Abstract
In human prostate adenocarcinoma, an association between loss of E-cadherin, increased Gleason score, and extracapsular dissemination has been observed. Further characterization of the E-cadherin/catenin phenotype of human prostate carcinoma cell lines showed loss of E-cadherin and expression of N-cadherin in poorly differentiated prostate carcinoma cell lines (PC-3N derived from PC-3, PC-3, and JCA1). We showed that N-cadherin is concentrated at sites of cell-cell contact in PC-3N cellular extensions. N-cadherin was also expressed in prostate stromal fibroblasts both in vitro and in prostate tissue. Co-cultures of prostate stromal fibroblasts and PC-3N cells showed the immunolocalization of N-cadherin in intercellular contacts. In addition, the isoform expression of the cadherin binding protein p120ctn differed in relation to the expression of E- versus N-cadherin by the prostate carcinoma cell lines. The p100 isoform was more highly expressed in E-cadherin-positive carcinoma cell lines, whereas p120 was predominantly expressed only in N-cadherin-positive prostate carcinoma cell lines and prostate stromal fibroblasts. The N-cadherin-positive carcinoma cell line, PC-3N, displayed aggressive invasion into the surface of the diaphragm muscle after intraperitoneal injection of SCID mice. The gain of N-cadherin and loss of E-cadherin by invasive prostate carcinoma cell lines suggests a progression from an epithelial to a mesenchymal phenotype, which may allow for their interaction with surrounding stromal fibroblasts and facilitate metastasis.
Prostate carcinomas display a high degree of biological diversity 1 and can be present as localized disease within the prostate or become highly invasive and metastasize to regional lymph nodes and bone. Although localized prostate carcinoma can be treated successfully, 2 the treatment success diminishes significantly when prostate tumor cells metastasize beyond the confines of the gland, mainly through perineural and stromal invasion. 3-5 The process of metastasis is multifaceted; however, the molecular mechanisms that promote dissemination of the metastatic phenotype in prostate carcinoma are not well understood. An early invasion event in prostate carcinoma is the loss or disruption of the basal cell component, followed by the gain or loss of genetic and biochemical functions in transformed luminal epithelial cells. 6 Some of the gains or losses of functions during prostate carcinoma progression are due to alterations in tissue organization between cells themselves, mediated by cell-cell adhesion receptors of the cadherin superfamily, and cell-extracellular matrix adhesion receptors of the integrin family and components of the extracellular matrix. 7
Epithelial cytoarchitecture and function in the prostate gland are maintained in part by the E-cadherin/catenin complex. 8 E-cadherin is a member of a family of Ca2+-dependent integral membrane cell-cell adhesion receptors. 9 E-cadherin is localized at the zonula adherens junction between epithelial cells 10 and is associated with peripheral basal-lateral actin filaments in a multiprotein complex with kinases, phosphatases, and catenins. 11 The cytoplasmic complex, which anchors E-cadherin to the actin cytoskeleton, 12 includes the intracellular proteins α-catenin, which has homology to vinculin, 13 and the armadillo family members β-catenin, γ-catenin/plakoglobin, 14 and p120ctn. 15 The p120ctn binding site in E-cadherin is different from the β-catenin/plakoglobin binding site and p120ctn does not bind to α-catenin. 16 After cell-cell contact, adhesion of the E-cadherin/catenin complex functions to establish epithelial cellular architecture by initiating formation of desmosomes, tight junctions, and gap junctions. 17
Alteration in E-cadherin/catenin function or expression is found in the neoplastic process as a step in metastasis. 18-21 This loss results, in part, in a transformation from the normal epitheloid morphology toward an invasive and less differentiated mesenchymal phenotype. 22,23 E-cadherin levels are reduced or absent in the more invasive tumor cell lines; this phenotype is reversed by transfection with full-length E-cadherin cDNA. 18 Immunohistochemical analysis of highly invasive tumors (breast, melanoma, prostate, non-small-cell lung carcinomas) indicates that these tissues have decreased E-cadherin levels, suggesting a decreased function for E-cadherin in organization of tissue structure. 24 Umbas et al 25 found that human prostate carcinomas with a Gleason score above 6 had decreased E-cadherin immunoreactivity compared to normal glandular epithelium, and tumors with a Gleason score of 9 or 10 had low E-cadherin immunoreactivity. Moreover, in prostate tumor cell lines (DU145, PC-3, PPC-1, and TSU-PR1), E-cadherin expression was also found to be decreased or absent. 26 These studies demonstrate that the down-regulation of the E-cadherin/catenin adhesion pathway is associated with loss of differentiation, and an increase in the invasive behavior of tumor cells observed in prostate carcinomas.
To better understand the molecular basis of variability of prostate carcinoma invasiveness, we examined the protein and mRNA expression levels of the E-cadherin/catenin complex in four human prostate adenocarcinoma cell lines: LNCaP, 27 DU145, 28 PC-3, 29 and JCA1. 30 We found that the prostate cell lines JCA1 and PC-3N, a derivative of PC-3, both of which lacked E-cadherin, expressed instead a larger molecular mass cadherin. We identified this cadherin as N-cadherin, and show here that N-cadherin is expressed in more invasive prostate adenocarcinomas cell lines and in prostate stromal fibroblasts. Moreover, the isoform expression (p120 and p100) of the cadherin-associated protein p120ctn was found to be dependent upon whether E- or N-cadherin was expressed in the prostate carcinoma cell lines. These results suggest that the loss of the epithelial phenotype in invasive prostate adenocarcinoma cell lines is followed by a gain of a N-cadherin/p120ctn phenotype.
Materials and Methods
Cell Culture
LNCaP and DU145 human prostate tumor cell lines were obtained from American Type Culture Collection (Manassas, VA). Isolation of JCA1 cells was previously described and established by Muraki et al. 30 The PC-3 cell line was originally obtained from American Type Culture Collection, and long-term passage in cell culture resulted in selection of a cell population with different growth, adhesive, and morphological phenotype (PC-3N). All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS; Intergen, Purchase, NY) and penicillin/streptomycin in a 37°C, 5% CO2 atmosphere at constant humidity.
Human prostate stromal fibroblasts (PSF) were cultured from surgical samples. Fibroblasts were isolated by cutting the prostate tissue into 1-mm 3 pieces, which were placed in 100-mm culture dishes and allowed to attach overnight. The tissue was maintained in DMEM with 10% FBS as cells migrated from the explants. After two passages with trypsin/EDTA, only prostate fibroblast cells remained in culture, which was shown by the absence of cytokeratin-positive cells. The cells were then maintained for another two passages before use in these studies.
Antibodies
A polyclonal antiserum (anti-pan-cadherin) was made to the deduced amino acid sequence of the COOH-terminus of mouse N-cadherin (residues 883–906) 31 using the peptide CDYDYLNDWGPRFKKLADMYGGGDD (Peptide Express, Fort Collins, CO). The peptide was coupled to keyhold limpet hemocyanin as described by Marcantonio and Hynes 32 and injected into New Zealand white rabbits with Freund’s incomplete adjuvant. Serum was harvested after booster injections of the antigen.
Mouse monoclonal antibodies used in the experiments were as follows: α-catenin clone 5 (Transduction Laboratories, Lexington, KY), E-cadherin clone HECD-1 (Zymed Laboratories, San Francisco, CA), N-cadherin (A-CAM clone GC-4; Sigma Chemical Co., St. Louis, MO), p120ctn clone 98 (Transduction Laboratories). A monoclonal mouse antibody to plakoglobin, PG5.1 33 was generously provided by Drs. Franke and Schmelz (Institute of Cell and Tumor Biology, German Cancer Research Center, Heidelberg, Germany). Rabbit cytokeratin 18A antibody was previously described by Nagle et al. 34 Secondary antibodies used in the experiments are as followed: Cy3-conjugated affinipure goat anti-mouse IgG (H + L) and fluorescein (FITC)-conjugated affinipure donkey anti-rabbit IgG (H + L) were purchased from Jackson ImmunoResearch Laboratories, Inc., (West Grove, PA). Anti-mouse IgG + HRP conjugate was purchased from Promega (Madison, WI) and anti-rabbit IgG-peroxidase conjugate from Boehringer Mannheim (Indianapolis, IN).
SDS-PAGE and Western Blot
Cell lysates were prepared and separated by 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) 35 and electrophoretically transferred to nitrocellulose. Monolayers of cells were washed with calcium- and magnesium-free phosphate buffered saline (CMF-PBS) containing 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). Cells were scraped in CMF-PBS, transferred to a microcentrifuge tube, and centrifuged. The pellet was lysed with 2× SDS sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 10% SDS, 25% glycerol) and 30 μg of cellular protein was loaded per lane. Protein concentrations were measured using the bicinchoninic acid (BCA) assay procedure (Pierce Chemical Co., Rockford, IL), with bovine serum albumin (BSA) as a standard. Antigens were detected by primary antibodies, followed with peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG. Protein bands were identified by chemiluminescence (NEN, Boston, MA) exposed on X-OMAT AR film (Kodak, Rochester, NY). Images of Western blots were captured using Metamorph Version 3.0 (Universal Imaging Corp., Westchester, PA), and quantitative densitometry was carried out by using One-D Scan Version 1.0 (Scanalytics, CSP Inc., Fairfax, VA).
To examine non-ionic detergent solubility of N-cadherin, PC-3N cells were grown to 90% confluency. Cells were washed 3× with CMF-PBS and cytoskeletal stabilization buffer (CSK buffer; 0.5% Triton X-100, 10 mmol/L PIPES, pH 6.8, 50 mmol/L NaCl, 3 mmol/L MgCl2, 0.3 mol/L sucrose) was added for 5 minutes at 4°C on a rocking platform. The cells were scraped and centrifuged, and detergent-soluble protein fractions were collected by acetone precipitation. The precipitate were collected by centrifugation at 10,000 rpm for 20 minutes air-dried, and resuspended in 2× SDS sample buffer. The insoluble fraction was collected by adding hot 2× SDS sample buffer to the cellular components remaining on the plate and syringing.
For immunoprecipitation, cells were lysed according to procedures by Reyolds et al 15 with 0.5% Nonidet P-40 (NP-40) in a buffer containing 10 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1 mmol/L PMSF, 1 mmol/L EDTA, 0.1 mmol/L sodium vanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin (Sigma). Proteins were immunoprecipitated from the lysates, separated by SDS-PAGE, and transferred to nitrocellulose, and antigen was detected as described above.
RNA Extraction and Northern Blot Analysis
Total RNA was prepared from cultured cells by acid guanidinium thiocyanate-pheno-chloroform extraction. 36 Twenty micrograms of each RNA sample were separated by electrophoresis in 1% agarose gel containing 1.85% formaldehyde and transferred onto a Hybond N+ nylon membrane (Amersham Life Science, Arlington Heights, IL). N-cadherin mRNA was detected by Northern blot analysis with a 300-bp EcoRI cDNA fragment isolated from full length N-cadherin (GenBank Accession X54315), which was obtained from Dr. John Hemperly (Becton Dickinson Research Center, Research Triangle Park, NC). 37 E-cadherin mRNA was detected using 1.7-kb SmaI fragment of mouse E-cadherin (GenBank Accession X06115). 12 Detection of plakoglobin (GenBank Accession M23410) was with a human cDNA obtained from Dr. Werner Franke. 38 Probes were random-primed with labeled α-32PdCTP (Amersham). Membranes were prehybridized for 18 hours at 42°C in a 6× SSC buffer consisting of 0.05 mol/L NaH2PO4, 5× Denhardt’s (50× = % Ficoll, 1% polyvinylpyrrolidone, 1% BSA), 1% SDS, 50% formamide, and 10 μg/ml salmon sperm DNA. 39 A denatured probe was added to blots and hybridized overnight. Blots were sequentially washed for 30 minutes at 65°C using the following conditions: 2× SSC/0.1% SDS, 0.3× SSC/0.5% SDS, and 0.1× SSC/1.0% SDS. Blots were then exposed to X-OMAT AR film (Kodak). Normalization for loading was compared to hybridization of a 1.2-kb PstI fragment of human GAPDH (GenBank Accession J04038).
Reverse Transcription-Polymerase Chain Reaction (RT/PCR) and DNA Sequencing
A PCR with cDNA generated by reverse transcription of total RNA from PC-3N cells was performed, using degenerate primers to amplify multiple cadherin subtypes. 40 PC-3N cDNA was amplified from 1 μg of DNase I-treated total RNA in 40 μl reaction mix containing random hexamer primers, 10 mmol/L DTT, 0.5 mmol/L dNTPs, 10 U RNasin, and 200 U of Maloney murine leukemia virus reverse transcriptase (Gibco BRL) for 60 minutes at 42°C. The cDNA product was then diluted with 80 μl of H2O, and 2.5 μl of this PC-3N cDNA product was used in a 25-μl PCR reaction using the 5′ oligonucleotide primer AATGAATTCGTNTTYGAYTAYGARGG and the 3′ primer AATGAATTCRTCNGCNAGYTTYTTRAA. The reaction products were next separated by a 4% agarose gel electrophoresis (3% Nusieve GTG agarose and 1% Seakem ME agarose, FMC BioProducts, Rockland, ME), and a cDNA fragment of about 150 bp was extracted, digested with EcoR1, and ligated into pBluescript (Stratagene, La Jolla, CA). Ligated products were transformed into XL-1 Blue E. coli (Stratagene), and sequences of 31 cDNA inserts were determined by dideoxy chain termination (Sequenase 2.0, United States Biochemical, Cleveland, OH).
SCID Mouse Model
BALB-c/B-17/IcrACCscid mice (Arizona Cancer Center SCID Colony) were maintained in a specific-pathogen-free environment in compliance with United States Public Health Service guidelines governing the care and maintenance of animals. Five-week-old male SCID mice were each inoculated intraperitoneally with 5 × 10 5 DU-145 or PC-3N cells resuspended in 0.25 ml of DMEM serum-free medium. Forty-two days after inoculation, mice were sacrificed and diaphragm tissues were fixed and processed according to McCandless et al. 41 Xenograft-fixed tissues were sectioned at 5 μm thickness. Sections were deparaffinized and stained with hematoxylin and eosin.
Preparation of Tissues and Immunocytochemistry
Human frozen prostate tissue was obtained from the University of Arizona Pathology Tissue Bank. Specimens were obtained at the time of surgery or autopsy, snap-frozen in isopentane, cooled by freon, and stored at −80°C. Frozen sections of 6 μm thick were placed onto poly-L-lysine-coated slides and fixed in acetone for 10 minutes at −20°C. Sections were then blocked with 2% BSA and 2% goat serum in CMF-PBS for 1 hour, then incubated with both rabbit polyclonal anti-cytokeratin 18 antibody and murine anti-N-cadherin for 1 hour. After washing, Cy3-conjugated anti-mouse IgG and FITC-conjugated donkey anti-rabbit IgG were applied for 1 hour. The slides were mounted with 2% n-propyl gallate/90% glycerol, pH 8.0. Human prostate tissues were viewed by laser scanning confocal microscope, LSM 410, equipped with He, Ne, and Ar lasers (Zeiss).
For immunofluorescence, cells were grown on glass coverslips to confluence. Cells were fixed for 5 minutes in 4% (w/v) paraformaldehyde in CMF-PBS and permeabilized in CSK buffer for 5 minutes at 4°C. Coverslips were incubated with 2% BSA and 2% goat serum in CMF-PBS and exposed to antibodies for 1 hour at 25°C. After washing, Cy3-conjugated secondary anti-mouse IgG was applied for 1 hour. For detection of N-cadherin in PC-3N and PSF co-cultures, PSF cells were grown to 50% confluency on glass coverslips overnight. PC-3N cells were labeled with 40 μg/ml of DiO (3,3′-dioctadecyloxacarbocyanine perchlorate (Molecular Probes, Eugene, OR) in ethanol) for 1 hour and washed extensively with CMF-PBS. Labeled PC-3N cells (104) were seeded with the PSF culture for 24 hours. Cells were then fixed, permeabilized with CSK buffer, and stained for N-cadherin as described above.
Cell Aggregation Assay
Cell-cell aggregation experiments were performed as described by Urushihara and Takeichi. 42 Monolayer cultures were treated with 0.01% trypsin (Worthington Biochemical Corp., Freehold, NJ) in the presence of 2 mmol/L calcium for 2 minutes. The trypsinized cells were washed gently by centrifugation in Hanks’ balanced salt solution (HBSS) containing 10 mmol/L HEPES, pH 7.4, and 1% BSA, and free of calcium and magnesium. Cells were dissociated thoroughly by trituration 10 times with a Pasteur pipette. 5 × 10 5 cells were then transferred to 24-well dishes in a final volume of 0.5 ml HEPES-buffered HBSS containing 1% BSA and 100 μg/ml of DNase I with or without 2 mmol/L CaCl2. The plates were previously coated with poly-hema (Sigma). Cell-cell adhesion was initiated with addition of calcium and the plates were rotated at 80 rpm at 37°C for 1 hour, and cells were then fixed with an equal volume of 8% paraformaldehyde in CMF-PBS, pH 7.4. For the mixed aggregation experiments, PC-3N cells were labeled for 1 hour at 37°C with 40 μg/ml DiO. Cell aggregation was done in the presence or absence of CaCl2 and/or N-cadherin specific blocking monoclonal antibody (A-CAM; clone GC4) at 80 rpm at 37°C for 1 hour. Cells were then fixed as previously described above. For analysis, 50 μl of the fixed aggregates were removed, placed on a slide, and covered with a coverslip. Aggregates were photographed under epifluorescence optics using the 20× objective (Zeiss) with both a FITC filter set and phase contrast.
Results
Invasive Characteristics of Prostate Carcinoma Cell Lines
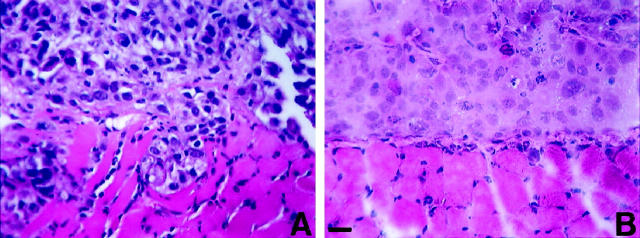
The human prostate adenocarcinoma cell lines LNCaP, DU145, PC-3, JCA1, and a subline, PC-3N, show distinct cellular morphologies in vitro. 27-30 DU145 and LNCaP cells displayed an epithelial phenotype, whereas PC-3, PC-3N, and JCA1 cells had, to varying degrees, a less organized and elongated, spindle-shaped, mesenchymal phenotype. PC-3N cells are a variant of parental PC-3 cell line 29 that displayed a more fibroblast-like phenotype after extensive subculturing. PC-3 has been previously shown to contain two distinct subpopulations. 43 One population expresses E-cadherin and displays an epithelial phenotype, and the other lacks E-cadherin expression and possesses a more scattered spindle-shaped phenotype, similar to PC-3N. Because the human prostate carcinoma cell lines PC-3N and DU145 have distinctly different growth characteristics, we sought to characterize the invasiveness using a human xenograph model of intraperitoneal inoculation in SCID mice. 41 Cross-sections of the diaphragm stained with hematoxylin and eosin show that after 5 weeks, PC-3N and DU145 cells had randomly attached to mesothelial surface of the diaphragm. PC-3N cells grew as small solid tumors on the surface of the diaphragm. There was also PC-3N cell invasion into the striated muscle of the diaphragm. Small clusters of invading PC-3N cells detected at multiple sites within the diaphragm muscle (Figure 1A) ▶ . These invading colonies of PC-3N human carcinoma were consistently only a few millimeters in diameter. DU145 grew as large, highly vascularized tumors on the diaphragm surface, but did not invade the diaphragm muscle (Figure 1B) ▶ .
Figure 1.
Photomicrographs of tumors of the prostate carcinoma cell lines PC-3N (A) and DU-145 (B) on the surface of diaphragms of SCID mice. SCID mice (n = 4) were injected intraperitoneally with 5 × 10 5 cells, sacrificed 5 weeks after injection, and the diaphragms fixed and processed in paraffin. DU145 tumors have penetrated the basement membrane, and PC-3N have penetrated through the murine straited muscle. Five-micron sections were cut and deparaffinized for hematoxylin and eosin staining. Scale, 60 μm.
Expression of N-Cadherin in PC-3, PC-3N, and JCA1 Prostate Cell Lines
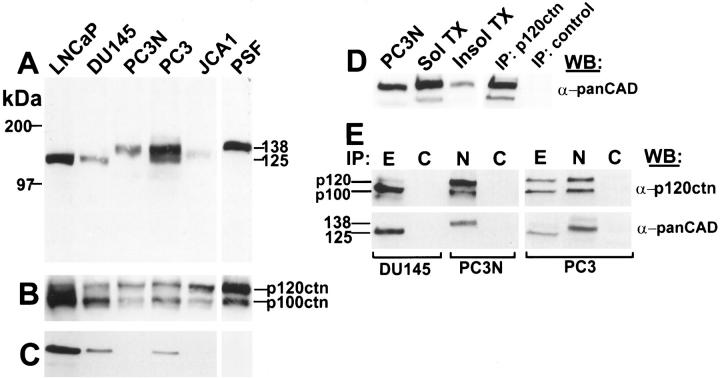
Because the growth and invasive characteristics of PC-3N prostate carcinoma cells in the SCID mouse diaphragm suggested weak cell-cell adhesion, we characterized the E-cadherin/catenin expression of PC-3N prostate carcinoma cells and the other four prostate carcinoma cell lines. The expression level of E-cadherin and α-, β-, and γ-catenins was assessed by immunoblotting equivalent amounts of cellular protein. Data are shown in Figure 2 ▶ , and summarized in Table 1 ▶ . To examine cadherins, we immunoblotted total prostate cell lysates with a pan-cadherin polyclonal antibody directed against the conserved cytoplasmic region of the classical cadherin family 44 (Figure 2A) ▶ . Antibodies prepared to this region have been shown to be immunoreactive with several members of the cadherin family. Immunoblot analysis indicated E-cadherin (MW = 125 kd) was present in LNCaP, DU145, and PC-3, but absent from PC-3N and JCA1 cell lines (Figure 2, A and C) ▶ . In addition, a higher molecular weight cadherin (138 kd) was detected in PC-3, PC-3N, and JCA1 adenocarcinoma cell lines. PC-3 cells showed expression of a mixed cadherin phenotype containing both E-cadherin and the larger cadherin. This unknown cadherin was not detected in LNCaP and DU145 (Figure 2A) ▶ .
Figure 2.
Immunoblot analysis of E-cadherin and p120ctn catenin in prostate carcinoma cell lines (LNCaP, DU-145, PC-3, PC-3N, JCA-1). Total cell lysates were extracted in 2× SDS-sample buffer, and 30 μg of protein/lane was analyzed by SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes and the filters were treated with either a pan cadherin polyclonal antibody which recognizes all classical cadherins (A), a mouse monoclonal antibody to p120ctn which recognizes both isoforms, p120ctn and p100ctn (B), or a mouse monoclonal antibody to E-cadherin (C). Non-ionic detergent solubility was determined by treating cultured PC-3N cells with CSK buffer. Triton X-100 soluble and insoluble fractions were collected. Equivalent amounts of total protein fractions were analyzed by SDS-PAGE and immunoblotted for N-cadherin using a polyclonal pan-cadherin antibody (D). PC-3N cells were extracted and immunoprecipitated with anti-p120ctn or normal mouse IgG. The immunoprecipitates were immunoblotted with anti-pan cadherin. Lane 1, PC-3N total protein lysate. Lane 2, soluble protein fraction of PC-3N. Lane 3, insoluble fraction of PC-3N cells. Lane 4, immunoprecipitation of p120ctn. Lane 5, control immunoprecipitation using mouse IgG antibody. (E) Immunoprecipitation of E- or N-cadherin in prostate carcinoma cell lines (DU-145, PC-3N, PC-3) using either a mouse monoclonal antibody to E-cadherin (E), a mouse monoclonal antibody to N-cadherin (N), or an irrelevant mouse IgG antibody (C). The immunoprecipitation fractions were separated on SDS-PAGE, transferred to a nitrocellulose membrane and immunoblotted with a monoclonal antibody to p120ctn (α-p120ctn) or a polyclonal cadherin antibody (α-panCAD). All immunoblots were developed with a chemiluminescence detection reagent.
Table 1.
Relative Expression Levels of E-Cadherin and Catenins in Human Prostate Adenocarcinoma Cell Lines
| DU-145* | LNCaP | PC-3 | PC-3N | JCA1 | |
|---|---|---|---|---|---|
| E-cadherin | 1.0 | 3.7 | 0.4 | 0.0 | 0.0 |
| α-catenin | 1.0 | 5.5 | ND§ | 0.0 | 0.2 |
| β-catenin | 1.0 | 3.1 | ND | 0.9 | 0.0 |
| plakoglobin | 1.0 | 1.7 | ND | 0.1 | 0.3 |
| p120ctn (p120+ p100)† | 1.0 | 2.5 | 0.5 | 0.4 | 0.8 |
| p120/p100‡ | 0.5 | 0.3 | 1.1 | 2.3 | 2.9 |
ND, not determined.
*DU-145 is a differentiated prostate carcinoma cell line which expresses equal levels of all catenins. Thus, all densitometry values are normalized to DU-145. Representative values are shown from at least three independent lysates.
†Immunoblot of p120ctn is shown in Figure 2B ▶ .
‡Values represent a ratio of p120 to p100.
§Loss of α-catenin has been previously reported by Morton et al. 26
Because the loss of catenins, especially α-catenin, has been shown to decrease E-cadherin mediated cell-cell interaction during the malignant progression of prostate cancer, 26,45 we characterized α-catenin, β-catenin, γ-catenin/plakoglobin (immunoblot not shown) and p120ctn expression levels in prostate carcinoma cell lines by immunoblotting of equivalent amounts of protein. The results are summarized in Table 1 ▶ . Because DU145 expressed approximately equal levels of all catenins and E-cadherin, densitometry values were normalized to DU145. Both LNCaP and DU145 expressed all catenins, consistent with a more differentiated phenotype. However, the catenin expression levels in both JCA1 and PC-3N cells showed dramatic differences. JCA1 lacked detectable β-catenin, and the level of α-catenin and plakoglobin was lower than in DU145. On the other hand, PC-3N cells showed reduced plakoglobin protein, and in agreement with Morton et al, 26 α-catenin also was not present.
Because the expression of the other catenins was abnormal in half of the prostate carcinoma cell lines studied, the expression of p120ctn isoforms was assessed by immunoblotting. The p120ctn monoclonal antibody used recognizes a common epitope in both the p120 and p100 isoforms. Both isoforms were present in all cell lines (Figure 2B) ▶ . However, expression of p100 was lower in PC-3N and JCA1 as compared to DU145 (Table 1) ▶ . This suggests expression of p120 and p100 is dependent on the type of cadherin present in the prostate cell lines. In the prostate carcinoma cell lines expressing only E-cadherin (DU145, LNCaP), p100 was the dominant isoform, approximately two-fold higher than p120. In contrast, p120 was the dominant isoform in PC-3N and JCA1, which lack E-cadherin and express a different cadherin. In PC-3 cells, which display a mixed cadherin population, both p120 and p100 isoforms were equally expressed (Table 1) ▶ .
To identify the unknown cadherin in PC-3N, PC-3, and JCA1 carcinoma cells, we amplified cadherin cDNAs by RT-PCR using degenerate oligonucleotide primers based on well-conserved amino acid sequences of the cadherin cytoplasmic domain. 40 A single cDNA band of approximately 150 bp was amplified from PC-3N cDNA, gel-purified, subcloned, and sequenced. The nucleotide sequence of 42% of the independent clones demonstrated 100% sequence identity with human N-cadherin. 37 None of the remaining clones demonstrated homology to E-cadherin or any other cadherin. These results suggest that the cadherin (MW = 138 kd) detected in PC-3N cells with the anti-pan cadherin polyclonal antibody is N-cadherin.
p120ctn Isoform Binds to N-Cadherin in PC-3N Cells
We assessed whether N-cadherin was distributed in the Triton X-100 insoluble fraction of confluent PC-3N cells, which presumably reflects N-cadherin associated with the cytoskeleton (Figure 2D) ▶ . Densitometric analysis showed approximately 25% of the N-cadherin was present in the detergent insoluble fraction of PC-3N cells (Figure 2D ▶ , lanes 2 and 3), suggesting that N-cadherin maybe associated with proteins of the cytoskeleton. We immunoprecipitated p120ctn from detergent lysates of PC-3N, transferred the immunoprecipitate to nitrocellulose, and blotted with a polyclonal pan-cadherin antibody. A cadherin of 138 kd was detected in the p120ctn immunoprecipitate, indicating the presence of N-cadherin (Figure 2D ▶ , lane 4). No cadherin band was detected in the nonimmune control.
To further determine if the ratio of p100 to p120 isoforms was associated with a particular cadherin subtype, we immunoprecipitated PC-3, DU145, and PC-3N cells with monoclonal antibodies specific to E-cadherin or N-cadherin and immunoblotted for p120ctn (Figure 2E) ▶ . In DU145 cells, which express only E-cadherin, p100 was the predominant isoform. In PC-3N cells, which express only N-cadherin, p120 was the predominant isoform. However, in PC-3 cells, which express both E- and N-cadherin, there were no differences in the binding of p100 vs. p120 in lysates immunoprecipitated with either E-cadherin or N-cadherin antibodies. This suggests that although both p120 and p100 isoforms bind N-cadherin and E-cadherin, the switch in the ratio of p120ctn isoforms in PC-3N and DU145 is due to differences in the isoform expression and not to differences in binding affinity.
Immunolocalization of N-Cadherin in PC-3N Cells
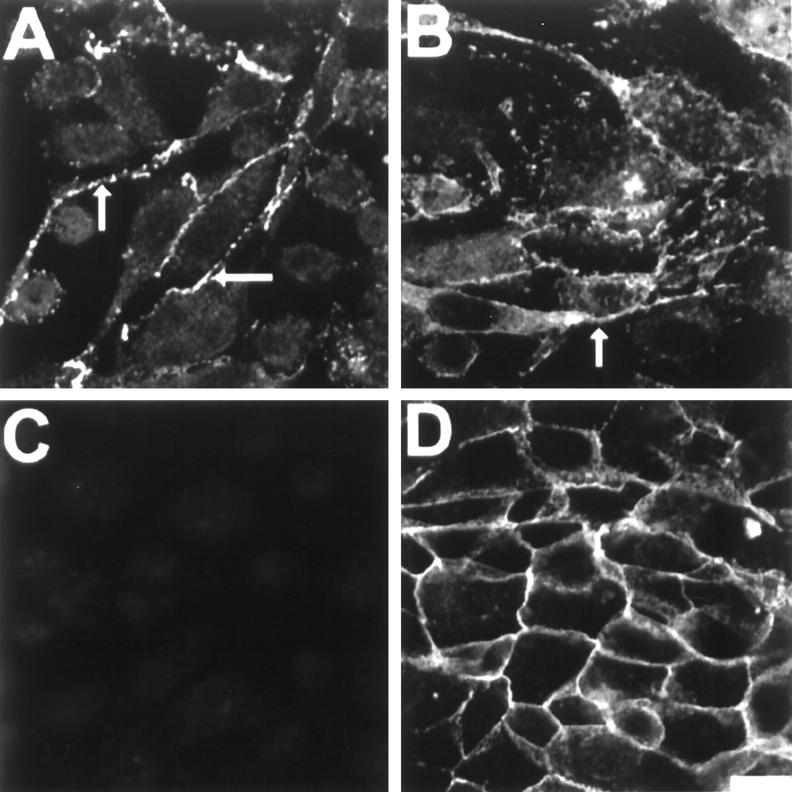
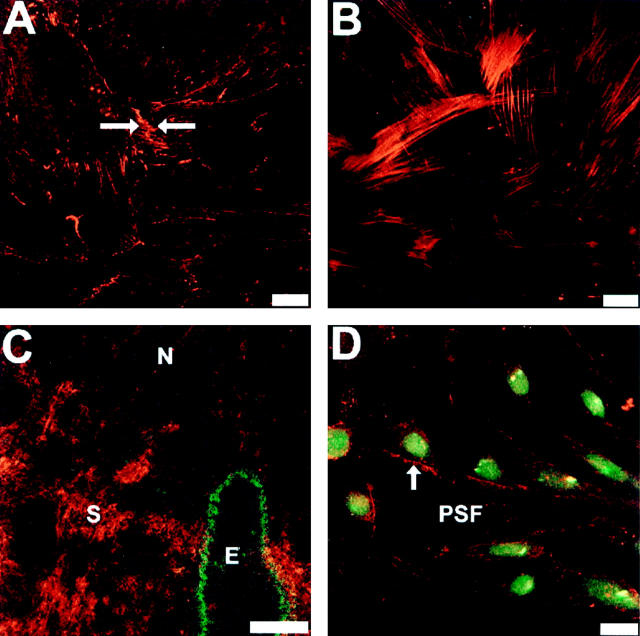
N-cadherin was localized to sites of cell-cell adhesive contacts in confluent cultures of PC-3N cells (Figure 3A) ▶ . The spindle-shaped PC-3N cells formed loose and extensive cellular contacts with their neighbors. The majority of N-cadherin immunoreactivity was localized in the cellular projections (Figure 3A and B ▶ , arrows). In addition, catenin p120ctn immunolabeling was similar to that of N-cadherin (Figure 3B) ▶ . p120ctn localized to similar regions as N-cadherin, with specific immunolocalization in the cellular projections. No E-cadherin immunoreactivity was observed in PC-3N, as expected (Figure 3C) ▶ . N-cadherin was also localized at cell-cell junctions in JCA1 and a subpopulation of PC-3 (data not shown). Moreover, E-cadherin was localized in the cellular junctions of DU145, which showed an epithelial morphology (Figure 3D) ▶ . E-cadherin expression is also similar in LNCaP cells, and no N-cadherin immunoreactivity was detected in either DU145 or LNCaP cells (data not shown).
Figure 3.
Immunolocalization of N-cadherin, E-cadherin and p120ctn in PC-3N and DU-145 carcinoma cell lines. Cells were grown on coverslips until confluency, then fixed with 4% PFA in CMF-PBS and permeabilized with CSK buffer. Immunofluorescence was carried out using either a mouse monoclonal antibody to N-cadherin on PC-3N (A), mouse monoclonal antibody to p120ctn on PC-3N cells (B), or mouse monoclonal antibody to E-cadherin on PC-3N (C) and DU-145 (D) cells. Rhodamine-coupled secondary antibody was used to reveal immunoreactivity, and photographs were taken by confocal microscopy. Arrows indicate cellular junctions and localization of N-cadherin and p120ctn to cellular extensions. Scale bar, 40 μm.
N-Cadherin mRNA Is Expressed in PC-3, PC-3N, JCA1, and Prostate Stromal Fibroblasts
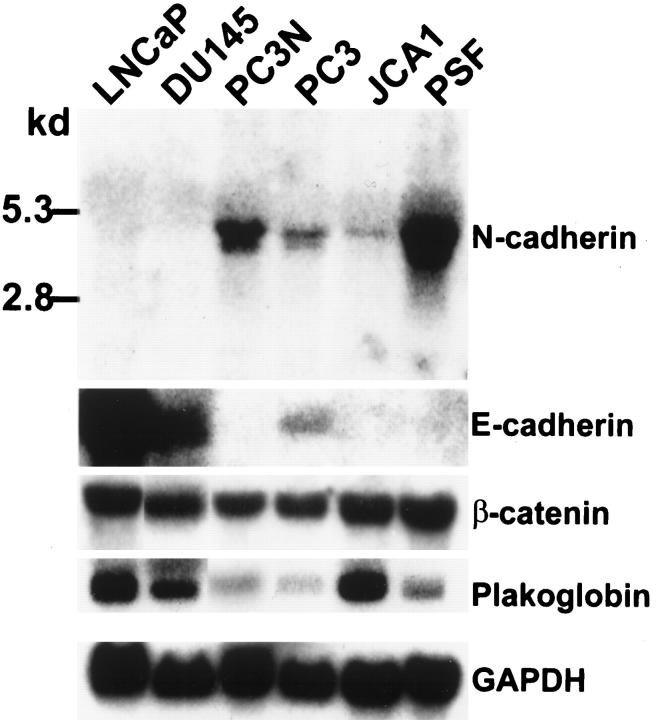
Differential expression of steady-state N-cadherin mRNA levels in the prostate carcinoma cell lines was determined by Northern blot analysis (Figure 4) ▶ . Similar to N-cadherin protein data, an N-cadherin mRNA transcript (4.2 kb) was detected in PC-3, PC-3N, and JCA1 cells, but was not detected in DU145 and LNCaP cells, even after long-term (1 week) exposure. In contrast, E-cadherin mRNA was expressed in LNCaP and DU145 cells, but not in PC-3N and JCA1 cells. Both E- and N-cadherin mRNAs were detected in PC-3 cells. Thus, in agreement with the protein expression, both PC-3N and JCA1 lack expression of E-cadherin, but alternatively express N-cadherin. N-cadherin mRNA, but not E-cadherin mRNA, was also detected in cultured prostate stromal fibroblasts (Figure 4) ▶ .
Figure 4.
Northern blot analysis of E- and N-cadherins and plakoglobin in prostate carcinoma cell lines and prostate stromal fibroblasts. Twenty micrograms per lane of total RNA from each cell line was blotted on a nylon membrane. A 300-bp EcoR I fragment to N-cadherin was used as a probe for N-cadherin detection. A 1.7-bp fragment to mouse E-cadherin was used as a probe for E-cadherin expression. Full-length plakoglobin and β-catenin cDNA were used to detect plakoglobin and β-catenin expression. A 1.2-kb GAPDH cDNA fragment was used as normalization standard. The hybridized membranes were exposed to X-ray films for 1 day (E-cadherin, β-catenin, plakoglobin, GAPDH) and 2 days (N-cadherin). This is a representative result of two independent experiments.
Moreover, steady-state mRNA expression levels of both β-catenin and plakoglobin mRNAs were not consistent with the protein expression in prostate cell lines. All prostate lines expressed detectable levels of plakoglobin mRNA (Figure 4) ▶ . However, although plakoglobin protein levels were lower in JCA1 than DU145 (Table 1) ▶ , plakoglobin mRNA appeared to be threefold higher in JCA1 versus DU145. Moreover, equal levels of β-catenin mRNA were expressed by all prostate carcinoma cell lines, even though protein levels varied (Figure 4) ▶ .
Immunolocalization of N-Cadherin in Prostate Stromal Fibroblasts in Vitro and in Situ
Immunolocalization of N-cadherin showed that both PC-3N cells and prostate stromal fibroblasts (PSFs) expressed N-cadherin and formed N-cadherin adherens junction contacts at cell-cell borders (arrow, Figure 5 ▶ ). Figure 5A ▶ shows that the expression of N-cadherin in cell-cell junctions of PSFs maintained in vitro. All cells were positive for N-cadherin and α-smooth muscle actin, which confirms the presence of only PSFs in the culture 46,47 (Figure 5B) ▶ . In addition, we immunolocalized N-cadherin in frozen sections of normal human prostate tissue. Immunoreactivity of N-cadherin in the prostate tissue sections was strongly detected in stromal cells and in nerve bundles that penetrate the gland, but N-cadherin was not detected in normal prostate epithelial glands, as shown by co-immunolocalization with antibodies to keratin 18 (Figure 5C) ▶ .
Figure 5.
Cellular localization of N-cadherin in prostate stromal fibroblast in vitro (A, B, and D) and in situ (C). PSF cells were grown on a coverslip until confluency. Cells were fixed with 4% PFA in CMF-PBS, permeabilized with CSK buffer and reacted with a mouse monoclonal antibody to N-cadherin (A) or mouse monoclonal antibody to α-smooth muscle actin (B). Arrow indicates N-cadherin mediated cellular junctions. C: N-cadherin immunoreactivity in stromal fibroblasts of normal prostate tissue (indicated by S). Fresh-frozen normal prostate tissues were sectioned at 6 μm and fixed with cold acetone. Coexpression of epithelial keratin 18 and N-cadherin was performed by co-immunoreacting the tissue with both monoclonal antibody to N-cadherin and polyclonal antibody to keratin 18. N-cadherin was detected by rhodamine-coupled secondary antibody whereas keratin 18 was detected by FITC-conjugated secondary antibody. N indicates N-cadherin expression in nerve and E indicates keratin 18 in a normal epithelial gland. D: Expression of N-cadherin in co-culture of PC-3N and PSF. PC-3 cells were labeled with 40 μg/ml of DiO (green membrane-labeled cells) before seeded on top of PSF cultured coverslip for 24. The arrow represents localization of N-cadherin in the cell-cell borders between PC-3N and PSF cells. Images were captured by confocal microscopy. Scale bars, 60 μm (A, B, and D) and 100 μm (C).
PC-3N prostate carcinoma cells and prostate stromal fibroblasts were cocultured to determine if N-cadherin was localized at sites of cell-cell contact. Stromal fibroblasts were cultured overnight before fluorescent dye DiO-labeled PC-3N cells were added, and cultured for an additional 24 hours. Immunolocalization of N-cadherin showed that there were sites of N-cadherin immunoreactivity at cell-cell contacts between PC-3N cells and stromal fibroblasts (arrow, Figure 5D ▶ ). There was no immunostaining detected in samples incubated with secondary antibodies alone (data not shown).
Cell-Cell Aggregation of PC-3N Cells and PSFs Is Mediated by N-Cadherin
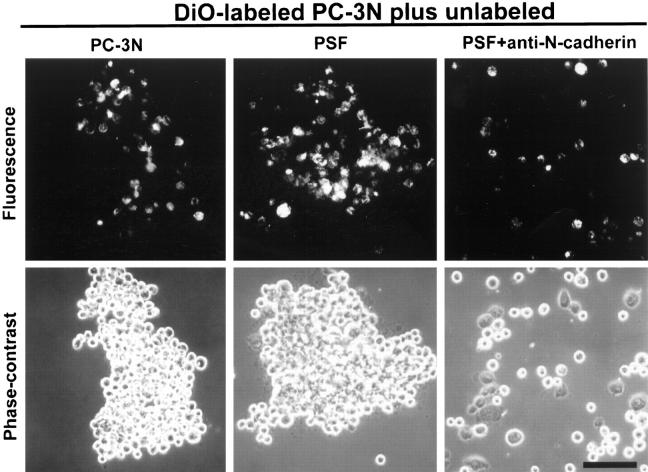
To examine whether the interaction between prostate carcinoma cells and prostate stromal fibroblasts was mediated by N-cadherin, a cell-cell aggregation assay 42 was performed. After dissociation of PC-3N cells by trypsinization in the presence of calcium into a single cell suspension, PC-3N cells labeled with DiO were mixed with unlabeled PC-3N cells and allowed to aggregate in the presence of calcium (Figure 6) ▶ . The calcium-dependent aggregation of PC-3N cells was time-dependent and blocked in the absence of Ca2+. Addition of function-blocking antibodies to N-cadherin inhibited the calcium-dependent aggregation of PC-3N carcinoma cells (data not shown).
Figure 6.
Cell-cell aggregation assay of PC-3N and prostate stromal fibroblasts. To distinguish PC-3N from PSF cells, PC-3N cells were labeled with 40 μg/ml of DiO for 1 hour. All cells were trypsinized in the presence of 2 mmol/L Ca2+ to prevent destruction of cadherins. Labeled PC-3N cells were mixed in an aggregation assay with either unlabeled PC-3N or PSF in the presence of Ca2+. Additionally, labeled PC-3N and PSF cells were mixed in an aggregation assay in the presence of anti-N-cadherin blocking antibody (n = 3). Cells were allowed to aggregate for 1 hour at 37°C on a gyrator shaker. Pictures of cell aggregation were taken both under fluorescence (top row) and phase contrast (bottom row) within same fields. Scale bar, 100 μm.
The homotypic interactions between PC-3N prostate carcinoma cells and stromal fibroblasts were also mediated by N-cadherin. Equivalent numbers of prostate stromal fibroblasts were mixed with PC-3N cells labeled with DiO. Large cell-cell aggregates, PC-3N cells, and PSF cells were observed in the presence of calcium. The calcium-dependent cell-cell aggregates consisted of all possible cell interactions: PC-3N/PC-3N, PC-3N/PSF, and PSF/PSF. This calcium-dependent cell-cell aggregation was largely abrogated in the presence of functional blocking antibody to N-cadherin. No aggregation was observed between DU145/PSF cells and DU145/PC-3N cells (data not shown). These findings demonstrate that homotypic interaction of N-cadherin mediates the interaction between the PC-3N prostate carcinoma cell line and prostate stromal fibroblasts.
Discussion
The results of this study demonstrate expression of N-cadherin in human prostate carcinoma cell lines (PC-3N and JCA1) that lack E-cadherin, and PC-3 cells, which have a mixed expression of E-cadherin and N-cadherin. In addition, we report the expression of N-cadherin in prostate stromal fibroblasts both in vitro and in situ. Co-aggregation of the prostate stromal fibroblasts with PC-3N carcinoma cells indicates that N-cadherin can mediate homotypic adhesion between these two cell populations. Prostate carcinoma invasion proceeds through stroma 3 with subsequent perineural migration and penetration of the capsule and escape from the prostate gland. 4,7 These data suggest that the presence of N-cadherin in stromal cells surrounding glandular epithelium and in nerve bundles extending into the prostate could facilitate prostate carcinoma cell invasion, and extracapsular metastasis. Furthermore, the less differentiated prostate cell lines that expressed only N-cadherin, PC-3N, and JCA1 also demonstrated a shift in isoform of the cadherin-associated catenin, p120ctn, a major substrate for src kinase and tyrosine kinase growth factor receptors. 15 Intercellular communication between stromal and carcinoma cells, through cell-cell adhesion molecules and growth factors, has been shown to be an important factor in neoplastic progression. 48,49 Umbas et al 25 found that 63% of prostate tumors that extended beyond the prostate capsule had decreased E-cadherin expression, compared to 33% of the tumors confined to the prostate. 25
Associated with the expression of N-cadherin in prostate carcinoma cell lines was the shift in p120ctn isoform expression of p120/p100. There are at least four p120ctn isoforms, which are thought to be generated by alternative splicing at the carboxy- and NH2-terminal ends of p120ctn. 15,50,51 The different p120ctn isoforms have been suggested to function in binding of distinct effectors regulating cell-cell adhesion or cell signaling. 52 Immunoprecipitation of PC-3, PC-3N, and DU145 cells indicated that although both p120 and p100 isoforms can bind E- and N-cadherin, the shift is a result of an increase in steady-state isoform protein level. In addition, high levels of the p120 isoform were also reported in cells that are highly motile, such as fibroblasts, whereas p100 is more abundant in epithelial cells. 50 This shift was also observed in epithelial cells transformed by src kinase. We localized p120ctn in the cellular extensions of PC-3N cells, and immunoprecipitated N-cadherin with a monoclonal antibody against p120ctn using conditions that preserve cadherin/catenin interactions. As p120ctn modulates cadherin adhesion, the differences in isoform expression may be important in the regulation of cadherin adhesion and metastatic potential of tumors. Reynolds et al 52 demonstrated that p120ctn was associated with all classical cadherin subtypes, and that overexpression of the p120 isoform in fibroblasts leads changes in cellular morphology with development of dendrite-like extension, with p120 isoform localized in these extensions.
The down-regulation of E-cadherin and/or catenins is a critical step for the progression of epithelial tumor invasion and metastasis. 53-55 In prostate adenocarcinomas, the aggressiveness of the tumor has been related to loss of E-cadherin. 25 The mechanism by which E-cadherin expression was lost may be mutation, 56 deletion, 57 hypermethylation, 55 and/or lost or altered catenin expression. 45 Our results demonstrate that the loss of E-cadherin in prostate cancer cell lines is accompanied by an unexpected expression of another classical type I cadherin subtype, N-cadherin. Whereas human E-cadherin has been mapped to chromosome 16q22.1 58 and is frequently deleted in prostate cancer, 59,60 N-cadherin has been mapped to human chromosome 18q11.2. 61
N-cadherin expression was not detected in normal prostate glandular epithelium, but it is found in neurons and stroma of the prostate. N-cadherin is found in a wide variety of cell types including neurons, skeletal and cardiac myocytes, fibroblasts, mesothelial cells, and some neoplastic epithelial cells. 62-66 Although growth factors and extracellular matrix are important contributors to prostate tumor progression, N-cadherin-mediated prostate carcinoma-stroma interaction may promote metastasis. Stromal mesenchymal-epithelial interactions reciprocally mediate the embryonic development and differentiation of the prostate. 67 In addition, prostate fibroblasts co-inoculated in athymic mice with prostate carcinoma cells have been found to accelerate tumor growth. 48,49 The role of N-cadherin in epithelium-derived tumor cell invasion is not restricted to prostate carcinomas. Islam et al 68 reported that there was an inverse expression of N- and E-cadherin in squamous cell carcinomas, and cells expressing high levels of N-cadherin were more invasive.
N-cadherin homotypic adhesion functions in distinct roles in different cell types. N-cadherin plays an important role in maintaining strong cell-cell adhesion in certain nonmotile cell types, such as in the intercalated discs of the myocardium. 69 In contrast, N-cadherin also plays an adhesive role in the dynamic growth of neurites, and the expression is also spatially diffuse throughout the cell surface of the neuron body. 70 Similar to neurons, N-cadherin localization in PC-3N carcinoma cells appears to be spatially diffuse and highly expressed in cellular extensions. A majority of the N-cadherin molecules are expressed diffusely throughout the cell membrane, and only a fraction of the N-cadherin molecules is concentrated at sites of cell-cell contact. This is similar to the reported distribution of N-cadherin 71 in neural crest cells migrating from the neural epithelium.
The expression of N-cadherin in invasive prostate carcinoma cell lines may be indicative of an epithelial/mesenchyme transition. In prostate carcinoma, the transformation of epithelium to invasive mesenchyme appears to involve a number of events in which certain carcinoma cells lose and gain functions, including cell-cell and cell-extracellular matrix interactions. Loss of E-cadherin and certain integrins are associated with loss of epithelial differentiation in prostate carcinoma. 7,72 This loss may lead to a gain of other adhesion molecules that may advance the development and aggressiveness of prostate carcinoma, as indicated by alterations in N-cadherin and catenin expression in the present studies. Furthermore, in prostate carcinoma, loss of β4 integrin with high expression of α6 and β1 integrins is associated with high invasive activity, implicating the heterodimer α6β1 integrins as leading candidates for conferring the invasive phenotype. 73
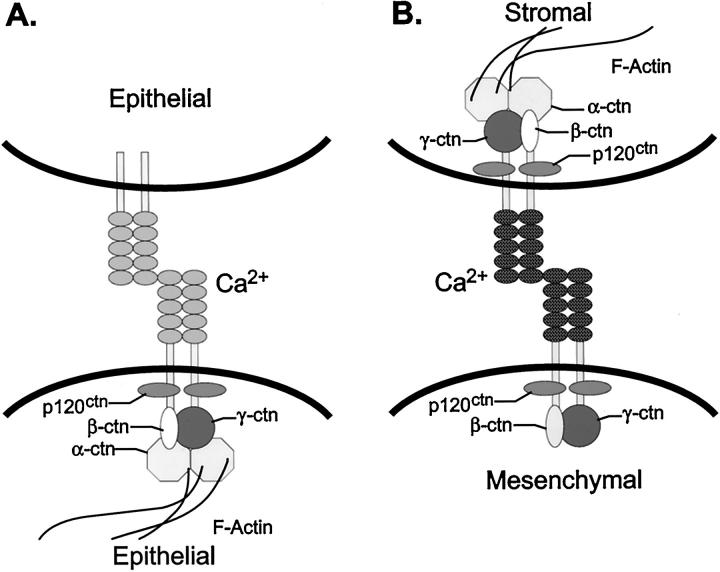
Stable adhesion for cadherins requires the homotypic protein-protein binding of the extracellular domains, and in addition, the cadherin cytoplasmic domain forms a complex with the actin cytoskeleton (Figure 7A) ▶ . Because α-catenin has actin binding activity, it probably links the cadherin/catenin complex to the actin cytoskeleton 74 and plays an important role in formation of a tight epithelial morphology. 75 The armadillo family members β-catenin and γ-catenin/plakoglobin function as an intermediate in linkage to α-catenin and the carboxy terminal cadherin cytoplasmic domain. 76 p120ctn binds in the juxtamembrane region 77 of the cadherin cytoplasmic domain and is likely to function in a different role than the other catenins.
Figure 7.
Two forms of cadherin cell-cell adhesion in human prostate carcinoma. E-cadherin mediated epithelial cell-cell adhesion (A) and N-cadherin mediated stromal:mesenchymal cell adhesion (B) are depicted. The cadherins are shown as a dimer. The armadillo catenins p100/p120ctn, β-catenin (βctn), and γ-catenin/plakoglobin (γctn) anchor E-cadherin to α-catenin (αctn), which links the complex to the peripheral actin cytoskeleton. In A, stable cadherin homotypic adhesion is depicted. B shows the known components of the N-cadherin/catenin complex in PC-3N carcinoma cells, which preferentially expresses the p120ctn isoform, and stromal fibroblasts. This cell-cell interaction is likely to be less stable and have a weaker affinity with the loss of α-catenin.
This work suggests that, although expression of N-cadherin may in part play a role in the progression of prostate carcinoma from epithelium to mesenchyme, it is likely that N-cadherin mediates a less stable cell-cell adhesion and may allow for carcinoma cell invasion and stromal interactions (Figure 7B) ▶ . Expression of N-cadherin in normal epithelial cells results in down regulation of E-cadherin expression and a scattered mesenchymal phenotype. 68 The PC-3N cell line has a spindle-shaped mesenchymal morphology, which expresses N-cadherin at sites of cell-cell contact. Because α-catenin is absent, 26 the N-cadherin adhesion between PC-3N cells is likely to be less stable. Our results show that N-cadherin mediates adhesion between α-catenin-deficient PC-3N cells and stromal fibroblasts, which contain normal levels of all of the catenins. N-cadherin in PC-3N cells may regulate the cellular outgrowth through cell-cell interactions, which may allow PC-3N to interact with surrounding prostate stromal fibroblasts. The adhesion by N-cadherin may explain the PC-3N invasive phenotype in the diaphragm striated muscle of xenograft tumors in SCID mice. Both PC-3N cells and the myocytes of the diaphragm express N-cadherin, potentially allowing the two cell populations to establish homotypic interactions. Our future direction is to determine whether N-cadherin is expressed in high-grade prostate carcinomas associated with capsular penetration through the perineural space, and is associated with metastasis.
Acknowledgments
We acknowledge the expert technical assistance of Colleen Forbes, Virginia Clark, and Karim Sallam. We thank Drs. T. Bowden and P. Gentry for helpful discussions.
Footnotes
Address reprint requests to Ronald L. Heimark, Department of Surgery, Arizona Health Sciences Center, P.O. Box 245084, 1501 N. Campbell, Tucson, AZ 85724. E-mail: rheimark@u.arizona.edu.
Supported by a Cancer Biology training grant (T32CA09213), NIH-CA 56666, and a grant to N. L. T. from the ARCS Foundation.
References
- 1.Cussenot O, Valeri A, Berthon P, Fournier G, Mangin P: Hereditary prostate cancer and other genetic predispositions to prostate cancer. Urologia Internationalis 1998, 60 Suppl 2:30-34 [DOI] [PubMed] [Google Scholar]
- 2.Gittes RF: Carcinoma of the prostate. N Engl J Med 1991, 324:236-245 [DOI] [PubMed] [Google Scholar]
- 3.Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA: The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol 1989, 142:763-768 [DOI] [PubMed] [Google Scholar]
- 4.McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA: Capsular penetration in prostate cancer. Significance for natural history and treatment. Am J Surg Pathol 1990, 14:240-247 [DOI] [PubMed] [Google Scholar]
- 5.Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, Nagle RB: Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol 1994, 145:167-174 [PMC free article] [PubMed] [Google Scholar]
- 6.Bostwick DG: Prostatic intraepithelial neoplasia (PIN). Urology 1989, 34:16-22 [PubMed] [Google Scholar]
- 7.Cress AE, Rabinovitz I, Zhu W, Nagle RB: The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev 1995, 14:219-228 [DOI] [PubMed] [Google Scholar]
- 8.Otto T, Rembrink K, Goepel M, Meyer-Schwickerath M, Rubben H: E-cadherin: a marker for differentiation and invasiveness in prostatic carcinoma. Urological Res 1993, 21:359-362 [DOI] [PubMed] [Google Scholar]
- 9.Takeichi M: Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 1995, 7:619-627 [DOI] [PubMed] [Google Scholar]
- 10.Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E: Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol 1996, 132:451-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozawa M, Baribault H, Kemler R: The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989, 8:1711-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M: Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 1987, 329:341-343 [DOI] [PubMed] [Google Scholar]
- 13.Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R: The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci USA 1991, 88:9156-9160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrea PD, Turck CW, Gumbiner B: A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 1991, 254:1359-1361 [DOI] [PubMed] [Google Scholar]
- 15.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z: Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 1994, 14:8333-8342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel JM, Reynolds AB: The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol 1995, 15:4819-4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boller K, Vestweber D, Kemler R: Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol 1985, 100:327-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schipper JH, Frixen UH, Behrens J, Unger A, Jahnke K, Birchmeier W: E-cadherin expression in squamous cell carcinomas of head and neck: inverse correlation with tumor dedifferentiation and lymph node metastasis. Cancer Res 1991, 51:6328-6337 [PubMed] [Google Scholar]
- 19.Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O: Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res 1989, 49:2128-2133 [PubMed] [Google Scholar]
- 20.Bussemakers MJ, Schalken JA: The role of cell adhesion molecules and proteases in tumor invasion and metastasis. World J Urol 1996, 14:151-156 [DOI] [PubMed] [Google Scholar]
- 21.Ruijter E, van dK, Aalders T, Ruiter D, Miller G, Debruyne F, Schalken J: Heterogeneous expression of E-cadherin and p53 in prostate cancer: clinical implications. BIOMED-II Markers for Prostate Cancer Study Group. Mod Pathol 1998, 11:276–281 [PubMed]
- 22.Birchmeier C, Birchmeier W, Brand S: Epithelial-mesenchymal transitions in cancer progression. Acta Anat 1996, 156:217-226 [DOI] [PubMed] [Google Scholar]
- 23.Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 24.Giroldi LA, Schalken J: Decreased expression of the intercellular adhesion molecule E-cadherin in prostate cancer: biological significance and clinical implications. Cancer Metast Rev 1993, 12:29-37 [DOI] [PubMed] [Google Scholar]
- 25.Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, Debruyne FM, Isaacs WB: Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res 1992, 52:5104-5109 [PubMed] [Google Scholar]
- 26.Morton RA, Ewing CM, Nagafuchi A, Tsukita S, Isaacs WB: Reduction of E-cadherin levels and deletion of the alpha-catenin gene in human prostate cancer cells. Cancer Res 1993, 53:3585-3590 [PubMed] [Google Scholar]
- 27.Horoszweicz JC, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim J, Chai LS, Kakati S, Arya SK, Sandberg AA: The LNCaP cell line—a new model for studies on human prostatic carcinoma. In: G.P. Murphy (ed.), Models for Prostate Cancer. 1980. New York, Alan R. Liss, Inc., pp 115–132 [PubMed]
- 28.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF: Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 1978, 21:274-281 [DOI] [PubMed] [Google Scholar]
- 29.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW: Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979, 17:16-23 [PubMed] [Google Scholar]
- 30.Muraki J, Addonizio JC, Choudhury MS, Fischer J, Eshghi M, Davidian MM, Shapiro LR, Wilmot PL, Nagamatsu GR, Chiao JW: Establishment of new human prostatic cancer cell line (JCA-1). Urology 1990, 36:79-84 [DOI] [PubMed] [Google Scholar]
- 31.Miyatani S, Shimamura K, Hatta M, Nagafuchi A, Nose A, Matsunaga M, Hatta K, Takeichi M: Neural cadherin: role in selective cell-cell adhesion. Science 1989, 245:631-635 [DOI] [PubMed] [Google Scholar]
- 32.Marcantonio EE, Hynes RO: Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol 1988, 106:1765-1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO: Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 1986, 46:1063-1073 [DOI] [PubMed] [Google Scholar]
- 34.Nagle RB, McDaniel KM, Clark VA, Payne CM: The use of antikeratin antibodies in the diagnosis of human neoplasms. Am J Clin Pathol 1983, 79:458-466 [DOI] [PubMed] [Google Scholar]
- 35.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 37.Reid RA, Hemperly JJ: Human N-cadherin: nucleotide and deduced amino acid sequence. Nucleic Acids Research 1990, 18:5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franke WW, Goldschmidt MD, Zimbelmann R, Mueller HM, Schiller DL, Cowin P: Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci USA 1989, 86:4027-4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roark EF, Paradies NE, Lagunowich LA, Grunwald GB: Evidence for endogenous proteases, mRNA level and insulin as multiple mechanisms of N-cadherin down-regulation during retinal development. Development 1992, 114:973-984 [DOI] [PubMed] [Google Scholar]
- 40.Suzuki S, Sano K, Tanihara H: Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regulat 1991, 2:261-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCandless JR, Cress AE, Rabinovitz I, Payne CM, Bowden GT, Knox JD, Nagle RB: A human xenograft model for testing early events of epithelial neoplastic invasion. Int J Oncol 1997, 10:279-285 [PMC free article] [PubMed] [Google Scholar]
- 42.Urushihara H, Takeichi M, Hakura A, Okada TS: Different cation requirements for aggregation of BHK cells and their transformed derivatives. J Cell Sci 1976, 22:685-695 [DOI] [PubMed] [Google Scholar]
- 43.Rokhlin OW, Cohen MB: Expression of cellular adhesion molecules on human prostate tumor cell lines. Prostate 1995, 26:205-212 [DOI] [PubMed] [Google Scholar]
- 44.Geiger B, Volberg T, Ginsberg D, Bitzur S, Sabanay I, Hynes RO: Broad spectrum pan-cadherin antibodies, reactive with the C-terminal 24 amino acid residues of N-cadherin. J Cell Sci 1990, 97:607-614 [DOI] [PubMed] [Google Scholar]
- 45.Shimazui T, Bringuier PP, van BH, Ruijter E, Akaza H, Debruyne FM, Oosterwijk E, Schalken JA: Decreased expression of alpha-catenin is associated with poor prognosis of patients with localized renal cell carcinoma. Int J Cancer 1997, 74:523-528 [DOI] [PubMed] [Google Scholar]
- 46.Ronnov-Jessen L, Celis JE, Van DB, Petersen OW: A fibroblast-associated antigen: characterization in fibroblasts and immunoreactivity in smooth muscle differentiated stromal cells. J Histochem Cytochem 1992, 40:475–486 [DOI] [PubMed]
- 47.Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE: Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA 1993, 90:999-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleave ME, Hsieh JT, von EA, Chung LW: Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol 1992, 147:1151-1159 [DOI] [PubMed] [Google Scholar]
- 49.Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, Zhau HE, von Eschenbach AC, Chung LW: Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci USA 1990, 87:75-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mo YY, Reynolds AB: Identification of murine p120 isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res 1996, 56:2633-2640 [PubMed] [Google Scholar]
- 51.Keirsebilck A, Bonne S, Staes K, van HJ, Nollet F, Reynolds A, Van RF: Molecular cloning of the human p120ctn catenin gene (CTNND1): expression of multiple alternatively spliced isoforms. Genomics 1998, 50:129–146 [DOI] [PubMed]
- 52.Reynolds AB, Daniel JM, Mo YY, Wu J, Zhang Z: The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp Cell Res 1996, 225:328-337 [DOI] [PubMed] [Google Scholar]
- 53.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S, Takeichi M: Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res 1993, 53:1696-1701 [PubMed] [Google Scholar]
- 54.Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M: Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol 1991, 139:17-23 [PMC free article] [PubMed] [Google Scholar]
- 55.Graff JR, Herman JG, Lapidus RG, Chopra H, Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davison NE, Baylin SB: E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res 1995, 55:5195-5199 [PubMed] [Google Scholar]
- 56.Giroldi LA, Bringuier PP, de WM, Jansen C, van BA, Schalken JA: Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Comm 1997, 241:453-458 [DOI] [PubMed] [Google Scholar]
- 57.Carter BS, Ewing CM, Ward WS, Treiger BF, Aalders TW, Schalken JA, Epstein JI, Isaacs WB: Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad Sci USA 1990, 87:8751-8755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bussemakers MJ, van BA, Voller M, Smit FP, Schalken JA: The genes for the calcium-dependent cell adhesion molecules P- and E-cadherin are tandemly arranged in the human genome. Biochem Biophys Res Comm 1994, 203:1291-1294 [DOI] [PubMed] [Google Scholar]
- 59.Pan Y, Matsuyama H, Wang N, Yoshihiro S, Haggarth L, Li C, Tribukait B, Ekman P, Bergerheim US: Chromosome 16q24 deletion and decreased E-cadherin expression: possible association with metastatic potential in prostate cancer. Prostate 1998, 36:31-38 [DOI] [PubMed] [Google Scholar]
- 60.Suzuki H, Komiya A, Emi M, Kuramochi H, Shiraishi T, Yatani R, Shimazaki J: Three distinct commonly deleted regions of chromosome arm 16q in human primary and metastatic prostate cancers. Genes Chromosomes Cancer 1996, 17:225-233 [DOI] [PubMed] [Google Scholar]
- 61.Wallis J, Fox MF, Walsh FS: Structure of the human N-cadherin gene: YAC analysis and fine chromosomal mapping to 18q11.2. Genomics 1994, 22:172-179 [DOI] [PubMed] [Google Scholar]
- 62.Hazan RB, Kang L, Whooley BP, Borgen PI: N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun 1997, 4:399-411 [DOI] [PubMed] [Google Scholar]
- 63.Hatta K, Takeichi M: Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 1986, 320:447-449 [DOI] [PubMed] [Google Scholar]
- 64.Duband JL, Dufour S, Hatta K, Takeichi M, Edelman GM, Thiery JP: Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol 1987, 104:1361-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatta K, Takagi S, Fujisawa H, Takeichi M: Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol 1987, 120:215-227 [DOI] [PubMed] [Google Scholar]
- 66.Wheelock MJ, Knudsen KA: N-cadherin-associated proteins in chicken muscle. Differentiation 1991, 46:35-42 [DOI] [PubMed] [Google Scholar]
- 67.Hayward SW, Rosen MA, Cunha GR: Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol 1997, 79 Suppl 2:18-26 [DOI] [PubMed] [Google Scholar]
- 68.Islam S, Cary TE, Wolf GT, Wheelock MJ, Johnson KR: Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol 1996, 135:1643-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goncharova EJ, Kam Z, Geiger B: The development of adherens junction components in myofibrillogenesis in cultured cardiac myocytes. Development 1992, 114:173-183 [DOI] [PubMed] [Google Scholar]
- 70.Letourneau PC, Shattuck TA, Roche FK, Takeichi M, Lemmon V: Nerve growth cone migration onto Schwann cells involves the calcium-dependent adhesion molecule, N-cadherin. Dev Biol 1990, 138:430-442 [DOI] [PubMed] [Google Scholar]
- 71.Monier-Gavelle F, Duband JL: Control of N-cadherin-mediated intercellular adhesion in migrating neural crest cells in vitro. J Cell Sci 1995, 108:3839-3853 [DOI] [PubMed] [Google Scholar]
- 72.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE: Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol 1995, 146:1498-1507 [PMC free article] [PubMed] [Google Scholar]
- 73.Rabinovitz I, Nagle RB, Cress AE: Integrin alpha 6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metast 1995, 13:481-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS: Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA 1995, 92:8813-8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watabe M, Nagafuchi A, Tsukita S, Takeichi M: Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol 1994, 127:247-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kemler R: From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genetic 1993, 9:317-321 [DOI] [PubMed] [Google Scholar]
- 77.Yap AS, Niessen CM, Gumbiner BM: The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol 1998, 141:779-789 [DOI] [PMC free article] [PubMed] [Google Scholar]