Abstract
Bacterial infection causes significant morbidity, mediated in part by the up-regulation of inflammatory cytokines. Cytokine induction is thought to stimulate osteolysis in conditions such as periodontal disease and otitis media. To establish the relative importance of interleukin-1 (IL-1) and tumor necrosis factor (TNF) in mediating the response to a mixed anaerobic infection, we used an in vivo model in which the dental pulp was inoculated with six anaerobic pathogens, in mice with functional deletions of receptors to IL-1 (IL-1RI−/−), TNF (TNFRp55−/−-p75−/−), or both (TNFRp55−/−-IL-1RI−/−). Polymorphonuclear and mononuclear phagocyte recruitment occurred to the greatest extent in TNFRp55−/−-IL-1RI−/− mice, and to a lesser extent in IL-1RI−/− or TNFRp55−/−-p75−/− mice, and the least in wild-type mice, demonstrating that recruitment of these phagocytes is not dependent on IL-1 or TNF receptor signaling. A similar pattern was observed for bacterial penetration into host tissue. Because it had recently been reported that TNF played a critical role in mediating lipopolysaccharide-induced bone loss, we anticipated that mice with targeted deletions of TNFRp55−/− would have reduced osteoclastogenesis. Surprisingly, osteolytic lesion formation was greatest in animals lacking TNF and/or IL-1 receptors. These results indicate that IL-1 or TNF receptor signaling is not required for bacteria-induced osteoclastogenesis and bone loss, but does play a critical role in protecting the host against mixed anaerobic infections.
Anaerobic bacteria initiate a series of host responses, including production of proinflammatory mediators, recruitment of inflammatory cells, elaboration of lytic enzymes, and, when bone is involved, activation of osteoclasts. 1-4 A body of evidence indicates that the inflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor (TNF) play an important role in these processes, particularly with regard to bone loss. 2-6 Although recent studies have uncovered several essential elements of the complex cytokine network that is involved in protection of the host from infection, 5-7 the relative contributions of IL-1 and TNF have not been thoroughly clarified.
IL-1 and TNF play an important role in initiating and coordinating the cellular events that make up the immune system’s response to infection. Many cell types are capable of producing IL-1 and TNF, and almost all are capable of responding to these cytokines. 5,6 The biological effects of IL-1 and TNF include activation of leukocytes such as lymphocytes (T and B cells), macrophages, and natural killer cells; fever induction; acute-phase protein release; cytokine and chemokine gene expression; and endothelial cell activation. Under physiological conditions, IL-1 and TNF are induced and released in restricted microenvironments where they have autocrine and paracrine activity. During infection, morbidity can result when the systemic levels are sufficiently high enough to induce shock.
Abu-Amer and colleagues have proposed that TNF activity is critical in mediating bone loss due to the gram-negative bacterial product lipopolysaccharide (LPS). 8 They suggest that LPS induces TNF expression, which in turn stimulates osteoclastogenesis and bone loss. This is supported by findings that TNFp55−/− receptor mice have greatly reduced in vivo and ex vivo formation of osteoclasts in response to LPS. Similarly, we have reported that inhibitors of IL-1 and TNF applied in combination significantly reduce osteoclast activity and bone loss in bacteria-induced experimental periodontitis. 9
IL-1 activity is conferred by two related proteins, IL-1α and IL-1β, both of which bind to IL-1 receptors, termed type I and type II. The type I IL-1 receptor (IL-1RI) is responsible for specific signaling, whereas the type II receptor functions as a nonsignaling decoy receptor. 10 Similarly, there are two molecules of TNF: TNFα and TNFβ, which have a high degree of structural and sequence homology and are able to interact with two distinct TNF receptors, type I (TNFRp55) and type II (TNFRp75). 11 Most of the well-documented inflammatory properties of TNF are mediated by TNFRp55, while signaling through TNFRp75 appears to reduce TNF-mediated inflammation. 12 IL-1 and TNF share several biological activities and often have similar effects on inflammatory processes. In many instances IL-1 and TNF act synergistically in both in vivo and in vitro studies. 13,14
Because IL-1 and TNF have several overlapping functions, it has been difficult to establish the importance of each of these cytokines in the process of inflammation and host resistance to infectious agents. The generation and availability of mice lacking cytokines or cytokine receptors through targeted gene mutation allows for a more exact determination of the role of a particular cytokine in physiological homeostasis and the pathogenesis of disease states. Recently, IL-1 receptor-mutant (IL-1RI−/−) and TNF receptor-mutant (TNFRp55−/− and/or TNFRp75−/−) mice have been generated. These mice do not exhibit gross abnormalities and are capable of developing antibodies to exogenous antigen stimulation. 12,15,16 However, TNFRp55−/− mice fail to develop germinal centers in their peripheral lymphoid organs.
To investigate the role of IL-1 and/or TNF in the host response to mixed anaerobic infection in both soft and hard connective tissue, a model that could develop and maintain a conducive growth environment for anaerobic pathogens was used. 17,18 Infection of the dental pulp and subsequent osseous lesion formation at the apex of the root fulfills these criteria and was used to examine osteoclastogenesis as well as bacterial penetration, leukocyte recruitment, and soft tissue necrosis. After surgical exposure of the dental pulp and inoculation by six putative pathogens, the infectious sequelae were assessed in control mice and three groups of experimental mice, which lacked IL-1 and/or TNF receptor signaling (IL-1RI−/−, TNFRp55−/−-p75−/−, or TNFRp55−/−-IL-1RI−/−). The results indicate that neither IL-1 or TNF receptor signaling is essential for bacteria-induced osteoclastogenesis and bone loss. However, both play a critical role in limiting the damage caused by a mixed anaerobic infection, and their activities together afford greater protection than either alone.
Materials and Methods
Mice
For each data point, specimens were obtained from five mice. A total of 150 animals (age 10–14 weeks), including experimental and wild-type mice, were examined. The experimental mice consisted of three different groups with targeted mutations, as have been described 12,15,16 : 1) IL-1 receptor type I (IL-1RI−/−); 2) TNF receptors type I and type II (TNFRp55−/−-p75−/−); and 3) type I receptors for both IL-1 and TNF (TNFRp55−/−-IL-1RI−/−) (generously donated by Immunex R&D Corp., Seattle, WA). The wild-type mice with similar genetic background were C57BL/6 × 129J hybrids (purchased from Jackson Lab, Bar Harbor, ME). Deletion of the targeted genes was verified by polymerase chain reaction (PCR) that could distinguish between homozygous or heterozygous mice with targeted deletions and wild-type mice.
Bacterial Strains and Growth Conditions
Surgically exposed dental pulps were inoculated with six putative oral pathogens, a facultative anaerobe (Streptococcus mutans) and five obligate anaerobes: Streptococcus mutans (American Type Culture Collection (ATCC) 25175), Streptococcus intermedius (ATCC 27335), Peptostreptococcus micros (ATCC 33270), Porphyromonas gingivalis (ATCC 33277), Prevotella intermedius (ATCC 25611), and Fusobacterium nucleatum (ATCC 49256). The bacteria were grown under anaerobic conditions. During the inoculation procedures, anaerobic conditions were maintained with an N2 environment.
Induction of Osteolytic Lesions
Animals were anesthetized with a combination of Xylazine-20 (Ben Venue Laboratory, Bedford, OH) and Ketaset (Fort Dodge Laboratory, Fort Dodge, IA) and placed on a retraction board, and the mesial cusps of the first mandibular molars were surgically removed to expose the dental pulp. One hundred microliters of a viscous bacterial mixture (containing 10 8 of each of the six bacteria strains in 2% methylcellulose) were placed on the tooth surface. Five mice from each group were sacrificed by CO2 asphyxiation at 0, 3, 7, 14, 21, and 38 days after pulp exposure. The mandibles were dissected, immersed in 4% paraformaldehyde at 4°C for 4 hours, decalcified in EDTA, and prepared for cryostat sectioning as described previously. 19 Some sections were stained with hematoxylin and eosin (H&E), and others were assessed for tartrate-resistant acid phosphatase (TRAP) activity, using a kit from Sigma (St. Louis, MO). In addition, immunostained (F4/80) cells were identified using a biotinylated secondary antibody and a detection kit from Vector Laboratories (Burlington, MA) as previously described. 19 This antibody recognizes peripheral monocytic cells, including monocytes, macrophages, and dendritic cells, but not osteoclast precursors. TRAP staining and F4/80 immunostaining were performed on frozen serial sections in coordination with the H&E-stained slides. Subsequent analysis was performed by “blinded” observers.
Image Analysis and Measurements
Analysis of osseous lesions was restricted to histological sections representing the maximum lesion size for a given specimen. Measurements were made on serial histological sections, using computer-assisted image analysis. All images of the slides were coded by one person and analyzed by another person, making the measurements double blind. The results were verified by a second examiner. Interexaminer and intraexaminer variation was generally less than 5%.
Degree of Tissue Necrosis
Necrosis in the pulpal tissue of the distal root of the mandibular first molar was assessed at a magnification of ×400. The tissues within the root canal were divided into three equal parts: coronal third, middle third, and apical third. The following scale was used to assess the spread of necrosis from the coronal to the apical third: 0, no necrosis; 1, partial necrosis of coronal third; 2, total necrosis of coronal third; 3, partial necrosis of middle third; 4, total necrosis of middle third; 5, partial necrosis of apical third; and 6, total necrosis of apical third. The highest score represented the necrosis status of that canal and was used in statistical analysis.
Osteoclastogenesis and Osteoclastic Activity
The number of osteoclasts was determined by counting multinucleated TRAP-positive cells in direct contact with bone at a magnification of ×200 and expressed as the number per millimeter of bone length. In addition, the relative depth of Howship’s lacunae was evaluated for each osteoclast at a magnification of ×400 as an indicator of osteoclast activity. The following scale was used for assessment of osteoclast activity: 1, shallow lacunae; 2, moderate lacunae (less than a half the diameter of the osteoclast); and 3, deep lacunae (more than a half the osteoclast diameter). The numbers of osteoclasts in shallow, moderate, or deep Howship’s lacunae were calculated separately for a given specimen. The osteoclast activity score, which was used in statistical analysis, was determined by calculating the sum of the number of osteoclasts in each major group multiplied by the value assigned to that group (1, shallow; 2, moderate; 3, deep lacunae).
Inflammatory Cell Recruitment
The number of polymorphonuclear leukocytes was counted manually from an image projected onto a color monitor at a magnification of ×1000. A counting template was centered at a fixed distance from the apical foramina of distal roots throughout the selected section of each different specimen of the H&E-stained slides. Within these designated areas, the number of polymorphonuclear leukocytes was counted using their identifying characteristics, such as darkly stained cells with multilobed, horseshoe-shaped nuclei. The number of F4/80 immunopositive monocytic cells, which had a distinctly round and darkly stained nucleus, was similarly counted. However, F4/80 immunopositive histiocytic cells with a dendritic appearance were excluded from these counts because of difficulty in obtaining accurate measurements and because they were found in relatively high numbers in noninflamed specimens.
Bacterial Penetration of Tissue
Inoculation of the dental pulp with six putative oral pathogens was carried out as described above. Six groups of mice were tested—three groups of mice with targeted deletions (IL-1RI−/−, TNFRp55−/−-p75−/−, TNFRp55−/−-IL-1RI−/−), matched wild-type control mice, and two groups of normal CD-1 mice, which served as negative controls for sterile technique. Eight days after inoculation, the mice were carefully disinfected with Povidone iodine (Baxter Health Care Corp., Deerfield, IL) followed by 70% ethanol, and the lower jaw was microdissected and immediately placed in a sterile petri dish. The apical third of the distal root of the experimental tooth was microdissected in a sterile lamina flow hood and then crushed while submerged in a liquid dental transport medium (Anaerobe Systems, San Jose, CA). Serial dilutions were performed, bacteria were grown under anaerobic conditions, and the number of bacterial colonies formed was determined 7 days later by “blinded” examiners.
Statistical Analysis
The degree of tissue necrosis, size of osteolytic lesions, osteoclastogenesis and osteoclast activity scores, number of polymorphonuclear and mononuclear phagocytes, and the number of bacterial colonies recovered from the apical third of each root were analyzed using one-way analysis of variance with Tukey-Kramer’s post hoc test to determine significance at a given time point. Significance was established at P < 0.05.
Results
Progression of Bacteria-Induced Necrosis
The model used is useful for following the spread of bacteria-induced tissue damage, which occurs reproducibly and predictably involves necrosis of soft tissue followed by formation of an osteolytic lesion. After inoculation with one facultative and five obligate anaerobic bacteria, little or no tissue necrosis was observed at day 7 in wild-type mice (Figure 1) ▶ . However, in mice lacking responses to IL-1 and TNF, the tissue within the root was completely necrotic at this time point. Quantitative assessment of the progression of tissue necrosis is shown in Figure 2 ▶ . There were no statistical differences between the experimental groups or the experimental and control groups on day 3. However, significant differences were noted between the three experimental groups of IL-1 or TNF receptor-deficient mice and the wild-type group at day 7 and later time points. By day 14, a maximum or nearly maximum necrosis score was observed for mice in each of the experimental groups, whereas the control group score was significantly less. In fact, the wild-type mice did not have a maximum necrosis score even at day 38.
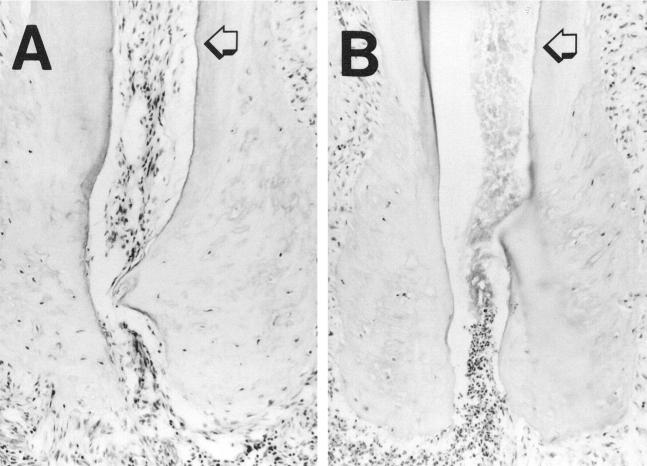
Figure 1.
Tissue necrosis is much more rapid in the absence of IL-1 and TNF activity. The dental pulp was surgically exposed and inoculated with 10 8 of five obligate anaerobes (Streptococcus intermedius, Peptostreptococcus micros, Porphyromonas gingivalis, Prevotella intermedius, and Fusobacterium nucleatum) and one facultative anaerobic bacteria (Streptococcus mutans), as described in Materials and Methods. Seven days later the animals were sacrificed and specimens were fixed in 4% paraformaldehyde, decalcified, prepared for cryostat sections, and stained with H&E. Intact tissues were still noted within the dental root in wild-type mice (A), whereas in the TNFRp55−/−-IL-1RI−/− mice (B), tissue necrosis had spread to the end of the root (original magnification ×200). The arrow indicates the junction between the middle and the apical third of the root.
Figure 2.
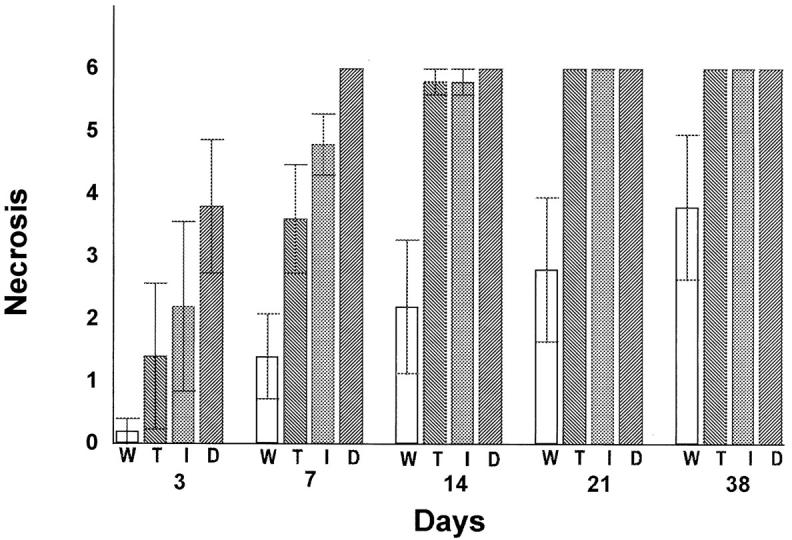
Quantitative analysis of tissue necrosis in mice lacking response to IL-1 and/or TNF. Surgical pulp exposure followed by inoculation with six oral pathogens was carried out as described in Figure 1 ▶ . H&E-stained cryostat sections were examined for the presence of tissue necrosis in the dental pulp. This tissue was divided into three equal parts: coronal third, middle third, and apical third, which follows the path of necrosis from the coronal third to the apical third of the dental root. Under microscopic examination (magnification, ×400), the following scale was used: 0, no necrosis; 1, partial necrosis of coronal third; 2, total necrosis of coronal third; 3, partial necrosis of middle third; 4, total necrosis of middle third; 5, partial necrosis of apical third; and 6, total necrosis of apical third. The highest score for the specimen represented the necrosis status of that tooth and was used in statistic analysis. Each value represents the mean ± SEM, n = 5 for each time point. Significant differences (P < 0.01) were noted between all receptor-deficient and wild-type mice at 7, 14, 21, and 38 days after bacterial challenge. There were no statistical differences between any of the groups at 3 days after pulpal insult. W, wild type; T, TNFRp55−/−-p75−/−; I, IL-1 RI−/−; D, TNFRp55−/−-IL-1RI−/−.
Bacterial Penetration of Tissues
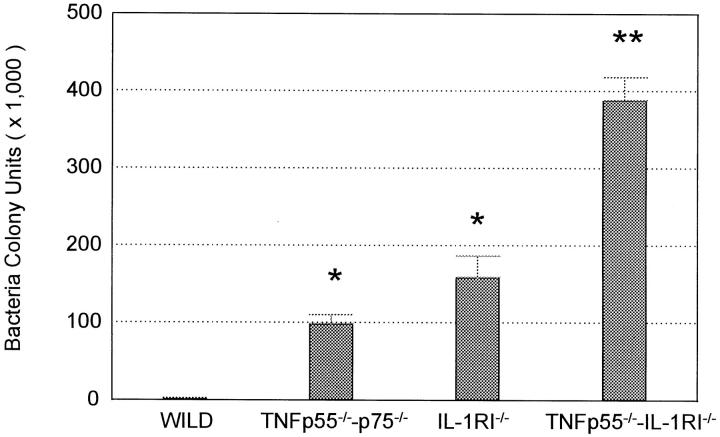
Bacteria that colonize the upper portion of the dental pulp eventually penetrate to the end of the tooth root. Because this progression is predictable and reproducible, it serves as an excellent model for the study of bacterial penetration of connective tissue. Figure 3 ▶ indicates that bacterial penetration is dramatically greater in mice lacking IL-1 and/or TNF responses. The mean number of bacterial colonies in receptor-deficient mice was significantly higher than that in wild-type mice; 4,700-fold for TNFRp55−/−-p75−/− mice, 7,600-fold for IL-1RI−/− mice, and 18,500-fold for TNFRp55−/−-IL-1RI−/− mice. Furthermore, bacterial penetration of the tissue was significantly greater in mice lacking both IL-1 and TNF responses compared to those lacking either IL-1 or TNF responses alone. There were no significant differences in the bacterial colonies formed between IL-1RI−/− and TNFRp55−/−-p75−/− mice. To establish that bacteria were not introduced into the specimens during dissection and bacterial sampling, bacterial colony counts were made for mice that had been treated in the same way as the experimental groups but did not undergo surgical exposure and bacterial inoculation of the dental pulp. As expected, no bacterial colonies were recovered from the dental pulp tissue of this negative control group (data not shown).
Figure 3.
Bacterial penetration at tissue is greater in mice lacking IL-1 and/or TNF activity. The number of bacterial colonies that could be cultured from the apical portion of the distal root 8 days after exposure and inoculation with six oral pathogens was determined. Values represent the mean ± SEM, n = 10 for each group. All receptor-mutant mice showed significant differences (P < 0.01) when compared with wild-type mice. **Significantly (P < 0.01) larger values were observed in TNFRp55−/−-IL-1RI−/− mice compared to IL-1RI−/− and TNFRp55−/−-p75−/− mice. *IL-1RI−/− and TNFRp55−/−-p75−/− mice had values that were not significantly different from each other (P > 0.05) but were significantly higher than those of wild-type mice (P < 0.01).
Formation of Osteolytic Lesions
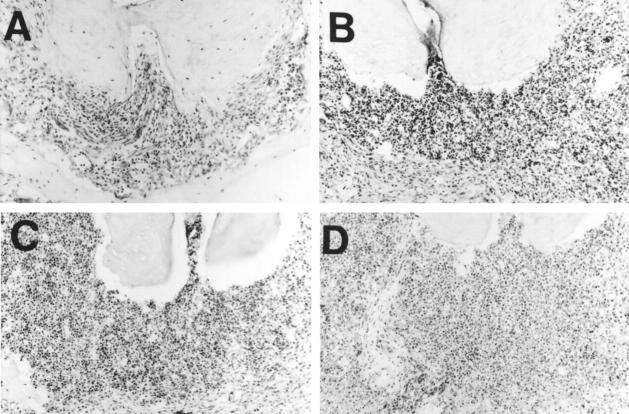
The photomicrographs shown in Figure 4 ▶ demonstrate the development of large osteolytic lesions in each of the experimental groups 38 days after bacterial inoculation. In the wild-type mice, there was a small osteolytic lesion with a light to moderate infiltration by inflammatory cells. Large osteolytic lesions in the IL/TNF receptor ablated groups were characterized by a severe inflammatory cell infiltrate with evidence of abscess formation.
Figure 4.
There is massive destruction of bone from osteolytic lesions in mice lacking IL-1 and/or TNF activity. The dental pulp was surgically exposed and inoculated with six different oral pathogens, as described in the legend to Figure 1 ▶ . Photomicrographs were taken of the osteolytic lesions from each group 38 days after inoculation. There was only slight bone loss in wild-type mice (A). In mice lacking either IL-1 (B) or TNF (C) activity, the amount of bone destruction was intermediate. Bone destruction was greatest in mice lacking both IL-1 and TNF activity (D). H&E-stained cryostat sections; original magnification, ×200.
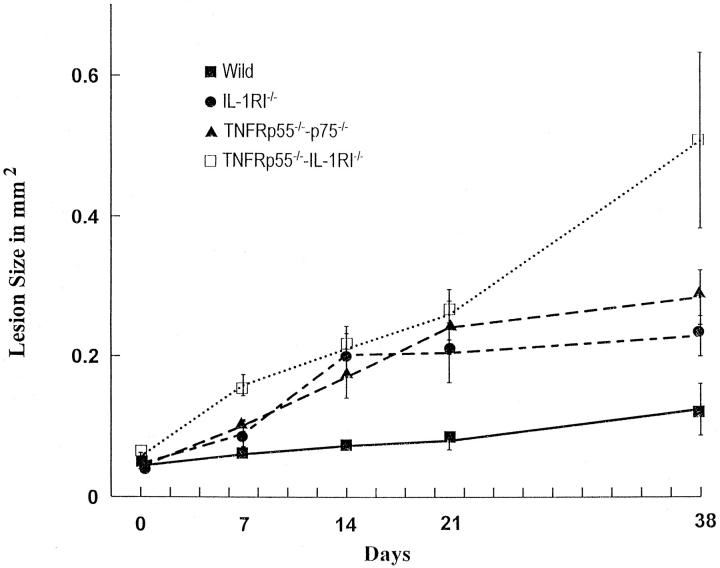
Figure 5 ▶ represents a quantitative assessment of the role of IL-1/TNF receptor signaling in the formation of osteolytic lesions at various time points. On day 7 the size of the osteolytic lesions was significantly greater in the TNFRp55−/−-IL-1RI−/− mice compared to wild-type mice, whereas on days 14, 21, and 38, all three experimental groups had osteolytic lesions that were significantly larger than those of the control group. Moreover, on day 38, the size of lesions in TNFRp55−/−-IL-1RI−/− mice was significantly greater than that observed in TNFRp55−/−-p75−/− or IL-1RI−/− mice, indicating the importance of both IL-1 and TNF in combination in limiting bacteria-induced osteolysis. At no time was there a statistical difference in the size of lesions between the mice with deleted responses to either IL-1 or TNF alone.
Figure 5.
Osteolytic lesions form rapidly and to a greater extent in mice lacking IL-1 and/or TNF activity. The size of the osteolytic lesions around the distal root end of experimental molars was determined at the indicated time points after exposure of the dental pulp and inoculation by six oral pathogens. At all time points after day 7, the size of lesions in the mice lacking receptors for IL-1, TNF, or both was significantly larger (P < 0.01) than in wild-type mice. There were no significant differences in lesion size between mice lacking receptors for IL-1 or TNF at any time point. Values represent the mean ± SEM, n = 5 for each data point.
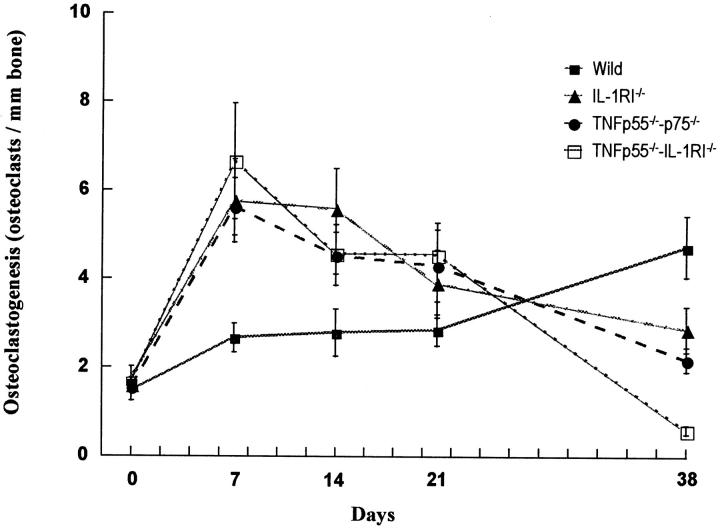
Osteoclastogenesis and Osteoclast Activity
Microscopic examination of TRAP-stained sections demonstrated that osteoclastogenesis proceeds rapidly in mice lacking IL-1/TNF receptor signaling, whereas it increases slowly and consistently in wild-type mice (Figure 6) ▶ . For the experimental groups after day 7, osteoclastogenesis reached a plateau and then gradually decreased, whereas it continued to increase for the wild-type mice. By day 38, osteoclastogenesis in the wild-type mice exceeded that found in the IL-1 and/or TNF receptor-deficient groups. A decline in osteoclast number at later time points in the experimental groups may be due to changes in osseous architecture as the lesions become very large. No significant differences in osteoclastogenesis were found between TNFRp55−/−-p75−/− and IL-1RI−/− mice at any time points.
Figure 6.
Osteoclastogenesis occurs rapidly in mice lacking IL-1 and/or TNF activity. Osteoclastogenesis was measured as the number of osteoclasts per millimeter length of bone at the root end, after exposure of the dental pulp and inoculation by six oral pathogens. Osteoclasts were identified as TRAP-positive multinucleated cells lining bone at ×200 magnification. Values represent the mean ± SEM, n = 5, for each data point. In wild-type mice, osteoclastogenesis increased slowly and gradually. In mice lacking receptors for IL-1, TNF, or both, osteoclastogenesis increased dramatically by day 7. Thereafter, it decreased gradually. At day 38, osteoclastogenesis in wild-type mice was greater than that in mice lacking receptors for IL-1, TNF, or both (P < 0.05). At any time point, there was no significant difference in osteoclastogenesis between mice lacking receptors for IL-1 or TNF (P > 0.05).
To provide a measure of osteoclast activity, the relative depth of Howship’s lacunae was assessed for each osteoclast, and the number of osteoclasts in shallow, moderate, or deep Howship’s lacunae were tabulated (Table 1) ▶ . Day 7 was chosen for this analysis because the osseous architecture was similar for all groups at that time point. In the wild-type mice, most of the Howship’s lacunae were shallow, whereas in the TNFRp55−/−-IL-1RI−/− group, osteoclasts were found in deep as well as moderate and shallow lacunae. The TNFRp55−/−-p75−/− and IL-1RI−/− mice had intermediate values between these two groups. Thus a greater proportion of osteoclasts were in moderate or deep lacunae in the mice lacking IL-1 or TNF responses compared to normals, and the greatest proportion in moderate or deep lacunae were observed in mice lacking both IL-1R1 and TNF-Rp55. The “osteoclast activity score,” which takes into account the activity per osteoclast and the number of osteoclasts, was significantly larger for TNFRp55−/−-IL-1RI−/− mice than for the two other experimental groups and wild-type mice. No significant differences were found between the scores of TNFRp55−/−-p75−/− or IL-1RI−/− mice compared to wild-type controls.
Table 1.
Osteoclast Activity is Greater in IL-1 or TNF Receptor-Deficient Mice Compared to Normal Mice
| Group | Distribution of osteoclasts in lacunae | Osteoclast activity score | ||
|---|---|---|---|---|
| Shallow lacunae | Moderate lacunae | Deep lacunae | ||
| Wild type | 3.00 | 0.11 | 0.00 | 3.30 ± 0.40 |
| TNFRp55−/−-p75−/− | 3.90 | 0.70 | 0.10 | 5.64 ± 0.92 |
| IL-1RI−/− | 2.91 | 1.45 | 0.27 | 6.60 ± 1.41 |
| TNFRp55−/−-IL-1RI−/− | 4.56 | 2.56 | 1.88 | 15.60 ± 2.32* |
Seven days after bacterial inoculation, osteoclast activity was assessed in TRAP-stained sections at ×400 magnification by evaluating the relative depth of Howship’s lacunae for each osteoclast, i.e. the number of osteoclasts were counted and categorized as to their distribution in shallow, moderate, and deep lacunae. The relative size of each Howship’s lacunae was given a numerical value as follows: 1, shallow lacunae; 2, moderate lacunae (less than a half-width of the osteoclast); 3, deep lacunae (more than a half-width of the osteoclast). The osteoclast activity score for each group was obtained by taking the sum of the product of the number of osteoclasts in each category times the value of that category, which is presented as the mean ± SEM, n = 5, for each data point.
* Significantly larger osteoclast activity score was noted in TNFRp55−/−-IL-1RI−/− mice compared to the other three groups (P < 0.01).
Inflammatory Cell Recruitment
Table 2 ▶ demonstrates that IL-1 or TNF receptor signaling is not required for rapid recruitment of polymorphonuclear and mononuclear phagocytes. In fact, at day 3, recruitment of phagocytes was observed for all groups, with TNFRp55−/−-IL-1RI−/− mice exhibiting a higher degree of mononuclear phagocyte recruitment compared to the others. By day 7 in the TNFRp55−/−-IL-1RI−/− mice and by day 14 in IL-1RI−/− or TNFRp55−/−-p75−/− mice, leukocyte counts could not be assessed because of the large degree of tissue necrosis, demonstrating the importance of IL-1/TNF receptor signaling in protecting the host from bacteria-mediated soft tissue necrosis.
Table 2.
Inflammatory Cell Recruitment Occurred in Osseous Lesions in Both IL-1 or TNF Receptor-Mutant Mice and in Normal Mice after Inoculation with Mixed Anaerobic Bacteria
| Time (day) | Polymorphonuclear leukocytes | Mononuclear phagocytes | ||||||
|---|---|---|---|---|---|---|---|---|
| W | T | I | D | W | T | I | D | |
| 03 | 0.31 ± 0.07 | 0.27 ± 0.07 | 0.40 ± 0.12 | 0.58 ± 0.09 | 0.18 ± 0.04 | 0.18 ± 0.04 | 0.29 ± 0.08 | 0.55 ± 0.09 |
| 07 | 0.82 ± 0.14 | 1.32 ± 0.34 | 0.97 ± 0.20 | Necrotic | 0.55 ± 0.20 | 0.80 ± 0.17 | 0.76 ± 0.20 | Necrotic |
| 14 | 1.06 ± 0.09 | Necrotic | Necrotic | Necrotic | 1.15 ± 0.17 | Necrotic | Necrotic | Necrotic |
| 21 | 1.04 ± 0.11 | Necrotic | Necrotic | Necrotic | 1.39 ± 0.21 | Necrotic | Necrotic | Necrotic |
The number of polymorphonuclear leukocytes and mononuclear phagocytes was counted manually from an image projected onto a color monitor at ×1000 magnification. Polymorphonuclear leukocytes were identified by their characteristic morphology on H&E-stained sections, and mononuclear phagocytes were identified with the F4/80 mAb on cryostat sections. A counting template was centered 0.1 mm from the root end as described in Materials and Methods. Values represent the mean ± SEM per 0.01 mm2 osseous lesion, n = 5, for each data point. In sections where the majority of fields had necrotic tissue, individual cells could not be accurately identified. If it was estimated that more than 30% of the cells were necrotic, the field was given that designation. If the majority of fields were necrotic, then the section was so designated. W, wild type; T, TNFRp55−/−-p75−/−; I, IL-1RI−/−; D, TNFRp55−/−-IL-1RI−/− mice.
Discussion
The dental pulp consists of loose connective tissue that can be colonized by bacteria after inoculation. Bacterial penetration can be assessed directly by culturing the infected dental pulp at the end of the root or indirectly by analyzing the spread of necrosis caused by infection. Once infection spreads, it initiates the formation of an osteolytic lesion, which is thought to result from the induction of cytokines, particularly IL-1α and TNFα. 20 We used this model to investigate the role of IL-1 and TNF activity in the protecting the host from a mixed, largely gram-negative, anaerobic infection. Furthermore, the experimental groups allowed us to directly assess whether IL-1 or TNF receptor signaling was essential for the induction of bacteria-induced osteoclastogenesis.
IL-1 and TNF are produced in response to infectious organisms, both in vitro and in vivo. Once produced, they may exert a beneficial or deleterious effect, depending on the quantity in which they are produced and the time period over which production is sustained. High systemic levels of IL-1 or TNF may create morbidity by the induction of shock. 5,6 We found that IL-1 and TNF individually represent critical cytokines in protecting the host from mixed anaerobic infections. Without IL-1 or TNF receptor signaling, bacteria were able to penetrate the loose connective tissue with much greater ease, as determined by bacterial colony counts from infected tissue and from observing the rate of necrosis caused by infection. Interestingly, IL-1 receptor signaling and TNF receptor signaling were both important, with little difference noted between them. However, in mice deficient in both IL-1 and TNF receptor signaling (IL-1R−/−-TNFR-p55−/−), the consequence of bacterial infection was more severe and more rapid. It should also be noted that the IL-1R−/−-TNFR-p55−/− mice possessed functional TNFR-p75, which has been shown to reduce inflammation. 12 A direct comparison of TNFR-p55−/− and TNFR-p75−/− mice, which was not undertaken here, would be required to establish the impact of TNFR-p75 on the progression of an anaerobic bacterial infection, the host response, osteoclastogenesis, and bone loss in this model.
IL-1 and TNF are prototypic cytokines that stimulate osteoclast formation and activity. In animals lacking functional IL-1 or TNF receptors there was a dramatic increase in bone destruction, as evidenced by enhanced osteoclastogenesis, a shift toward deeper Howship’s lacunae, and loss of bone mass. From these results it is clear that bone resorption can occur independently of IL-1R-1 and TNF-Rp55/p75 receptor signaling. The finding was surprising given that IL-1 and TNF have been shown to play prominent roles in bone loss associated with estrogen deficiency in mice 21 and that TNF has been suggested to be an essential mediator of LPS-induced bone loss. 8 Furthermore, we have recently reported that IL-1 and/or TNF have been shown to play central roles in bacteria-induced experimental periodontitis. 9 Thus results presented here indicate that a mixed anaerobic infection can induce the production of mediators other than IL-1 or TNF that are capable of initiating severe destruction of bone. Thus it is likely that IL-1 or TNF participates in osteoclastogenesis in many inflammatory processes, but under certain conditions such as anaerobic infection, other mediators are more important. The process of osteoclast differentiation is not fully understood, but numerous factors have the potential to induce osteoclast differentiation and bone resorption, including arachidonic acid metabolites, IL-3, IL-6, IL-11, granulocyte-macrophage colony-stimulating factor and osteoclast differentiation factor. 3,4
Polymorphonuclear leukocytes are particularly important in protecting the host from bacterial infection. The area over which polymorphonuclear leukocytes accumulate typically reflects the degree to which bacterial contamination has spread. 22 We noted that polymorphonuclear leukocyte recruitment in normal mice was concentrated in an area close to the apex of the tooth root, whereas in IL-1 receptor- and/or TNF receptor-deficient mice, a dense polymorphonuclear leukocyte infiltrate was spread over a relatively large area. This would suggest that IL-1 and/or TNF are important in localizing an infection and restricting its spread. We also found that there was rapid and equivalent accumulation of both mononuclear and polymorphonuclear leukocytes in normal and experimental mice. Thus formation of an inflammatory cell infiltrate occurs efficiently without IL-1 or TNF activity and suggests that endothelial changes necessary for leukocyte recruitment occur without activation by these cytokines. However, in their absence, there was greater tissue damage and bacterial penetration, indicating a diminished capacity to clear a mixed anaerobic infection. In vitro studies suggest that monocyte products such as IL-1 or TNF may represent important components in stimulating the antimicrobial activity of neutrophils during infection. 23,24 Future studies may determine whether there is a diminished antimicrobial function of neutrophils in IL-1 receptor- or TNF receptor-deficient mice in vivo, and whether this plays a role in their enhanced susceptibility to mixed anaerobic infection.
Acknowledgments
We thank Dr. Jacques Peschon at Immunex for generously donating the IL-1 and TNF receptor-deficient mice, and Dr. John Richardson, Boston University School of Dental Medicine, for helpful discussions regarding the identification of leukocytes in histological sections.
Footnotes
Address reprint requests to Dr. Dana T. Graves, Boston University Medical Center, Rm. W-202, 700 Albany Street, Boston, MA 02118. E-mail: dgraves@acs.bu.edu.
Supported by a grant from the National Institute of Dental and Craniofacial Research (DE07559).
Dr. Chen’s present address is Dental Department, Tri-Service General Hospital and School of Dentistry, National Defense Medical Center, Taipei, Taiwan, Republic of China.
References
- 1.Finlay BB, Falkow S: Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev 1997, 61:136-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkedal-Hansen H: Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res 1993, 28:500-510 [DOI] [PubMed] [Google Scholar]
- 3.Reddy SV, Roodman GD: Control of osteoclast differentiation. Crit Rev Eukaryot Gene Expr 1998, 8:1-17 [DOI] [PubMed] [Google Scholar]
- 4.Boyce BF, Hughes DE, Wright KR, Xing L, Dai A: Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab Invest 1999, 79:83-94 [PubMed] [Google Scholar]
- 5.Dinarello CA: Biologic basis for interleukin-1 in disease. Blood 1996, 87:2095-2147 [PubMed] [Google Scholar]
- 6.Tracey KJ, Cerami A: Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med 1994, 45:491-503 [DOI] [PubMed] [Google Scholar]
- 7.Kunkel S, Standiford T, Chensue SW, Kasahara K, Strieter RM: Cellular and molecular mechanisms of cytokine networking. Agents Actions Suppl 1991, 32:205-218 [DOI] [PubMed] [Google Scholar]
- 8.Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL: LPS-stimulated osteoclastogenesis is mediated by TNF via its p55 receptor. J Clin Invest 1997, 100:1557-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assuma R, Oates T, Cochran D, Amar S, Graves DT: IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol 1998, 160:403-409 [PubMed] [Google Scholar]
- 10.Colotta F, Dower SK, Sims JE, Mantovani A: The type II “decoy” receptor: a novel regulatory pathway for interleukin-1. Immunol Today 1994, 15:562-566 [DOI] [PubMed] [Google Scholar]
- 11.Tartaglia LA, Goeddel DV: Two TNF receptors. Immunol Today 1992, 13:151-153 [DOI] [PubMed] [Google Scholar]
- 12.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM: TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol 1998, 160:942-943 [PubMed] [Google Scholar]
- 13.Okusawa S, Gelfland JA, Ikejima T, Conolly RJ, Dinarello CA: Interleukin 1 induces a shock-like state in rabbits: synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest 1988, 81:1162-1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stashenko P, Dewhirst FE, Peros WJ, Kent RL: Synergistic interaction between IL-1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol 1987, 138:1464-1468 [PubMed] [Google Scholar]
- 15.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Peschon PJ: Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol 1997, 159:3364-3371 [PubMed] [Google Scholar]
- 16.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD: Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 1996, 271:1289-1291 [DOI] [PubMed] [Google Scholar]
- 17.Kakehashi S, Stanley GR, Fitzgerald RJ: The exposed germ-free pulp: effects of topical corticosteroid medication and restoration. Oral Surg Oral Med Oral Pathol 1965, 20:340-349 [DOI] [PubMed] [Google Scholar]
- 18.Fabricius L, Dahlen G, Dahlen SE, Moller AJ: Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res 1982, 90:200-206 [DOI] [PubMed] [Google Scholar]
- 19.Volejnikova S, Laskari M, Marks SC, Jr, Graves DT: Monocyte recruitment and expression of monocyte chemoattractant protein-1 are developmentally regulated in remodeling bone in the mouse. J Pathol 1997, 150:1711-1721 [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CY, Tani-Ishii N, Stashenko P: Bone-resorptive cytokine gene expression in periapical lesions in the rat. Oral Microbiol Immunol 1997, 12:65-71 [DOI] [PubMed] [Google Scholar]
- 21.Kimble RB, Matayoshi AB, Vannice JL, Kung VT, Williams C, Pacifici R: Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology 1995, 136:3054-3061 [DOI] [PubMed] [Google Scholar]
- 22.Smith JA: Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 1994, 56:672-686 [DOI] [PubMed] [Google Scholar]
- 23.Ferrante A, Harvey DP, Bates EJ: Staphylococcus aureus-stimulated mononuclear leucocyte-conditioned medium increases the neutrophil bactericidal activity and augments oxygen radical production and degranulation in response to the bacteria. Clin Exp Immunol 1989, 78:366-371 [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrante A, Martin AJ, Bates EJ, Goh DHB, Harvey DP, Parsons D, Rathjen DA, Russ G, Dayer JM: Killing of Staphylococcus aureus by TNFα-activated neutrophils. J Immunol 1993, 151:4821-4828 [PubMed] [Google Scholar]