Abstract
Human vascular adhesion protein-1 (VAP-1) is a dual-function molecule with adhesive and enzymatic properties. In addition to synthesis in endothelial cells, where it mediates lymphocyte binding, VAP-1 is expressed in smooth muscle cells. Here we studied the expression, biochemical structure, and function of VAP-1 in muscle cells and compared it to those in endothelial cells. VAP-1 is expressed on the plasma membrane of all types of smooth muscle cells, but it is completely absent from cardiac and skeletal muscle cells. In tumors, VAP-1 is retained on all leiomyoma cells, whereas it is lost in half of leiomyosarcoma samples. In smooth muscle VAP-1 predominantly exists as a ∼165-kd homodimeric glycoprotein, but a trimeric (∼250 kd) form of VAP-1 is also found. It contains N-linked oligosaccharide side chains and abundant sialic acid decorations. In comparison, in endothelial cells dimeric VAP-1 is larger, no trimeric forms are found, and VAP-1 does not have N-glycanase-sensitive oligosaccharides. Unlike endothelial VAP-1, VAP-1 localized on smooth muscle cells does not support binding of lymphocytes. Instead, it deaminates exogenous and endogenous primary amines. In conclusion, VAP-1 in smooth muscle cells is structurally and functionally distinct from VAP-1 present on endothelial cells.
Vascular adhesion protein-1 (VAP-1) supports the adherence of blood-borne lymphocytes to the vascular lining under conditions of shear. 1 In endothelial cells, VAP-1 is localized both in conspicuous cytoplasmic granules and on the luminal surface. Under physiological conditions, the synthesis of VAP-1 is most prominent in high endothelial venules (HEVs) of peripheral lymph node-type lymphatic organs, but it is also inducible in vessels at other locations in the setting of inflammation. 2 In endothelial cells VAP-1 is expressed as a homodimer of two 90-kd subunits. VAP-1 isolated from HEVs is an abundantly sialylated glycoprotein, and the sialic acid decorations are a prerequisite to the adhesive function of VAP-1. 3 Molecularly VAP-1 shows sequence similarity to semicarbazide-sensitive amine oxidases (SSAOs). 4 These enzymes oxidatively deaminate amines in a reaction whose biological function is still obscure, but which has been implicated in the pathogenesis of vasculopathies. 5 It should be noted that SSAOs are different in respect to cellular localization, substrates, cofactors, and inhibitors when compared to better characterized monoamine oxidases (MAOs) A and B.
In addition to endothelial cells, VAP-1 is also present in smooth muscle cells. 1,2 However, there is no detailed knowledge of the expression pattern of VAP-1 in different types of muscle cells, and the regulation of VAP-1 in pathological states involving smooth muscle cells, like tumorigenesis and atherogenesis, has not been assessed. Moreover, the structural similarity between VAP-1 in endothelial cells and VAP-1 in muscle cells has not been studied, and the function of VAP-1 at nonendothelial sites has remained elusive. There is at least one other endothelial adhesion molecule, vascular cell adhesion molecule-1 (VCAM-1, CD106), which is also expressed on these two distinct cell types. 6-8 Interestingly, expression of VCAM-1 in endothelial cells and muscle cells is differentially regulated, 9,10 and VCAM-1 serves different functions in these two cell types. 6,8 To understand the role of VAP-1 in muscle cells, we undertook a study to evaluate the localization, structure, and function of VAP-1 in different types of muscle cells.
We report here that VAP-1 is selectively expressed in smooth muscle among different types of muscle cells. In these cells, it is present on specialized plasma membrane domains as dimeric and trimeric forms, and it is a heavily glycosylated protein. VAP-1 expression is modulated during malignant transformation of smooth muscle cells. Functionally, smooth muscle VAP-1 displays clear differences from endothelial VAP-1 in serving only an enzymatic but not an adhesive function. Together, these data imply that VAP-1 molecules in different cell types are structurally distinct and serve different functions.
Materials and Methods
Normal Cells and Tissues
Muscle samples (the major saphenous vein, aorta, heart, gut from small intestine, appendix and colon, and bladder wall) were obtained from surgical operations, and skeletal muscle and large vessels (vena cava) were from autopsies. All of these tissues were macroscopically and microscopically normal. Tonsil samples were from tonsillectomies. Aortic smooth muscle cells (CRL 1999) and smooth muscle cell lines HISM (human intestinal smooth muscle, CRL 1692) and SK-LMS-1 (leiomyosarcoma, HTB 88) were from the American Type Culture Collection and cultured in RPMI 1640 containing 10% fetal calf serum (Gibco BRL, Paisley, UK), 2 mmol/L l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Peripheral blood lymphocytes (PBLs) were isolated from normal volunteers, using Ficoll-Hypaque gradient centrifugations.
Tumors, Atheromatous Samples, and Inflamed Tissue Specimens
Benign leiomyomas were from uterine tissue, and malignant leiomyosarcomas were collected from different locations as detailed in Table 1 ▶ . A portion of the specimens was snap-frozen in liquid nitrogen, and the rest was submitted to routine histopathological analyses. The samples from atheromatous arteries were taken during five surgical correction procedures of atheromatous aortic aneurysms. The specimens were taken from the borderline between aneurysmal and normal areas of arteries. The control specimens representing normal arterial wall were from macroscopically and microscopically nonatheromatous arteries of five patients (four renal arteries and one splenic artery) who underwent surgery for nonvascular indications. In addition, surgical samples from severely inflamed gut with massive leukocyte infiltrations (ulcerative colitis) were obtained from colectomy operations.
Table 1.
VAP-1 Expression in Leiomyosarcomas
| Patient | Grade | Location | VAP-1 expression |
|---|---|---|---|
| 1 | G4 | Soft tissue, rec. | − |
| 2 | G4 | Soft tissue, rec. | ++ |
| 3 | G4 | Soft tissue | − |
| 4 | G3 | Soft tissue | ++ |
| 5 | G3 | Subcutaneous | ++ |
| 6 | G4 | Kidney | − |
| 7 | G3 | Soft tissue, rec. | − |
| 8 | G4 | Soft tissue | ++ |
| 9 | G4 | Subcutaneous | − |
| 10 | G4 | Subcutaneous | − |
The intensity of VAP-1 expression was scored on a semiquantitative scale as follows: −, negative (identical to a negative control mAb); +, faintly positive; ++, moderately positive; +++, strongly positive (as bright as in normal smooth muscle). rec., recurrent disease.
mAbs
A function-inhibiting mouse mAb 1B2 against human VAP-1 and a control mAb against chicken T cells have been described. 1 A new mAb 2D10 (mouse IgG1) was raised against immunoaffinity-purified VAP-1. 11 Because neither 1B2 nor 2D10 works in formalin-fixed, paraffin-embedded sections, use of frozen sections was mandatory in all experiments.
Function blocking mAbs TS1/18 against β2-integrins (CD18), 12 HP2/1 against α4-integrin (CD49d), 13 and Hermes-1 and Hermes-3 against the hyaluronan-binding and endothelial-binding epitopes of CD44 14 have been described. To identify endothelial cells mAb 2C8 against CD31 15 and a polyclonal rabbit anti-human Factor VIII (Dakopatts, Glostrup, Denmark) were used. Peroxidase-conjugated sheep anti-mouse Ig was from Dakopatts, and fluorescein isothiocyanate-conjugated sheep anti-mouse Ig was from Sigma.
Immunolabeling and Electron Microscopy
Immunocytochemical reactions on acetone-fixed frozen sections were made by using an indirect immunoperoxidase method for light microscopy as described earlier. 1 After detachment of adherent cells with 5 mmol/L EDTA, analyses of smooth muscle cells in suspension were done with and without permeabilization (−20°C acetone, 2 minutes) by indirect immunofluorescence and fluorescence-activated cell sorter, using an earlier described protocol. 16 Reactions of adherent cells were carried out on chamber slides as described. 16
For immunolabeling electron microscopy, 1–2-mm-thick slices were cut from the tissue samples under a stereomicroscope. Selected regions were fixed for 3 hours in 2% paraformaldehyde fixative buffered with Sörensen’s phosphate buffer (pH 7.4). Then the specimens were incubated in 2.1 mol/L sucrose in water for 48 hours, embedded in O.C.T. (Tissue-Tec; Miles, Elkhart, IN), and frozen in liquid nitrogen. Sixty-micron frozen sections were cut, and the immunocytochemical reactions were made in free floating sections in test tubes. Anti-VAP-1 mAbs 1B2 and 2D10 were used at 5–10 μg/ml, and 2% normal mouse serum was used as a negative control. Incubation time was 48 hours for primary antibodies and 24 hours for the peroxidase-conjugated secondary antibody at 4°C. The washes between the antibody incubations were 3 × 45 minutes. The reaction was visualized using 3,3′-diamino-benzidine (DAB)-tetrahydrochloride (Polysciences, Warrington, PA) as an electron donor. Although the DAB precipitate becomes electron opaque in the osmium fixative, in several specimens the immunoreaction was further intensified with silver enhancement, as described. 17,18 In brief, precipitated DAB was first treated for 5 minutes with 0.1% sodium sulfide and washed in 250 mmol/L acetate buffer (pH 7.0). Then the reaction was developed in a solution containing sodium carbonate, ammonium nitrate, silver nitrate, tungstosilicic acid, and formaldehyde. The reaction was stopped with 1% acetic acid, and the product was stabilized by a 3-minute treatment with 0.05% gold chloride. After a 5-minute incubation in 0.3% sodium thiosulfate, the specimens were washed in phosphate-buffered saline (PBS) and fixed in 5% glutaraldehyde in 0.16 mol/L s-collidin-HCl buffer (pH 7.4) overnight. Selected specimens containing blood vessels were then postfixed for 2 hours in 1% aqueous OsO4 and processed for embedding in epoxy resin (Glycidether 100; Merck AG, Darmstadt, Germany) as described. 19 Thin sections were cut with a Reichert Ultracut E (Reichert-Jung Optische Werke, Vienna, Austria) ultramicrotome, and the grids were examined with a Jeol JEM 100 SX transmission electron microscope.
Immunoblotting and Glycosidase Digestions
Blood vessels were opened with scissors, and they were denuded from the endothelial layer by mechanical scraping. Lamina propria cells were stripped from gut samples by mechanical dissection and scraping. Thereafter, samples from vessel and gut wall, tonsil stroma, heart, and skeletal muscle were minced and lysed in a buffer containing 1% NP-40, 1.5 mmol/L MgCl2, 10 mmol/L Tris-base (pH 7.4), and 150 mmol/L NaCl or in PBS containing 1% NP-40. After clarification by centrifugation, the supernatants were treated with exo- and endoglycosidases or mock treated using an earlier described protocol. 3 Vibrio cholerae sialidase is active against α2,3, α2,6, and α2,8 linked sialic acids, 20 whereas Newcastle disease virus neuraminidase only hydrolyzes α2,3 (and α2,8)-linked sialic acids. 21 O-Sialoglycoprotease endopeptidase degrades mucin-like glycoproteins containing adjacent O-linked sialylated side chains. 22,23 O-Glycans were removed from sialidase-treated samples with recombinant O-glycanase and for N-glycanase treatment peptide: N-glycosidase F was added to PBS-lysate. The specificity and effectiveness of these digestions were documented earlier. 3
Metabolic Labelings
Small fragments of smooth muscle from intestinal wall were labeled with [35S]methionine/cysteine, lysed, and used for immunoprecipitations as described earlier for labeling of lymphoid tissues. 3 After washings, the antigens were eluted in Laemmli’s reducing sample buffer and separated by 5–12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were finally fixed, soaked in Enlightning, dried, and autoradiographed.
Adhesion Assays
In a modification of the Stamper-Woodruff endothelial cell binding assay, 24 frozen sections of appendices from normal and inflamed intestine and tonsil were cut and overlaid with saturating concentrations of anti-VAP-1 mAb 1B2 or a control mAb. Freshly isolated PBLs without or with phorbol myristate acetate activation (50 ng/ml for 30 minutes at 37°C followed by two washes) were resuspended in RPMI 1640 containing 10% fetal calf serum and 10 mmol/L HEPES (= binding medium). After filtering through a silk mesh, 3 × 10 6 cells were applied to the sections and allowed to bind for 30 minutes at +7°C under rotation (60 rpm on an orbital shaker) or at 37°C under stationary conditions. The nonadherent cells were then tilted off, and the adherent ones were fixed to the sections with ice-cold 1% glutaraldehyde in PBS. The number of cells bound to HEVs and to smooth muscle cells was counted under dark-field microscopy. Although the VAP-1 dependence of PBL adhesion to endothelial cells is much more pronounced in tonsil than in appendix, 1 the use of appendix allowed us to analyze simultaneously the binding of PBLs to HEVs and to muscular wall under identical experimental conditions.
Alternatively, vascular explants (∼2–3 cm-long fragments of saphenous vein) were cut open, and the endothelial cell layer was stripped off with gentle scraping. The explants were attached from their corners to the bottom of soft wells by fine needles and preincubated with an anti-VAP-1 mAb 1B2 or with a negative control mAb 3G6 (100 μg/ml) for 30 minutes. After washing with PBS, 4 × 10 6 lymphocytes were added into each well in 2 ml of the binding medium and allowed to adhere to smooth muscle cells under static or rotatory conditions for 30 minutes at room temperature. The nonadherent cells were removed by gently inverting the assembly, and the bound cells were fixed to the tissue by an overnight incubation in 5% glutaraldehyde in PBS. After Diff-Quick staining the number of adherent lymphocytes was counted from at least seven random microscopic fields per explant (magnification: ×200; 361 ± 89 (mean ± SEM) bound cells in the negative control mAb-treated explants in four independent assays). As a positive control, PBLs were preincubated with mAbs against CD44, CD18, and α4-integrins (each mAb at 25 μg/ml concentration, 0.5 ml for 10 × 10 6 cells), washed, and resuspended in the assay medium, and the adhesion assay was performed as described above.
Enzymatic Analyses
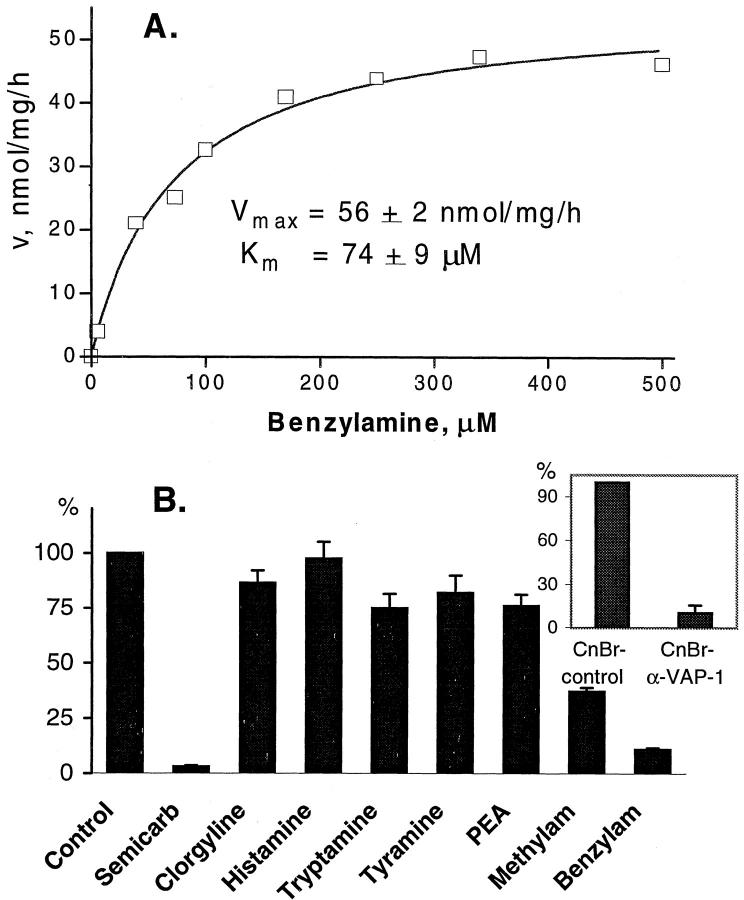
Amine oxidase activity in smooth muscle was determined radiochemically based on the method of Fowler and Tipton, 25 using labeled benzylamine ([7-14C]benzylamine hydrochloride, sp. act. 57 mCi/mmol; Amersham) as a substrate. The standard assay was performed at 37°C for 60 minutes in a final volume of 500 μl of 0.1 mol/L Na-phosphate buffer (pH 7.4) containing smooth muscle lysate (60–100 μg protein); various concentrations of benzylamine with tracer [14C]benzylamine (40,000 dpm); and 1 mmol/L unlabeled tyramine, tryptamine, histamine, methylamine, or β-phenylethylamine as a competitor. In the inhibitory studies, the lysate was preincubated with 1 mmol/L clorgyline or semicarbazide, preferential inhibitors of SSAO and MAOA/B activity, respectively, 5 at 37°C for 30 minutes before benzylamine was added. In certain experiments, smooth muscle lysate was depleted of VAP-1 by immunochromatography. In brief, aliquots of lysate were incubated three times with CnBr-activated Sepharose 4CL beads to which either an anti-VAP-1 or a control antibody had previously been coupled. A complete and specific depletion of VAP-1 by anti-VAP-1 but not by control beads was confirmed by immunoblotting. In all enzyme assays the catalytic reaction was stopped by 120 μl of 2 mol/L citric acid, and aldehyde reaction products were extracted from the analyzed mixture into toluene containing 0.35 g/L diphenyloxazole. The amount of 14C-labeled benzaldehyde was quantified by scintillation counting with a Wallac-1409 β-counter (Turku, Finland). The activity of the enzyme was expressed as nanomoles of benzaldehyde oxidized by 1 mg lysate protein per hour. Protein concentrations were determined with a bicinchoninic acid assay (Pierce). The kinetic parameters for smooth muscle monoamine oxidase (Km and Vmax) were calculated using a nonlinear curve-fitting program based on the Michaelis-Menten equation (Enzkin, GraphPad Prism).
Results
VAP-1 Is Expressed in Smooth Muscle
Tissue distribution of VAP-1 was determined using two different anti-VAP-1 mAbs, 1B2 and 2D10. The two mAbs gave similar results, but mAb 2D10 displayed higher intensity. Immunohistochemistry showed that VAP-1 is absent from skeletal and cardiac muscle cells. In these tissues only endothelial cells of small vessels and medial cells of the small arteries and veins were VAP-1 positive (Figure 1, A and C) ▶ . In larger veins VAP-1 was abundantly present in smooth muscle cells of the medial layer (Figure 1E) ▶ . In the largest vessels (aorta and veins draining into heart), the endothelium and intimal layer were VAP-1 negative, and this molecule was absent from the outermost adventitial layer as well. In contrast, the smooth muscle cells of the medial layer were VAP-1 positive (Figure 1G) ▶ . In other sources of smooth muscle (small intestine, appendix (Figure 1I) ▶ , skin, bladder wall, uterus) VAP-1 was expressed in a similar pattern on all smooth muscle cells. VAP-1 reactivity appeared to surround the surface of the myocyte, being most evident in the cross sections of smooth muscle fascicles (Figure 1I) ▶ . The reaction pattern would be compatible with the VAP-1 expression on the plasma membrane of the myocyte, in the basement membrane, which surrounds each smooth muscle cell, or in the adjacent extracellular components of the smooth muscle fibers.
Figure 1.
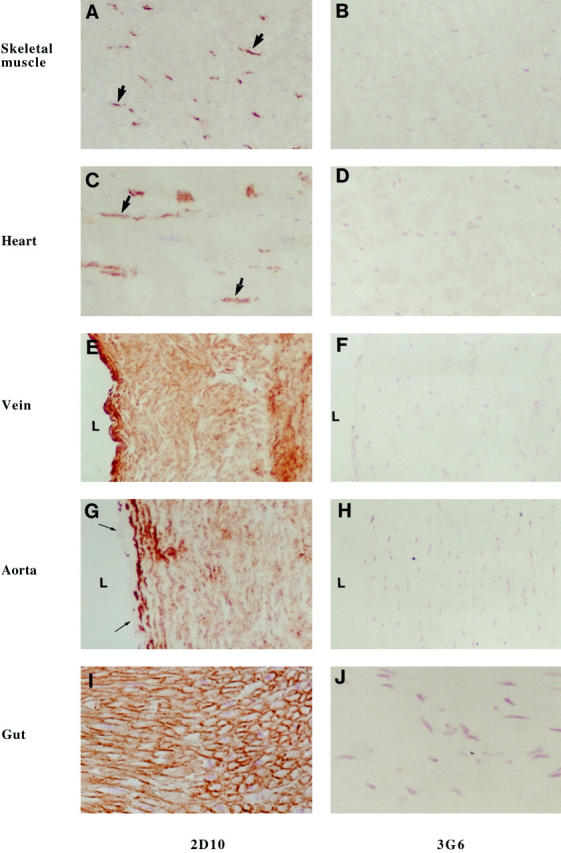
VAP-1 is selectively found in smooth muscle cells but not in striated or cardiac muscle cells. Immunocytochemical reactions on frozen sections from specimens from the indicated tissues were made using mAbs against VAP-1 (2D10) and against a negative control antigen (3G6) as the first-stage antibodies. Thick arrows point to positively stained endothelial cells in skeletal and heart muscle samples, and thin arrows to the VAP-1-negative endothelial cell layer in aorta. L, lumen of the vessel. Magnifications: A–H, ×200; I and J, ×400.
Immunolabeling electron microscopy was used to study the subcellular localization of VAP-1 in detail. In smooth muscle cells of blood vessels, VAP-1 was clearly localized on the plasma membrane (Figure 2A) ▶ . It was also present in small cup-like invaginations of the membrane called caveolae, which, characteristically of micropinocytotic vesicles, lack any evident cytoplasmic coat (Figure 2B) ▶ . Thus VAP-1 is expressed in smooth muscle cells, and it is present on the surface of the cells.
Figure 2.
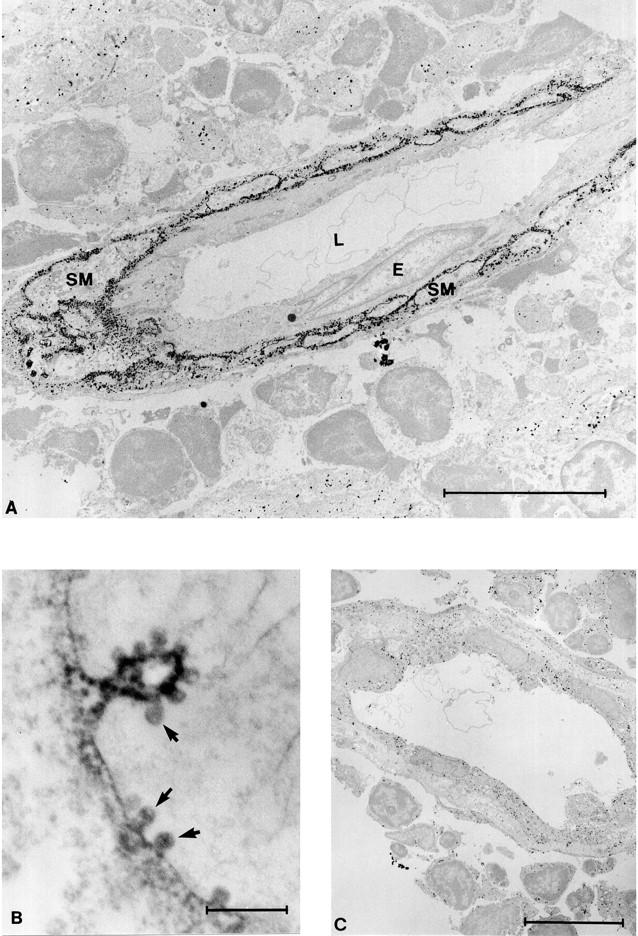
VAP-1 localizes to the plasma membrane in smooth muscle and is present in caveolae. A: In immunolabeling electron microscopy an anti-VAP-1 MAb reacts with vascular smooth muscle cells in tonsil. In this particular vessel VAP-1 is absent from the endothelial cells (E), and only the subendothelial smooth muscle cells (SM) are VAP-1 positive. L, lumen of the vessel. B: At high magnification the localization of VAP-1 into plasma membrane and caveolae (arrows) is readily apparent. C: A control reaction with normal mouse serum. Nonspecific grains apparently resulting from the very sensitive silver intensification of DAB used in A and C are seen in the background. Scale bars: A and C, 10 μm; B, 0.5 μm.
Smooth Muscle VAP-1 Expression Is Not Induced in Inflammation
In endothelial cells VAP-1 is an inflammation-inducible adhesion molecule in vivo. 2 To see whether VAP-1 is similarly regulated in smooth muscle cells, we studied atheromatous samples. In these specimens equal VAP-1 positivity was seen in apparently normal smooth muscle cells surrounding the lesion and in those of the atheromatous areas. Moreover, the intensity of VAP-1 in smooth muscle cells of the lesion was comparable to those in the noninvolved segment of the same vessels. The endothelial lining covering the normal and atheromatous large arteries was VAP-1 negative. In another model of inflammation involving smooth muscle cells, specimens from inflammatory bowel disease showed no induction of VAP-1 on gut wall muscle cells, although it was up-regulated in endothelial cells of lamina propria. Thus smooth muscle VAP-1, in contrast to endothelial VAP-1, is not induced on inflammation.
Smooth Muscle Cells Synthesize VAP-1 Only in Vivo
VAP-1 was also searched for in different cell lines of smooth muscle origin. However, both the cell surface and the cytoplasm of intestinal and aortic smooth muscle cells and a leiomyosarcoma line were constantly negative for VAP-1, as detected by immunofluorescence with and without permeabilization. Moreover, smooth muscle cell monolayers derived from tissue explants or by enzymatic dispersion did not display any VAP-1 (data not shown).
Smooth Muscle VAP-1 Is Down-Regulated during Malignant Transformation
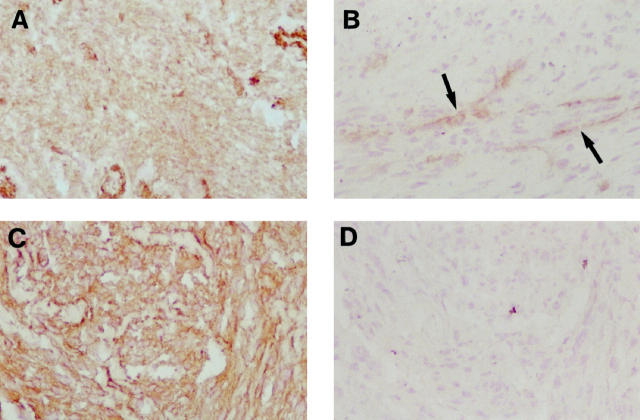
Because smooth muscle cell lines derived from tumors were VAP-1 negative, we analyzed the expression of this molecule in situ in tumor specimens (Table 1 ▶ and Figure 3 ▶ ). In leiomyomas, the expression of VAP-1 was retained at normal levels. In contrast, two different expression patterns were observed in leiomyosarcoma samples. In four of 10 specimens, the VAP-1 was clearly present on malignant cells. In six of 10 samples, however, VAP-1 was completely lost from the leiomyosarcoma cells, although it was still present on smooth muscle cells of the vessels, which served as a useful internal standard of the staining intensity. Thus, at a certain stage of malignant transformation, smooth muscle cells can stop synthesis of VAP-1.
Figure 3.
VAP-1 expression is modulated in tumors. VAP-1 expression in leiomyomas (A) and leiomyosarcomas (B and C) was analyzed by immunoperoxidase staining. D: The specimen in C, stained with a negative control MAb. In B, VAP-1-positive vessels are indicated by arrows. Magnification, ×200.
Multimeric Forms of VAP-1 in Smooth Muscle
In endothelium, VAP-1 is a dimeric protein consisting of two identical 90-kd subunits. 3 To see whether VAP-1 in smooth muscle cells is biochemically similar, small pieces of smooth muscle from the gut were labeled in vitro with [35S]methionine/[35S]cysteine. Immunoprecipitations with mAb 1B2 showed specific bands at ∼90 and ∼170 kd under reducing conditions (Figure 4) ▶ . The low-molecular-mass bands precipitated with 1B2 but not with a control mAb probably are not specific, inasmuch as they were not seen with anti-VAP-1 mAb 2D10. In contrast, faint but specific signals detected with both anti-VAP-1 mAbs were also apparent at higher molecular masses.
Figure 4.
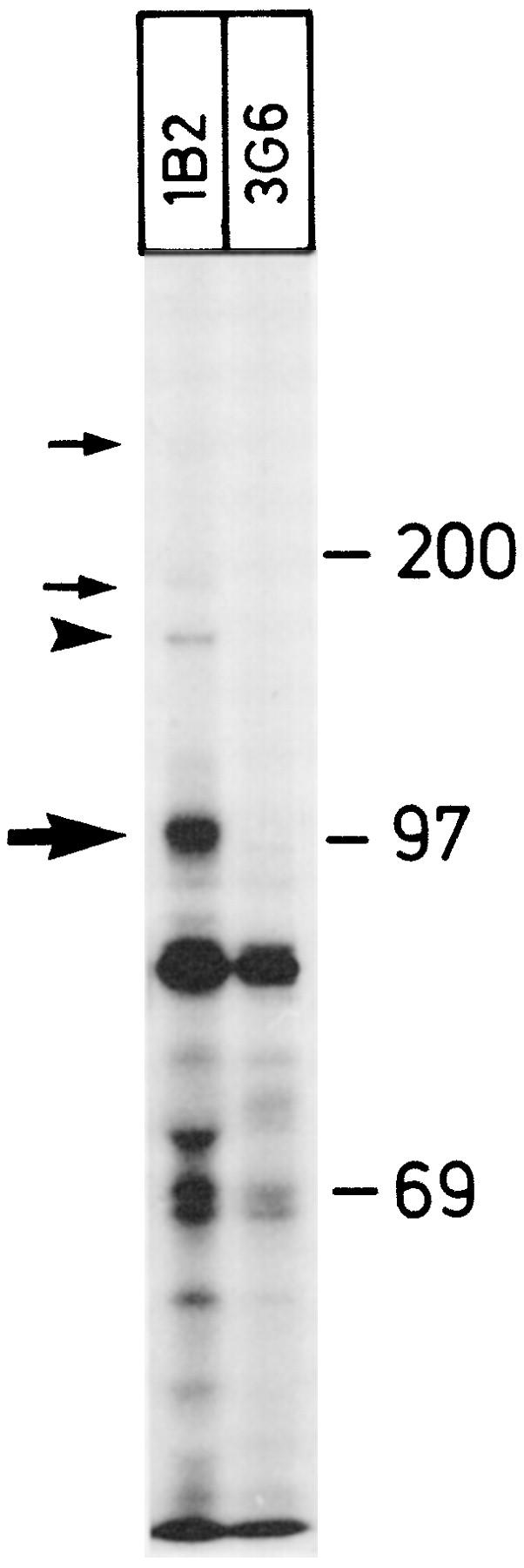
Dimeric and multimeric forms of VAP-1 are present in smooth muscle cells. [35S]Methionine/cysteine-labeled fragments of smooth muscle were precipitated with an anti-VAP-1 mAb 1B2 and with a negative control mAb 3G6. Under reducing conditions, the monomeric 90–100-kd form of VAP-1 (thick arrow) and the dimeric VAP-1 (arrowhead) are most prominent. Fainter but specific VAP-1 bands are also visible at higher molecular mass (thin arrows). Molecular mass standards in kd are shown on the right.
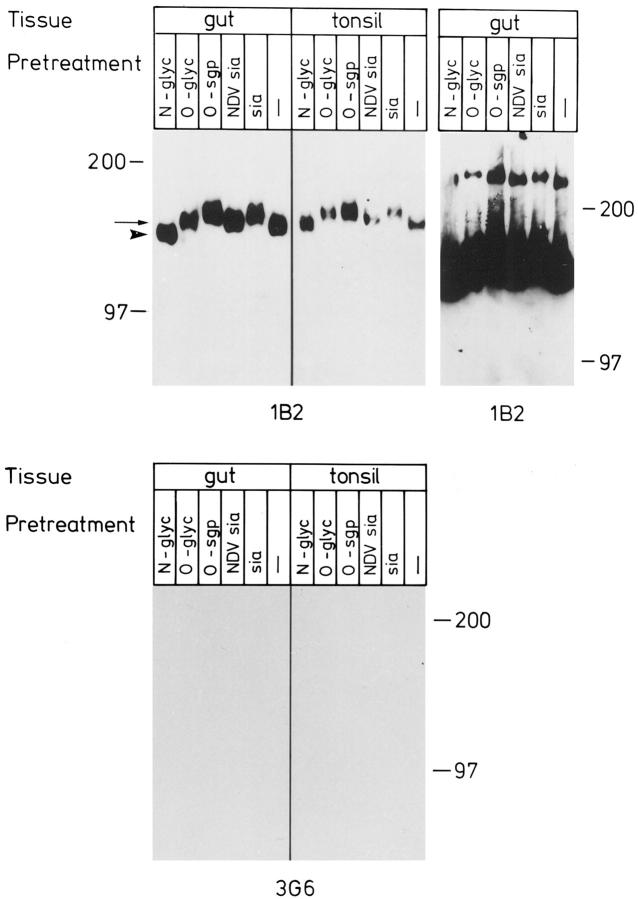
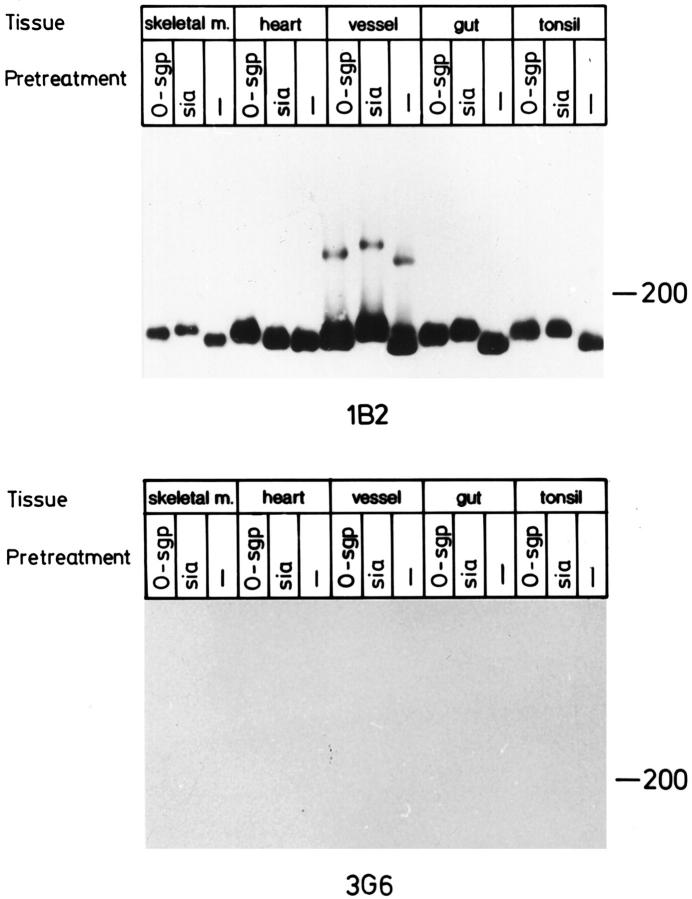
mAb 1B2 also readily detected the dimeric and trimeric forms of VAP-1 in immunoblotting of 1% NP-40 lysates of smooth muscle from vessel and gut wall under nonreducing conditions (Figures 5–7) ▶ ▶ ▶ . The dimeric form migrates as a 160–185-kd band and the trimeric form as a 240–260-kd band; hereafter these will be called the 165-kd and 250-kd forms of VAP-1, respectively, according to the estimated size of the most intense signal. The majority of VAP-1 in skeletal muscle, heart, and tonsil is of endothelial origin, and it has a slightly slower electrophoretic mobility than that of smooth muscle preparations of vessels and gut (Figure 5) ▶ . It should be noted that although the size differences in immunoblots appear quite small, all samples have been analyzed in parallel lanes in one gel, and the results were constant in several independent analyses. Moreover, a small shift in the electrophorectic mobility at the high-molecular-mass range in SDS-PAGE already translates into a marked difference in the actual size of the molecule. Neither mAb 1B2 nor 2D10 reacts with the 90-kd monomer of VAP-1 in immunoblotting. 3 Thus VAP-1 in smooth muscle cells appears to be mainly in a dimeric form like that in tonsil HEVs. However, the size difference of smooth muscle VAP-1 and endothelial VAP-1 suggests that either the protein core is different or there are alterations in the posttranslational modifications of VAP-1 between the two cell types.
Figure 5.
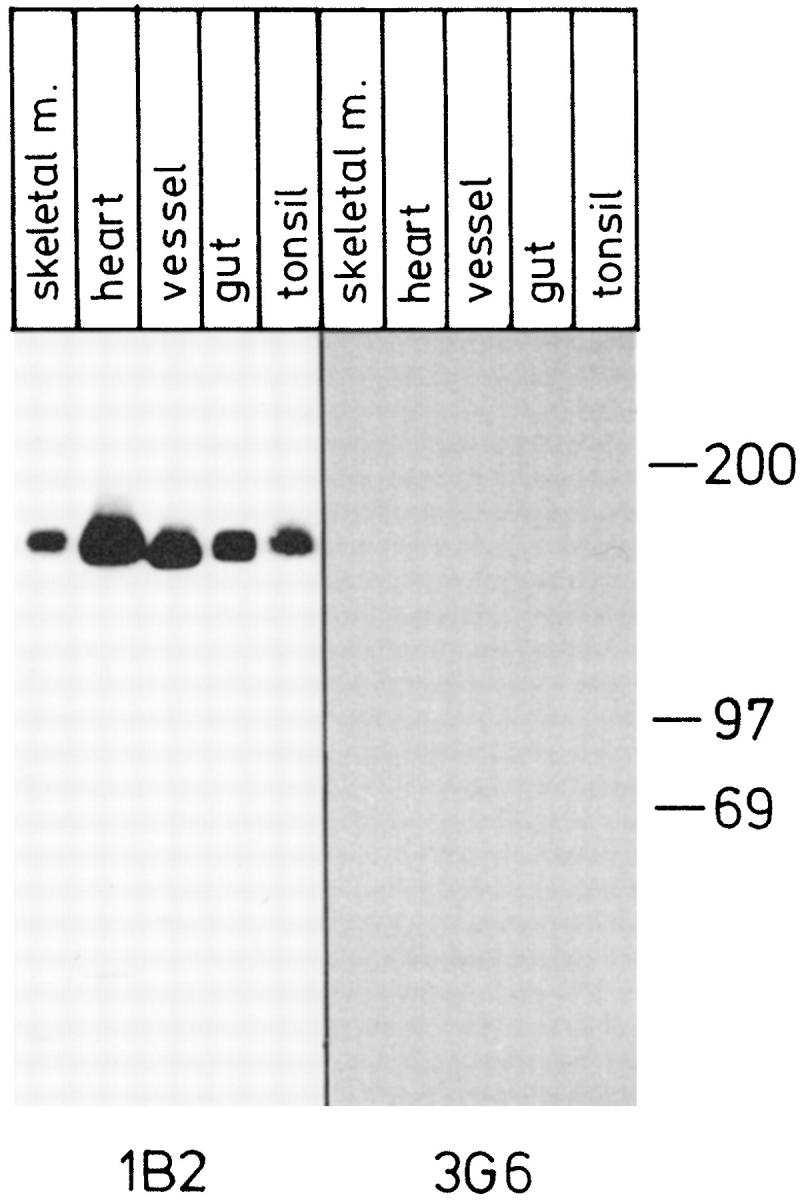
VAP-1 dimer in smooth muscle is smaller than that in endothelial cells. Lysates from the indicated tissues were prepared, separated in SDS-PAGE under nonreducing conditions, and subjected to immunoblotting with an anti-VAP-1 mAb 1B2 and with a negative control mAb 3G6. Samples from skeletal muscle, heart, and tonsil contain mostly endothelial VAP-1, whereas vessel and gut samples are of smooth muscle origin. All samples were analyzed in the same gel, which is a representative of three independent analyses. Note that the negative control was exposed 4 times longer than the 1B2 filter.
Figure 6.
VAP-1 is an N-glycosylated sialoglycoprotein in smooth muscle cells. Tissue lysates from the indicated sources were digested with the enzymes as described in Materials and Methods and analyzed by immunoblotting with an anti-VAP-1 mAb 1B2 and with a negative control mAb 3G6. All digests were analyzed in the parallel lanes of the same gel. The arrow points to untreated smooth muscle VAP-1 and the arrowhead to the N-glycanase-digested smooth muscle VAP-1. An intentionally overexposed film from the gut is also included to demonstrate the effects of the glycosidase digestions on the trimeric form of VAP-1. No signal was seen from molecules of the same size in prolonged exposures of tonsil samples or from the strip probed with the negative control mAb. These data are representative of three independent experiments with similar results.
Figure 7.
Sialoglycoprotein nature of VAP-1. VAP-1 from different sources was analyzed with enzymatic digestions and immunoblotting with an anti-VAP-1 mAb 1B2 and with a negative control mAb 3G6. To allow direct comparison of the molecular masses, all samples were analyzed in parallel lanes of one gel. The results are representative of two independent experiments.
Smooth Muscle VAP-1 Is a Sialoglycoprotein
Smooth muscle VAP-1 was digested with different exo- and endoglycosidases to study the extent of posttranslational modifications in VAP-1 from this source. After V. cholerae sialidase treatment, which cleaves sialic acids in all common linkages, VAP-1 displayed retarded electrophoretic mobility (Figure 6) ▶ . This paradoxical decrease in migration in SDS-PAGE after sialidase treatment is characteristic of abundantly sialylated proteins, and it is attributed to the removal of large quantities of negatively charged sialic acids from the molecule. Specific removal of α2,3 (and α2,8)-linked sialic acids with Newcastle disease virus sialidase also increased the apparent molecular mass of VAP-1, but to a lesser extent. Both the 165-kd dimeric and the higher molecular mass multimer showed altered migration in SDS-PAGE after removal of sialic acids (Figure 6) ▶ . O-Sialoglycoprotein endopeptidase digestion also increased the molecular mass of both VAP-1 species (Figure 6) ▶ .
Smooth muscle VAP-1 has no major O-linked side chains, inasmuch as further digestion of sialidase-treated VAP-1 with O-glycanase had only a minor additional effect (decrease) on the mobility of VAP-1 when compared to the sialidase treatment only (Figure 6) ▶ . In contrast, cleavage of N-linked sugars with recombinant N-glycanase markedly decreased (about 15 kd) the apparent molecular mass of smooth muscle VAP-1 (Figure 6 ▶ ; the arrow and arrowhead indicate the mobilities of untreated and N-glycanase-treated smooth muscle VAP-1, respectively). Together these data indicate that VAP-1 has abundant sialic acid decorations and more than one N-linked oligosaccharide side chain.
The effect of these enzymatic treatments was similar in smooth muscle lysates from all types of origins tested, ie, muscular wall of the small intestine and wall of medium-sized vein (Figure 7 ▶ and data not shown). These data suggest that smooth muscle VAP-1 in different tissues and organs is similarly modified. However, it displays several differences from tonsil VAP-1. The behavior of tonsil VAP-1 is shown as an internal control in Figures 5–7 ▶ ▶ ▶ to allow direct comparison of VAP-1 of smooth muscle and HEV origin in the same experiments and in the same SDS-PAGE gels. Notably, tonsillar VAP-1 constantly had a slower electrophoretic mobility, the trimeric VAP-1 was never seen in tonsil, and, under these same conditions, tonsil VAP-1 was not sensitive to N-glycanase.
Smooth Muscle VAP-1 Does Not Bind Lymphocytes
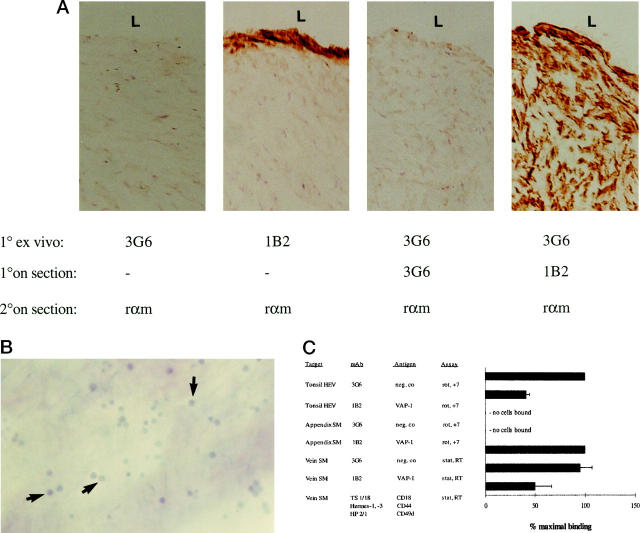
Because endothelial VAP-1 is a sialic acid-dependent adhesion molecule for blood-borne lymphocytes, 3 we determined whether smooth muscle VAP-1, which appeared to carry the critical proadhesive modifications, would also support lymphocyte binding. When PBLs were preincubated on frozen sections under conditions that resulted in a VAP-1-dependent adherence to vessel endothelium in both tonsil and appendix, we saw no specific adhesion to smooth muscle cells in sections of either vascular or gut origin (Figure 8) ▶ . Because these data may reflect the physiological absence of normal PBLs from these locations, we preactivated PBLs with phorbol myristate acetate to simulate conditions of inflammation where lymphocytes can be found in vessel or gut wall. However, not even under these conditions, at either 7°C or 37°C, was any significant binding of PBLs to smooth muscle detectable.
Figure 8.
Different function of VAP-1 in endothelial and smooth muscle cells. A: Tissue explants used for the smooth muscle cell adhesion assays. After the endothelial layer was denuded from the luminal aspect (L) of the vein, the tissue was incubated with the primary mAbs 1B2 against VAP-1 and 3G6 as a negative control (1° ex vivo), washed, snap-frozen, sectioned, and subjected to immunoperoxidase reactions with the indicated mAbs (1° on section), and with the second-stage HRP-conjugated rabbit anti-mouse Ig (rαm, 2° on section). Note that the anti-VAP-1 MAb penetrates only about 10 μm from the surface of the specimen (second panel), even though the antigen is present throughout the tissue (fourth panel). B: Binding of PBLs to smooth muscle cells. PBLs were incubated with the de-endothelized vascular explant, the nonadherent cells were removed by 1× gravitation, and the adherent cells (some pointed out by arrows) were fixed to the sections and counterstained. Magnification, ×100. C: VAP-1 mediates PBL binding to HEV but not to smooth muscle cells. The results of the in vitro HEV and smooth muscle (SM) cell binding assays and of the ex vivo vascular explant (vein) assays after mAb blocking of the indicated molecules are presented as mean ± SEM of four or more independent experiments. Analyses were made under rotatory (rot) or static (stat) conditions at +7°C or at room temperature (RT).
To circumvent the use of frozen target tissue in functional analyses, we developed an ex vivo explant model. The endothelial layer of larger vessels was gently removed mechanically, which exposed the VAP-1-positive subintimal smooth muscle surface (Figure 8A) ▶ . Specific interactions of single lymphocytes with the smooth muscle substratum were readily discernible (Figure 8B) ▶ in the presence of a control mAb. However, no VAP-1-inhibitable adhesion was seen in this model either (Figure 8C) ▶ . As a positive control, lymphocytes were preincubated with a pool of mAbs against CD44, LFA-1, and α4-integrins, adhesion molecules known to be involved in PBL-smooth muscle cell interaction. 26 A 50% reduction of PBL binding to smooth muscle cells with these antibodies was observed. These functional data indicate that smooth muscle VAP-1 may not play a role in PBL adhesion.
Smooth Muscle VAP-1 Is a Monoamine Oxidase
Cloning and sequencing of VAP-1 revealed that it belongs to the SSAO family. 4 Therefore, we investigated whether the smooth muscle expressing high levels of VAP-1 possesses monoamine oxidase activity and whether it is derived from VAP-1. Lysates made of gut smooth muscle cells exhibited a significant monoamine oxidase activity, which displays Michaelis-Menten kinetics, with a Km for benzylamine of 74 μmol/L (Figure 9A) ▶ . Since benzylamine can be metabolized by both SSAO and MAO (especially MAO-B), the lysates were pretreated with selective inhibitors of these enzymes. 5 As shown in Figure 9B ▶ , smooth muscle monoamine oxidase activity was practically abolished by specific SSAO inhibitor semicarbazide, whereas about 90% of the enzyme remained insensitive to clorgyline, a MAO inhibitor.
Figure 9.
Smooth muscle VAP-1 is an amine oxidase. A: Kinetics of smooth muscle monoamine oxidase activity. The rate of [14C]benzylamine deamination (nanomoles of substrate oxidized by mg lysate protein per hour) by smooth muscle lysate versus substrate concentration is plotted. Data are the mean of duplicate assays from a representative experiment that was repeated twice. B: Substrate specificity of smooth muscle monoamine oxidase. The enzyme activity in smooth muscle lysate is arbitrarily set at 100% (control). PEA, β-phenylethylamine. Inset: The smooth muscle lysate was precleared with cyanogen bromide-activated Sepharose beads to which a control mAb (CnBr-contr, set as 100%) or anti-VAP-1 MAb (CnBr-α-VAP-1) was coupled before the enzyme assay was performed. Data represent the mean ± SEM of three experiments performed in duplicate.
To evaluate the substrate specificity of smooth muscle monoamine oxidase, the relative rate of [14C]benzylamine deamination was measured in the presence of various amines. The preferential substrates were benzylamine and methylamine, whereas histamine, tryptamine, tyramine, and β-phenylethylamine were poorly oxidized (Figure 9B) ▶ .
To study the contribution of VAP-1 to the overall SSAO activity in the smooth muscle cells, VAP-1 was depleted from the lysate by immunoaffinity chromatography. As shown in the inset of Figure 9B ▶ , 89% of SSAO activity was concomitantly abolished, indicating that VAP-1 is the major but not the only SSAO species in human smooth muscle cells.
Discussion
We have shown here that among all muscle types VAP-1 is present only in smooth muscle cells. Smooth muscle VAP-1 is structurally and functionally different from endothelial VAP-1. The noninducibility, lower molecular mass, the presence of trimeric forms of VAP-1, and existence of abundant N-linked oligosaccharide modifications distinguish smooth muscle VAP-1 from endothelial VAP-1. From a functional point of view, endothelial VAP-1 is involved in binding of PBLs, whereas no such function could be attributed to smooth muscle VAP-1. Nevertheless, smooth muscle VAP-1 was completely intact as a monoamine oxidase.
VAP-1 was found in smooth muscle cells in all tissues and organs tested. In contrast, all samples from skeletal muscle and heart only displayed VAP-1 reactivity in blood vessels, whereas striated and cardiac muscle cells were absolutely VAP-1 negative. Therefore, VAP-1 appears to be a useful marker of normal cells of smooth muscle origin. In smooth muscle tumors, VAP-1 was present in benign lesions. In malignant cases VAP-1 expression was either retained or lost. Disappearance of VAP-1 did not appear to correlate with the anatomical location or the differentiation level of the tumor, although the small number of samples of these rare malignancies prevented any statistical analyses. It will be interesting to see in larger series whether VAP-1 positivity can serve as an independent factor in the prognosis of leiomyosarcomas. In smooth muscle cells VAP-1 was clearly localized on the cell surface. Moreover, it was seen in caveolae, bottle-shaped invaginations of the plasma membrane. Caveolae are specialized microdomains of plasma membrane that may have a role in transcytosis of macromolecules, in potocytosis, and in signal transduction. 27 Caveolar localization of VAP concentrates this molecule in a discrete region of the plasma membrane.
There are distinct structural differences in VAP of endothelial and smooth muscle origin. First, smooth muscle VAP-1 displayed faster apparent electrophoretic mobility than endothelial VAP-1. Second, only the monomeric and dimeric forms of VAP-1 are found in endothelial cells. 3 In addition to these two forms, trimeric VAP-1 species were detected in smooth muscle. These species of VAP-1 apparently represent multimeric complexes of VAP-1 that are only partially sensitive to the reduction of disulfide bonds with 5% 2-mercaptoethanol. Third, VAP-1 in smooth muscle cells contained readily cleavable N-linked glycans, which were absent from the VAP-1 of endothelial cell origin under the same conditions. Both endothelial and smooth muscle VAP-1 are abundantly sialylated glycoproteins that result in aberrant migration in SDS-PAGE gels. Therefore, the reduction of VAP-1 molecular mass by about 15 kd after N-glycanase treatment does not allow direct estimation of the number of N-linked glycans in smooth muscle cells, because retarded mobility caused by removal of N-linked oligosaccharides may be counteracted by a paradoxical increase in mobility by simultaneous deletion of sialic acids on these glycans. Smooth muscle VAP-1 has both α2,3- and α2,6-linked sialic acids, inasmuch as the apparent shift in molecular mass was less with an α2,3-linkage-specific Newcastle disease virus neuraminidase than with V. cholerae neuraminidase, which has a broad substrate specificity. Smooth muscle VAP-1 was also cleaved by O-sialoglycoprotease, suggesting that VAP-1 may contain O-linked oligosaccharide side chains that are decorated by sialic acids.
VAP-1 requires an intact smooth muscle microenvironment for its expression. This was evident from experiments that showed an absence of VAP-1 in primary and transformed smooth muscle cells. Endothelial VAP-1 is not as sensitive to disruption of vascular architecture, inasmuch as collagenase-digested, immunoaffinity-isolated HEV cells retain VAP-1 expression. 28 Moreover, transformed endothelial cell lines and human umbilical vein endothelial cells lack all cell surface VAP-1, but nevertheless they do express low levels of intracellular VAP-1. 29 These data suggest that in endothelial cells and in smooth muscle cells VAP-1 expression is differently regulated.
VCAM-1 shares similar features with VAP-1 in the expression in both endothelial and muscle cells. The two cell types use different transcriptional mechanisms to control VCAM-1 expression. On endothelial cells VCAM-1 is an inducible protein, 30 whereas muscle cell VCAM-1 is ontogenically expressed on developing secondary myotubes. 8 Endothelial VCAM-1 is induced, for example, with interleukin-1, tumor necrosis factor-α, and lipopolysaccharide, which may act via NF-κB binding sites that override the constitutively active inhibitory element. Instead, on cultured myocytes these transcription factors do not regulate VCAM-1 expression, because in these cells a specific enhancer is overriding the effect of the silencers, resulting in constitutive VCAM-1 expression. 9,10 However, increased expression of VCAM-1 is found in vivo on smooth muscle cells in atherosclerotic plaques. 31 VAP-1 on endothelial cells is also inducible in the setting of inflammation in vivo, 2 and in vitro certain cytokines induce a modest increase in endothelial VAP-1 expression in an organ culture model. 28 Interestingly, there are also two functional NF-κB elements in the promoter of human VAP-1. In animal models endothelial VAP-1 can be induced at the site of skin inflammation (our unpublished observations). In contrast, the level of smooth muscle VAP-1 remains the same during in vitro induction of tonsil with cytokines and other proinflammatory mediators, and in vivo smooth muscle VAP-1 induction is not seen, even in the areas of most extreme inflammation involving smooth muscle (in the severely inflamed gut). Furthermore, in atheromatous lesions, where induction of other endothelial adhesion molecules like ICAM-1 and VCAM-1 is seen on smooth muscle cells, 31-33 no evidence of up-regulation of VAP-1 was seen. These data further indicate that the regulation of VAP-1 expression in endothelial cells and in smooth muscle cells is different.
VAP-1 in smooth muscle could be envisaged to be involved in adhesion of PBLs. These mobile cells could make sequential contacts first with VAP-1 on endothelial cells and then proceed to use the same molecule for guiding migration through the smooth muscle layer. Although majority of lymphocyte trafficking takes place via HEVs, which do not have a smooth muscle layer around them, pathological accumulation of leukocytes into vessel wall in atherogenesis and vasculitis and into bronchioles in asthma clearly involves intimate contacts between leukocytes and smooth muscle cells. In vitro activated smooth muscle cells can bind activated PBLs in a VCAM-1-, ICAM-1-, and CD44-dependent manner. 26,34 However, smooth muscle VAP-1 showed no appreciable PBL binding under any conditions we tested. Thus smooth muscle cells may lack some other crucial adhesion molecule(s) that is needed to stabilize VAP-1 adhesion. In the case of endothelial cells, a paradigm has arisen in which at least three sequential steps are required to secure the binding of a lymphocyte to an endothelial cell. 35,36 In HEVs, VAP-1 mediates the initial stage of adhesion under conditions of shear. 3 Therefore, our inability to find an adhesive function for VAP-1 on smooth muscle cells may reflect the absence of the subsequent chemokine activation or a lack of immunoglobulin superfamily members (ICAM-1, ICAM-2, and VCAM-1) on our smooth muscle cells. On the other hand, the differences in posttranslational modifications between smooth muscle cell and endothelial VAP-1 may render the smooth muscle form incapable of lymphocyte adhesion. In particular, the N-linked sugars may interfere with ligand-binding motifs of VAP-1 in smooth muscle. Finally, the most likely explanation for the lack of adhesive function for smooth muscle VAP-1 is that the plasma membrane of each smooth muscle cell is surrounded by a basement membrane. This makes a ∼50-nm physical barrier which all integral membrane proteins have to penetrate before they are available for contacts with other cells. Because VAP-1 monomer is a 90-kd mushroom-shaped molecule, 37 it may well be that not even the oligosaccharide residues of VAP-1 extend far enough to be seen by PBLs.
Smooth muscle VAP-1 turned out to be a semicarbazide-sensitive monoamine oxidase. The kinetic parameters of SSAO vary substantially between various species and tissues. 5 However, the Km and Vmax values determined here are in close agreement with those reported earlier for SSAO in human aorta, umbilical artery, and other tissues. SSAO catalyzes oxidative deamination of primary exogenous and endogenous amines in a reaction that produces the corresponding aldehyde, hydrogen peroxide, and ammonium. 5,38 The biological substrates and the function of SSAO enzymes have remained elusive. Whereas benzylamine is not believed to occur physiologically, methylamine can be formed endogenously from certain metabolic pathways and act as a possible physiological substrate for SSAO, but not for MAO, in various tissues, including vasculature. SSAO-mediated generation of aldehydes and hydrogen peroxide has been implicated in the pathogenesis of different diseases. In particular, inhibition of SSAO activity has alleviated vasculopathic changes in animal models of diabetes. 39,40 Moreover, there is a role emerging for hydrogen peroxide as a signaling molecule, which regulates the expression of other adhesion molecules, cell growth, and apoptosis. 41 Because smooth muscle VAP-1 is present in arterial media and in smooth muscle tumors and it is the major SSAO species in humans, it is likely to be crucial in these multiple biological functions.
In conclusion, we have shown that VAP-1 is exclusively expressed only on smooth muscle cells among different muscle cells. Smooth muscle VAP-1 has several similarities but also distinct differences in structure with the endothelial VAP-1. Most notably, the trimeric forms and N-linked posttranslational modifications of VAP-1 are only encountered on smooth muscle cells. Smooth muscle VAP-1 is not directly involved in lymphocyte binding, but instead it accounts for the most semicarbazide-sensitive monoamine oxidase activity in humans and may thus be important in the pathogenesis of multiple vasculopathies.
Acknowledgments
We thank Drs. Riitta Aho and Pertti Rajala for tissue samples, Ms. Riikka Lehvonen for excellent technical assistance, and Mrs. Anne Sovikoski-Georgieva for secretarial help.
Footnotes
Address reprint requests to Dr. Marko Salmi, MediCity Research Laboratory, University of Turku, Tykistökatu 6A, 20520 Turku, Finland. E-mail: marko.salmi@utu.fi.
Supported by the Finnish Academy, the Sigrid Juselius Foundation, the Finnish Cancer Union, the Paulo Foundation, and the Finnish Cultural Foundation.
The first two authors contributed equally to this work.
References
- 1.Salmi M, Jalkanen S: A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 1992, 257:1407-1409 [DOI] [PubMed] [Google Scholar]
- 2.Salmi M, Kalimo K, Jalkanen S: Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med 1993, 178:2255-2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmi M, Jalkanen S: Human vascular adhesion protein-1 (VAP-1) is a unique sialoglycoprotein that mediates carbohydrate-dependent binding of lymphocytes to endothelial cells. J Exp Med 1996, 183:569-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DJ, Salmi M, Bono P, Hellman J, Leu T, Jalkanen S: Cloning of vascular adhesion protein-1 reveals a novel multifunctional adhesion molecule. J Exp Med 1998, 188:17-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyles GA: Mammalian plasma and tissue-bound semicarbazide-sensitive amine oxidases: biochemical, pharmacological and toxicological aspects. Int J Biochem Cell Biol 1996, 28:259-274 [DOI] [PubMed] [Google Scholar]
- 6.Rice GE, Bevilacqua MP: An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science 1989, 246:1303-1306 [DOI] [PubMed] [Google Scholar]
- 7.Rice GE, Munro JM, Corless C, Bevilacqua MP: Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol 1991, 138:385-393 [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC: Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 1992, 69:1107-1119 [DOI] [PubMed] [Google Scholar]
- 9.Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T: Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med 1992, 176:1583-1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iademarco MF, McQuillan JJ, Dean DC: Vascular cell adhesion molecule 1: contrasting transcriptional control mechanisms in muscle and endothelium. Proc Natl Acad Sci USA 1993, 90:3943-3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurkijärvi R, Adams DH, Leino R, Möttönen T, Jalkanen S, Salmi M: Circulating form of human vascular adhesion protein-1 (VAP-1): increased serum levels in inflammatory liver diseases. J Immunol 1998, 161:1549-1557 [PubMed] [Google Scholar]
- 12.Sánchez-Madrid F, Krensky AM, Ware CF, Robbins E, Strominger JL, Burakoff SJ, Springer TA: Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA 1982, 79:7489-7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez-Madrid F, de Landázuri MO, Morago C, Cebrián M, Acevedo A, Bernabeu C: VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol 1986, 16:1343-1349 [DOI] [PubMed] [Google Scholar]
- 14.Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC: Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol 1987, 105:983-990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Airas L, Salmi M, Jalkanen S: Lymphocyte-vascular adhesion protein-2 is a novel 70-kd molecule involved in lymphocyte adhesion to vascular endothelium. J Immunol 1993, 151:4228-4238 [PubMed] [Google Scholar]
- 16.Salmi M, Grön-Virta K, Sointu P, Grenman R, Kalimo H, Jalkanen S: Regulated expression of exon v6 containing isoforms of CD44 in man: downregulation during malignant transformation of tumors of squamocellular origin. J Cell Biol 1993, 122:431-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liposits Z, Sétáló G, Flerkó B: Application of the silver-gold intensified 3,3′-diaminobenzidine chromogen to the light and electron microscopic detection of the luteinizing hormone-releasing hormone system of the rat brain. Neuroscience 1984, 13:513-525 [DOI] [PubMed] [Google Scholar]
- 18.Smiley JF, Goldman-Rakic PS: Silver-enhanced diaminobenzidine-sulfide (SEDS): a technique for high-resolution immunoelectron microscopy demonstrated with monoamine immunoreactivity in monkey cerebral cortex and caudate. J Histochem Cytochem 1984, 41:1393-1404 [DOI] [PubMed] [Google Scholar]
- 19.Fröjdman K, Paranko J, Virtanen I, Pelliniemi LJ: Intermediate filaments and epithelial differentiation of male rat embryonic gonad. Differentiation 1992, 50:113-123 [DOI] [PubMed] [Google Scholar]
- 20.Varki A, Diaz S: A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J Biol Chem 1983, 258:12465-12471 [PubMed] [Google Scholar]
- 21.Paulson JC, Weinstein J, Dorland L, van Halbeek H, Vliegenthart JFG: Newcastle disease virus contains a linkage-specific glycoprotein sialidase. Application to the localization of sialic acid residues in N-linked oligosaccharides of α1-acid glycoprotein. J Biol Chem 1982, 257:12734-12738 [PubMed] [Google Scholar]
- 22.Sutherland DR, Abdullah KM, Cyopick P, Mellors A: Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. J Immunol 1992, 148:1458-1464 [PubMed] [Google Scholar]
- 23.Norgard KE, Moore KL, Diaz S, Stults NL, Ushiyama S, McEver RP, Cummings RD, Varki A: Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem 1993, 268:12764-12774 [PubMed] [Google Scholar]
- 24.Jalkanen ST, Butcher EC: In vitro analysis of the homing properties of human lymphocytes: developmental regulation of functional receptors for high endothelial venules. Blood 1985, 66:577-582 [PubMed] [Google Scholar]
- 25.Fowler CJ, Tipton KF: Concentration dependence of the oxidation of tyramine by the two forms of rat liver mitochondrial monoamine oxidase. Biochem Pharmacol 1981, 30:3329-3332 [DOI] [PubMed] [Google Scholar]
- 26.Lazaar AL, Albelda SM, Pilewski JM, Brennan B, Puré E, Panettieri RA, Jr: T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J Exp Med 1994, 180:807-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parton RG: Caveolae and caveolins. Curr Opin Cell Biol 1996, 8:542-548 [DOI] [PubMed] [Google Scholar]
- 28.Arvilommi A-M, Salmi M, Jalkanen S: Organ-selective regulation of vascular adhesion protein-1 expression in man. Eur J Immunol 1997, 27:1794-1800 [DOI] [PubMed] [Google Scholar]
- 29.Salmi M, Jalkanen S: Different forms of human vascular adhesion protein-1 (VAP-1) in blood vessels in vivo and in cultured endothelial cells: implications for lympohocyte-endothelial cell adhesion models. Eur J Immunol 1995, 25:2803-2812 [DOI] [PubMed] [Google Scholar]
- 30.Wellicome SM, Thornhill MH, Pitzalis C, Thomas DS, Lanchbury JSS, Panayi GS, Haskard DO: A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol 1990, 144:2558-2565 [PubMed] [Google Scholar]
- 31.O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, Lobb R, Alpers CE: Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest 1993, 92:945-951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poston RN, Haskard DO, Coucher JR, Gall NP, Johnson-Tidey RR: Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol 1992, 140:665-673 [PMC free article] [PubMed] [Google Scholar]
- 33.Printseva OY, Peclo MM, Gown AM: Various cell types in human atherosclerotic lesions express ICAM-1. Further immunocytochemical and immunochemical studies employing monoclonal antibody 10F3. Am J Pathol 1992, 140:889-896 [PMC free article] [PubMed] [Google Scholar]
- 34.Panettieri RA, Jr, Lazaar AL, Puré E, Albelda SM: Activation of cAMP-dependent pathways in human airway smooth muscle cells inhibits TNF-α-induced ICAM-1 and VCAM-1 expression and T lymphocyte adhesion. J Immunol 1995, 154:2358-2365 [PubMed] [Google Scholar]
- 35.Butcher EC, Picker LJ: Lymphocyte homing and homeostasis. Science 1996, 272:60-66 [DOI] [PubMed] [Google Scholar]
- 36.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994, 76:301-314 [DOI] [PubMed] [Google Scholar]
- 37.Salminen TA, Smith DJ, Jalkanen S, Johnson MS: Structural model of the catalytic domain of an enzyme with cell adhesion activity: human vascular adhesion protein-1 (HVAP-1) D4 domain is an amine oxidase. Protein Eng 1998, 11:1195-1204 [DOI] [PubMed] [Google Scholar]
- 38.Klinman JP, Mu D: Quinoenzymes in biology. Annu Rev Biochem 1994, 63:299-344 [DOI] [PubMed] [Google Scholar]
- 39.Yu PH, Zuo DM: Aminoguanidine inhibits semicarbazide-sensitive amine oxidase activity: implications for advanced glycation and diabetic complications. Diabetologia 1997, 40:1243-1250 [DOI] [PubMed] [Google Scholar]
- 40.Yu PH, Deng YL: Endogenous formaldehyde as a potential factor of vulnerability of atherosclerosis: involvement of semicarbazide-sensitive amine oxidase-mediated methylamine turnover. Atherosclerosis 1998, 140:357-363 [DOI] [PubMed] [Google Scholar]
- 41.Finkel T: Oxygen radicals and signaling. Curr Opin Cell Biol 1998, 10:248-253 [DOI] [PubMed] [Google Scholar]