Abstract
Centrosomes are the major microtubule organizing center in mammalian cells and establish the spindle poles during mitosis. Centrosome defects have been implicated in disease and tumor progression and have been associated with nullizygosity of the p53 tumor suppressor gene. In the present ultrastructural analysis of 31 human breast tumors, we found that centrosomes of most tumors had significant alterations compared to centrosomes of normal breast tissue. These alterations in included 1) supernumerary centrioles, 2) excess pericentriolar material, 3) disrupted centriole barrel structure, 4) unincorporated microtubule complexes, 5) centrioles of unusual length, 6) centrioles functioning as ciliary basal bodies, and 7) mispositioned centrosomes. These alterations are associated with changes in cell polarity, changes in cell and tissue differentiation, and chromosome missegregation through multipolar mitoses. Significantly, the presence of excess pericentriolar material was associated with the highest frequency of abnormal mitoses. Centrosome abnormalities may confer a mutator phenotype to tumors, occasionally yielding cells with a selective advantage that emerge and thrive, thus leading the tumor to a more aggressive state.
Checkpoints monitor the nuclear cycle and signal progression after proper completion of earlier stages of the cell cycle. 1 Differentiation, cell proliferation, and programmed cell death are normal outcomes of checkpoint surveillance. In cancer, disregulation of the cell cycle can result in either a decrease in the rate of cell death or an increase in the rate of cell division, and thereby lead to tumor growth. The orderly duplication of the centrosome once, and only once, in each cell cycle and the formation of a bipolar mitotic spindle are key cell cycle checkpoints leading to successful cell division. The importance of the centrosome in the development of malignant tumors was suspected first by Boveri 2 nearly 100 years ago. More recently, centrosome defects have been implicated in disease and tumor progression. 3-13 Defects in centrosome duplication, alteration in centrosome microtubule nucleation capacity, and inappropriate phosphorylation of centrosome proteins were first described for human breast tumors 14 and subsequently, centrosome anomalies were reported for other tumors. 15-17 Recent evidence suggests that elevated Aurora kinase or Serine/Threonime kinase-15 (STK15) activity may play a key role in acquisition of at least some of these centrosome defects during tumor progression. 18
The centrosome is the major microtubule-organizing center in mammalian cells; it regulates the number, stability, polarity, and spatial arrangement of microtubules in interphase cells. 19,20 Thereby, the centrosome and microtubules play a role in maintaining overall cell polarity, provide an architectural framework for directed organelle transport, and participate in cell shape and movement.
The interphase centrosome consists of a pair of orthogonally oriented centrioles surrounded by a pericentriolar matrix. Duplication of the centrosome begins during S phase of the cell cycle when the two centrioles lose their orthogonal arrangement before the formation of a procentriole (or bud) closely associated with the proximal end of each of the original centrioles. The procentrioles lengthen during S and G2, so that by prophase the cell contains two diplosomes, that is, two orthogonal pairs of full-length centrioles. 21-24 At the onset of prophase, the diplosomes, along with associated pericentriolar material, move to opposite sides of the nucleus and establish the bipolar mitotic spindle. 25
We recently have shown that the centrosomes of high-grade breast cancers do not follow this program of events. 14 In breast tumor cells, centrosome duplication is uncoupled from the cell cycle, resulting in cells with numerous centrosomes, many of which are larger than normal. Tumor centrosomes typically show inappropriate levels of phosphorylated proteins, in contrast to normal centrosomes, which contain increased levels of phosphorylated proteins during mitosis.
Here we compare the ultrastructure of centrosomes of normal breast epithelial tissues and breast adenocarcinomas. These studies reveal dramatic abnormalities in the centrioles and centrosomes of breast tumor cells. These abnormalities include 1) supernumerary centrioles, 2) excess pericentriolar material, 3) disrupted centriole barrel structure, 4) unincorporated microtubule complexes, 5) centrioles of unusual length, 6) centrioles functioning as ciliary basal bodies, and 7) mispositioned centrosomes. Structural centrosome abnormalities, most notably excess pericentriolar material, were associated with an increased frequency of abnormal mitoses as assessed by Ki-67-immunolabeled paraffin sections of the same tumors. The relevance of centrosome structure with regard to cell polarity, differentiation, bipolar and multipolar mitosis, and tumor progression is discussed.
Materials and Methods
Tissues
Tissues from 45 consecutive mastectomy and lumpectomy surgeries were collected according to an Institutional Review Board-approved protocol. Tissues were omitted from the analysis if patients had received previous chemotherapy or radiation therapy (n= 6), did not include primary invasive tumor (n = 4), were poorly preserved (n = 3), or were from male patients (n = 1). The remaining 31 tumors, which included two grade 2, nine grade 3, and twenty grade 4 specimens (Mayo histological grading scale), were analyzed. Six normal tissues from breast reduction surgeries were also analyzed.
Transmission Electron Microscopy Processing and Observation
Tissues were cut into small pieces and placed in fixative (4% formaldehyde, 1% glutaraldehyde in sodium phosphate buffer, pH 7.2) at 4°C for up to 36 hours. Tissues were further processed by postfixation in osmium tetroxide, en bloc staining with uranyl acetate, dehydration in ethanol, and embedding in epoxy resin. Thin sections were poststained with lead citrate and examined using a Philips CM10 Biotwin transmission electron microscope (Philips Electronic Instruments, Mahwah, NJ). Tissues were categorized according to centrosome location, number of centrioles in thin section, qualitative level of pericentriolar material, presence and arrangement of centriolar appendages, presence of primary cilia, variations on centriolar structure, and multipolar mitotic figures.
Light Microscopy and Mitotic Index Determination
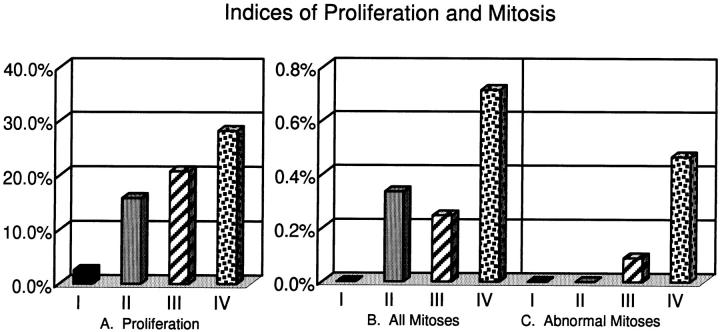
Portions of tissues also were formalin-fixed and paraffin-embedded for light microscopy. Sections were immunostained using MIB-1 antibody against Ki-67 (Dako Corp., Carpinteria, CA). Ki-67 is a nuclear antigen that is present in late G1, S, G2, and mitotic cells, but is lacking in G0 and early G1 cells. Condensed chromosomes are stained intensely with this antibody, allowing for easy quantification of proliferative and mitotic cells and identification of abnormal mitotic figures. Proliferative index (PI) was calculated as the percentage of Ki67-positive cells out of the total number of epithelial cells. A minimum of 200 cells was counted in defined fields of view using a 40× objective. Likewise, mitotic index (MI) was calculated as the percentage of mitotic cells in the same fields of view. When no mitotic cells were observed, the MI was calculated as <1 mitotic cell per the total number of cells observed. Because the frequency of abnormal mitotic figures is very low in most tissues, the abnormal mitotic index (AMI) was determined by scanning the entire section and counting the total number of mitotic cells and the total number of abnormal mitotic figures. The ratio of abnormal to total mitoses was then multiplied by the mitotic index to yield the AMI. These data are summarized in Figure 7 ▶ . All tissues were scored blindly. Photographs were made using a Nikon FXA photomicroscope.
Figure 7.
Indices of proliferation and mitosis. Tissues were placed in categories as follows: I (solid black bars), six normal tissues from reduction mammoplasties with normal centriole/centrosome structure (all normal tissues examined fell in this category); II (solid gray bars), nine tumor tissues with normal centrioles/centrosomes; III (striped bars), twelve tumor tissues with abnormal centrioles (this includes tissues with supernumerary centrioles and those with centriole defects, but excludes those with excess pericentriolar material); and IV (stippled bars), seven tumor tissues with excess pericentriolar material, regardless of centriole defects. The Wilcoxon Rank Sum Test was used to determine statistical significance. Median values are plotted. A: The proliferation index of category I (normal tissue) is significantly lower than the other three categories and that of category IV (tumors with excess pericentriolar material) is significantly greater than categories I and II, but not III. Categories II and III are not significantly different from each other. B and C: Category IV has significantly higher frequencies of mitosis and abnormal mitosis than the other three categories. The other categories were not significantly different from each other.
Centrin Immunofluorescence
A subset of tissues was selected for immunofluorescence studies. These tissues included one tumor with normal centrosome ultrastructure, one tumor with clusters of extra centrioles, two tumors with extra pericentriolar material, and two tumors with inverted polarity. Normal tissue used for immunofluorescence was from a different patient than that used in the ultrastructure studies. All tissues were frozen in liquid nitrogen within 30 minutes of surgical removal and stored at −70°C until use. Cryosections were immunostained with a monoclonal antibody against centrin, a centrosomal protein, as previously described. 14 Sections were examined and photographed using a Nikon FXA epifluorescence microscope.
Results
Normal Breast Epithelium
Normal breast epithelial tissues were organized with a high cuboidal layer of luminal cells separated at intervals from the basement membrane by a discontinuous layer of myoepithelial cells (Figure 1, A and B) ▶ . The nuclei of the luminal epithelial cells tended to be basal and the centrioles apical. Although apical, most often the position of the centrioles was eccentric; that is, they were located near the lateral junctional complexes of adjacent cells (Figure 1B) ▶ . Although centrioles usually did not maintain an orthogonal orientation, they were typically close to each other (Figure 1, A and C) ▶ . Occasionally, an extremely short primary cilium extended from the distal end of the mature centriole (Figure 1C) ▶ . Fine striated rootlets infrequently were observed extending from the proximal ends of centrioles toward the base of the cell (Figure 1D) ▶ . The striated rootlets were quite variable in extent and were not observed with most centrioles. Other than distal and subdistal appendages on the mature centriole and fine fibrillar material along the outer walls of the centriole barrels, little pericentriolar material was noted with the centrioles of normal luminal epithelial cells (Figure 1, A ▶ -D). Subdistal appendages were slightly more developed on the centrioles of the myoepithelial cells, and their primary cilia were longer than those of luminal epithelial cells (Figure 1, B and E) ▶ . Unlike luminal epithelial cells, diplosomes of myoepithelial cells were located close to the nuclei. Filaments extended from the myoepithelial diplosome to the nucleus (Figure 1E) ▶ ; this was never observed in luminal epithelial cells. No centrosome abnormalities were observed in normal epithelial cells of the four reduction mammoplasties examined by electron microscopy.
Figure 1.
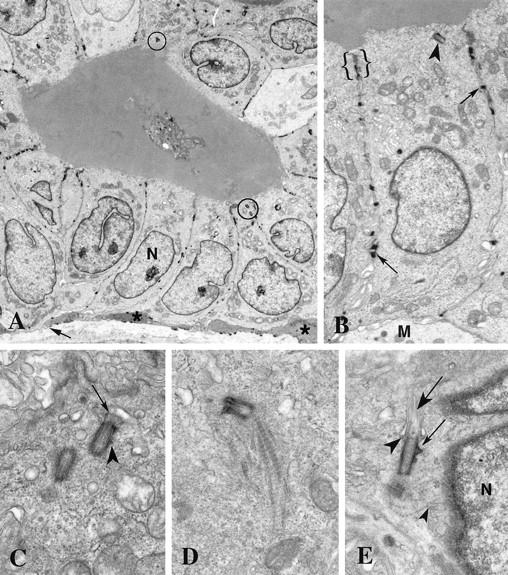
Normal breast epithelium. A: The normal breast ductal epithelium consists of a high cuboidal layer of luminal cells subtended by a discontinuous layer of myoepithelial cells (*) and basement membrane (arrow). The nuclei (N) are basal and the centrosomes (circled) are apical. B: Adjacent luminal epithelial cells are joined by lateral junctional complexes (brackets) near the apical membrane and desmosomes (arrows) between their lateral membranes. A single centriole (arrowhead) is located at the apex next to a junctional complex. A portion of a myoepithelial cell (M) is seen at the base of the luminal epithelial cell. C: The mature centriole of this nonorthogonal diplosome bears a short primary cilium (arrow) at its distal end in this luminal epithelial cell. A small subdistal appendage (arrowhead) is present on the mature centriole, whereas the immature centriole lacks appendages. Although very little pericentriolar material is present, the centrioles do have a coating of fine fibers. D: A striated rootlet extends from the proximal end of this mature centriole toward the base of the luminal epithelial cell. E: Fine fibers (small arrowhead) extend between the diplosome and the nearby nucleus (N) in this myoepithelial cell. Distal appendages (large arrowhead) extend between the centriole and the plasma membrane at the site of primary cilium (large arrow) emergence. Subdistal appendages (small arrow) are prominent on the mature centriole. The immature centriole is seen in oblique section. Original magnifications, ×3500 (A), ×8850 (B), ×27,500 (C), ×25,600 (D), ×21,200 (E).
Invasive Breast Tumors
Twenty-four of 31 invasive tumors contained centrosomes and that differed from those of normal breast cells in a variety of ways. Eleven tumors were characterized by centrosomes with more than two centrioles (Figures 2 and 3 ▶ ▶ , A-C). In thin sections, these supernumerary centrioles ranged from a pair of centrioles with a single extra procentriole to a field of 9 centriole profiles (Figure 2, A ▶ -F). Often the extra centrioles were arranged in a group and were closely linked by fine fibers extending between subdistal appendages (Figure 2, C, E, and F) ▶ . Appendages normally associated with only the mature centriole were seen frequently with more than one centriole in these groups (Figure 2, C ▶ -F, and Figure 3A ▶ ). Centrosomes with extra centrioles were most often located adjacent to the nucleus (Figure 2, B, E, and F) ▶ , in contrast to normal luminal epithelial cells, in which the centrioles tended to be closer to the apical plasma membrane (Figure 1, A and B) ▶ .
Figure 2.
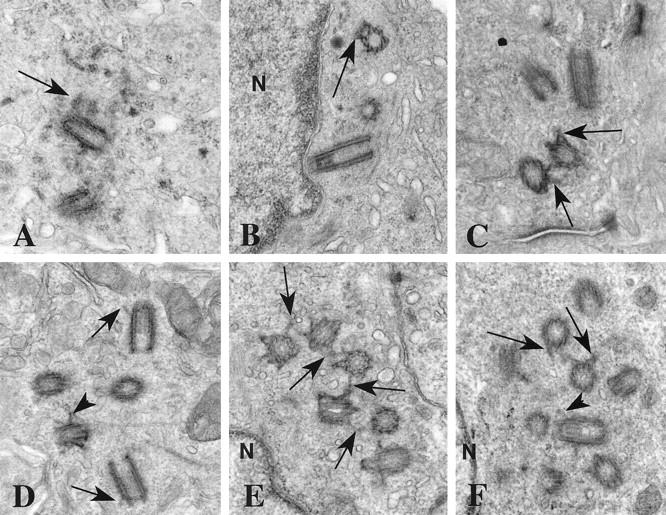
Supernumerary centrioles in breast tumors. A: A procentriole (arrow) is present at the proximal end of one of the two centrioles in this section. This procentriole is identifiable by its orthogonal orientation relative to the full length centriole and by the width of its lumen. Notice the electron opaque pericentriolar satellites surrounding the centrioles. B: Two centrioles are seen in cross section and a third is in longitudinal section. One centriole has subdistal appendages (arrow). All three are close to the nucleus (N). There is no orthogonal relationship between any of the three centrioles. C: At least two of these four centrioles have subdistal appendages (arrows). D: The barrels of these five centrioles are coated with a fine electron opaque material. Two centrioles have distal appendages (arrows) and at least one also has subdistal appendages (arrowhead). E: This group of six centrioles is linked by fine fibers between their subdistal appendages (arrows). The group is next to the nucleus (N). F: At least nine centriole profiles are present in this thin section. Subdistal (arrows) and distal (arrowhead) appendages are seen on many of the centrioles. The nucleus (N) is adjacent to this cluster of centrioles. Original magnifications, ×27,500 (A and B), ×32,300 (C and F), ×31,000 (D), ×34,150 (E).
Figure 3.

Excess pericentriolar material in breast tumors. A: Centrosomes in two adjacent cells are seen. Desmosomes (small arrows) tether the plasma membranes. All of the centriole profiles include subdistal appendages that are characteristic of mature centrioles (large arrows). Electron opaque fibrogranular material is present around both centrosomes. B: The barrels of these centrioles are coated with a dark granular material and pericentriolar satellites are present. One centriole has distal and subdistal appendages (arrow) while the other has a procentriole (arrowhead) associated with it. C: Fine electron opaque fibers coat the five centriole profiles seen in this section. Two orthogonal centrioles are connected by a dense parallel array of fibers (arrow). D: Two centrioles with numerous dark granules are present in this section. E: This centrosome contains one centriole and several masses (arrows) similar to generative complexes visible in this section. Original magnifications, ×17,900 (A), ×31,650 (B), ×28,000 (C), ×28,700 (D), ×27,650 (E).
The amount of pericentriolar material and satellites associated with tumor centrosomes was variable, ranging from low levels similar to normal centrosomes (Figure 2, B ▶ -F), to moderate (Figure 2A) ▶ and excessive levels (Figure 3) ▶ . In all, nine tumors had excess pericentriolar material, often in addition to extra centrioles. In some tumors this pericentriolar material had a distinct fibrogranular appearance (Figures 2A and 3) ▶ ▶ reminiscent of material associated with basal body formation in ciliated cells. Large granular masses, similar to generative complexes involved in ciliary basal body formation, were also observed in the pericentriolar material in some tumor cells (Figure 3E) ▶ . Many centrioles were encased in electron opaque material pressed directly to the barrel of the centriole (Figure 3, B and C) ▶ .
In addition to excessive pericentriolar material, two tumors had centrioles that were structurally defective in various aspects (Figure 4) ▶ . Normal centrioles are composed of nine sets of triplet microtubules in which the A microtubule is complete and the B and C microtubules share protofilaments with A and B, respectively. 26 Unusual microtubule complexes were observed near complete centrioles in some tumors (Figure 4A) ▶ . These microtubule complexes were not assembled into normal triplets nor arranged in a barrel shape; rather they were an assortment that included five or more microtubules with shared protofilaments embedded in amorphous electron-opaque material (Figure 4A) ▶ . In one instance a centriolar microtubule triplet was displaced away from the centriole barrel, resulting in what has been termed an open ring centriole (Figure 4B) ▶ . Unusually long centrioles (Figure 4D) ▶ were observed in one tumor. Primary cilia ranged from very short to well developed (Figure 4C) ▶ .
Figure 4.
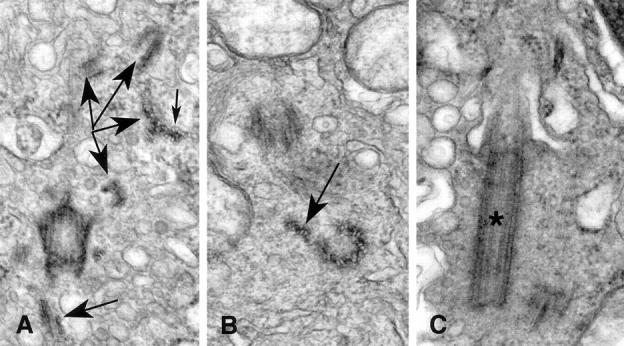
Abnormal centriole structure in breast tumors. A: Subdistal appendages are seen in this oblique section through a centriole. Numerous microtubule complexes (large arrows) are seen in various planes of section throughout the cytoplasm near the centriole. As is seen in cross section of the complexes, the individual microtubules share a portion of the wall of the neighbor microtubules (small arrow). B: The open-ring configuration of this centriole is shown in cross section. Two of the nine triplet microtubule complexes are splayed away from the centriole barrel (arrow). C: This centriole bearing a primary cilium (*) is nearly twice as long as normal centrioles. Original magnifications, ×54,500 (A), ×59,625 (B), ×47,700 (C).
Some tumors had regions of apocrine metaplasia in which luminal epithelial cells maintained normal apical/basal polarity, but had cytoplasmic beaks that projected into the lumen (Figure 5A) ▶ . The beaks were bordered by the apical plasma membrane that protruded well past the junctional complexes that mark the apical limit of the lateral plasma membrane. Beak cytoplasm contained numerous secretory vesicles, endoplasmic reticulum, and mitochondria. The centrosomes in these cells were near the junctional complexes and just apical to the nucleus, but not adjacent to the lumen as in normal luminal epithelial cells (Figure 5A) ▶ . In one well differentiated grade 2 tumor with apocrine metaplasia, the beaked apocrine cells were mixed with ciliated cells. The ciliated cells also maintained apical/basal polarity, but along their apical membrane were numerous cilia with centrioles functioning as ciliary basal bodies (Figure 5B) ▶ . These cilia and basal bodies were similar in location and appearance to those of normally ciliated cells such as ciliated respiratory epithelium. Microvilli also were located along the apical membranes of the ciliated cells (Figure 5B) ▶ . The apical membranes of the ciliated cells did not protrude into the lumen as did the nonciliated beaked cells (Figure 5A) ▶ . Both the ciliated and the beaked cells were in regions of tumors that were well differentiated.
Figure 5.
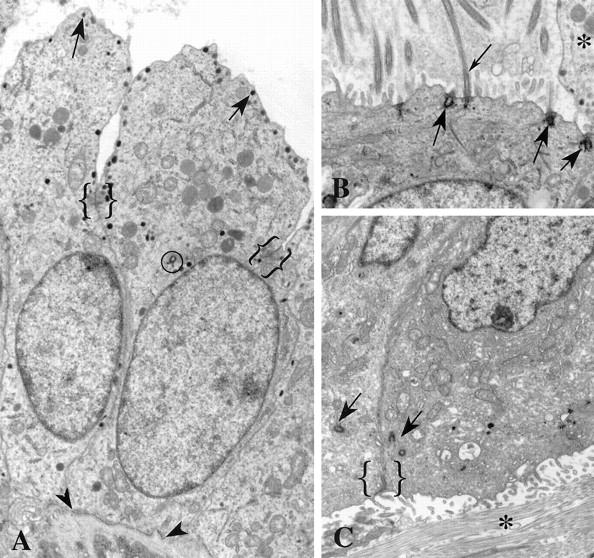
Positional centrosomal anomalies in breast tumors. A: Secretory granules (arrows) are present at the apical membrane of these cells displaying apocrine metaplasia. Junctional complexes (brackets) mark the transition from lateral to apical membrane domains. Apocrine beaks extend into the lumen of the duct. Notice the centriole (circled) near the apical end of the nucleus. These cells have apical/basal polarity and rest on a basement membrane (arrowheads). B: Extra centrioles in this cell are inserted at the apical plasma membrane where they function as basal bodies (large arrows) for cilia (small arrow). Microvilli and cilia project into the lumen. The beak of an adjacent apocrine cell (*) is visible. The ciliated cell does not protrude into the lumen, as does the apocrine cell; but like its apocrine neighbor, it has apical/basal polarity and rests on a basement membrane (not visible in this figure). C: The two centrosomes (arrows) seen in adjacent cells are located near the junctional complex between these polarized cells (bracket). However the apical membrane domain with microvilli faces collagen (*) of the stromal tissue rather than the lumen of a duct. This invasive group of cells has ramified through the breast stroma and is not subtended by a basement membrane. The polarity of these cells is inverted, with the basal domains abutting the basal domains of other cells and the apical domains facing the stroma rather than a lumen. Original magnifications, ×8150 (A), ×10,000 (B), ×7900 (C).
Two tumors contained regions in which cells still maintained apical/basal polarity even in poorly differentiated and highly invasive tumors lacking a basement membrane (Figure 5C) ▶ . The apical and lateral membranes were identified by their location relative to junctional complexes and the presence of microvilli on the apical membrane. In these instances, the cell apices often did not face a lumen, but instead faced collagen fibrils of the stromal connective tissue (Figure 5C) ▶ . The centrosomes of these cells were normal in structure and were located next to the junctional complexes near the apical plasma membrane, but, because the apices face the stroma, the cell polarity was inverted.
Mitosis in Tumor Cells
Although mitotic figures were not observed in normal breast tissues, there were numerous mitotic figures present in four of the tumors examined transmission electron microscopy. Some mitotic figures appeared normal in thin section, having a typical metaphase plate and bipolar spindle (not shown), whereas others had significant abnormalities (Figure 6) ▶ . A tripolar mitosis is shown in Figure 6A ▶ . Tracings of microtubules, spindle poles, and condensed chromosomes from six nonadjacent serial sections through the cell in Figure 6A ▶ are presented in Figure 6B ▶ . Analysis of the reconstruction in three dimensions revealed that one spindle pole was composed of two distinct but adjacent foci of microtubules, which perhaps resulted from their coalescence in prometaphase. Each spindle pole had at least two centrioles recognizable as distinct structures in these six nonadjacent thin sections. Many division figures were too bizarre for analysis in thin section.
Figure 6.
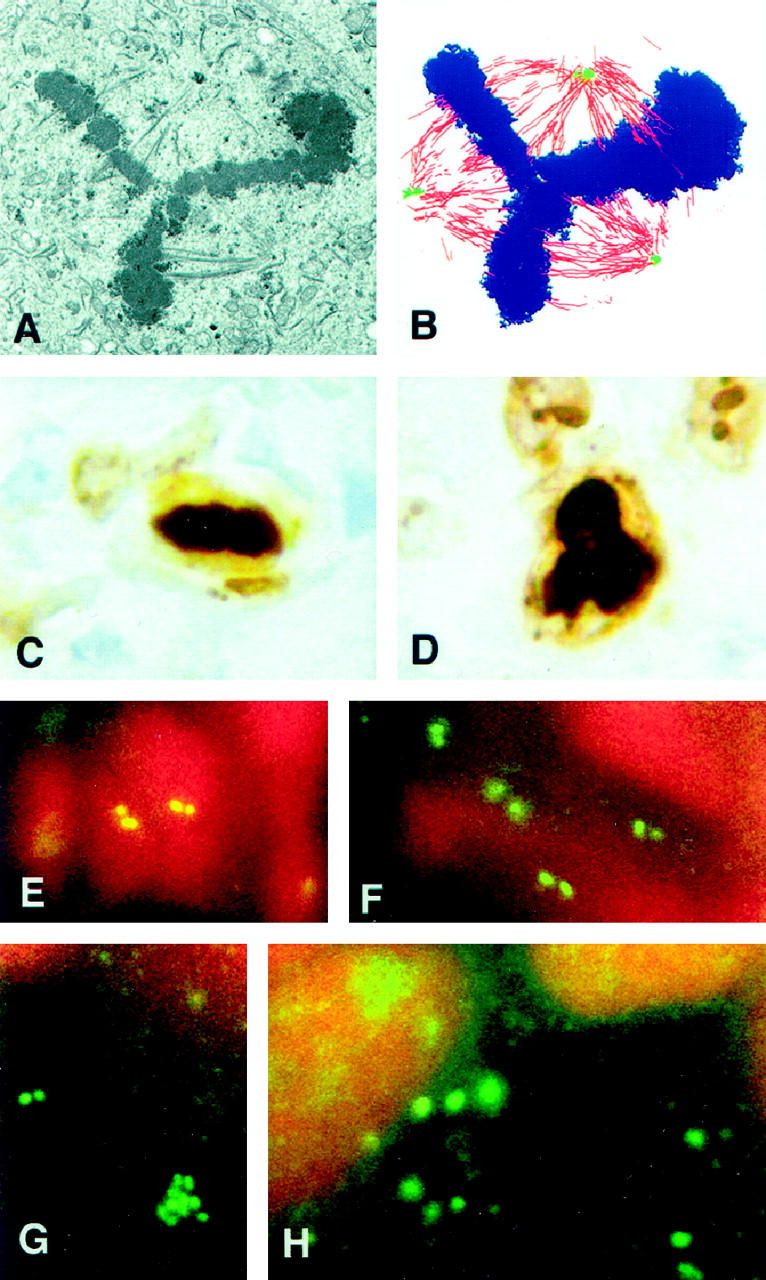
Multipolar mitoses and centrin immunofluorescence. A: This section through a symmetrical tripolar mitotic cell shows part of the metaphase plate and portions of the tripolar spindle. B: Tracings of microtubules (red), spindle poles (green), and condensed chromosomes (blue) from six nonadjacent serial sections through the cell shown in A are shown in this overlay. The upper spindle pole appears to contain two separate, but adjacent, microtubule foci that have coalesced. C: A normal metaphase plate is shown in this Ki-67 immunostained paraffin section of a breast tumor. D: A tripolar metaphase cell immunolabeled with Ki-67 is shown in this tumor section. E: In normal breast epithelium, the centrosomes appear as distinct pairs of spots when labeled with antibodies against centrin. Centrosomes of two adjacent cells are shown in this cryosection. F: In this tumor characterized with normal centrosome ultrastructure, the centrosomes are similar to those of normal tissue when immunolabeled using antibodies against centrin. G: Centrin immunofluorescence of the same tumor shown in Figure 2, E and F ▶ , reveals a cluster of centriole-sized spots as well as a normal looking pair of spots. By transmission electron microscopy this tumor had up to 9 centrioles in a single thin section, but no excess pericentriolar material. H: Centrin immunofluorescence of the same tumor as shown in Figures 3D and 6A ▶ ▶ reveals numerous large, amorphous spots. By transmission electron microscopy, centrosomes of this tumor contain excess pericentriolar material and extra centrioles. Original magnifications, ×160 (A and B), × 925 (C and D), ×2050 (E-H).
Centrin Immunofluorescence
As previously described, 14 normal breast tissues have an apically positioned pair of immunolabeled spots that correspond to the centrioles (Figure 6E) ▶ . Pairs of spots also were observed in cells of the tumor with normal centrosome ultrastructure, although the tissue was anaplastic and centriole location appeared random (Figure 6F) ▶ . Many cells in the tumor with numerous centrioles closely linked by fine fibers contained clusters of spots the size and shape of centrioles (Figure 6G) ▶ , whereas spots of various sizes and shapes were present in cells of the tumors characterized by extra pericentriolar material (Figure 6H) ▶ .
Proliferation and Mitotic Indices
Indices of proliferation, mitosis, and abnormal mitosis are summarized in Figure 7 ▶ . Tissues were placed in one of four categories on the basis of tissue type and centriole/centrosome structure. Category I is comprised of all normal tissues from reduction mammoplasty. All six of these tissues had normal centrosome structure as assessed by immunofluorescence and/or electron microscopy. Category II consists of the nine tumors that have normal centriole/centrosome structure as assessed by immunofluorescence and electron microscopy. Category III contains twelve tumors with abnormal centriole/centrosome structure such as supernumerary centrioles or structurally defective centrioles. Tumors with excess pericentriolar material in addition to centriole abnormalities are excluded from this category and placed in Category IV. Category IV contains seven tumors with excess pericentriolar material, regardless of other centriole/centrosome characteristics.
The six normal breast tissues (Category I, Figure 7 ▶ ) examined by light microscopy had a median PI of 5.3% as determined by Ki67 immunostaining. These normal tissues had a median MI of 0.00% (mean mitotic index = 0.03%) based on the total of 4238 epithelial cells observed. On examination of entire histological sections from all six tissues, only two contained identifiable mitotic figures, and no abnormal mitotic figures were observed. Of the nine tumors with normal centriole/centrosome ultrastructure (Category II, Figure 7 ▶ ), five contained no abnormal mitotic figures and four did, yielding a median AMI of 0.00% (mean = 0.16%). The median PI, MI, and AMI of Category II tumors were not significantly different from Category III tumors.
The Category IV tumors, characterized by the presence of excess pericentriolar material, had the highest median frequencies of proliferation, mitosis, and abnormal mitosis (28.2%, 0.71%, and 0.46%, respectively). Category IV values, with the exception of the PI relative to Category III, were significantly different from the values of all other categories.
Discussion
The centrosome functions to nucleate and organize microtubules; during interphase the centrosome is the primary microtubule organizing center, and during mitosis duplicated centrosomes serve as mitotic spindle poles. 19 We found that centrosomes in normal breast tissue are apical and usually adjacent to the junctional complex, whereas nuclei are basal. Very little pericentriolar material is associated with these centrosomes. As is seen in other polarized epithelial cells, 27 centrioles may separate a short distance from each other after losing their orthogonal orientation, and the mature centriole may form a short primary cilium. In addition, centrioles occasionally bear a striated rootlet.
Only by selecting breast biopsy tissue from premenopausal women in the luteal phase of the menstrual cycle was Ferguson 28 able to investigate mitosis in normal breast parenchyma. In these normal cells, very little pericentriolar material was associated with the spindle poles. The normal tissues in the present study were not selected according to the phase of menstrual cycle, and no mitoses were observed by transmission electron microscopy or by light microscopy. However, normal breast epithelium does maintain a population of proliferating cells that immunostain with antibodies to Ki67; our median PI value of 5.3% in normal breast epithelium is within the range of published values. 29 In agreement with our observations on interphase cells by immunofluorescence and by transmission electron microscopy, Ferguson 28 noted that centrioles of normal interphase cells were apical and not associated with the basal nuclei. Likewise, primary cilia have previously been noted in myoepithelial cells. 30
Centrosomes undergo changes throughout the cell cycle. 21-24 The nuclear and centrosome cycles are synchronized by checkpoints that prevent DNA reduplication before karyokinesis and prevent centrosome reduplication before anaphase. In certain normal cell types such as binuclear mouse hepatocytes 31 and human megakaryocytes, 32 synchrony between the nuclear and centrosome cycles is maintained even in the absence of cytokinesis, resulting in polyploid cells with centrosome numbers appropriate for the level of ploidy. Due to the numerous centrosomes arranged around the polyploid nucleus, megakaryocytes lack apical/basal polarity, although they do have a radial organization. In contrast, cancer cells have asynchronous nuclear and centrosome cycles, often resulting in multicentrosomal aneuploid cells that lack apical/basal polarity and appear disorganized.
We have shown that centrosomes and centrioles of most human breast tumors (24 of 31 analyzed) display a range of significant structural and functional abnormalities. Breast tissues can be divided into four categories: normal tissue with structurally normal centriole/centrosomes (Category I), tumors with structurally normal centriole/centrosomes (Category II), tumors with centriole-based abnormalities (Category III), and tumors with excess pericentriolar material (Category IV). Category IV tumors are associated with significantly increased frequencies of both normal and abnormal mitoses. Cells having no visible centrosome abnormality are also present in all tumors. Some abnormalities may be related to loss of synchrony between the centrosome cycle and nuclear cycle.
Tumor cells that become ciliated retain apical/basal polarity and tend to be well differentiated. These tumors are included in Category III. Ciliated cells have been described infrequently in breast carcinomas. 33 These multiple centrioles probably arise through the same acentriolar basal body neogenesis that occurs in normal ciliated epithelial cells. 34-37 In effect, these cells differentiate into the wrong cell type, resulting in metaplasia rather than anaplasia. These ciliated breast tumors have PI and MI of 20% and 0.2%, respectively, similar to normal breast epithelium. The ciliated cells, like normal ciliated epithelial cells, probably are terminally differentiated and remain in G0 of the cell cycle. Therefore, the production of centrioles that function as ciliary basal bodies may be a relatively harmless structural alteration with no adverse implications for genetic stability.
Open-ring centrioles and centrioles missing triplet microtubles (MTs) occur in some Category III tumors. Although these structures are similar to those present during basal body formation in hamster ciliogenesis, 38 no cilia are present in these tumors. Disrupted centriole barrels similar to open-ring centrioles have also been observed as a consequence of infection with and treatment with DNA-binding dyes, 39 and DNA-binding dyes have been shown to induce multipolar mitoses in cultured cells. 39 However, in the present study, open ring centrioles are not associated with an increase in the frequency of multipolar mitoses.
Unusual microtubule complexes embedded in dark amorphous material were also noted in one Category III tumor. The PI, MI, and AMI of this tumor are not significantly different from those of tumors with normal centrosome structure. These novel structures have not been described previously, and their importance is not understood. They may be a further indication that the mechanics, as well as timing, of centriole formation is not well regulated in tumors.
Some tumors (11 of 31 studied) produce extra centrioles that do not serve as ciliary basal bodies. In some cells of these Category III tumors, centrioles often appear linked closely together by fine fibers and remain near the nucleus. These tumors are anaplastic; ie, they are not as differentiated as tumors that produce cilia and do not retain apical/basal cell polarity. The presence of procentrioles along the proximal walls of mature centrioles indicates that these extra centrioles arose through template driven duplication rather than through acentriolar neogenesis typical of basal body production in ciliated cells. 35 Fine fibers linking the centrioles in tumors are similar to those described linking the pair of centrioles of a diplosome, 40 further supporting the idea that they originate as procentrioles associated with a mature centriole. Because template-driven centriole duplication normally occurs only once per nuclear cycle, these cells have lost the synchrony between the nuclear cycle and the centrosome cycle. As long as the centrioles remain linked together, they may function as one large centrosome in an interphase cell. However, if these large centrosomes separate into more than two spindle poles at the onset of mitosis, it is likely that chromosomal missegregation will occur, resulting in aneuploidy. Indeed, the frequency of abnormal mitoses is quite variable among these tumors, indicating that most cells with extra centrioles are capable of forming bipolar spindles.
Other tumors (9 of 31 studied, 7 of which were available for proliferation and mitotic index determination) accumulate excess pericentriolar material with their centrosomes and variable numbers of extra centrioles (Category IV tumors). The nature of the pericentriolar material is reminiscent of fibrogranular material and generative complexes associated with acentriolar as well as centriolar basal body formation. 34-37,41 However, no cilia are observed and the randomly positioned centrioles are not located near the plasma membrane. This accumulation of excess pericentriolar material may be the result of overexpression of centrosomal proteins or the reorganization of material that is normally dispersed within the cytoplasm. 14,42,43 Increased levels of γ-tubulin, 14,17 pericentrin, 15 and centrin 14 have been demonstrated in abnormal centrosomes in human tumors, and it is likely that other centrosomal proteins are present in increased levels as well. γ-tubulin-containing complexes located in the pericentriolar material are the site of microtubule nucleation, and as such are key to centrosome function. 44 We have shown that tumors with excess pericentriolar material are highly anaplastic and have lost cell polarity. These Category IV tumors tend to have higher median frequency of abnormal mitoses (0.46%) compared to tumors with other centrosome abnormalities (0.09%). This higher frequency of abnormal mitoses in tumors with extra pericentriolar material suggests that the regulation of accumulation of centrosomal proteins is more critical than regulation of centriole duplication for proper centrosome function during the cell cycle.
Some cells have more than two centrosomes that can function as spindle poles, yielding atypical multipolar mitoses. Atypical mitoses have been observed in breast tumors and other pathological specimens such as ulcerative colitis 7 and a mouse model of pancreatic cancer. 3 Multipolar mitoses were observed in several breast tumors in the present study. Aberrant mitoses such as these may arrest in metaphase, with the cells eventually undergoing apoptosis. In some instances, however, a selective advantage may be conferred to one of the daughter cells, leading to a clone of cells with chromosome gains and/or losses.
Serial sectioning through mitotic tumor cells showed that spindle poles are sometimes composed of more than one focus of microtubules. These spindle poles likely resulted from the coalescence of two or more centrosomes before metaphase. Coalescence of centrosomes could allow the formation of a bipolar spindle in a cell having extra centrosomes. Coalescence of extra centrosomes may be a mechanism by which cells can minimize the rate at which aneuploidy develops in tumors. Because compounded aneuploidy ultimately would be a self-limiting characteristic of tumors, a proportion of bipolar mitoses must be maintained for tumor growth.
The centrosomal abnormalities described here in breast tumor cells reflect changes in the status of cell and tissue differentiation of the tumors. Differentiated tumors have centrosomes of more normal appearance that are either mislocated, as in the tumors with inverted cell polarity, or perform a normal function not typical of mammary epithelial cells, such as producing ciliary basal bodies in tumors displaying apocrine metaplasia. Centrosome abnormalities are characteristic of poorly differentiated anaplastic tumors that have lost checkpoint synchronization of nuclear and centrosome cycles. This loss is reflected in centrosome defects and multipolar mitoses. As recognized by Boveri 2 earlier in this century, defective centrosomes may decrease the fidelity of chromosome segregation during multipolar mitoses. Consequently, centrosome abnormalities such as those described here may confer a mutator phenotype to tumor cells. As is the case for the molecular mutator phenotype, most mutated progeny will not be viable, but occasionally progeny with a selective advantage will emerge and thrive, and thus the tumor progresses to a more aggressive state.
Acknowledgments
We thank Ms. Denise Morgan and Ms. Belinda Hoebing of the Surgical Pathology Laboratory and the staff of the Electron Microscopy Core Facility for collecting and processing tissues used in this study and Ms. Linda Murphy for preparation of the Ki-67-immunostained slides. Dr. Vera Suman, Cancer Center Statistics, analyzed the proliferation and mitosis data.
Footnotes
Address reprint requests to Wilma L. Lingle, Tumor Biology Program, Division of Experimental Pathology, Mayo Foundation, 200 First St. S.W., Rochester, MN 55905. E-mail: lingle@mayo.edu.
Supported by grants from the National Cancer Institute (CA72836 and CA09441) and the Department of Defense (DAMD17-98-1-8122) and by the Mayo Clinic Foundation.
References
- 1.Hartwell LH, Weinert TA: Checkpoints: controls that ensure the order of cell cycle events. Science 1989, 246:629-634 [DOI] [PubMed] [Google Scholar]
- 2.Boveri T: Zur Frage der Emtstehung Maligner Tumoren. Fischer Verlag, Jena, 1914. English translation by Boveri M, The Origin of Malignant Tumors. Baltimore, Waverly Press, 1929
- 3.Levine DS, Sanchez CA, Rabinovitch PS, Reid BJ: Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer. Proc Natl Acad Sci USA 1991, 88:6427-6431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR: Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol 1995, 130:105-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ: A p53-dependent mouse spindle checkpoint. Science 1995, 267:1353-1356 [DOI] [PubMed] [Google Scholar]
- 6.Fukasawa K, Choi T, Kuriyama R, Rulong S, van de Woude GF: Abnormal centrosome amplification in the absence of p53. Science 1996, 271:1744-1747 [DOI] [PubMed] [Google Scholar]
- 7.Rubio CA, Befrits R: Atypical mitoses in colectomy specimens from patients with long standing ulcerative colitis. Anticancer Res 1997, 17:2721-2726 [PubMed] [Google Scholar]
- 8.Bystrevskaya VB, Lobova TV, Smirnov VN, Makarova NE, Kushch AA: Centrosome injury in cells infected with human cytomegalovirus. J Struct Biol 1997, 120:52-60 [DOI] [PubMed] [Google Scholar]
- 9.Pittman S, Geyp M, Fraser M, Ellem K, Peaston A, Ireland C: Multiple centrosomal microtubule organising centres and increased microtubule stability are early features of VP-16-induced apoptosis in CCRF-CEM cells. Leuk Res 1997, 21:491-499 [DOI] [PubMed] [Google Scholar]
- 10.Gualberto A, Aldape K, Kozakiewicz K, Tlsty TD: An oncogenic form of p53 confers a dominant, gain-of-function phenotype that disrupts spindle checkpoint control. Proc Natl Acad Sci USA 1998, 95:5166-5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH: Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J 1998, 17:159-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinchcliffe EH, Cassels GO, Rieder CL, Sluder G: The coordination of centrosome reproduction with nuclear events of the cell cycle in the sea urchin zygote. J Cell Biol 1998, 140:1417-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu YH, Xu FJ, Peng HQ, Fang XJ, Zhao SL, Li Y, Cuevas B, Kuo WL, Gray JW, Siciliano M, Mills GB, Bast RC: NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc Natl Acad Sci USA 1999, 96:214-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL: Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci USA 1998, 95:2950-2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, Doxsey SJ: Centrosome defects and genetic instability in malignant tumors. Cancer Res 1998, 58:3974-3985 [PubMed] [Google Scholar]
- 16.Weber RG, Bridger JM, Benner A, Weisenberger D, Ehemann V, Reifenberger , Lichter P: Centrosome amplification as a possible mechanism for numerical chromosome aberrations in cerebral primitive neuroectodermal tumors with TP53 mutations. Cytogenet Cell Genet 1998, 83:266–269 [DOI] [PubMed]
- 17.Carroll PE, Okuda M, Horn HF, Biddinger P, Stambrook PJ, Gleich LL, Li YQ, Tarapore P, Fukasawa K: Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene 1999, 18:1935-1944 [DOI] [PubMed] [Google Scholar]
- 18.Zhou HY, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR: Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet 1998, 20:189-193 [DOI] [PubMed] [Google Scholar]
- 19.Kellogg DR, Moritz M, Alberts BM: The centrosome and cellular organization. Annu Rev Biochem 1994, 63:639-674 [DOI] [PubMed] [Google Scholar]
- 20.Rose MD, Biggins S, Satterwhite LL: Unravelling the tangled web at the microtubule-organizing center. Curr Opin Cell Biol 1993, 5:105-115 [DOI] [PubMed] [Google Scholar]
- 21.Chretien D, Buendia B, Fuller SD, Karsenti E: Reconstruction of the centrosome cycle from cryoelectron micrographs. J Struct Biol 1997, 120:117-133 [DOI] [PubMed] [Google Scholar]
- 22.Vorobjev IA, Chentsov YS: Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol 1982, 93:938-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvey PL: An investigation of the centriole cycle using 3T3 and CHO cells. J Cell Sci 1985, 78:147-162 [DOI] [PubMed] [Google Scholar]
- 24.Robbins E, Jentzsch G, Micali A: The centriole cycle in synchronized HeLa cells. J Cell Biol 1968, 36:329-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compton DA: Focusing of spindle poles. J Cell Sci 1998, 111:1477-1481 [DOI] [PubMed] [Google Scholar]
- 26.Wheatley DN: Ultrastructure. I. The basic centriole. The Centriole: A Central Enigma of Biology. 1982:pp 21-49 Elsevier Biomedical Press, Amsterdam,
- 27.Reinsch S, Karsenti E: Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J Cell Biol 1994, 126:1509-1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson DJ: An ultrastructural study of mitosis and cytokinesis in normal ‘resting’ human breast. Cell Tissue Res 1988, 252:581-587 [DOI] [PubMed] [Google Scholar]
- 29.Olsson H, Jernstrom H, Alm P, Kreipe H, Ingvar C, Jonsson PE, Ryden S: Proliferation of the breast epithelium in relation to menstrual cycle phase, hormonal use, and reproductive factors. Breast Cancer Res Treat 1996, 40:187-196 [DOI] [PubMed] [Google Scholar]
- 30.Stirling JW, Chandler JA: Ultrastructural studies of the female breast. I. 9+0 cilia in myoepithelial cells. Anat Rec 1976, 186:413-416 [DOI] [PubMed] [Google Scholar]
- 31.Onishchenko GE: On the consistence between the number of centrioles and the ploidy in the hepatocytes in the mouse liver. Tsitologiia 1978, 20:395-399 [PubMed] [Google Scholar]
- 32.Nagata Y, Muro Y, Todokoro K: Thrombopoietin-induced polyploidization of bone marrow megakaryocytes is due to a unique regulatory mechanism in late mitosis. J Cell Biol 1997, 139:449-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reilova-Velez J, Seiler MW: Abnormal cilia in a breast carcinoma. An ultrastructural study. Arch Pathol Lab Med 1984, 108:795-797 [PubMed] [Google Scholar]
- 34.Sorokin SP: Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 1968, 3:207-230 [DOI] [PubMed] [Google Scholar]
- 35.Anderson RG, Brenner RM: The formation of basal bodies (centrioles) in the Rhesus monkey oviduct. J Cell Biol 1971, 50:10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirksen ER: Ciliary basal body morphogenesis: the early events. Symp Soc Exp Biol 1982, 35:439-463 [PubMed] [Google Scholar]
- 37.Dirksen ER: Centriole and basal body formation during ciliogenesis revisited. Biol Cell 1991, 72:31-38 [DOI] [PubMed] [Google Scholar]
- 38.van den Steen P, van Lommel A, Lauweryns JM: Presence and possible implications of open-ring centrioles, multiple basal centrioles and basal cilia in neonatal hamster bronchioles. Acta Anat 1995, 153:85-95 [DOI] [PubMed] [Google Scholar]
- 39.McGill M, Highfield DP, Monahan TM, Brinkley BR: Effects of nucleic acid specific dyes on centrioles of mammalian cells. J Ultrastruct Res 1976, 57:43-53 [DOI] [PubMed] [Google Scholar]
- 40.Tournier F, Komesli S, Paintrand M, Job D, Bornens M: The intercentriolar linkage is critical for the ability of heterologous centrosomes to induce parthenogenesis in Xenopus. J Cell Biol 1991, 113:1361-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemullois M, Klotz C, Sandoz D: Immunocytochemical localization of myosin during ciliogenesis of quail oviduct. Eur J Cell Biol 1987, 43:429-437 [PubMed] [Google Scholar]
- 42.Callaini G, Marchini D: Abnormal centrosomes in cold-treated Drosophila embryos. Exp Cell Res 1989, 184:367-374 [DOI] [PubMed] [Google Scholar]
- 43.Baron AT, Suman VJ, Nemeth E, Salisbury JL: The pericentriolar lattice of PtK2 cells exhibits temperature and calcium-modulated behavior. J Cell Sci 1994, 107:2993-3003 [DOI] [PubMed] [Google Scholar]
- 44.Moritz M, Zheng YX, Alberts BM, Oegema K: Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J Cell Biol 1998, 142:775-786 [DOI] [PMC free article] [PubMed] [Google Scholar]