Abstract
There is little information regarding the status of cell cycle regulators in malignant peripheral nerve sheath tumors (MPNSTs) and neurofibromas (NFs). In this study, we investigated patterns of expression of p53 and pRB, cyclin-dependent kinase inhibitors (CKIs) p21 and p27, as well as cyclins D1 and E, in a cohort of 35 well-characterized MPNSTs and 16 NFs. These phenotypes were correlated with proliferative index, as assessed by Ki-67, as well as clinicopathological parameters of poor outcome. p53 nuclear overexpression was found in 10 of 35 (29%) MPNSTs, and it was lacking in NFs (P = 0.02). There were no differences in the patterns of expression of pRB, cyclin D1, and p21 between MPNSTs and NFs. However, p27 nuclear expression was present in most NFs, but it was absent in the majority of MPNSTs, which displayed cytoplasmic staining (P < 0.001). Nuclear cyclin E expression was more pronounced in MPNSTs than in NFs. We observed inverse patterns of expression for nuclear p27 and nuclear cyclin E expression. The staining profiles of cytoplasmic p27 and nuclear cyclin E expression were found to be statistically associated (P = 0.01). High Ki-67 expression was found in 20 of 34 (59%) MPNSTs but was absent in NFs (P < 0.001). Furthermore, detection of cytoplasmic p27 expression was found to be a prognostic factor for poor survival in MPNSTs (P = 0.03, relative risk = 2.4).
Cell cycle transitions, critical in homeostasis and tumorigenesis, depend on the functional status of cyclin-dependent kinases (CDKs), enzymes subject to positive and negative regulation by cyclins and cyclin-dependent kinase inhibitors (CKIs). The major role in controlling the progression from G1 to S phase is attributed to heterodimeric complexes formed by Cdk4-Cdk6 and D-type cyclins, as well as Cdk2 and cyclins A and E. 1-3 These active enzymatic complexes mediate the phosphorylation of key molecules, such as the protein encoded by retinoblastoma (RB) gene, also known as pRB. pRB is a nuclear phosphoprotein that appears to be active in its hypophosphorylated state by binding to and sequestering transcription factors involved in the induction of the S-phase, such as those of the E2F family. 4,5 Phosphorylation of pRB results in the release of E2F molecules, which then bind to and activate the promoter sites of various genes crucial for DNA replication, including thymilate synthase and dihydrofolate reductase. 6
Cyclins primarily involved in the G1-S transition include D-type cyclins (D1, D2, and D3) and cyclin E. 7,8 It has been reported that E2F1 promotes the activation of the cyclin E gene, resulting in its synthesis during late G1. 9 Cyclin E associates with Cdk2, targets pRB phosphorylation, and is considered the rate-limiting factor for the transition into S phase. 10
CKIs bind to and inhibit cyclin-Cdk complexes, exerting a negative regulatory function. CKIs are classified into two groups: the KIP family, which includes p21 (WAF1, Cip1, Sdi1), 11-12 p27KIP1 13 , and p57KIP2, 14 and the INK4 family, which includes p16INK4A, 15 p15INK4B, 16 p18INK4C, and p19INK4D. 17-18 p21 is transactivated by p53 in response to recognition of DNA damage or cellular stress, producing a transient G1 arrest. 19 However, p27 is mainly regulated through signals originating in the membrane, 13,20 also inhibiting progression to S phase.
The molecular events involved in the tumorigenesis of malignant peripheral nerve sheath tumors (MPNSTs) are still largely unknown. Deletions affecting chromosome 17p, including the p53 locus, as well as mutations of the p53 gene, have been described in MPNSTs. 21-22 Furthermore, p53 nuclear overexpression has been demonstrated frequently in MPNSTs but not in neurofibromas (NFs). 23-24 This is of particular interest, because MPNSTs often arise from NFs, mainly in the clinical setting of neurofibromatosis type 1 (NF1). A specific aim of our study was to address not only p53, but also downstream events of its pathway, such as p21 expression. We also analyzed alterations affecting the RB pathway, including G1 cyclins and p27. Because these proteins are responsible for the functional status of pRB and entry into S phase, we also analyzed proliferative activity by assessing Ki-67 expression. The final goal was to compare the phenotypes of these two distinct tumor types, as well as to conduct clinicopathological correlations.
Materials and Methods
Patients and Tissue Samples
The cases studied included 35 well-characterized, high-grade, deep-seated MPNSTs diagnosed at our center for which paraffin blocks were available. Information regarding tumor size, type of surgical treatment, and clinical outcome (ie, intervals to recurrence, metastasis, and overall survival) was available for each case. Seven of the 35 patients were alive at the end of the follow-up period for 17–276 months (median 35 months). The overall survival was 2–276 months (median overall survival 21 months). A control group consisting of 16 randomly chosen NFs, including four cellular NFs, was also studied. Cellular NFs are characterized by a moderately increased cellularity with absence of nuclear atypia and generally do not show mitotic activity, although on occasion they contain a rare mitotic figure.
Immunohistochemical Analysis
Formalin-fixed, paraffin-embedded, 5-μm sections mounted on superfrost slides were deparaffinized, rehydrated in graded alcohols, and processed using the avidin-biotin immunoperoxidase method. Briefly, sections were submitted to antigen retrieval by microwave oven treatment for 15 minutes in 0.01 mol/L citric acid at pH 6.0. This procedure was performed for all antibodies under study. For anti-Ki-67 antibodies, an additional step of incubation in preheated 0.05% trypsin, 0.05% CaCl2 in Tris-HCl (pH 7.6) for 2 minutes at 37°C was followed by microwave oven treatment, as described above. Slides were subsequently incubated for 15 minutes with 10% normal horse serum (10% whole goat serum for anti-cyclin E staining), followed by overnight incubation at 4°C with appropriately diluted primary antibody. The antibodies used were anti-p53 mouse monoclonal antibody (mAb) PAb1801 (clone Ab2; Oncogene Research Products/Calbiochem, Cambridge, MA; 1:500 dilution), anti-pRB mouse mAb (clone 3C8; QED Bioscience, San Diego, CA; 1:700 dilution), anti-cyclin D1 mouse mAb (Ab3; Oncogene Research Products/Calbiochem; 1:100 dilution), anti-p21 mouse mAb (Ab1; Oncogene Research Products/Calbiochem; 1:20 dilution), anti-p27 mouse mAb (Ab2; Oncogene Research Products/Calbiochem; 1:1000 dilution), anti-cyclin E purified rabbit antiserum (gift from Dr. A. Koff, 1:500 dilution), and anti-Ki-67 mouse mAb (clone MIB1; Immunotech, Westbrook, ME; 1:50 dilution). After antibody washing, the sections were sequentially incubated for 30 minutes with either biotinylated horse anti-mouse IgG (Vector Laboratories, Burlingame, CA; 1:500 dilution) or biotinylated goat anti-rabbit IgG (Vector Laboratories; 1:1000 dilution) antibodies, followed by avidin-biotin peroxidase complexes (Vector Laboratories; 1:25 dilution). Diaminobenzidine (0.06%) was used as a chromogen and hematoxylin for nuclear counterstain.
Immunohistochemical Evaluation and Scoring
Immunohistochemically stained slides were evaluated by one pathologist (HPK), without knowledge of the clinical outcome or other immunohistochemical results. Each histological section was screened and assessed for the percentage of tumor nuclei displaying staining. When homogeneous distribution of the immunoreactivity was observed, 20 HPF were evaluated with a 40× objective. In heterogeneous distribution of the immunoreactivity the entire tissue section was screened using a 40× objective to arrive at the estimated degree of positivity. NFs, neoplasms shown by electron microscopy to contain Schwann cells along with minor populations of perineurial-like cells, fibroblasts, and cells with intermediate features, 25-26 were evaluated in a similar fashion. In these tumors we evaluated immunohistochemical staining in the entire cellular population, acknowledging a minor standard error in the comparison of NFs with MPNSTs. Intensity of the staining was recorded as 1+ (weak), 2+ (moderate), or 3+ (strong). Cases were considered positive for p53, p21, p27 (p27N), and Ki-67 if nuclear staining was observed in ≥20% tumor cells. For p27, the presence of cytoplasmic staining (p27C) was also recorded and considered positive if the cytoplasm of ≥20% tumor cells displayed staining. Cyclin D1 and cyclin E were considered positive if staining was present in ≥10% of tumor nuclei. Interpretation of pRB immunoreactivities was conducted by stratifying cases in those showing undetectable (0%) to very low levels (1–3%), low levels (>3–19%), and pRB overexpression (≥20%) in tumor nuclei. pRB expression was considered positive if noted in more than 3% of tumor nuclei. Intraobserver variability was tested by random reevaluation of 25% of the cases. In no case was there a change in category (positive/negative result) or a difference greater than 10%. The variation in most cases was in the 5% range.
Statistical Analysis
Fisher’s exact test was used to study the association between clinical parameters. The effect of the factors examined by immunohistochemistry and clinical outcome parameters (recurrence, metastasis, and survival) was analyzed by the Cox regression model log-rank test. Survival was measured in months beginning from the date of the first pathological diagnosis. Statistical significance was set at P ≤ 0.05.
Results
Table 1 ▶ summarizes data regarding the patterns of expression of the examined cell cycle regulators in MPNSTs and NFs. Figure 1 ▶ illustrates the features of typical neurofibroma, cellular neurofibroma, and MPNST. Figures 2–8 ▶ ▶ ▶ illustrate alterations occurring in these tumors in relation to the markers analyzed.
Table 1.
Immunohistochemical Expression of Cell Cycle Regulators and Ki-67 in MPNSTs and NFs
| p53* (%) | pRb* (%) | p21* (%) | p27N* (%) | p27C* (%) | CyclinD1* (%) | Cyclin E* (%) | Ki-67* (%) | |
|---|---|---|---|---|---|---|---|---|
| MPNST | 10/35 | 31/35 | 16/35 | 3/35 | 19/35 | 10/35 | 14/33 | 20/34 |
| (N = 35) | (29%) | (89%) | (46%) | (9%) | (54%) | (29%) | (42%) | (59%) |
| NF | 0/16 | 15/16 | 11/16 | 15/16 | 12/16 | 5/16 | 5/16 | 0/16 |
| (N = 16) | (94%) | (69%) | (94%) | (75%) | (31%) | (31%) |
*(%), Number of positive cases/total number of cases examined, (percentage of positive cases); p27C, cytoplasmic expression of p27; p27N, nuclear expression of p27; ND, not detected.
Figure 1.
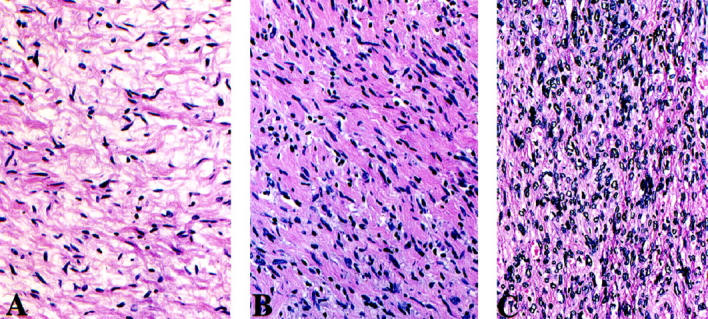
Hematoxylin and eosin (H&E)-stained typical NF (A), cellular NF (B), and MPNST (C).
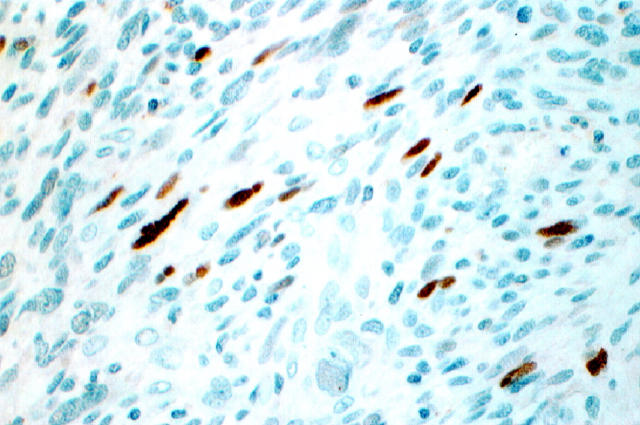
Figure 2.
Nuclear p53 expression in MPNST.
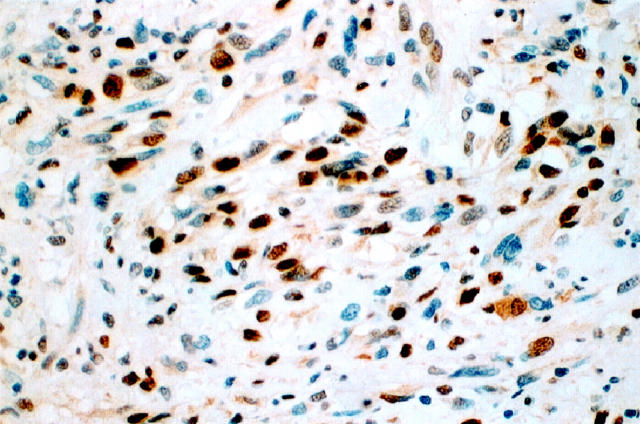
Figure 3.
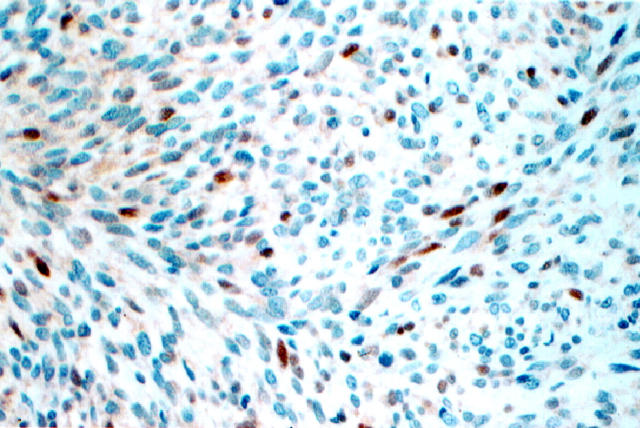
pRb expression in MPNST.
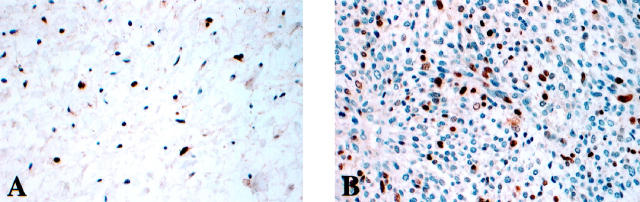
Figure 4.
p21 nuclear expression in typical NF (A) and MPNST (B).
p53 nuclear overexpression was observed in 10 of 35 (29%) MPNSTs (Figure 2) ▶ ; however, it was not detected in the typical and cellular neurofibromas analyzed. There was a statistically significant difference between p53 detection in MPNSTs and that in NFs (P = 0.02).
pRB nuclear immunoreactivities were identified in 31 of 35 (89%) MPNSTs (Figure 3) ▶ . The intensity of staining in this group of lesions was moderate to strong. Seven cases showed low pRB levels, whereas 24 cases displayed pRB nuclear overexpression. The percentage of tumor cells expressing pRB ranged from 5% to 80%. pRB nuclear immunostaining was noted in 15 of 16 (94%) NFs. The intensity of staining in typical NFs was weak. However, in the four cellular NFs nuclear pRB expression was more pronounced in both intensity and percentage of positive cells, resembling the pattern seen in MPNSTs.
p21 nuclear immunoreactivity was displayed in 16 of 35 (46%) MPNSTs and 11 of 16 (69%) NFs (Figure 4) ▶ . The intensity of p21 staining was similar in the two tumor types and scored as moderate to strong. The cellular NFs exhibited higher levels of p21 expression and were scored as displaying stronger staining signals.
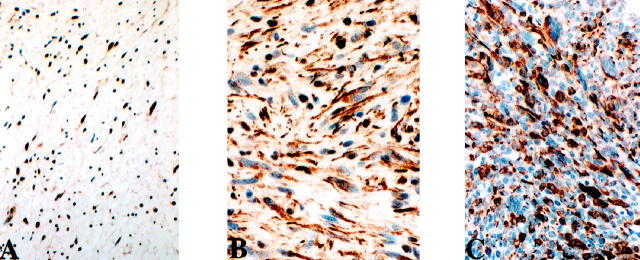
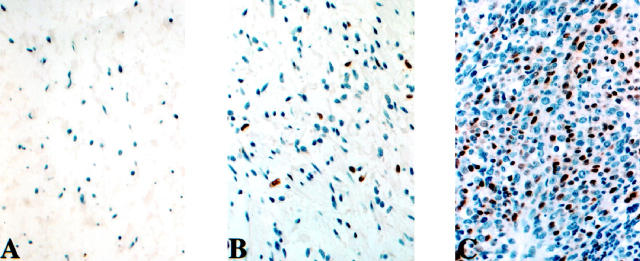
Scoring of p27 was conducted by separately recording nuclear and cytoplasmic expression. Nuclear p27 immunoreactivities were noted in 3 of 35 (9%) MPNSTs (in 30–70% of the cells, mean 43%) and 15 of 16 (94%) NFs, (in 30–90% of the cells, mean 55%), a finding of statistical significance (P < 0.001). In contrast, cytoplasmic p27 expression was identified in 19 of 35 (54%) MPNSTs (in 20–80% of the cells, mean 41%) and 12 of 16 (75%) NFs (in 20–50% of the cells, mean 28%). Cellular NFs showed decreased nuclear p27 expression and more intense cytoplasmic staining, resembling the pattern noted in MPNSTs (Figure 5) ▶ .
Figure 5.
Expression of p27 is prominent in nuclei of NF (A) and decreased in nuclei and prominent in cytoplasm of both cellular NFs (B) and MPNSTs (C).
Cyclin D1 nuclear expression was identified in some tumors of both categories, including 10 of 35 (29%) MPNSTs and 5 of 16 (31%) NFs (Figure 6) ▶ . In contrast, cyclin E immunoreactivities were noted in 14 of 33 (42%) MPNSTs (in 10–50% of the cells, mean 24%) and 5 of 16 (31%) NFs (in 10–20% of the cells, mean 18%). Cellular NFs also displayed significant cyclin E levels (Figure 7) ▶ . Moreover, if cellular NFs were to be excluded from the statistical analysis, a significant difference would then be noted between cyclin E expression in MPNSTs and typical NFs (P = 0.03).
Figure 6.
Nuclear cyclin D1 in MPNST.
Figure 7.
Nuclear expression of cyclin E is undetectable in NF (A), present at low levels in cellular NF (B), and significant in MPNST (C).
High proliferative index, as assessed by Ki-67 expression, was found in 20 of 34 (59%) MPNSTs (Figure 8) ▶ ; however, Ki-67 was either undetectable or scored as low (ranging from 1% to 2%) in all tested NFs (P < 0.001).
Figure 8.
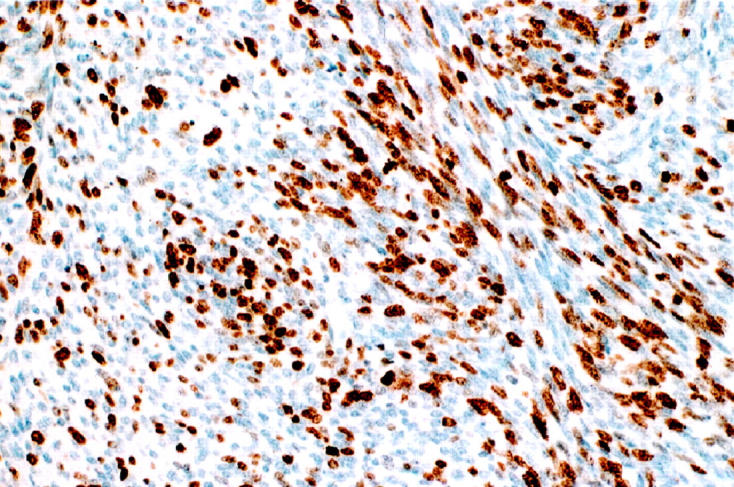
Ki-67 expression in MPNST.
A comparative analysis of the distinct patterns of expression identified significant associations between p27 cytoplasmic immunoreactivity and nuclear cyclin E overexpression (P = 0.01). There was also a significant association between p53 nuclear overexpression and detection of p21 nuclear immunoreactivities (P = 0.03). Analysis of expression between the other cell cycle regulators failed to reveal statistically significant associations.
Among the patients with MPNST, 26 died of disease during follow-up. The median follow-up of the nine survivors was 24 months (16–276 months). Thirteen patients developed recurrent disease (median time to recurrence 15 months), and 19 patients developed metastases (median time to metastasis 22 months). Tumor sizes ranged from 4 cm to 40 cm (median and mean 13 cm). We observed a statistically significant association between p27 cytoplasmic expression and shortened survival, by univariate analysis (P = 0.03, RR = 2.4, CI = 1.0–5.6). In addition, we found that undetectable levels of pRB were significantly associated with a higher rate of local recurrence (P = 0.004, relative risk = 0.2, CI = 0.06–0.7), but not with shortened survival.
Discussion
Malignant peripheral nerve sheath tumors are uncommon neoplasms 27 but arise frequently in patients affected by neurofibromatosis type 1. 27-29 In this setting, they are likely to develop within a preexisting neurofibroma. This suggests that a multistage sequence occurs for tumor progression in these neoplasms. The molecular events involved in such a scheme, leading from a NF to a MPNST, are still unknown.
p53 nuclear overexpression has previously been reported to be detected in 13 of 26 (50%) MPNSTs, but in only one of 12 NFs. 23 Similar findings were independently reported by another group of investigators, who found p53 overexpression in 16 of 28 (57%) MPNSTs and one of 27 (4%) NFs. 24 In the present study, we used a cutoff value of ≥20% positive tumor cell nuclei to categorize a case as displaying the positive phenotype. Based on this value, we observed p53 nuclear immunoreactivities in 10 of 35 (29%) MPNSTs. The difference in percentage of p53 positive cases is probably due to the selection of the cutoff point, as well as methodological differences such as distinct primary antibody clones and immunostaining protocols. We selected the cutoff point of ≥20% positive tumor cell nuclei based on previous experience from our laboratory and other groups that compared the sensitivity of immunohistochemistry, using clone PAb 1801, and sequencing as a gold standard. In this context, our result closely approximates the frequency of p53 mutations in two of seven MPNSTs reported by Menon et al. 21 It is known that not all p53 mutations are detected by immunohistochemical analyses, because those fail to identify mutations that result in truncated proteins. Interestingly, although p53 nuclear overexpression has been associated with poor outcome, in the present study no such correlation was observed.
Unlike other soft tissue malignancies, 30 expression of pRB was generally maintained in both MPNSTs and NFs. However, we noticed that all four pRB-negative MPNSTs recurred. In contrast, the rate of recurrence for pRB-positive MPNSTs was 65%, a finding that reached statistical significance. No correlation of immunohistochemical pRB expression with the examined cell cycle regulators or Ki-67 was identified. It is of interest to note that whereas only one of seven (14%) patients with MPNSTs of low pRB expression died of the disease, 8 of 24 (33%) patients with tumors showing pRB overexpression did so. This difference, however, did not reach statistical significance. It has been observed that pRB overexpression identifies a subgroup of tumors with poor outcome. 31-32 The functional status of these overexpressed pRB products is at present unknown. Immunohistochemistry using antibodies that would specifically recognize the functional, underphosphorylated pRB or the application of Western blotting for detecting this form of pRB may help in resolving this issue.
Although p21 expression was noted in a minority of MPNSTs versus a majority of NFs, this difference did not reach statistical significance. p21 expression in MPNSTs was associated with a positive p53 phenotype. This finding supports the postulate that p21 could be induced via pathways that are independent of p53 transactivation. 33 In this setting, p21 expression may be related to mitogenic stimuli via growth factor signaling. There is abundant evidence regarding the up-regulation of growth factor receptor/ligand activity in soft tissue sarcomas. The intense p21 immunoreactivity observed in cellular neurofibromas might represent either a response to cellular stress, induced by a functional p53, or the influence of mitogenic signals produced by growth factors acting on these lesions.
Distinct patterns of immunoreactivity were identified for p27. A nuclear p27 phenotype was observed in 15 of 16 (94%) NFs versus only 3 of 35 (9%) MPNSTs, a statistically significant finding. These data support the postulate that p27 might be involved in tumor progression in the NF-to-MPNST pathway. In contrast, the majority of MPNSTs displayed strong cytoplasmic p27 staining. Because no tumor-specific p27 mutations have been identified in large series of solid tumors, 34-35 the p27 phenotypes identified in MPNSTs are likely to be related to epigenetic phenomena. In this regard, it has been reported that p27 is a target of degradation via the ubiquitin pathway. 36 Thus it is possible that the cytoplasmic localization of p27 reflects delayed proteasome degradation and cytoplasmic accumulation. Alternatively, recent work has suggested that the cytoplasmic p27 mislocalization relates to the loss of the tuberin protein, 37-38 encoded by the tuberous sclerosis gene-2 (TSC-2). Whether such abnormalities are also present in MPNSTs remains unknown. Interestingly, there was no association between p27 phenotypes and the Ki-67 proliferative index.
Complexes formed by cyclin E and Cdk2 can directly down-regulate p27 by promoting p27 phosphorylation at ATP concentrations approaching physiological levels, leading to subsequent p27 elimination from the cell. 39 The significant association between cyclin E and p27 is evidenced in this study, relating p27 cytoplasmic accumulation to cyclin E nuclear overexpression. Appreciable differences in cyclin E expression were noted among the two tumor types under study. Many MPNSTs, up to 40% of the cases, expressed nuclear cyclin E, whereas only one of 12 typical NFs exhibited such a pattern. It is noteworthy that all four cellular NFs showed strong nuclear cyclin E immunoreactivity, paralleling the finding in MPNSTs. These data indicate that cyclin E may also participate in the pathway of NF-to-MPNST progression.
Taken together, data from this study suggest that two distinct patterns of expression of cell cycle regulators are associated with the two tumor types, namely NF and MPNST. Typical NFs express nuclear p27 and lack cyclin E, whereas MPNSTs have undetectable nuclear p27, acquire p27 cytoplasmic expression, and display high levels of nuclear cyclin E. These events reflect the disregulation of the G1 transition and appear to contribute to progression in the NF-to-MPNST pathway, as well as to the process of tumor progression in MPNSTs. Furthermore, detection of cytoplasmic p27 expression was found to be a prognostic factor for poor survival in MPNSTs (P = 0.03, relative risk = 2.4). It is conceivable that such biological determinants may be of clinical significance in the prognostication of this group of neoplasms.
Footnotes
Address reprint requests to Dr. Helen P. Kourea, Department of Pathology, University of Patras Hospital, Patras–Rion 26500, Greece. E-mail: kouma@otenet.gr.
Supported by Grant CA47179 from the National Institutes of Health.
References
- 1.Matsushime H, Ewen ME, Strom DK, Kato J, Hanks SK, Roussel MP, Sherr CI: Identification and properties of an atypical catalytic subunit (p34 PSK-J3/Cdk4) for mammalian D-type cyclins. Cell 1992, 71:323-334 [DOI] [PubMed] [Google Scholar]
- 2.Meyerson M, Harlow E: Identification of G1 kinase activity for Cdk6, a novel cyclin D partner. Mol Cell Biol 1994, 14:2077-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordon-Cardo C: Mutation of cell cycle regulators: biological and clinical implications for human neoplasia. Am J Pathol 1995, 147:545-560 [PMC free article] [PubMed] [Google Scholar]
- 4.Lee W-H, Shew J-Y, Hong FD, Sery TW, Donoso LA, Young LJ, Bookstein R, Lee EY: The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature 1987, 329:642-645 [DOI] [PubMed] [Google Scholar]
- 5.Nevins JR: E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 1992, 258:424-429 [DOI] [PubMed] [Google Scholar]
- 6.Hirama T, Koeffler HP: Role of cyclin-dependent kinase inhibitors in the development of cancer. Blood 1995, 86:841-854 [PubMed] [Google Scholar]
- 7.Sherr C: G1 phase progression: Cycling on cue. Cell 1994, 79:551-555 [DOI] [PubMed] [Google Scholar]
- 8.Dowdy SF, Hinds PW, Loule K, Reed SI, Arnold A, Weinberg RA: Physical interaction of the retinoblastoma protein with human D cyclins. Cell 1993, 73:499-511 [DOI] [PubMed] [Google Scholar]
- 9.Geng Y, Ng Eaton E, Picon M, Roberts JM, Lundberg AS, Gifford A, Sardet C, Weinberg RA: Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene 1996, 12:1173-1180 [PubMed] [Google Scholar]
- 10.Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M: Human cyclin E, a nuclear protein essential for G1 to S phase transition. Mol Cell Biol 1995, 15:2612-2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 5:805-816 [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D: P21 a universal inhibitor of cyclin kinases. Nature 1993, 366:701-704 [DOI] [PubMed] [Google Scholar]
- 13.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 14.Lee M-H, Reynisdottir I, Massague J: Cloning of p57kip2, a cyclin dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev 1995, 9:639-649 [DOI] [PubMed] [Google Scholar]
- 15.Serrano M, Hannon GJ, Beach D: A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366:704-707 [DOI] [PubMed] [Google Scholar]
- 16.Hannon GJ, Beach D: P15INK4B is a potential effector of TGF-b induced cell cycle arrest. Nature 1994, 371:257-261 [DOI] [PubMed] [Google Scholar]
- 17.Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, O’Keefe CL, Matera AG, Xiong Y: Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild type pRb function. Genes Dev 1994, 8:2939-2952 [DOI] [PubMed] [Google Scholar]
- 18.Chan FKM, Zhang J, Cneng L, Shapiro DN, Winoto A: Identification of human and mouse p19, a novel CDK4and CDK6 inhibitor with homology to p16INK4. Mol Cell Biol 1995, 15:2682-2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer E, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumour suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 20.Polyak K, Lee M-H, Erjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J: Cloning of p27kip, a cyclin-dependent kinase inhibitor amd potential mediator of extracellular antimitogenic signals. Cell 1994, 78:59-66 [DOI] [PubMed] [Google Scholar]
- 21.Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW, Farmer GE, Freiman RN, Lee JK, Li FP, Barker DF, Ledbetter DH, Kleider A, Martuza RL, Gusella JF, Seizinger BR: Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci USA 1990, 87:5435-5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legius E, Dierick H, Wu R, Hall BK, Marynen P, Cassiman JJ, Glover TW: TP53 mutations are frequent in malignant NF1 tumors. Genes Chromosom Cancer 1994, 10:250-255 [DOI] [PubMed] [Google Scholar]
- 23.Kindblom LG, Ahlden M, Meis-Kindblom JM, Stenman G: Immunohistochemical and molecular analysis of p53, MDM2, proliferating cell nuclear antigen and Ki67 in benign and malignant nerve sheath tumors. Virchows Arch 1995, 427:19-26 [DOI] [PubMed] [Google Scholar]
- 24.Halling KC, Scheithauer BW, Halling A, Nascimento AG, Ziesmer SC, Roche PC, Wollan PC: p53 expression in neurofibroma and malignant peripheral nerve sheath tumor: an immunohistochemical study of sporadic and NF1-associated tumors. Am J Clin Pathol 1996, 106:282-288 [DOI] [PubMed] [Google Scholar]
- 25.Erlandson RA, Woodruff JM: Peripheral nerve sheath tumors: an electron microscopic study of 43 cases. Cancer 1982, 49:273-287 [DOI] [PubMed] [Google Scholar]
- 26.Woodruff JM: Pathology of major peripheral nerve sheath tumors. Weiss SW Brooks JSJ eds. Soft Tissue Tumors, International Academy of Pathology Monograph. 1996, :pp 129-161 Williams and Wilkins, Baltimore [PubMed] [Google Scholar]
- 27.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM: Malignant peripheral nerve sheath tumor. A clinicopathologic study of 120 cases. Cancer 1986, 57:2006-2021 [DOI] [PubMed] [Google Scholar]
- 28.Hruban RH, Shiu MH, Senie RT, Woodruff JM: Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer 1990, 66:1253-1265 [DOI] [PubMed] [Google Scholar]
- 29.Kourea HP, Bilsky MH, Leung DH, Lewis JJ, Woodruff JM: Subdiaphragmatic and intrathoracic paraspinal malignant peripheral nerve sheath tumor. A clinicopathologic study of 25 patients and 26 tumors. Cancer 1998, 82:2191-2203 [DOI] [PubMed] [Google Scholar]
- 30.Karpeh MS, Brennan MF, Cance WG, Woodruff JM, Pollack D, Casper ES, Dudas ME, Latres E, Drobnjak M, Cordon-Cardo C: Altered expression of retinoblastoma gene product in adult soft tissue sarcomas. Br J Cancer 1995, 72:986-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cote RJ, Dunn MD, Chatterjee SJ, Stein JP, Shi SR, Tran QC, Hu SX, Xu HJ, Groshen S, Taylor CR, Skinner DG, Benedict WF: Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p531. Cancer Res 1998, 58:1090-1094 [PubMed] [Google Scholar]
- 32.Stefanaki K, Tzardi M, Kouvidou C, Chaniotis V, Bolioti M, Vlychou M, Zois M, Kakolyris S, Delides G, Rontogianni D, Georgoulias V, Kanavaros P: Expression of p53, p21, mdm2, Rb, bax and Ki67 proteins in lymphomas of the mucosa-associated lymphoid (MALT) tissue. Anticancer Res 1998, 18:2403-2408 [PubMed] [Google Scholar]
- 33.Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE, Givol D: Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res 1994, 54:3391-3395 [PubMed] [Google Scholar]
- 34.Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S: Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 1995, 55:2266-2269 [PubMed] [Google Scholar]
- 35.Ponce-Castaneda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Sheinfeld J, Massague J, Cordon-Cardo C: P27 Kip1: chromosomal mapping to 12p12–12p13.1 and absence of mutations in human tumors. Cancer Res 1995, 55:1211-1214 [PubMed] [Google Scholar]
- 36.Pagano M, Tam SW, Theodoras AN, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 37.Soucek T, Yeung RS, Hengstshlager M: Inactivation of the cyclin-dependent kinase inhibitor p27 upon loss of the tuberous sclerosis complex gene-2. Proc Natl Acad Sci USA 1998, 95:15653-15658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clurman BE, Porter P: New insights into the tumor suppressor function of p27KIP1. Proc Natl Acad Sci USA 1998, 95:15158-15160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE: Cyclin E-CDK2 is a regulator of p27kip1. Genes Dev 1997, 11:1464-1478 [DOI] [PubMed] [Google Scholar]