishment was retrieved when the membrane strips were treated by the chelating autoclave method after the incubation with AlCl3 (Figure 4D) ▶ , but not by the hydrated autoclaving without DFO (Figure 4D ▶ , lane 3) or by incubation at 37°C with DFO overnight (not shown). The immunoreactive retrieval by the chelating autoclave method was dependent on DFO because the retrieved immunoreactive bands of PHFτ increased in intensity with the concentration of DFO.
Figure 4.

Immunoblot analysis of the Al binding to PHFτ versus dephosphorylated PHFτ and autopsy-derived normal adult human τ as well as the effect of the chelating autoclave method on the Al binding to PHFτ. A-C: Aliquots of each of PHFτ (the Sarkosyl-PHFτ) (lanes 1–4 in A and lanes 1 and 2 in B), dephosphorylated PHFτ (lanes 3 and 4 in B and lanes 1 and 3 in C), and autopsy-derived normal adult human τ (lanes 5 and 6 in B and lanes 2 and 4 in C) were subjected to 10% SDS-PAGE and transferred to PVDF membranes. Each of the membrane strips was preincubated with control buffer without Al (lanes 1 and 3 in A, lanes 1, 3, and 5 in B, and lanes 1 and 2 in C) or buffer containing 10 mmol/L AlCl3 (lanes 2 and 4 in A, lanes 2, 4, and 6 in B, and lanes 3 and 4 in C) with shaking at room temperature for 1 hour, followed by probing with AT8 (lanes 1 and 2 in A), PHF1 (lanes 3 and 4 in A), T14 (lanes 1–6 in B), and Tau1 (lanes 1–4 in C). D: The immunoblot membrane strips containing PHFτ was preincubated with control buffer without Al (lane 1) or buffer containing 10 mmol/L AlCl3 (lanes 2–6) with shaking at room temperature for 1 hour. Following washing with TTBS after the incubation with Al, the membrane strips were incubated with control buffer without DFO (lanes 1 and 2), or treated by the autoclave procedure using deionized water (lane 3) or using DFO concentrations of 10 (lane 4), 30 (lane 5), and 100 mmol/L (lane 6). Each pair of lanes, 1–2 and 3–4 in A, 1–2, 3–4, and 5–6 in B, and 1 and 3 and 2 and 4 in C, as well as all lanes in D, are loaded with the same amount of the corresponding protein samples. Note that the incubation with Al totally abolished the immunoreactive bands and smear corresponding to those of PHFτ when probed with AT8 and PHF1 but not with T14. In contrast, no immunoreactive alterations were observed for dephosphorylated PHFτ and autopsy-derived normal adult human τ as revealed by T14 and Tau1. The Al-induced abolition of the immunoreactive bands of PHFτ was reversed by the chelating autoclave method in a DFO-concentration dependent manner. The position of Mr markers is indicated in kilodaltons at the left of each panel.
In Vitro Al-Induced Aggregation of PHFτ Is Mediated by the Phosphorylation of PHFτ and Is Prevented by DFO
In our previous study 14 we observed the in vitro effect of AlCl3 to induce aggregation on PHFτ but not on normal adult human τ. To demonstrate further that this effect of Al depends entirely on the phosphorylation of PHFτ, AlCl3 was reacted in vitro with PHFτ before and after dephosphorylation. Following incubation of the protein sample containing PHFτ with increasing concentrations of AlCl3, the solubility properties and electrophoretic mobility of the PHFτ were altered (Figure 5A) ▶ as previously shown, 14 reflecting Al-induced aggregation of PHFτ. In contrast, no changes were noted for the dephosphorylated PHFτ under the same conditions (Figure 5B) ▶ . The in vitro Al-induced aggregation of PHFτ was significantly inhibited by DFO. Specifically, following co-incubation of PHFτ and Al with increasing concentrations of DFO, increasing amounts of PHFτ remained soluble in the supernatant. Thus, DFO is an effective inhibitor of the formation of in vitro Al-induced aggregates of PHFτ by co-incubation with DFO.
Figure 5.
Immunoblot analysis of the in vitro effects of Al on PHFτ versus dephosphorylated PHFτ as well as the effect of DFO on the in vitro interaction between PHFτ and Al. A: Aliquots of each of the buffer-PHFτ and dephosphorylated PHFτ were incubated with 0 mmol/L (lanes 1, 2, 9, and 10), 0.1 mmol/L (lanes 3 and 4), 0.3 mmol/L (lanes 5 and 6), and 1 mmol/L (lanes 7, 8, 11, and 12) AlCl3 at 37°C for 1 hour, followed by centrifugation at 100,000 × g for 30 minutes. For each of these samples, the resulting supernatants (lanes 1, 3, 5, 7, 9, and 11) and pellets (lanes 2, 4, 6, 8, 10, and 12) were subjected to 10% SDS-PAGE. After transfer to PVDF membrane, the gel replicas were probed with PHF1 (lanes 1–8) and Tau1 (lanes 9–12). B: Aliquots of the buffer-PHFτ were co-incubated with 1 mmol/L AlCl3 plus DFO concentrations of 0.1 mmol/L (lanes 1 and 2), 0.3 mmol/L (lanes 3 and 4), 1 mmol/L (lanes 5 and 6), and 2 mmol/L (lanes 7 and 8) at 37°C for 1 hour, followed by centrifugation at 100,000 × g for 30 minutes. For each of these samples, the resulting supernatants (lanes 1, 3, 5, and 7) and pellets (lanes 2, 4, 6, and 8) were analyzed using 10% SDS-PAGE and immunoblots probed with PHF1. Note that increasing amounts of PHFτ were altered in its solubility and electrophoretic mobility by increasing concentrations of AlCl3, whereas dephosphorylated PHFτ was unaffected by AlCl3. The in vitro aggregation of PHFτ by Al was significantly inhibited by co-incubation with DFO; increasing amount of PHFτ remained soluble in the supernatants with the concentration of DFO. The arrows at the top of the Western blot in A and B indicate the position of the top of the stacking gel. The arrowhead at the right of A indicates immunoreactivity-excluding band corresponding to alkaline phosphatase, which was used to dephosphorylate PHFτ. The position of Mr markers is indicated in kilodaltons at the left of A.
Discussion
A number of studies using various analytic techniques have demonstrated increased level of Al in NFTs of the AD brain, 34-38 although there are other reports of studies that failed to demonstrate this. 12 Using the Morin fluorescnece stain established as a histochemical detector for Al, previous 29,36 and present studies have also shown that Al is present in association with NFTs. In the Morin histochemical procedures no labeling of brain sections occurred after removal of Al. When sections were incubated with Al after Al removal, this metal was reassociated with NFTs. Thus, the Morin staining procedures used here did not produce an artifact which might result from Al contaminants in reagents used. The Morin-positive illumination of NFTs is therefore considered to reflect the presence of endogenous Al in these lesions.
Al is known to bind avidly to phosphate groups in proteins, and this binding can modify the conformation of the proteins. 39-41 Therefore, it is plausible to assume that Al in the NFTs interacts with the hyperphosphorylated PHFτ. Lending support for this hypothesis is our previous demonstration that interaction of Al with PHFτ led to its aggregation in vitro and retarded in vivo proteolysis. 14 These data explored the possible role of Al as a cofactor in the formation of the NFD.
The current study was aimed at demonstrating that Al interacts with PHFτ in the NFD of the AD brain. The newly developed chelating autoclave method was the key tool to accomplish this purpose. Application of this method to AD brain sections induced the Al chelation from NFTs and presumably other neurofibrillary lesions and also the immunoreactive enhancement in the phosphorylated epitopes such as Ser202, Thr205, Ser396, Ser404, and Ser422 of PHFτ in these lesions. The observation that these two phenomena converged to occur concurrently in the same brain sites, ie, the NFD where PHFτ and Al are colocalized, indicates intimate association of Al with PHFτ, especially at its phosphorylated epitopes. However, for a given phosphorylated epitope of PHFτ, such Al-association contributes to its partial fraction, and the rest fraction of the epitope which is demonstrable without the Al chelation remains unassociated with Al. The immunoreactive abolition after the Al incubation indicates the presence of the Al-free fraction, which also retains an ability to interact with Al. Thus, there appear to be Al-associated and Al-free fractions in each of the phosphorylated epitopes of PHFτ in the NFD. The PHFτ-Al interaction was analyzed using the immunoblot system, in which electrophoretically-resolved PHFτ was also shown to have an ability to bind with exogenous Al at its phosphorylated sites. These findings indicate that Al can bind directly to PHFτ without relying on the conformation of native PHFτ adopted in the NFD or without mediation of other factors which presumably might be associated with PHFτ in these lesions. Based on the following two observations, it appears that the interaction between PHFτ and Al is largely mediated by the phosphorylation of PHFτ. First, the phosphorylation-dependent but not phosphorylation-independent anti-τ antibodies revealed immunoreactive alterations in PHFτ of the NFD following the Al chelation as well as the Al incubation. Second, dephosphorylation of PHFτ eliminated its interaction to Al observed on the sections and immunoblots, as well as its aggregation inducible by Al in vitro. Taken together with our previous study, 14 the intracellular interaction of Al with PHFτ is likely to induce the aggregation of PHFτ in affected neurons. The aggregated PHFτ-Al complexes might accumulate and constitute the NFD during the events leading to the neurodegeneration in AD brain.
DFO is an effective chelator for trivalent cations, including Al, with no known satisfactory alternative. 42 This chelator was, however, ineffective to dissociate Al from PHFτ of the NFD as well as from electrophoretically resolved PHFτ on the immunoblots when used for mere incubation at 37°C of the AD brain sections. In combination with the autoclave method, DFO exhibited effective chelation of Al from PHFτ. The hydrated autoclave pretreatment for tissue sections 16 is the first antigen retrieval method, which is now widely used for the immunoreactive enhancement of a wide variety of antigens in formalin-fixed neuronal and non-neuronal tissues. Its potential effect is believed to derive from high-temperature heating of antigens, because subsequent reports using microwave 43 and even simple boiling water 44 were shown to elicit similar effects. The possible mechanisms of immunoreactive enhancement by the antigen heating could be ascribed partly to enhanced rehydration of tissue sections, resulting in enhanced access of antibodies to their antigens. 45 Thus the Al chelation attainable by the chelating autoclave method might be based on enhanced access of DFO to the Al-associated antigens in PHFτ. The chelating autoclave method must chelate not only Al but also Fe (III) from tissue sections because DFO is a chelator of both of the metals. Indeed iron accumulation was found with association to NFTs and SPs in the AD brain, and this metal was removed by incubation with DFO at room temperature. 46 Incubation with FeCl3 was shown to have negligible effect on the τ immunolabeling of the NFD, but this finding does not necessarily mean that Fe is not involved in interaction with PHFτ. Significantly, Fe and Al share the same protein ligands and distribute intracellularly by the same mechanisms. 42,47 Possible involvement of Fe in the pathology of PHFτ remains to be determined. A divalent cationic chelator EDTA also exhibits a chelating effect for Al, though with stability constant for Al lower than DFO. 42 We found that EDTA effectively removed Al added to PHFτ on the sections and the immunoblots. No apparent effects of CaCl2 and ZnSO4 on the τ immunolabeling of the NFD appears to be against that Ca2+ and Zn2+ are involved in such interaction with PHFτ as seen for Al. We consider that the similar enhancing effect of EDTA as that of DFO on the τ immunolabeling of the NFD possibly occurred via Al removal. Yet this hypothesis does not exclude the possibility that these divalent cations are involved in the formation of the NFD. 48,49
This study provides important insights into therapeutic approaches to PHFτ-associated pathology. Though the Al-induced aggregation of PHFτ represents the secondary event that follows the hyperphosphorylation of τ in the neurofibrillary pathology, prevention of the Al interaction with PHFτ could consequently inhibit the formation and maturation of the NFD. Results described in this study suggest that, when administered to AD patients, DFO will not remove Al that is already involved in the aggregation and deposition of PHFτ, whereas this agent is effective to prevent soluble PHFτ from aggregating and participating in the formation of the NFD. In fact, the in vitro Al-inducible aggregation of PHFτ was significantly prevented when DFO was co-incubated with PHFτ and Al. These findings might form a theoretical basis for explaining the modest therapeutic response to clinical trials of the treatment of AD patients with DFO. 50 Nevertheless, as DFO is not an ideal chelator, particularly for long-term prevention, due to its practical limitations such as the need to administer it via injection and its high cost, the search for effective orally administered alternatives should be encouraged. We believe the hypothesis for the PHFτ-Al interaction involved in the formation of the NFD will be eventually verified by the advent of an Al chelation therapy that is shown to be clinically beneficial for patients with AD.
Acknowledgments
We thank Dr. K. Sagisaka for providing some of the brain samples used here, Drs. J. Q. Trojanowski and V. M.-Y. Lee for providing T14, T46, TA51, RMO24, and RMdO20, Drs. P. Davies and S. Greenberg for providing PHF1, and Drs. Y. Ihara and M. Hasegawa for providing AP422. We also thank Drs. S. Yoshida, T. Kihira, I. Wakayama, and H. Arai for helpful discussions and Ms. H. Kudo, Ms. Y. Murayama, and Ms. M. Kimura for technical assistance.
Footnotes
Address reprint requests to Dr. Ryong-Woon Shin, Department of Neurological Science, Tohoku University School of Medicine, 2–1 Seiryo-machi, Sendai 980-8575, Japan. Tel.: 81-22-717-8147; Fax: 81-22-717-8148; E-mail: shin@mail.cc.tohoku.ac.jp.
Supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education (R.-W. S, T. K.), and grants from Japan Brain Foundation(R.-W. S, T. K.) and Life Science Foundation of Japan (R.-W. S).
References
- 1.Trojanowski JQ, Schmidt ML, Shin R-W, Bramblett GT, Rao D, Lee VM-Y: Altered τ and neurofilament proteins in neurodegenerative disease: diagnostic implications for Alzheimer’s disease and Lewy body dementia. Brain Pathol 1993, 3:45-54 [DOI] [PubMed] [Google Scholar]
- 2.Trojanowski JQ, Schmidt ML, Shin R-W, Bramblett GT, Goedert M, Lee VM-Y: PHFτ (A68): from pathological markers to potential mediator of neuronal dysfunction and degeneration in Alzheimer’s disease. Clin Neurosci 1993, 1:184-191 [Google Scholar]
- 3.Goedert M, Trojanowski JQ, Lee VM-Y: The neurofibrillary pathology of Alzheimer’s disease. Rosenberg RN Prusiner SB DiMauro D Barchi RL eds. The Molecular and Genetic b\/Basis of Neurological Diseases. 1997, :pp 613-627 Butterworth-Heinemann, Boston [Google Scholar]
- 4.Matsuo ES, Shin R-W, Billingsley ML, deVoorde AV, O’Connor M, Trojanowski JQ, Lee VM-Y: Biopsy-derived adult human brain τ is phosphorylated at many of the same site as Alzheimer’s disease paired helical filament τ. Neuron 1994, 13:989-1002 [DOI] [PubMed] [Google Scholar]
- 5.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM: Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res 1989, 477:90-99 [DOI] [PubMed] [Google Scholar]
- 6.Shin R-W, Kitamoto T, Tateishi J: Modified tau is present in younger nondemented persons: a study of subcortical nuclei in Alzheimer’s disease and progressive supranuclear palsy. Acta Neuropathol 1991, 81:517-523 [DOI] [PubMed] [Google Scholar]
- 7.Braak E, Braak H, Mandelkow E-M: A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil thread. Acta Neuropathol 1994, 87:554-567 [DOI] [PubMed] [Google Scholar]
- 8.Shin R-W, Ogomori K, Kitamoto T, Tateishi J: Increased tau accumulation in senile plaques as a hallmark in Alzheimer’s disease. Am J Pathol 1989, 134:1365-1371 [PMC free article] [PubMed] [Google Scholar]
- 9.Bramblett GT, Trojanowski JQ, Lee VM-Y: Regions with abundant neurofibrillary pathology in human brain exhibit a selective reduction in levels of binding-competent τ and accumulation of abnormal τ-isoforms (A68 proteins). Lab Invest 1992, 66:212-222 [PubMed] [Google Scholar]
- 10.Shin R-W, Iwaki T, Kitamoto T, Sato Y, Tateishi J: Massive accumulation of modified tau and severe depletion of normal tau characterize the cerebral cortex and white matter of Alzheimer’s disease: demonstration using the hydrated autoclaving method. Am J Pathol 1992, 140:937-945 [PMC free article] [PubMed] [Google Scholar]
- 11.Markesbury WR, Ehmann WD: Aluminum and Alzheimer’s disease. Clin Neurosci 1993, 1:212-218 [Google Scholar]
- 12.Mclachlan DRC: Aluminum and the risk for Alzheimer’s disease. Environmetrics 1995, 6:233-275 [Google Scholar]
- 13.Shin R-W, Lee VM-Y, Trojanowski JQ: Neurofibrillary pathology and aluminum in Alzheimer’s disease. Histol Histopathol 1995, 10:969-978 [PubMed] [Google Scholar]
- 14.Shin R-W, Lee VM-Y, Trojanowski JQ: Aluminum modifies the properties of Alzheimer’s disease PHFτ proteins in vivo and in vitro. J Neurosci 1994, 14:7221-7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savory J, Huang Y, Wills MR, Herman M: Reversal by desferrioxamine of tau protein aggregates following two days of treatment in aluminum-induced neurofibrillary degeneration in rabbit: implications for clinical trials in Alzheimer’s disease. Neurotoxicology 1998, 19:209-214 [PubMed] [Google Scholar]
- 16.Shin R-W, Iwaki T, Kitamoto T, Tateishi J: Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Lab Invest 1991, 164:693-702 [PubMed] [Google Scholar]
- 17.Goedert M, Jakes R, Crowther RA, Six J, Lubke U, Vandermeeren M, Cras P, Trojanowski JQ, Lee VM-Y: The abnormal phosphorylation of τ proteins at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci USA 1993, 90:5066-5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goedert M, Jakes R, Vanmechelen: Monoclonal antibody AT8 recognizes tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 1995, 189:167-170 [DOI] [PubMed] [Google Scholar]
- 19.Greenberg SG, Davies P, Schein JD, Binder LI: Hydrofluoric acid-treated PHFτ proteins display the same biochemical properties as normal τ. J Biol Chem 1992, 267:564-569 [PubMed] [Google Scholar]
- 20.Lang E, Szendrei GI, Lee VM-Y, Otvos L, Jr: Immunological and conformational characterization of a phosphorylated immunodominant epitope of the paired helical filaments found in Alzheimer’s disease. Biochem Biophys Res Commun 1992, 187:783-790 [DOI] [PubMed] [Google Scholar]
- 21.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y: Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem 1995, 13:823-829 [DOI] [PubMed] [Google Scholar]
- 22.Kosik KS, Orecchio LD, Binder LI, Trojanowski JQ, Lee VM-Y, Lee G: Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1988, 1:817-825 [DOI] [PubMed] [Google Scholar]
- 23.Trojanowski JQ, Schuck T, Schmidt ML, Lee VM-Y: Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem 1989, 37:209-215 [DOI] [PubMed] [Google Scholar]
- 24.Binder LI, Frankfurter A, Rebhun LI: The distribution of tau in the mammalian central nervous system. J Cell Biol 1985, 101:1371-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goedert M, Spillantini MG, Cairns NJ, Crowther RA: Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 1992, 8:159-168 [DOI] [PubMed] [Google Scholar]
- 26.Lee VM-Y, Carden MJ, Schlaepfer WW, Trojanowski JQ: Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci 1987, 7:3474-3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Boni U, Scott JW, Crapper DR: Intracellular aluminum binding; a histochemical study. Histochemistry 1974, 40:31-37 [DOI] [PubMed] [Google Scholar]
- 28.Wen GY, Wisniewski HM: Histochemical localization of aluminum in the rabbit CNS. Acta Neuropathol 1985, 68:175-184 [DOI] [PubMed] [Google Scholar]
- 29.Kihira T, Yoshida S, Mitani K, Yasui M, Yase Y: ALS in the Kii peninsula of Japan, with special reference to neurofibrillary tangles and aluminum. Neuropathol 1993, 13:125-136 [Google Scholar]
- 30.Martin RB: The chemistry of aluminum as related to biology and medicine. Clin Chem 1986, 32:1797-1806 [PubMed] [Google Scholar]
- 31.Greenberg SG, Davies P: A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 1990, 87:5827-5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee VM-Y, Balin BJ, Otvos L, Jr, Trojanowski JQ: A68: a major subunit of paired helical filaments and derivatized forms of normal τ. Science 1991, 251:675-678 [DOI] [PubMed] [Google Scholar]
- 33.Shin R-W, Bramblett GT, Lee VM-Y, Trojanowski JQ: Alzheimer disease A68 proteins injected into rat brain induce codeposits of β-amyloid, ubiquitin, and α1-antichymotrypsin. Proc Natl Acad Sci USA 1993, 90:6825-6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perl DP, Brody AR: Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 1980, 208:297-299 [DOI] [PubMed] [Google Scholar]
- 35.Garruto RM, Fukatsu R, Yanagihara R, Gajdusek DC, Hook G, Fiori CE: Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci USA 1984, 81:1875-1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccardo P, Yanagihara R, Garruto RM, Gibbs CJ, Jr, Gajdusek DC: Histochemical and X-ray microanalytical localization of aluminum in amyotrophic lateral sclerosis and parkinsonism dementia of Guam. Acta Neuropathol 1988, 77:1-4 [DOI] [PubMed] [Google Scholar]
- 37.Good PF, Perl DP, Bierer LM, Schmeidler J: Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer’s disease: a laser microprobe (LAMMA) study. Ann Neurol 1992, 31:286-292 [DOI] [PubMed] [Google Scholar]
- 38.Perl DP, Good PF: Aluminum and the neurofibrillary tangle: results of tissue microprobe studies. Aluminum in Biology and Medicine: Ciba Found Symp 169. 1992, :pp 217-236 John Wiley & Sons, Chichester, UK [DOI] [PubMed] [Google Scholar]
- 39.Birchall JD, Chappell JS: Aluminum, chemical physiology, and Alzheimer’s disease. Lancet 1988, ii:1008-1010 [DOI] [PubMed] [Google Scholar]
- 40.Siegel N, Haug A: Aluminum interaction with calmodulin: evidence for altered structure and function from optic and enzymatic studies. Biochem Biophys Acta 1983, 744:36-45 [DOI] [PubMed] [Google Scholar]
- 41.Webb J, Multani JS, Saltman P, Beach NA, Gray HB: Spectroscopic and magnetic studies of iron (III) phosvitins. Biochemistry 1973, 12:1797-1802 [DOI] [PubMed] [Google Scholar]
- 42.Yokel RA: Aluminum chelation: chemistry, clinical, and experimental studies and the search for alternatives to desferrioxamine. J Toxicol Environ Health 1994, 41:131-174 [DOI] [PubMed] [Google Scholar]
- 43.Shi S-R, Key ME, Kalra KL: Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining base on microwave oven heating of tissue section. J Hisotochem Cytochem 1991, 39:741-748 [DOI] [PubMed] [Google Scholar]
- 44.Kawai K, Serizawa A, Hamana T, Tsutsumi Y: Heat-induced antigen retrieval of proliferating cell nuclear antigen and p53 protein in formalin-fixed, paraffin-embedded sections. Pathol Int 1994, 44:759-764 [DOI] [PubMed] [Google Scholar]
- 45.Suurmeijer AJH, Boon ME: Notes on the application of microwaves for antigen retrieval in paraffin and plastic tissue sections. Eur J Morphol 1993, 31:144-150 [PubMed] [Google Scholar]
- 46.Smith MA, Harris PLR, Sayre LM, Perry G: Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA 1997, 94:9866-9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roskams AJ, Connor JR: Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci USA 1990, 87:9024-9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattson MP: Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamic and Ca2+ influx in cultured hippocampal neurons. Neuron 1990, 4:105-117 [DOI] [PubMed] [Google Scholar]
- 49.Perry G, Siedlak SL, Richey P, Kawai M, Cras P, Kalaria RN, Galloway PG, Scardina JM, Cordell B, Greenberg BD, Ledbetter SR, Gambetti P: Association of heparan sulfate proteoglycan with the neurofibrillary tangles of Alzheimer’s disease. J Neurosci 1991, 11:3679-3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crapper-McLachlan DR, Dalton AJ, Kruck TPA, Bell MY, Smith WL, Kalow W, Andrews DF: Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337:1304-1308 [DOI] [PubMed] [Google Scholar]
- 51.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]