Abstract
This study was designed to explore the possible functional relationships between apolipoprotein E (apoE) and the protease inhibitor α-1-antichymotrypsin in the aging mouse brain and in Alzheimer’s disease. For this purpose, levels of EB22/5 (the mouse homologue to human α-1-antichymotrypsin) mRNA expression was studied in apoE-deficient mice. These mice showed an age-dependent increase of EB22/5 mRNA expression in the brain. Furthermore, overexpression of allele 3 of human APOE gene in transgenic mice (in an apoE-deficient background) resulted in normalization of levels of EB22/5 mRNA expression compatible with levels found in control mice. In contrast, overexpression of human APOE4 allele or down-regulation of the apoE receptor low density lipoprotein receptor-related protein by deletion of the receptor-associated protein was associated with elevated levels of EB22/5 similar to apoE-deficient mice. Consistent with the findings in murine models, human α-1-antichymotrypsin protein was increased in brain homogenates from patients with Alzheimer’s disease, and levels of this serpin were the highest in patients with the APOE4 allele. In summary, the present study showed evidence supporting a role for apoE in regulating α-1-antichymotrypsin expression. This is relevant to Alzheimer’s disease because these two molecules appear to be closely associated with the pathogenesis of this disorder.
Apolipoprotein E (apoE) is a polymorphic protein that binds and transports cholesterol and other lipids. 1 Three alleles (APOE2, APOE3, and APOE4) on human chromosome 19 code for the different isoforms of apoE, and increased frequency of APOE 4 is associated with late onset familial 2 and sporadic 3 Alzheimer’s disease (AD). Moreover, findings from some studies suggest that patients with AD and the APOE4 allele showed increased levels of amyloid deposition in the cortex. 4 Although apoE binds Aβ-peptide and promotes β-amyloid fibrillogenesis 5 in vitro, it is unclear whether the APOE4 genotype is associated with accelerated cognitive deterioration. 6,7 Thus, the physiopathological role(s) of apoE in AD remains to be clarified.
α-1-Antichymotrypsin (ACT) is one of the serpins that has been shown to be altered in AD. 8 ACT, as well as other serpins, are important components of amyloid deposits which represent one neuropathological hallmark of AD. 9 ACT binds Aβ-peptide and can affect the rate of amyloid fibril formation in vitro. 10-12 The above findings, together with the observation that mRNA expression of ACT is increased in astrocytes surrounding the neurodegenerative lesions of AD brains, 13,14 has led to the hypothesis that ACT may play a role in the pathogenesis of neurodegeneration associated with AD. ApoE and ACT share some functional characteristics, since they both colocalize in β-amyloid deposits, bind to Aβ-peptides, and affect fibril formation, which may be important in the pathogenesis of the disease. Recently, we found that ACT levels in AD brains correlated with activated astrocytes scattered in the neuropil and these scattered astrocytes were increased in AD patients with the APOE4 allele. 15 However, possible relationships between these two molecules in AD pathogenesis are still largely to be determined. In this context, the main objective of the present study was to better understand the functional relationship between apoE and ACT during aging of the brain and in AD. For this purpose, levels of EB22/5 (also known as contrapsin-2) which is the murine homologue to human ACT 16,17 were evaluated in the brains of young and aged apoE-deficient mice, transgenic mice overexpressing human apoE3 and apoE4, as well as in mice where the apoE receptor low density lipoprotein receptor-related protein (LRP) was down-regulated by deleting the 39-kd receptor-associated protein (RAP). 18 To further validate the results observed in the experimental animal models, levels of ACT were determined in the brains of control and AD cases bearing the APOE3 and/or APOE4 alleles. Our study supports a role for apoE in regulating ACT in aging and in AD.
Materials and Methods
Colony Breeding and Characterization
ApoE-deficient homozygous mice (n = 3, 3 months; n = 5, 6 months; n = 5, 8 months; n = 4, 9 months; n = 2, 15 months; n = 2, 24 months) were obtained by cross-breeding heterozygous mutants that were kindly provided by Dr. J. Breslow (Rockefeller University, New York, NY). 19 Characterization of homozygosity or heterozygosity for apoE-deficient mice was carried out by polymerase chain reaction (PCR) with DNA extracted from the tail, as previously described. 20 In addition, a total of 10 wild-type mice were used for the study (n = 2, 3 months; n = 5, 6 months; n = 5, 9 months; n = 2, 15 months; n = 2, 24 months).
ApoE3 heterozygous (n = 5, 12 months) and apoE4 heterozygous (n = 5, 12 months) transgenic mice were obtained by cross-breeding heterozygous transgenic mice in a homozygous apoE-deficient background. Briefly, mice were obtained by microinjecting genomic sequences for human APOE3 or APOE4 into single-cell embryos from apoE-deficient mice. 21 Confirmation of the presence of human APOE and absence of endogenous mouse APOE gene was done by PCR as previously described. 21
RAP-deficient homozygous mice (n = 5, 12 months) were obtained by cross-breeding heterozygous mutants from Jackson Laboratories (Bar Harbor, ME). Briefly, as previously described 18 these mice were generated by recombination of the neo cassette with exon 1 of the RAP gene. Confirmation of the genotype was done by PCR as previously described. 22
Murine Tissue Preparation
Anesthetized mice were perfused with cold saline and their brains removed. The left hemibrain was frozen in liquid nitrogen and the right hemibrain was immersion-fixed in 4% paraformaldehyde in phosphate-buffered saline, pH 7.4. Poly(A)+ RNA was isolated as previously described 23 from frozen hemibrains. Fixed hemibrains were paraffin-embedded and serially sectioned (10 μm) for hematoxylin and eosin/cresyl violet staining or in situ hybridization.
Analysis of RNA Expression in Mouse Brain Samples
For the analysis of gene product expression, RNase protection assays (RPA) were performed as previously described. 23 Multiprobe sets were used that permitted simultaneous detection of various cytokine mRNAs, such as interleukin (IL)-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, or IL-6, interferon-γ, tumor necrosis factor (TNF)-α and TNF-β, or the mRNA expression of the following cellular response genes: EB22/5, glial fibrillary acid protein (GFAP), intercellular adhesion molecule-1 (ICAM-1), inducible nitric oxide synthase (iNOS), and the macrophage adhesion molecule-1 (Mac-1). The development and characterization of the cytokine 24 and cellular response 23 RPA probe sets were described previously. 23 A cloned fragment of the ribosomal protein RPL32 (L32) cDNA was used as a control of RNA loading. 23
In situ hybridization was carried out using 35S-labeled cRNA corresponding to EB22/5 sense and antisense riboprobes essentially as described previously. 23 The probe for EB22/5 was synthesized from the pGEM4 vector that contained a 1.8-kb EcoRI fragment of EB22/5. 16 Briefly, paraffin sections were treated with proteinase K, incubated in prehybridization solution and hybridized with riboprobes at 60°C. Sections were exposed to film and developed 3 days to 2 weeks later. These sections were then dipped in Kodak NTB-2 emulsion and developed 10 days later.
Characterization and Processing of Human Brain Specimens
Twenty-nine autopsy cases from the Alzheimer’s Disease Research Center at UCSD were used for the present study. Of them, 25 were clinically and histopathologically diagnosed 25 as AD and 4 as controls (Table 1) ▶ . For all cases, the postmortem delay was approximately 6 hours. APOE genotyping was performed by PCR as previously described 26 (Table 1) ▶ . Paraffin sections from cortical and subcortical regions were stained with hematoxylin and eosin, thioflavin-S, and cresyl violet for routine histopathological examination and morphometric analysis, as previously described. 27 Additional paraffin sections were immunostained with the monoclonal antibody against amyloid β-protein (clone 10D5, Athena Neurosciences, San Francisco, CA), as previously described 28 and analyzed with the Quantimet 570C to determine the percent area of the neuropil covered by amyloid (Table 1) ▶ .
Table 1.
Neuropathological Features of Control and Alzheimer’s Disease Patients with Different APOE Genotypes
| Cases | N | APOE genotype | Age (years) | Blessed score | Area covered by amyloid (%) |
|---|---|---|---|---|---|
| Controls | 4 | 3 /3 | 76 ± 7 | 1 ± 0.3 | |
| Alzheimer’s disease | 11 | 3 /3 | 78 ± 3 | 24 ± 3 | 11.8 ± 2.2 |
| 9 | 3 /4 | 77 ± 3 | 23 ± 2 | 33.2 ± 6.9 | |
| 5 | 4 /4 | 74 ± 4 | 22 ± 7 | 31.9 ± 5.6 |
Determination of ACT Levels in Human Brain Homogenates
Brain homogenates of the mid frontal cortex were prepared as follows: 0.1 g of specimens were dissected from mid-frontal area of frozen tissues and homogenized in 0.9 ml of cold buffer (1 mmol/L HEPES, 5 mmol/L benzamide, 2 mmol/L 2-mercaptoethanol, 3 mmol/L EDTA, 3 mmol/L EDTA, 0.5 mmol/L MgSO4, 0.05% sodium azide, pH 8.8) containing 0.01 mg/ml leupeptin. Homogenates were centrifuged (5000 × g) for 10 minutes at 4°C, and supernatants were collected and centrifuged again (100,000 × g) for 1 hour at 4°C. Protein determination was performed according to the method of Lowry 29 and samples were stored at −80°C.
Levels of human ACT, inhibitor of the complement C1 component (C1-I), and C-reactive protein (CRP) were measured by a radial immunodiffusion kit (NANORID; the Binding Site, Birmingham, UK) as previously described. 30 The lowest detectable level of ACT, C1-I, or CRP was 0.1 mg/l.
Statistical Analysis
Both murine and human samples were examined blind and the diagnosis and genotype were assigned only after the experiments were completed. Comparisons among groups of mouse or human samples were performed using one-way analysis of variance followed by post hoc comparisons using the Fisher test. All results were expressed as means ± SEM.
Results
Expression of EB22/5 Is Increased in Brains of apoE-Deficient Mice
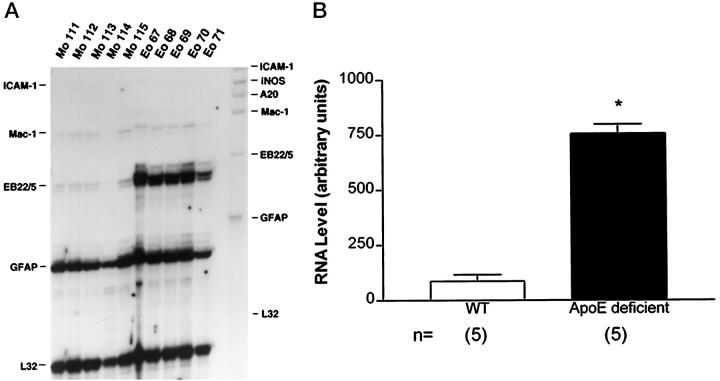
Since the main objective of the present study was to determine whether apoE regulates the expression of ACT, levels of EB22/5 (murine homologue of human ACT) were determined by RPA in the brains of apoE-deficient mice. EB22/5 mRNA was detected at low levels in wild-type samples. In contrast apoE-deficient mice showed a sevenfold increase in EB22/5 mRNA levels (Figure 1, A and B) ▶ .To determine whether the increased expression of EB22/5 mRNA in apoE-deficient mice was the result of either a direct interaction between apoE and ACT or a secondary response to brain injury, levels of GFAP, ICAM-1, iNOS, A20, and Mac-1 mRNA were determined. No significant difference in levels of expression for all these molecules associated with glial cell reactivity was observed between wild-type and apoE-deficient mice (Figure 1A) ▶ . Additional RPA analysis for IL-1 and IL-6 mRNAs showed that these molecules were not detectable in either wild-type or apoE-deficient animals (data not shown).
Figure 1.
Levels of EB22/5 mRNA expression in apoE-deficient mice. A: RPA analysis of the expression level of different genes (ICAM-1, iNOS, A20, Mac-1, EB22/5, GFAP, and L32) in brains of 5 control (Mo 111–115) and 5 apoE-deficient (Eo 67–71) 8-month-old mice. Solution hybridization was done for 18 hours at 55°C in a volume of 10 μl. B: EB22/5 expression in brains of apoE-deficient compared to wild-type control (WT) mice was significantly increased (Student’s t-test, P < 0.05) (*).
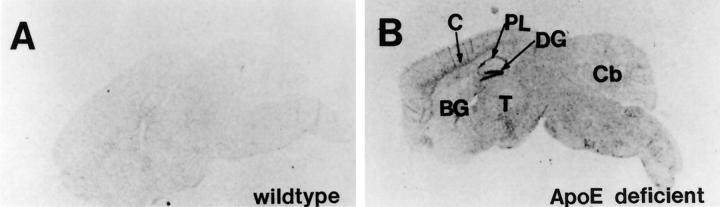
Further analysis of EB22/5 mRNA expression and distribution in the brain was assessed by in situ hybridization (Figure 2) ▶ . Consistent with RPA results this study showed that in apoE-deficient mice (Figure 2A) ▶ compared to wild-type control mice (Figure 2B) ▶ there was a dramatic increase in the levels of EB22/5 expression. EB22/5 was widely distributed throughout the brain with the highest levels detected in the neocortex and hippocampus (Figure 2) ▶ . The patterns of hybridization in the dentate gyrus and pyramidal cell layer in the hippocampus indicates a neuronal expression for this gene (Figure 2) ▶ .
Figure 2.
Analysis of EB22/5 gene expression by in situ hybridization. Levels of EB22/5 expression were increased throughout the brain in the apoE-deficient (A) compared to wild-type control (B) mice. DG, dentate gyrus; PL, pyramidal layer; C, cortex; Cb, cerebellum; T, thalamus; BG, basal ganglia.
Effect of Age on EB22/5 mRNA Expression in apoE-Deficient Mice
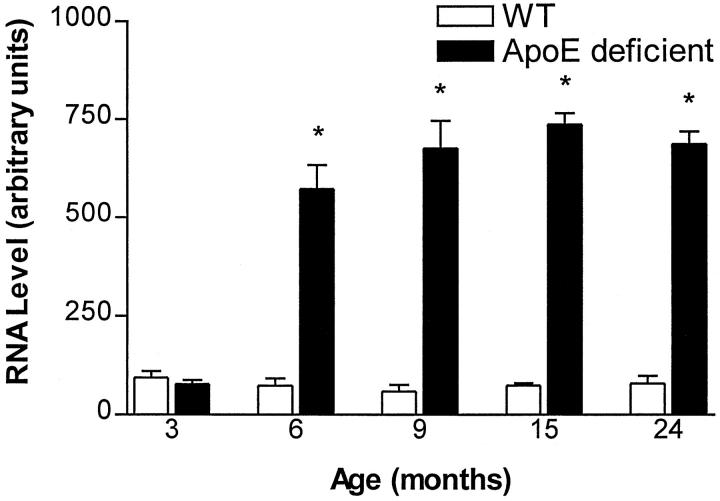
To determine whether the increase in EB22/5 levels if apoE-deficient mice was developmental or related to the aging process, levels of EB22/5 were determined in wild-type and apoE-deficient mice ranging in age from 3 to 24 months. At 3 months of age levels of EB22/5 were similar in wild-type and apoE-deficient mice (Figure 3) ▶ . However, at 6 months of age there was a dramatic sixfold increase in EB22/5 mRNA expression in apoE-deficient mice, which remained elevated during their life spans (Figure 3) ▶ .
Figure 3.
Age-dependent increase in EB22/5 expression in apoE-deficient mice by RPA. EB22/5 expression was not different at 3 months of age but was up-regulated beginning at 6 months of age in apoE-deficient mice compared to wild-type controls (WT). For each age time point n = 3, 3 months; n = 5, 6 months; n = 5, 8 months; n = 4, 9 months; n = 2, 15 months; n = 2, 24 months old mice. Statistical analysis was done by one-factor analysis of variance with post hoc Scheffé test, P < 0.05 (*).
EB22/5 mRNA Expression in apoE3 and apoE4 Transgenic and RAP-Deficient Mice
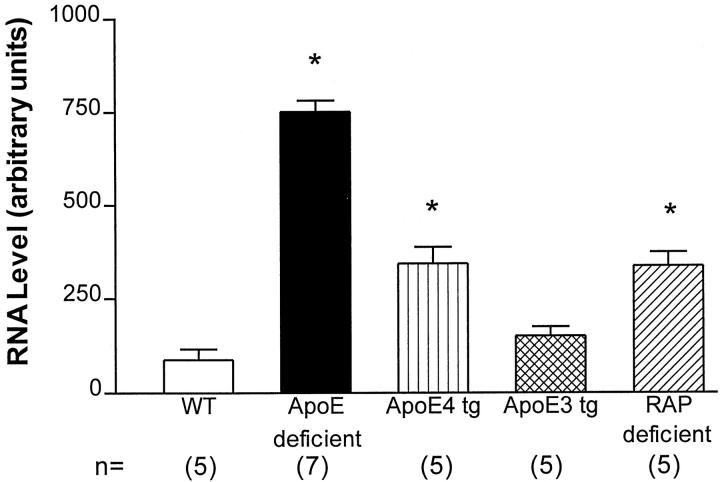
To determine whether the elevation in the apoE-deficient mice was more efficiently corrected by apoE3 versus apoE4, levels of EB22/5 mRNA expression were analyzed in the brains of apoE3- and apoE4- transgenic mice. This study showed that apoE3 was more effective in reestablishing the normal levels of EB22/5 expression in the endogenous apoE-deficient background, when compared to apoE4 transgenic mice (Figure 4) ▶ . Levels of EB22/5 mRNA were still moderately elevated in apoE4 transgenic mice, when compared to both wild-type and apoE3 transgenic mice (Figure 4) ▶ . Since previous studies have shown that the cellular effects of apoE are mediated by LRP, 31,32 we wanted to determine whether the effects of apoE on ACT were mediated by this receptor. For this purpose, levels of EB22/5 were determined in RAP-deficient mice. These mice were selected, as previous studies have shown, these mice are viable despite a 75% reduction in the levels of LRP. 18 Homozygous LRP-deficient mice cannot be used for these experiments because they are not viable. 18 Compared to the wild-type mice, levels of EB22/5 were elevated in the RAP-deficient mice (Figure 4) ▶ . However, these levels were lower compared to the apoE-deficient mice, but comparable to levels observed in apoE4 transgenic mice (Figure 4) ▶ . This supports the possibility that the effects of apoE on ACT might be mediated by LRP.
Figure 4.
EB22/5 mRNA expression in apoE3 and apoE4 transgenic and RAP-deficient mice by RPA. ApoE3 was more effective in reestablishing the normal levels of EB22/5 expression in the endogenous apoE-deficient background when compared to apoE4 transgenic mice. Levels of EB22/5 mRNA were still moderately elevated in apoE4 transgenic mice when compared to both wild-type (WT) and apoE3 transgenic mice. Compared to the wild-type mice, levels of EB22/5 were elevated in the RAP-deficient mice. However, these levels were lower compared to the apoE-deficient mice, but comparable to levels observed in apoE4 transgenic mice. Statistical analysis was done by one-factor analysis of variance with post hoc Scheffé test, P < 0.05 (*).
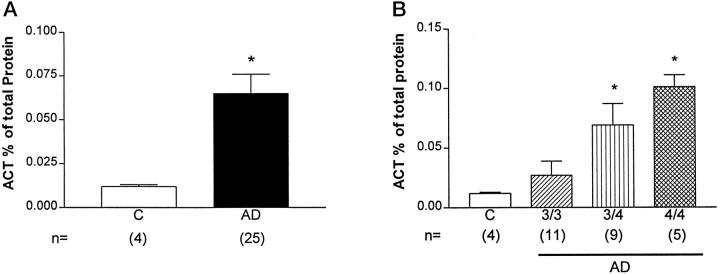
Determination of ACT Concentration in Human Brain Homogenates
Since the presence of the allele 4 of APOE is associated with risk for AD 33 and studies in apoE4 transgenic mice showed that apoE4 was less effective than apoE3 in reducing levels of EB22/5 expression in apoE-deficient mice (Figure 4) ▶ , we wanted to determine whether a similar APOE allele effect could be observed in AD brains. For this purpose, levels of ACT were determined in brain cytosolic fractions from AD and non-demented controls with different APOE genotypes (Figure 5) ▶ . Overall levels of ACT were significantly increased in AD compared to controls (Figure 5A) ▶ . However, among AD cases, those with APOE 4/4 genotype showed the highest ACT levels when compared to controls (Figure 5B) ▶ . Differences in ACT brain levels between AD patients with APOE 4/4 or APOE 3/4 and those with APOE 3/3 were also significant (Figure 5B) ▶ . Differences of ACT levels between patients with APOE 4/4 and those with APOE 3/4 were not significant (Figure 5B) ▶ . To further determine whether changes in ACT expression in AD cases were related to apoE effects or to a systemic reactive condition, levels of C1-I and CRP were determined. This analysis showed no significant differences between control and AD (not shown).
Figure 5.
ACT expression in the brains of Alzheimer’s disease (AD) patients. A: Compared to controls (C), AD cases showed a significant increase in ACT expression in the frontal cortex. B: Presence of allele 4 of APOE was associated with a significant increase in ACT levels in the frontal cortex compared to AD cases with allele 3 or controls. Statistical analysis was done by one-factor analysis of variance with post hoc Scheffé test, P < 0.05 (*).
Discussion
The present study showed that apoE plays and important role in regulating the in vivo expression of ACT in the brain. One possible interpretation for this result is that under physiological conditions apoE might have a suppressive effect on the ACT gene or protein expression and this regulation might occur at the transcriptional or post-transcriptional level. Since we observed that apoE had significant effects on ACT mRNA expression it is reasonable to propose that apoE might negatively regulate ACT gene by blocking the expression of transcription factors necessary to trigger the expression of this gene. Recent studies have shown that regulation of the ACT gene might be mediated by two binding elements, namely STAT1 and STAT3 (signal transducer and activator of transcription). 34 In astrocytes, oncostatin M and IL-1β are potent stimulators of ACT mRNA expression via STAT1 and STAT3 induction. IL-6 is also a potent stimulator of ACT if the appropriate receptor is present in the cell surface. 34 This suggests that up-regulation of IL-1, IL-6, or oncostatin M in apoE-deficient mice could trigger the up-regulation of ACT. However, the RPA showed that at least levels of IL-1 and IL-6 were unaltered in the apoE-deficient mice compared to controls. We did not measure levels of oncostatin M for the present study and this analysis is currently underway. ApoE might also regulate the ACT gene via induction of STAT inhibitory factors. In this regard, recent studies have shown that factors such as SOCS-1 are capable of inhibiting IL6-induced receptor phosphorylation and STAT activation. 35 The SOCS family of factors may act in a classic negative feedback loop to regulate cytokine signal transduction. 35 It is possible that while apoE might induce (through a yet unknown pathway) the production of anti-STAT factors, the absence of apoE will be followed by STAT1- and STAT3- mediated activation of the ACT gene.
Alternatively, post-transcriptional effects of apoE on ACT might be related to alterations in cholesterol levels associated with the apoE-deficient state of the mice, 13 since apoE is a well known cholesterol carrier that is produced by astrocytes within the brain. 1 In apoE-deficient mice, there is a significant increase in plasma cholesterol levels, 13 and cholesterol content in the neuronal membrane is abnormal. 36 Similarly, ACT is known to bind cholesterol as well as other hydrophobic molecules. 37 Also, serpins have been shown to bind cholesterol and other lipids. 38 This could indicate that abnormal cholesterol levels might trigger ACT up-regulation as a compensatory response to bind and transport cholesterol in the apoE-deficient mice.
Cholesterol transport in the CNS might be necessary to maintain synaptic plasticity and neuronal repair after injury or during aging. 39-41 In some models, apoE deficits are associated with neurodegeneration, abnormal repair after injury and neurophysiological deficits, 20,42-45 However, there is some variability in the results observed with these paradigms in apoE-deficient mice. 42,46,47 Then, in models such as ours where neurodegeneration is observed, an alternative explanation for the increased EB22/5 in the apoE-deficient mice might be related with astroglial response to neuronal injury.
Since previous studies have shown that higher risk for AD is associated with the presence of the allele 4 of APOE 33 and in vitro and in vivo models apoE3 appears to be more bioactive than apoE, 44,48 we seek to determine whether one isoform was more effective than the other. In this respect, it is of interest that apoE4 was less effective than apoE3 at reestablishing the baseline levels of EB22/5 expression in apoE-deficient mice. This data are in accordance with the in vivo studies in human brain showing significant increase in ACT cases with APOE4 alleles and with other studies showing that apoE4 was less effective that apoE3 in promoting in vitro neurite extension. 49,50 Furthermore, overexpression of apoE4 has deleterious effects in the CNS of transgenic mice when compared to apoE3 or non-transgenic littermates. 51 This suggests that apoE4 might represent a metabolically abnormal isoform, since in humans apoE4 showed a faster catabolism then apoE3, induced a rapid catabolism of cholesterol-enriched particles and was associated with increased plasma and LDL cholesterol concentrations. 52
Finally, to gain more knowledge as to the potential mechanisms involved in ACT regulation by apoE, we determined the levels of EB22/5 in RAP-deficient mice and showed a persistent elevation of EB22/5 in RAP-deficient mice. ApoE is a ligand for LRP 32 and RAP is a 39-kd molecule that regulates LRP transport to the cell membrane and functional properties of this receptor. 32 In fact, RAP-deficient mice showed a reduced expression of LRP 18 associated with altered neurotransmitter expression and behavioral deficits. 22 Furthermore, LRP has been recently shown to mediate the physiological effects of apoE. 49,53,54 Taken together, these studies suggest that the effects of apoE on ACT might be mediated via LRP. However, the precise intracellular events involved are unclear since LRP has not yet been linked to an intracellular signaling pathway.
In summary, the present study showed evidence supporting a role for apoE in regulating ACT expression via LRP, which is relevant to AD since these molecules appear to be closely associated with the pathogenesis of this neurodegenerative disorder.
Footnotes
Address reprint requests to Dr. E. Masliah, Department of Neurosciences, University of California, San Diego, La Jolla, CA 92093-0624. E-mail: emasliah@ucsd.edu.
Supported by grants from the Italian Ministero dell’Universita e della Recercia Scientifica e Tecnologia and Associazione de Ricerca e Assistenze delle Demenze (to FL), National Institutes of Health Grants AG5131 and AG10869, The Broad Foundation, Alzheimer’s Association, and EBEWE (to EM).
References
- 1.Mahley RW: Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988, 240:622-630 [DOI] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261:921-923 [DOI] [PubMed] [Google Scholar]
- 3.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S: Apolipoprotein E polymorphism, and Alzheimer’s disease. Lancet 1993, 342:697-699 [DOI] [PubMed] [Google Scholar]
- 4.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD: Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA 1993, 90:9649-9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisniewski T, Haltia M, Ghiso J, Frangione B: Lewy bodies are immunoreactive with antibodies raised to gelsolin related amyloid-Finnish type. Am J Pathol 1991, 138:1077-1083 [PMC free article] [PubMed] [Google Scholar]
- 6.Feskens EJM, Havekes LM, Kalmijn S, De Knijff P, Launer LJ, Krombout D: Apolipoprotein E4 allele, and cognitive decline in elderly men. Br Med J 1994, 309:1202-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plassman BL, Breitner JC: Apolipoprotein E, and cognitive decline in Alzheimer’s disease. Neurology 1996, 47:317-320 [DOI] [PubMed] [Google Scholar]
- 8.Turgeon VL, Houenou LJ: The role of thrombin-like (serine) proteases in the development, plasticity and pathology of the nervous system. Brain Res Brain Res Rev 1997, 25:85-95 [DOI] [PubMed] [Google Scholar]
- 9.Abraham CR, Selkoe DJ, Potter H: Immunochemical identification of the serine protease inhibitor α1-antichemotripsin in the brain amyloid deposits of Alzheimer disease. Cell 1988, 52:487-501 [DOI] [PubMed] [Google Scholar]
- 10.Fraser PE, Nguyen JT, McLachlan DR, Abraham CR, Kirschner DA: a1-Antichymotripsin binding to Alzheimer Aβ peptides is sequence specific and induces fibril disaggregation in vitro. J Neurochem 1993, 61:298-305 [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Yee A, Brewer HB, Jr, Das S, Potter H: Amyloid-associated proteins α1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer β-protein into filaments. Nature 1994, 372:92-94 [DOI] [PubMed] [Google Scholar]
- 12.Eriksson S, Janciauskiene S, Lannfelt L: α1-antichymotrypsin regulates Alzheimer β-amyloid peptide fibril formation. Proc Natl Acad Sci USA 1995, 92:2313-2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasternack JM, Abraham CR, Van Dyke BJ, Potter H, Younkin SG: Astrocytes in Alzheimer’s disease gray matter express α-1-antichymotrypsin messenger RNA. Am J Pathol 1989, 135:827-834 [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham CR: The role of acute-phase α-1-antichymotrypsin in brain dysfunction and injury. Res Immunol 1992, 143:631-636 [DOI] [PubMed] [Google Scholar]
- 15.Licastro F, Mallory M, Hansen LA, Masliah E: Increased levels of α-1-antichymotrypsin in brain of patients with Alzheimer’s disease correlate with activated astrocytes and are affected by APOE4 genotype. J Neuroimmunol 1998, 88:105-110 [DOI] [PubMed] [Google Scholar]
- 16.Inglis JD, Lee M, Davidson DR, Hill RE: Isolation of two cDNAs encoding novel α-1-antichymotrypsin-like proteins in a murine chondrocytic cell line. Gene 1991, 106:213-220 [DOI] [PubMed] [Google Scholar]
- 17.Campbell I, Abraham CR, Masliah E, Kemper Ph, Inglis JD, Oldstone MBA, Mucke L: Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin-6. Proc Natl Acad Sci USA 1993, 90:10061-10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willnow TE, Armstrong SA, Hammer RE, Herz J: Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc Natl Acad Sci USA 1995, 92:4537-4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plump AS, Smith JD, Hayek T, Aaalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL: Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992, 71:343-353 [DOI] [PubMed] [Google Scholar]
- 20.Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD: Neurodegeneration in the CNS of apoE-deficient mice. Exp Neurol 1995, 136:107-122 [DOI] [PubMed] [Google Scholar]
- 21.Xu P-T, Schmechel D, Rothrock-Christian T, Burkhart DS, Qiu H-L, Popko B, Sullivan P, Maeda N, Saunders AM, Roses AD, Gilbert JR: Human apolipoprotein E2, E3, and E4 isoform-specific transgenic mice: human-like pattern of glial and neuronal immunoreactivity in central nervous system not observed in wild-type mice. Neurobiol Dis 1997, 1:1-18 [DOI] [PubMed] [Google Scholar]
- 22.Van Uden E, Veinbergs I, Mallory M, Orlando R, Masliah E: A novel role for receptor-associated protein in somatostatin modulation: implications for Alzheimer’s disease. Neuroscience 1999, 88:687-700 [DOI] [PubMed] [Google Scholar]
- 23.Chiang CS, Stalder A, Samini A, Campbell IL: Reactive gliosis as a consequence of IL-6 expression in the brain: studies in transgenic mice. Dev Neurosci 1994, 16:212-221 [DOI] [PubMed] [Google Scholar]
- 24.Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly RJ, Koch RJ, Cardenas GJ, Ernst DN: Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol 1993, 150:3602-3614 [PubMed] [Google Scholar]
- 25.Hansen LA, Masliah E, Galasko D, Terry RD: Plaque only Alzheimer disease is usually the Lewy body variant, and vice versa. J Neuropathol Exp Neurol 1993, 52:648-654 [DOI] [PubMed] [Google Scholar]
- 26.Galasko D, Saitoh T, Xia Y, Thal LJ, Katzman R, Hill LR, Hansen L: The apolipoprotein E allele 4 is over-represented in patients with the Lewy body variant of Alzheimer’s disease. Neurology 1994, 44:1950-1951 [DOI] [PubMed] [Google Scholar]
- 27.Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS: Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol 1981, 10:184-192 [DOI] [PubMed] [Google Scholar]
- 28.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemes J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J: Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 1995, 373:523-527 [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ: Protein measurement with Folin phenol reagent. Biol Chem 1951, 193:265-272 [PubMed] [Google Scholar]
- 30.Tanaka S, Chen X, Xia Y, Kang DE, Matoh N, Sundsmo M, Thomas RG, Katzman R, Thal LJ, Trojanowski JQ, Saitoh T, Ueda K, Masliah E: Association of CYP2D microsatellite polymorphism with Lewy body variant of Alzheimer’s disease. Neurology 1998, 50:1556-1562 [DOI] [PubMed] [Google Scholar]
- 31.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE: Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science 1994, 264:850-852 [DOI] [PubMed] [Google Scholar]
- 32.Strickland DK, Kounnas MZ, Argraves WS: LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J 1995, 9:890-898 [DOI] [PubMed] [Google Scholar]
- 33.Saunders AM, Strittmatter WJ, Schmechel D, St. George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD: Association of apolipoprotein E allele E4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43:1467-1472 [DOI] [PubMed] [Google Scholar]
- 34.Kordula T, Rydel RE, Brigham EF, Horn F, Heinrich PC, Travis J: Oncostatin M, and the interleukin-6, and soluble interleukin-6 receptor complex regulate α-1-antichymotrypsin expression in human cortical astrocytes. J Biol Chem 1998, 273:4112-4118 [DOI] [PubMed] [Google Scholar]
- 35.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA: A family of cytokine-inducible inhibitors of signalling. Nature 1997, 387:917-921 [DOI] [PubMed] [Google Scholar]
- 36.Igbavboa U, Avdulov NA, Chochina SV, Wood WG: Transbilayer distribution of cholesterol is modified in brain synaptic plasma membranes of knockout mice deficient in the low-density lipoprotein receptor-apolipoprotein E, or both proteins. J Neurochem 1997, 69:1661-1667 [DOI] [PubMed] [Google Scholar]
- 37.Janciauskiene S, Eriksson S: In vitro complex formation between cholesterol and α-1-proteinase inhibitor. FEBS Lett 1993, 316:269-272 [DOI] [PubMed] [Google Scholar]
- 38.Licastro F, Davis LJ, Pedrini S, Galasko D, Masliah E: Prostaglandin E2 induced polymerization of human α-1-antichymotrypsin, and suppressed its protease inhibitory activity: implications for Alzheimer’s disease. Biochem Biophys Res Commun 1998, 249:182-186 [DOI] [PubMed] [Google Scholar]
- 39.Poirier J, Baccichet A, Dea D, Gauthier S: Cholesterol synthesis and lipoprotein reuptake during synaptic remodeling in hippocampus in adult rats. Neuroscience 1993, 55:81-90 [DOI] [PubMed] [Google Scholar]
- 40.Poirier J: Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci 1994, 17:525-530 [DOI] [PubMed] [Google Scholar]
- 41.Masliah E, Mallory M, Alford M, Veinbergs I, Roses AD: Apolipoprotein E role in maintaining the integrity of the aging central nervous system. Christen Y Roses AD eds. Apolipoprotein E and Alzheimer’s Disease. 1995, :pp 59-73 Springer-Verlag, New York [Google Scholar]
- 42.Veinbergs I, Masliah E: Synaptic alterations in apolipoprotein E knockout mice. Neuroscience 1999, 91:401-403 [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Lomnitski L, Michaelson DM, Shohami E: Motor and cognitive deficits in apolipoprotein E deficient mice after closed head injury. Neuroscience 1997, 80:1255-1262 [DOI] [PubMed] [Google Scholar]
- 44.Laskowitz DT, Sheng H, Bart RD, Joyner KA, Roses AD, Warner DS: Apolipoprotein E-deficient mice have increased susceptibility to focal cerebral ischemia. J Cereb Blood Flow Metab 1997, 17:753-758 [DOI] [PubMed] [Google Scholar]
- 45.Veinbergs I, Jung MW, Young SJ, Van Uden E, Groves PM, Masliah E: Altered long-term potentiation in the hippocampus of apolipoprotein E-deficient mice. Neurosci Lett 1998, 249:1-4 [DOI] [PubMed] [Google Scholar]
- 46.Anderson R, Higgins GA: Absence of central cholinergic deficits in apoE knockout mice. Psychopharmacology 1997, 132:135-144 [DOI] [PubMed] [Google Scholar]
- 47.Anderson R, Barnes JC, Bliss TVP, Cain DP, Cambon K, Davies HA, Errington ML, Fellows LA, Gray RA, Hoh T, Stewart M, Large CH, Higgins GA: Behavioral, physiological, and morphological analysis of a line of apolipoprotein E knockout mice. Neuroscience 1998, 85:93-110 [DOI] [PubMed] [Google Scholar]
- 48.Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB, Jr: Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest 1986, 78:815-821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, Fagan AM, Holtzman DM: Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci 1998, 18:3261-3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE: Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem 1995, 270:27063-27071 [DOI] [PubMed] [Google Scholar]
- 51.Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L: Isoform-specific effects of human apolipoprotein E on brain function revealed in APOE knockout mice: increased susceptibility of females. Proc Natl Acad Sci USA 1998, 95:10914-10919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cullen P, Cignarella A, Brennhausen B, Mohr S, Assmann G, von Eckardstein A: Phenotype-dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte-derived macrophages. J Clin Invest 1998, 101:1670-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL: Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA 1995, 92:9480-9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM: Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth, and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem 1996, 271:30121-30125 [DOI] [PubMed] [Google Scholar]