Abstract
Different ethnic groups with a high human leukocyte antigen (HLA)-A11 prevalence have been shown to experience a high rate of Epstein-Barr virus (EBV) infection, EBV-associated malignancies, and Epstein-Barr nuclear antigen (EBNA)-4 mutations. The epitopes 399-408 and 416-424 of EBNA-4 are major antigenic epitopes that elicit an HLA-A11 cytotoxic T lymphocyte (CTL) response to EBV infection. Mutations selectively involving one or more nucleotide residues in these epitopes affect the antigenicity of EBNA-4, because the mutant EBV strains are not recognized by the HLA-A11-restricted CTLs. To investigate these mutations in common EBV-associated malignancies occurring in different populations, we studied the mutation rate of epitopes 399-408 and 416-424 of EBNA-4 in 25 cases of EBV-associated Hodgkin’s disease (HD), nine cases of AIDS-related non-Hodgkin’s lymphoma, and 37 cases of EBV-associated gastric carcinoma (GC) from the United States, Brazil, and Japan. We found one or more mutations in these two epitopes in 50% (6/12) of United States HD, 15% (2/13) of Brazilian HD, 50% (6/12) United States GC and 28% (7/25) Japanese GC, and 22% (2/9) of United States AIDS-lymphoma. Similar mutations were found in 30% (3/10) of United States reactive, 0% (0/6) of Brazilian reactive, and 25% (2/8) Japanese reactive tissues. The most frequent amino acid substitutions were virtually identical to those seen in previously reported isolates from EBV-associated nasopharyngeal carcinomas and Burkitt’s lymphomas occurring in high prevalence HLA-A11 regions. However, only 2/28 (7%) mutations occurred in HLA-A11-positive patients. Our studies suggest that: 1) EBNA-4 mutations are a common phenomenon in EBV-associated HD, GC, and AIDS-lymphoma; 2) the mutation rate does not vary in these geographic areas and ethnic groups; 3) EBNA-4 mutations in EBV-associated United States and Brazilian HD, United States and Japanese GC, and United States AIDS lymphomas are not related to patients’ HLA-A11 status.
Epstein-Barr virus (EBV), a member of the human herpesvirus family, is ubiquitous worldwide, with more than 90% of adults having evidence of past EBV infection. EBV-specific cytotoxic T lymphocytes (CTLs) are found in virtually all healthy virus-carrying individuals. 1,2 These CTLs play a primary effector role in the immune system by controlling the proliferation of EBV-infected cells in vivo. 3-5 In healthy individuals, EBV-specific memory CTL responses are usually Class I-restricted and are directed against EBV nuclear antigens EBNA-2-6, and the latent membrane proteins (LMP1, 2), which are expressed in latently infected cells.
An individual’s human leukocyte antigen (HLA) Class I genotype strongly influences the EBV-specific CTL response; strong CTL responses are frequently associated with restriction through certain HLA Class I alleles. For example, CTLs restricted through the HLA-A11 allele are often dominant in polyclonal CTL cultures reactivated in vitro from HLA-A11-positive individuals. Investigators have shown this A11-restricted response to be directed largely to the transformation-associated viral antigen EBNA-4, which contains multiple HLA-A11-restricted epitopes with differing immunogenicities. 6
Investigators have determined the relative immunogenicities of overlapping truncated EBNA-4 (also known as EBNA-3B) fragments (14-15 amino acids in length) by a cytotoxic assay. 6 Among those fragments, HLA-A11-restricted CTLs have demonstrable reactivities against peptides of epitopes 399-408 and 416-424 of the EBNA-4 antigen, whereas reactivities against other fragments were either never detected or had a tendency to be lost on serial passage. 6 Lymphoblastoid cell lines with mutant EBNA-4 antigen, particularly within epitopes 416-424, are capable of escaping from HLA-A11-restricted CTLs. 7-9
It has recently been proposed that a high prevalence of EBNA-4 mutations serves as a mechanism of escaping the CTL response in certain HLA types. Previous studies of a high HLA-A11-prevalence population in Papua New Guinea found the same EBNA-4 point mutation at residue 424 in several Type A EBV isolates. 7 Study of another high HLA-A11-prevalence population in southern China revealed only two point mutations in 23 different EBV isolates, at epitopes 417 or 424 of the EBNA-4 antigen. 10 These findings suggested that immune pressure selected for resident EBV strains lacking immunodominant HLA-A11-restricted CTL epitopes. In contrast, two recent polymerase chain reaction (PCR) studies from two geographically distinct Papua New Guinea populations identified epitope-loss variants of EBV with the identical amino acid substitutions in all EBV isolates restricted through several class I HLA types. 11 Furthermore, the substitutions did not correlate with the contemporary distribution of HLA types in the different Papua New Guinea populations, suggesting that immune pressure plays a minimal role in the long-term evolution of EBV.
EBNA-4 has not been consistently identified in EBV-associated tumors in immunocompetent patients. However, it is possible that EBNA-4 may be expressed in early tumorigenesis, perhaps prior to or concurrent with neoplastic transformation, at or around the time that immune selection occurs, since this protein has been shown to up-regulate and down-regulate a variety of host proteins, including the Burkitt’s lymphoma-associated antigen CD77. 12,13 EBNA-4 mutations have not been previously extensively investigated in tumor tissues. To test the hypothesis that EBNA-4 mutations are a mechanism of escaping the normal immune surveillance, we examined EBNA-4 mutations in several different EBV-associated malignancies, including Hodgkin’s disease (HD) from the United States and Brazil, AIDs-related non-Hodgkin’s lymphoma from the United States, and gastric carcinoma (GC) from the United States and Japan, as well as normal tissues from all three countries. We found that i) mutations in positions 1 and 2 of epitope 399-408 and positions 2 and 9 of epitope 416-424 are very common (67% of all mutations detected) in HD and GC; ii) the substituted amino acids in these positions are virtually identical to those seen in endemic EBV-associated nasopharyngeal carcinoma (southern China) and Burkitt’s lymphoma (Papua New Guinea); iii) the reactive tissues show similar mutations to their neoplastic counterparts; iv) the mutation rate may vary in different geographic areas; and v) the mutations show no relationship to HLA-A11 positivity.
Materials and Methods
Cases
We studied 37 cases of known EBV-associated GC (12 from the United States, 25 from Japan), 25 cases of known EBV-associated Hodgkin’s disease (HD) (12 from the United States and 13 from Brazil), and 9 cases of acquired immunodeficiency syndrome (AIDS)-related non-Hodgkin’s lymphomas (from the United States). We also studied 24 reactive lymphoid tissues (10 from the United States, 8 from Japan, and 6 from Brazil). The EBV status in GC, HD, AIDS lymphoma, and reactive lymphoid tissues have been previously reported. 14-17 Briefly, all cases in this study were previously shown to be EBV-positive by in situ hybridization. 14-17 As previously reported, all 37 GC cases, all 24 reactive lymphoid tissues, 8/9 AIDS lymphoma cases, and 23/25 HD cases had Type A EBV. Only two United States HD and one AIDS lymphoma case had type B EBV. 14,17
PCR Studies for Epitopes 399-408 and 416-424 of EBNA-4
For each case, viral genomic DNA was extracted from 5-μm sections cut from formalin-fixed, paraffin-embedded tissue blocks, using 0.2 mg/ml protease K digestion buffer overnight, followed by denaturation by boiling. PCR studies were performed with 2 μl of extracted DNA in a 30-μl mixture containing 50 mmol/L KCl, 10 mmol/L Tris buffer (pH 8.3), 50 μm of each deoxynucleotide triphosphate, 2.5 mmol/L MgCl2, 1 unit of Taq polymerase (Perkin Elmer, Foster City, CA), and 20 pmol of each primer. Two sets of appropriate oligonucleotide primers were chosen, flanking the DNA region coding for epitopes 399-408 and 416-424 EBNA-4 of the prototype B95.8 EBV virus: 18 EBNA-4+ primer 5′-GAGGAGGAAGACAAGAGTGG-3′ and EBNA-4− primer 5′-GATTCAGGCGTGGCTCTTGG-3′; nested EBNA-4 PCR primer+ 5′-TACCGCAAACACTGCCGTAC-3′ and nested EBNA-4 primer− 5′-CTGTTCTGGCTGCCTTCTTC-3′. After initial denaturation for 3 minutes at 95°C, 45 amplification cycles were performed as follows: denaturing at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 40 seconds. A final extension at 72°C for 3 minutes completed the PCR amplification. The PCR setup and the post-PCR work were performed in separate laboratories to minimize the possibility of contamination.
Bidirectional PCR Amplification of Specific Alleles (Bi-PASA) for the HLA-A11 Locus
PCR amplification of specific alleles (PASA) was originally developed for assaying a known mutation in one allele. 19 Bi-PASA was designed for heterozygous gene analysis, such as HLA type determination. 20 HLA-Class I genes are located in human chromosome 6 and are highly polymorphic, with each of the HLA genes having multiple alleles in the population.
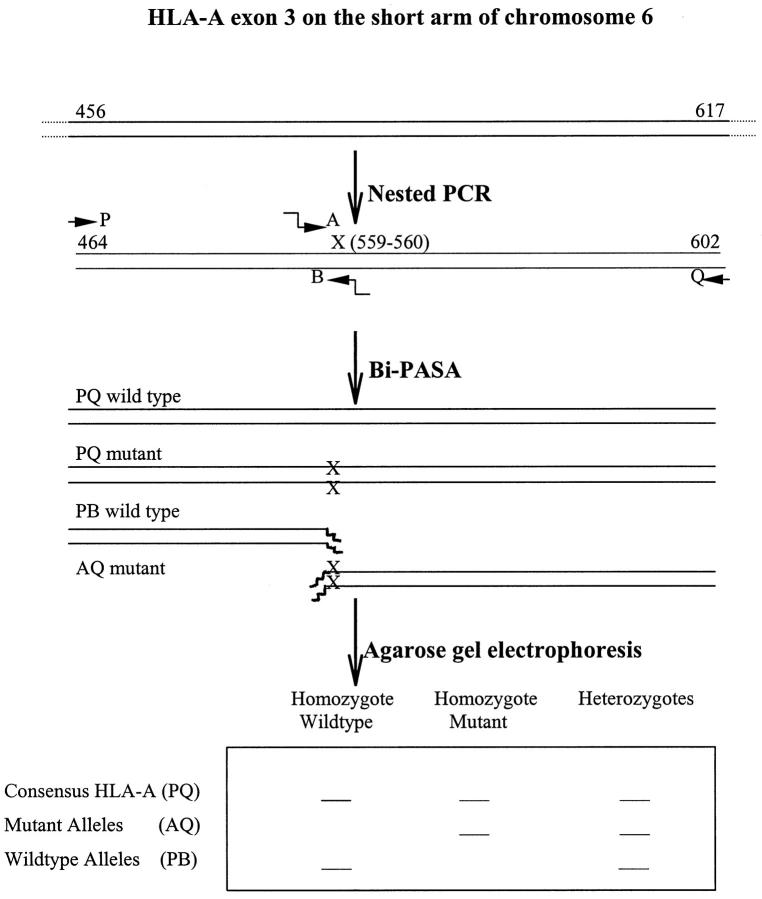
Genomic DNA was extracted as above. A unique HLA-A11 sequence was previously identified in HLA-A exon-3 from 524-560, which is specific to HLA-A1101, A1102 and A1103. 21 This fragment was first amplified by PCR from 456-617 using HLA-A11 PCR primer+ 5′-GGACCTGCGCTCTTGGAC-3′ and HLA-A11 PCR primer− 5′-GTGCGCTGCAGCGTCTCC-3′. The HLA-A11-specific sequence was determined by Bi-PASA method (Figure 1) ▶ . 19,20,22 Briefly, two outer (P 464-481 and Q 585-602) and two inner (A 543-560 and B 559-574) HLA-A-11-specific primers were made. The two outer primers were P (primer+ 5′-ATGGCAGCTCAGATCACC-3′) and Q (primer− 5′-TCCTTCCCGTTCTCCAGG-3′). The two inner primers were A (wild-type) (primer+ 5′-ggggcgggcAGCCTACCTGGAGGGCAC-3′) and B (mutant) (primer− 5′-gcgggcgggGCCACTCCACGCACCG-3′). The italic letters in primers A and B represent the noncomplementary 5′ tail sequences that switch from inefficient amplification of genomic DNA to efficient amplification of previously amplified template by PCR. 18 The Bi-PASA products were run on an agarose gel. The mutant bands were cut out for DNA sequencing (Figure 1) ▶ .
Figure 1.
Schematic of nested PCR and Bi-PASA. The genomic HLA-A11 specific region was amplified by nested PCR. cg nucleotides at 559 and 560 were used as mutant alleles, which are exclusively seen in HLA-A1101, A1102 and A1103 and few other HLA-As (also see Figure 6). The positions of the two outer (P,Q) and the two inner (A,B) Bi-PASA primers are indicated. The solid arrows indicate the direction of Bi-PASA. The wavy arrows in the A and B primers represent 5′ tails. The bands containing the mutant alleles (AQ) were cut out for DNA sequencing.
DNA Sequencing
The sequencing primers were: for EBNA-4: EBNA-4+ 5′-ACTGCCGTACAATCCAACAG-3′ and EBNA-4− 5′-CCTTCTTCTTTCTGTGTTCC-3′, and for HLA-A11:HLA-A11 5′-ATGGCAGCTCAGATCAAC. Thirty microliters of the PCR products were run on an agarose gel and the product bands were cut out, purified using a Qiaex gel extraction kit (Qiagen, Hilden, Germany), and resuspended in 30 μl of water. The products were sequenced with an AmpliCycle sequencing kit (Perkin Elmer), using the manufacturer’s recommended conditions. The products of the sequencing reaction were then separated by gel electrophoresis, dried, and exposed to film. The gel consisted of 7 mol/L urea and 8% polyacrylamide.
Results
Using PCR to amplify the EBNA-4 gene fragment of the EBV prototype B95.8 flanking the 399-408 and 416-424 epitopes, followed by gene sequencing, EBNA-4 genomic DNA was identified from all of the cases, confirming the presence of EBV infection in all studied cases. DNA sequencing of the PCR products revealed that 28/95 (29%) study cases had EBNA-4 mutations, including 6/12 cases of United States HD, 2/13 Brazilian HD, 6/12 cases of United States GC, 7/25 Japanese GC, 2/9 AIDS-lymphoma, 3/10 United States reactive tissues, 2/8 Japanese reactive tissues, and 0/6 Brazilian reactive tissues. There were no statistically significant differences in the percentage of EBNA-4 mutations between malignant and reactive tissues, or when comparing geographic location, although the percentage of EBNA-4 mutations was slightly higher in United States HD than Brazilian HD (P = 0.06) (Table 1) ▶ .
Table 1.
Summary of EBNA-4 Mutations in HD, GC, and AIDS-Lymphoma
| Total cases | Mutations detected (% of cases) | Location of Mutations | ||
|---|---|---|---|---|
| Epitope 399-408 | Epitope 416-424 | |||
| U.S. | ||||
| HD | 12 | 6 (50%) | 3 | 4* |
| U.S. | ||||
| Reactive | 10 | 3 (30%) | 1 | 3*† |
| Brazilian | ||||
| HD | 13 | 2 (15%) | 0 | 2 |
| Brazilian | ||||
| Reactive | 6 | 0 (0%) | 0 | |
| U.S. | ||||
| GC | 12 | 6 (50%) | 1 | 6* |
| U.S. | ||||
| Reactive | 10 | 3 (30%) | 1 | 3*† |
| Japanese | ||||
| GC | 25 | 7 (28%) | 1 | 6 |
| Japanese | ||||
| Reactive | 8 | 2 (25%) | 0 | 2 |
| U.S. | ||||
| AIDS-lymphoma | 9 | 2 (22%) | 4 | 4 |
*Some cases have mutation in both epitopes.
†These are the same cases.
Forty-five mutations, as compared to the prototype B95.8 sequence, were identified in the 28 cases. Among the 28 cases with mutations, 22 had only one mutation (81%), and 6 had two or more mutations (19%). One case of United States HD had five mutations (Table 1 ▶ , Figures 2 and 3 ▶ ▶ ) and one case of United States AIDS lymphoma had seven mutations (Figure 4) ▶ .
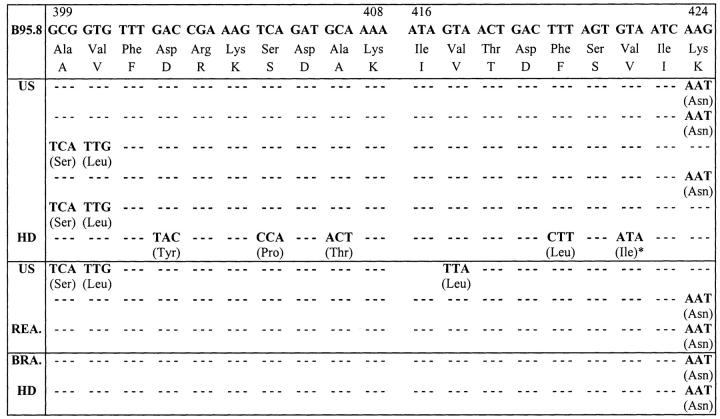
Figure 2.
Sequence of epitopes 399-408 and 416-424 of A11-restricted EBNA-4 epitopes in HD. Corresponding nucleotides and amino acids of prototype B95.8 are at top. Changes of nucleotides and amino acids and their positions of HD cases relative to B95.8 sequence are indicated. Only epitopes 399-408 and 416-424 of EBNA-4 are shown. *This HD case was type B EBV-positive. All other cases were type A EBV positive. US: United States; HD: Hodgkin’s disease; GC: Gastric carcinoma; BRA: Brazilian; REA: Reactive lymphoid lesions.
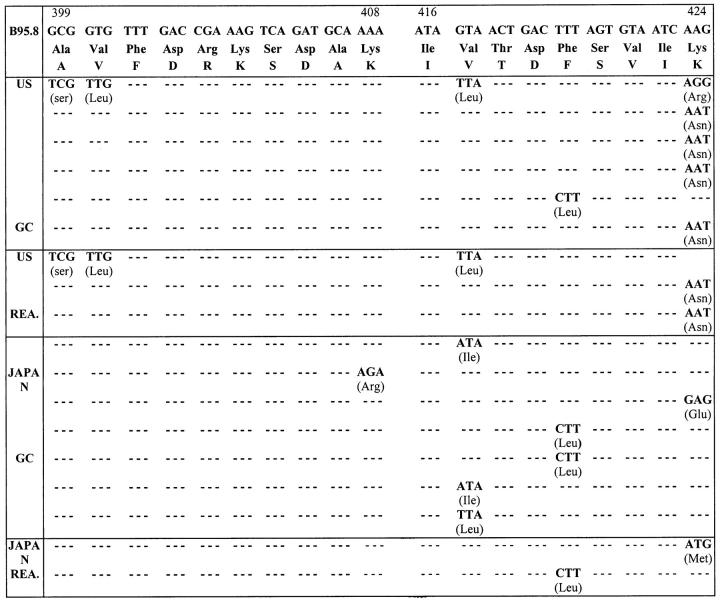
Figure 3.
Sequence of epitopes 399-408 and 416-424 of A11-restricted EBNA-4 epitopes in GC. Corresponding nucleotides and amino acids of prototype B95.8 are at top. Changes of nucleotides and amino acids and their positions of GC cases relative to B95.8 sequence are indicated. Only epitopes 399-408 and 416-424 of EBNA-4 are shown. All cases are type A EBV-positive. The three US reactive cases are the same cases as those in Figure 2 ▶ . US: United States; HD: Hodgkin’s disease; GC: Gastric carcinoma; REA: Reactive lymphoid lesions.
Figure 4.
Sequence of epitopes 399-408 and 416-424 of A11-restricted EBNA-4 epitopes in US AIDS-related non-Hodgkin’s lymphoma. Corresponding nucleotides and amino acids of prototype B95.8 are at top. Changes of nucleotides and amino acids and their positions of GC cases relative to B95.8 sequence are indicated. Only epitopes 399-408 and 416-424 of EBNA-4 are shown. The first case is type A EBV-positive and the second case is type B EBV-positive. US: United States; AIDS: acquired immunodeficiency syndrome.
Twelve different mutant amino acids were observed. However, 67% (30/45) of the substitutions selectively involved four positions: positions 1 and 2 of first epitope 399-408, which we have termed F1 and F2, respectively (Figures 2–5) ▶ ▶ ▶ , and positions 2 and 9 of second epitope 416-424, which we have termed S2 and S9, respectively (Figures 2–4) ▶ ▶ ▶ . Among the 45 detected mutations, 15 occurred in S9 (33%), six in S2 (13%), five in F1 (11%), and four in F2 (9%). All except two of these mutations were identified in type-A EBV-positive cases. The F1/F2 mutations occurred in 2/6 cases of United States HD, 1/3 United States reactive tissues, 1/6 United States GC, and 1/2 United States AIDS lymphoma cases. It was not observed in any of the tissues from Brazil or Japan. The difference between the incidence of F1/F2 mutations in tissues from the United States compared to other countries was not statistically significant (P = 0.06). The S2 mutations occurred in 1/3 United States reactive tissues, 1/6 United States GC cases, 1/2 United States AIDS-lymphoma cases, and 3/7 Japanese GC. The difference between the incidence of S2 mutations in tissues from the United States compared to other countries was not statistically significant (all tissues, P = 0.37; malignant tissues only, P = 0.11). The S9 substitutions were observed in all tissue types, from all countries, and included 3/6 United States HD, 2/3 United States reactive, 2/2 Brazilian HD, 5/6 United States GC, 1/2 United States AIDS-lymphomas, 1/7 Japanese GC, and 1/2 Japanese reactive tissues.
In five of six cases involving an amino acid substitution at S2, Val was replaced by Leu or Ile. The other was a silent mutation involving no amino acid substitution. In 12 of 15 cases involving an amino acid substitution at S9, Lys was replaced by Asn. F1 and F2 mutations from Ala-Val to Ser-Leu occurred together in four cases involving those loci (Figures 2 and 3) ▶ ▶ . In the case involving an F1 mutation without F2 mutation, the amino acid substitution was also Ser-for-Ala. Substitution of position 5 (S5) with or without substitution of position 7 (S7) of second epitope 416-424 was detected in six cases. Four out of six S5-mutated cases were type A EBV-positive and two were type B EBV-positive.
All 28 cases with EBNA-4 mutations had HLA-A locus Bi-PASA-amplifiable products. Only two of the 28 patients were shown to be HLA-A11-positive, including one case each of United States reactive and United States GC. Fourteen of the 28 (50%) cases had no AQ band, con-sistent with no HLA-A11 amplification. Fourteen of the 28 (50%) cases had AQ bands, which were sequenced. However, only two of 14 AQ bands were demonstrated to have the HLA-A11 sequence (data not shown).
Discussion
Epstein Barr virus induces a strong cytotoxic T lymphocyte (CTL) response to multiple antigens. EBV viruses may escape immune surveillance in vivo in several ways: i) by altering the amino acid sequence within epitopes recognized by virus specific CTLs; ii) via down-regulation of the peptide transporters, thus restricting the endogenous loading of MHC class I molecules with peptide derived from intracellular antigens; 23 iii) via down-regulation of CTL immunodominant EBV proteins of EBNA-3, -4, and -6 as in some EBV-associated malignancies, such as Hodgkin’s disease; 24-26 or iv) via strong CTL response by certain dominant HLA alleles, such as B8, which mounts a strong response to an EBNA-3 epitope, 27 or B27, which mounts a strong response to an EBNA-6 epitope, 28 or A11, which mounts a strong response to an epitope in EBNA-4. 6 EBNA-4 is a transformation-associated EBV nuclear antigen that has been shown to contain multiple HLA-A11-restricted epitopes with different immunogenicities. A high prevalence of EBNA-4 mutations has been proposed as mechanism of escaping the CTL response in certain HLA types. 6-8
Recent epidemiological studies have shown that mutation of the antigenically determined epitope of EBNA-4 may play a key role in the development of EBV-associated malignancies. The Papua New Guinea and southern Chinese populations have an unusually high HLA-A11 prevalence, with a frequency of ∼60%. 7,10 HLA-A11 epitope-loss variants of EBV were first identified from six Papua New Guinea Type A EBV isolates by PCR studies. 7 De Campos-Lima et al 10 reported 23/23 southern Chinese NPC isolates to show mutations in EBNA-4 at epitope positions S2 and S9. These studies suggested that the viral strains lacking the major A11-restricted CTL epitope enjoyed a selective advantage in this particular population due to their failure to elicit a strong CTL response.
We examined EBNA-4 mutations in different EBV-associated malignancies, including Hodgkin’s disease from the United States and Brazil and gastric carcinoma from the United States and Japan, as well as AIDS-related non-Hodgkin’s lymphomas from the United States and normal tissues from all three countries. Many AIDS lymphomas are known to express a latency pattern consistent with EBNA-4 expression, whereas the other two neoplasms are thought to not express EBNA-4, at least at the time of biopsy. However, one could hypothesize that EBNA-4 may be expressed early in transformation, because it has been shown to up-regulate and down-regulate a variety of host proteins, including the Burkitt’s lymphoma-associated antigen CD77. 13 We found that approximately one-third of our malignant cases, regardless of tumor type or geographic origin, had EBNA-4 mutations. In addition, the reactive tissues showed a similar percentage of EBNA-4 mutations.
Two-third of mutations detected in United States and Brazilian HD, US and Japanese GC and US AIDS-lymphoma occurred in the same EBNA-4 epitope positions as those reported in Papua New Guinea and Southern China (Figures 2 and 3) ▶ ▶ . 7,10 We also found a limited number of specific EBNA-4 mutations that accounted for a high percentage (67% of all mutations detected) of mutations in cases of HD and GC from all three countries. The amino acid substitutions at the four commonly mutated epitopes in our cases were similar to those found in isolates of nasopharyngeal carcinoma patients from southern China and Burkitt’s lymphoma patients from Papua New Guinea. 7,10,29 In five of our six cases involving an amino acid substitution at S2, Val was substituted by Leu or Ile. In the southern Chinese isolates, one-half of the cases had the Val at S2 substituted by Leu. 7 In 12 of our 15 cases involving an amino acid substitution at S9, Lys was substituted by Asn. All of the Papua New Guinea isolates showed the single-point mutation Lys to Thr at S9. 7 In the southern Chinese cases, the Lys at S9 was substituted by Asn or more rarely by Arg or Thr. 10 F1 and F2 mutations from Ala-Val to Ser-Leu occurred together in all four of our cases involving those loci (Figures 2 and 3) ▶ ▶ and the single case with F1 mutation without F2 mutation also had Ser replacement of Ala. The Papua New Guinea isolates all showed Ser-Leu or Ser-Phe mutation in F1 and F2. 10 In addition, 13/23 Chinese isolates also showed mutations that altered F1 and F2 from Ala-Val to either Ser-Leu, Pro-Leu, or Ser-Phe. 10 Experiments have shown that A11-positive LCLs carrying these mutations are not lysed by B95.8-induced CTL clones because the substitutions of Val by Leu at S2 and Lys by Asn at S9 are likely to abrogate binding of the peptide to the HLA-A11 groove. 7,10 Epitope positions S2 and S9 of EBNA-4 are known to be important determinants for HLA-A11 and peptide interaction because both residues are positively charged and form a small groove for HLA-A11-peptide binding. 29 Thus, the CTLs of susceptible individuals cannot eliminate mutant EBV strains before viral persistence is established. Furthermore, the viral strains lacking the target peptides may have a greater chance of successful transmission to such individuals.
In our study, although the observed amino acid substitutions were nearly identical to those seen in isolates from EBV-associated nasopharyngeal carcinoma and Burkitt’s lymphoma, the large majority of our cases were not in a high HLA-A11 prevalence population. The phenomenon that epitope loss variants of EBV have enjoyed a selective advantage in HLA-A11 high prevalence populations was not observed in our EBV-associated HD, GC, and AIDS-lymphoma cases. This is in contrast to earlier studies of a high HLA-A11-prevalence population in Papua New Guinea that found the same EBNA-4 point mutation at residue 424 in several Type A EBV isolates. 7,10 Study of another high HLA-A11-prevalence population in Southern China revealed only two point mutations in 23 different EBV isolates, at epitopes 417 or 424 of the EBNA-4 antigen. These findings suggested that immune pressure selected for resident EBV strains lacking immunodominant HLA-A11-restricted CTL epitopes. In their original studies, 7,10 de Compos-Lima et al predicted HLA-A11 prevalence based on population data, not on HLA-A11 phenotyping of individual cases as performed in the current study. Several recent studies on EBV isolates from coastal and highland Papua New Guinea showed that sequence analysis within the CTL epitope regions was identical in isolates from these two regions, whereas the prevalence of HLA-A11 is >50% in the coastal population and <5% in the highland population. 11 The substituted amino acids were virtually identical to those seen in a recent PCR study from two geographically distinct Papua New Guinea populations that identified epitope-loss variants of EBV with the identical amino acid substitutions in all EBV isolates restricted through several class I HLA types. 11 The substitutions did not correlate with the contemporary distribution of HLA types in the different Papua New Guinea populations, suggesting that immune pressure plays a minimal role in the long-term evolution of EBV. These studies strongly suggested that the mutations within CTL epitope sequences are not due to selective pressure exerted by the immune system, but as a result of local dissemination of a single origin. Although we did not observe identical mutations in GC and HD of different ethnic groups in the current study, the positions and substitutions of mutated amino acids in epitopes 399-408 and 416-424 of EBNA-4 are very similar among the cases from different ethnic groups and geographical locations with no correlation with the contemporary distribution of HLA-A11. 11,30 Our results further support that amino acid changes within the CTL epitope regions of the EBV are fortuitous events of a random genetic drift and are not influenced by the host genetic background and immune system. 30 Similar results were also documented in some cases of Hodgkin’s disease, from which no relationship was found between LMP-2 mutation and HLA-A0201, though HLA-A0201 allele is known to present one epitope of LMP-2 protein and generate a CTL response. 31 EBV is a virus that is highly stable through evolution. It is possible that EBV strains detected in EBV-associated malignancies or in EBV-associated reactive conditions are similar regardless of ethnic groups and geographical locations.
The incidence of EBV-positive HD in Brazil and elsewhere in South America has been shown to be much higher (70–90%) than that in the United States. 32,33 It is very interesting to note that the Brazilian HDs have a borderline significantly lower EBNA-4 mutation rate than United States HD cases (P = 0.06). However, the HLA-A11 antigen frequency (AF) in South America is about the same as United States (AF < 10). 10
Two cases showed substitution at epitope position S5, with Phe by Leu and at epitope position S7, with Phe by Ile. Both of these cases had type B EBV. Identical amino acid substitutions were previously detected in three type B EBV isolates. 7 Type B EBV, whether wild-type or mutant at these epitopes, does not bind to HLA-A117; instead, type B EBV interacts with the T cell receptor. 34 It remains to be determined whether the peptide actually represents a CTL epitope in individuals infected with type B EBV strains. 7
Although the precise role of EBV in the etiology of many EBV-associated tumors remain unclear, the presence of this virus in malignant cells offers a potential target for a CTL-based therapy, because immunodominant epitopes are likely to elicit an effective response in vivo. 35-38 Different EBV-associated malignancies express different set of EBV antigens. 39 Although individuals show clear differences (HLA background) in target antigen choice, the most frequent target antigens are EBNA-3, -4 and -6. Other latency antigens, including EBNA-2, EBNA-LP, LMP-1, and LMP-2 are sometimes targeted. 40,41 EBV-specific CTLs to LMPs have already been used to treat EBV-associated malignancies. 37,42 Before initiating such studies, an immunodominant epitope must be demonstrated for a given intracellular pathogen. For EBV-associated neoplasms that express EBNA-4, epitope 412-424 of EBNA-4 could be a candidate for such purpose. However, it is important to first determine whether the gene is mutated such that it no longer constitutes a target for CTLs. For those patients with specific epitope mutations, both vaccination and immunotherapy using these epitopes are likely to fail.
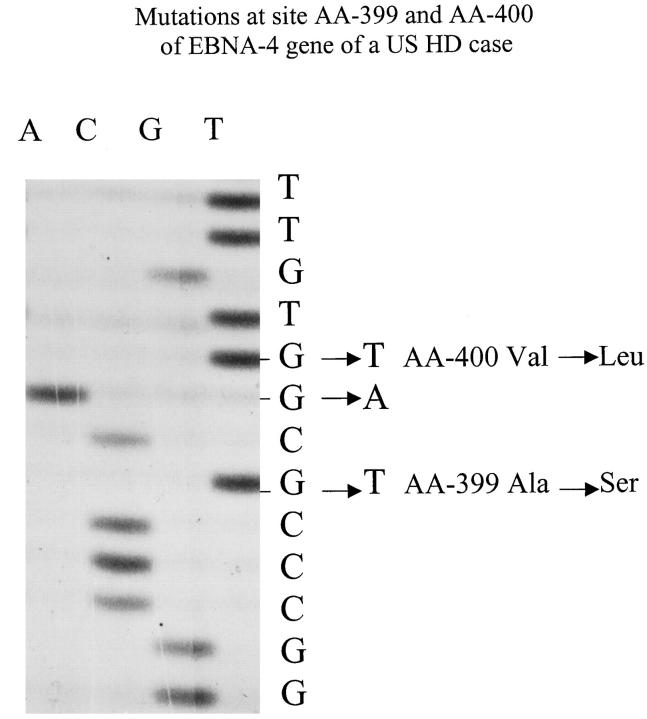
Figure 5.
F1 and F2 mutations seen in a US HD case. Part of sequencing gel shows substitutions of Ala in the prototype AA-399 (F1) (GCG) by Ser (TCA), and Val in AA-400 (F2) (GTG) by Leu (TTG). The mutant DNA sequence is shown in the first column, and prototype DNA sequence showed in the second column.
Footnotes
Address reprint requests to Lawrence M. Weiss, M.D., Division of Pathology, City of Hope National Medical Center, 1500 East Duarte Road, Duarte, CA 91010-0269.
References
- 1.Moss DJ, Rickinson AB, Pope JH: Long-term T-cell-mediated immunity to Epstein-Barr virus in man. III. Activation of cytotoxic T cells in virus-infected leukocyte cultures. Int J Cancer 1979, 23:618-625 [DOI] [PubMed] [Google Scholar]
- 2.Wallace LE, Rickinson AB, Rowe M, Epstein MA: Epstein-Barr virus-specific cytotoxic T-cell clones restricted through a single HLA antigen. Nature 1982, 297:413-415 [DOI] [PubMed] [Google Scholar]
- 3.Khanna R, Burrows SR, Moss DJ: Immune regulation in Epstein-Barr virus-associated diseases. Microbiol Rev 1995, 59:387-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray PG, Constandinou CM, Crocker J, Young LS, Ambinder RF: Analysis of major histocompatibility complex class I, TAP expression, and LMP2 epitope sequence in Epstein-Barr virus-positive Hodgkin’s disease. Blood 1998, 92:2477-2483 [PubMed] [Google Scholar]
- 5.Nowak MA, Bangham CR: Population dynamics of immune responses to persistent viruses. Science 1996, 272:74-79 [DOI] [PubMed] [Google Scholar]
- 6.Gavioli R, Kurilla MG, de Campos-Lima PO, Mallace LE, Dolcetti R, Murray RJ, Rickinson AB, Masucci MG: Multiple HLA-A11-restricted cytotoxic T-lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J Virol 1993, 67:1572-1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Campos-Lima PO, Gavioli R, Zhang Q-J, Wallace LE, Dolcetti R, Rowe M, Rickinson AB, Masucci MG: HLA-A11 epitope loss isolates of Epstein-Barr virus from a highly A11+ population. Science 1993, 260:98-100 [DOI] [PubMed] [Google Scholar]
- 8.Gavioli R, de Campos-Lima PO, Kurilla MG, Kieff E, Klein G, Masucci MG: Recognition of the Epstein-Barr virus-encoded nuclear antigens EBNA-4 and EBNA-6 by HLA-A11-restricted cytotoxic T lymphocytes: implications for down-regulation of HLA-A11 in Burkitt lymphoma. Proc Natl Acad Sci USA 1992, 89:5862-5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitsky V, Zhang QJ, Levitskaya J, Masucci MG: The life span of major histocompatibility complex-peptide complexes influences the efficiency of presentation and immunogenicity of two class I-restricted cytotoxic T lymphocyte epitopes in the Epstein-Barr virus nuclear antigen 4. J Exp Med 1996, 183:915-926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Campos-Lima PO, Levitsky V, Brooks J, Lee SP, Hu LF, Rickinson AB, Masucci MG: T cell responses and virus evolution: loss of HLA-A11-restricted CTL epitopes in Epstein-Barr virus isolates from highly A11-positive population by selective mutation of anchor residues. J Exp Med 1994, 179:1297-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows JM, Burrows SR, Poulsen LM, Sculley TB, Moss DJ, Khanna R: Unusually high frequency of Epstein-Barr virus genetic variants in Papua New Guinea that can escape cytotoxic T-cell recognition: implications for virus evolution. J Virol 1996, 70:2490-2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson ES: The Epstein-Barr virus EBNA 3 protein family as regulators of transcription. Epstein-Barr Virus Report 1997, 4:143-150 [Google Scholar]
- 13.Silins SL, Sculley TB: Modulation of vimentin, the CD40 activation antigen and Burkitt’s lymphoma antigen (CD77) by the Epstein-Barr virus nuclear antigen EBNA 4. Virology 1994, 202:16-24 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Chen WG, Chen YY, Bacchi MM, Bacchi CE, Alvarenga M, Abreu S, Chang KL, Weiss LM: Deletion of the latent membrane protein 1 in United States and Brazilian Hodgkin’s Disease and reactive lymphoid tissue: high frequency of a 30-base pair deletion. Hum Pathol 1997, 28:1408-1414 [DOI] [PubMed] [Google Scholar]
- 15.Hayashi K, Chen WG, Chen YY, Murakami I, Chen HL, Ohara N, Nose S, Hamaya K, Matui S, Bacchi MM, Bacchi CE, Chang KL, Weiss LM: Deletion of Epstein-Barr Virus latent membrane protein 1 gene in Japanese and Brazilian gastric carcinomas, metastatic lesions, and reactive lymphocytes. Am J Pathol 1998, 152:191-198 [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YY, Chang KL, Chen WG, Shibata D, Hayashi K, Weiss LM: Epstein-Barr virus-associated nuclear antigen-1 carboxy-terminal gene sequences in Japanese and American patients with gastric carcinoma. Lab Invest 1998, 78:1-6 [PubMed] [Google Scholar]
- 17.Shibata D, Weiss LM, Hernandez AM, Nathwani BN, Bernstein L, Levine AM: Epstein-Barr virus-associated non-Hodgkin’s lymphoma in patients infected with the human immunodeficiency virus. Blood 1993, 83:618-619 [PubMed] [Google Scholar]
- 18.Baer R, Bankier AT, Biggin MD, Beininger PL, Farrell PJ, Gibson TJ, Hatfull G, Hudson GS, Satchwell SC, Sequin C, Tuffnell PS, Barrell BG: DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature 1984, 310:297-311 [DOI] [PubMed] [Google Scholar]
- 19.Sommer SS, Cassady JD, Sobell JL, Bottema CDK: A novel method for detecting point mutations or polymorphisms and its application to population screening for carriers of phenylketonuria. Mayo Clin Proc 1989, 64:1361-1372 [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Thorland EC, Heit JA, Sommer SS: Overlapping PCR for bidirectional PCR amplification of specific alleles: a rapid one-tube method for simultaneously differentiating homozygotes and heterozygotes. Genome Res 1997, 7:389-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason PM, Parham P: HLA Class I nucleotide sequences, 1998. Tissue Antigens 1998, 51:417-466 [DOI] [PubMed] [Google Scholar]
- 22.Bottema CDK, Sommer SS: PCR amplification of specific alleles: rapid detection of known mutations and polymorphisms. Mutation Res 1993, 288:93-102 [DOI] [PubMed] [Google Scholar]
- 23.Niedermann G, Butz S, Ihlenfeldt HG, Grimm R, Lucchiari M, Hoschutzky H, Jung G, Maier B, Eichmann K: Contribution of proteasome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity 1995, 2:289-299 [DOI] [PubMed] [Google Scholar]
- 24.Pallesen G, Hamilton-Dutoit SJ, Rowe M, Young LS: Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin’s disease. Lancet 1991, 337:320-322 [DOI] [PubMed] [Google Scholar]
- 25.Grasser FA, Murray PG, Kremmer E, Klein K, Remberger K, Feiden W, Reynolds G, Niedobitek G, Young LS: Monoclonal antibodies directed against the Epstein-Barr virus-encoded nuclear antigen 1 (EBNA1): immunohistologic detection of EBNA1 in the malignant cells of Hodgkin’s disease. Blood 1994, 84:3792-3798 [PubMed] [Google Scholar]
- 26.Deacon EM, Pallesen G, Niedobitek G, Crocker J, Brooks L, Rickinson AB, Young LS: Epstein-Barr virus and Hodgkin’s disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med 1993, 177:339-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrows SR, Gardner J, Khanna R, Steward T, Moss DJ, Rodda S, Suhrbier A: Five new cytotoxic T cell epitopes identified within Epstein-Barr virus nuclear antigen 3. J Gen Virol 1994, 75:2489-2493 [DOI] [PubMed] [Google Scholar]
- 28.Brooks JM, Murray RJ, Thomas WA, Kurilla MG, Rickinson AB: Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J Exp Med 1993, 178:879-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang QJ, Gavioli R, Klein G, Masucci MG: An HLA-A11-specific motif in nonamer peptides derived from viral and cellular proteins. Proc Natl Acad Sci USA 1992, 90:2217-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna R, Slade RW, Poulsen L, Moss DJ, Burrows SR, Nicholls J, Burrows JM: Evolutionary dynamics of genetic variation in Epstein-Barr virus isolates of diverse geographical origins: evidence for immune pressure-independent genetic drift. J Virol 1997, 71:8340-8346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryden H, MacKenzie J, Andrew L, Alexander FE, Angus B, Krajewski AS, Armstrong AA, Gray D, Cartwright RA, Kane E, Wright DH, Taylor P, Jarrett RF: Determination of HLA-A*02 antigen status in Hodgkin’s disease and analysis of an HLA-A*02-restricted epitope of the Epstein-Barr virus LMP-2 protein. Int J Cancer 1997, 72:614-618 [DOI] [PubMed] [Google Scholar]
- 32.Chang KL, Albujar PF, Chen YY, Johnson RM, Weiss LM: High prevalence of Epstein-Barr virus in the Reed-Sternberg cells of Hodgkin’s disease occurring in Peru. Blood 1993, 81:496-501 [PubMed] [Google Scholar]
- 33.Ambinder RF, Browning PJ, Lorenzana I, Leventhal BG, Cosenza H, Mann RB, MacMahon EM, Medina R, Cardona V, Grufferman S: Epstein-Barr virus and childhood Hodgkin’s disease in Honduras and the United States. Blood 1993, 81:462-467 [PubMed] [Google Scholar]
- 34.Davis MM, Bjorkman PJ: T-cell antigen receptor genes and T-cell recognition. Nature 1988, 334:395-402 [DOI] [PubMed] [Google Scholar]
- 35.Lee SP, Tierney RJ, Thomas WA, Brooks JM, Rickinson AB: Conserved CTL epitopes with EBV latent membrane protein 2. a potential target for CTL-based tumor therapy. J Immunol 1997, 158:3325-3334 [PubMed] [Google Scholar]
- 36.Khanna R, Burrows SR, Neisig A, Neefjes J, Moss DJ, Silins SL: Hierarchy of Epstein-Barr virus-specific cytotoxic T-cell responses in individuals carrying different subtypes of an HLA allele: implications for epitope-based antiviral vaccines. J Virol 1997, 71:7429-7435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN, Young JW, Kernan NA, O’Reilly RJ: Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 1994, 330:1185-1191 [DOI] [PubMed] [Google Scholar]
- 38.Sing AP, Ambinder RF, Hong DJ, Jensen M, Batten W, Petersdorf E, Greenberg PD: Isolation of Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes that lyse Reed-Sternberg cells: implications for immune-mediated therapy of EBV+ Hodgkin’s disease. Blood 1997, 89:1978-1986 [PubMed] [Google Scholar]
- 39.Weiss LM, Chang KL: Association of the Epstein-Barr virus with hematolymphoid neoplasia. Adv Anat Pathol 1996, 3:1-15 [Google Scholar]
- 40.Murray RJ, Kurilla MG, Brooks JM, Thomas WA, Rowe M, Kieff E, Rickinson AB: Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med 1992, 176:157-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khanna R, Burrows SR, Kurilla MG, Jacob CA, Misko IS, Sculley TB, Kieff E, Moss DJ: Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med 1992, 176:169-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roskrow MA, Suzuki N, Gan YJ, Sixbey JW, Ng CY, Kimbrough S, Hudson M, Brenner MK, Heslop HE, Rooney CM: Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin’s disease. Blood 1998, 91:2925-2934 [PubMed] [Google Scholar]