Abstract
Alteration of psoriasin (S100A7) expression has previously been identified in association with the transition from preinvasive to invasive breast cancer. In this study we have examined persistence of psoriasin mRNA and protein expression in relation to prognostic factors in a cohort of 57 invasive breast tumors, comprising 34 invasive ductal carcinomas and 23 other invasive tumor types (lobular, mucinous, medullary, tubular). We first developed an IgY polyclonal chicken antibody and confirmed specificity for psoriasin by Western blot in transfected cells and tumors. The protein was localized by immunohistochemistry predominantly to epithelial cells, with both nuclear and cytoplasmic staining, as well as occasional stromal cells in psoriatic skin and breast tumors; however, in situ hybridization showed that psoriasin mRNA expression was restricted to epithelial cells. In breast tumors, higher levels of psoriasin measured by reverse transcriptase-polymerase chain reaction and Western blot (93% concordance) were significantly associated with estrogen and progesterone receptor-negative status (P < 0.0001, P = 0.0003), and with nodal metastasis in invasive ductal tumors (P = 0.035), but not with tumor type or grade. Psoriasin expression also correlated with inflammatory infiltrates (all tumors excluding medullary, P = 0.0022). These results suggest that psoriasin may be a marker of aggressive behavior in invasive tumors and are consistent with a function as a chemotactic factor.
Earlier diagnosis of breast cancer has increased the need for the identification of molecular alterations that might serve as tissue markers to predict the risk of progression to metastatic disease. Among the most important of these alterations are likely to be those associated with the development of the invasive phenotype and the transition from preinvasive to invasive cancer with the capability for subsequent metastasis.
We have recently identified psoriasin (S100A7) as a gene that is frequently overexpressed in preinvasive ductal carcinoma in situ (DCIS) relative to adjacent invasive carcinoma, suggesting a role in breast tumor progression. 1 Other members of the S100 gene family of calcium-binding proteins have been implicated in a range of biological processes, including tumor metastasis. 2 In particular, S100A2 has been shown to be down-regulated in breast tumor cells relative to their normal epithelial cell counterparts, 3 whereas up-regulation of S100A4 has been strongly implicated in breast tumor metastasis. 4-6 In the latter case this may reflect the ability of S100A4 to influence cell motility, 7 the cytoskeleton 6,8,9 or cell adhesion molecules. 10 Psoriasin was initially identified as a highly abundant protein belonging to the S100 gene family, 11 expressed by abnormally proliferating keratinocytes in psoriatic epidermis. 12,13 It has subsequently been shown to be a secreted protein that can exert an effect as a chemotactic factor for inflammatory cells. 14,15 However, the function of psoriasin in breast cancer remains to be determined. 16 In this study we have developed a psoriasin-specific antibody and evaluated the persistence of psoriasin expression in invasive breast tumors with different invasive and metastatic potential as well as host inflammatory response.
Materials and Methods
Human Breast Tissues and Cell Lines
All breast tumor cases used for this study were selected from the NCIC–Manitoba Breast Tumor Bank (Winnipeg, Manitoba, Canada). As has previously been described, 17 tissues accrue to the Bank from cases at multiple centers within Manitoba and are rapidly collected and processed to create matched formalin-fixed embedded and frozen tissue blocks for each case, with mirror-image surfaces oriented by colored inks. The histology of every sample in the Bank is uniformly interpreted in hematoxylin/eosin (H&E)-stained sections from the face of the paraffin tissue block by a pathologist. This information is available in a computerized database along with relevant pathological and clinical information and was used as a guide for the selection of specific paraffin and frozen blocks from cases for this study. For each case interpretations included an estimate of the cellular composition (including the percentage of invasive epithelial tumor cells, collagenous stroma, and fatty stroma), tumor type, and tumor grade for ductal tumors (Nottingham score). 18,19 The inflammatory host response was scored semiquantitatively on a scale of 1 (low) to 5 (high). Steroid receptor status was determined for all cases by ligand binding assay performed on an adjacent portion of tumor tissue. Tumors with estrogen and progesterone receptor levels above 20 fmol/mg and 15 fmol/mg of total protein, respectively, were considered ER- or PR-positive.
Two cohorts of tumors were selected. The first cohort comprised 35 invasive ductal carcinomas selected to include six subgroups differing with respect to estrogen receptor status (ER-positive and ER-negative) and tumor grade (low, intermediate, high). Additional selection criteria also included high tissue quality, presence of invasive tumor within >35% of the cross section of the frozen block for invasive ductal cases, and minimal (<5%) normal or in situ epithelial components. The second cohort comprised 23 invasive tumors selected to include four subgroups of different tumor types 18 that vary in differentiation and metastatic potential, including invasive lobular (six), medullary (five), tubular (six), and colloid (six). Similar secondary criteria were also used for this cohort.
For analysis of antibody specificity and for positive controls for tumor assays, MCF7 human breast cancer cells obtained from the American Type Culture Collection (Manassas, VA) were used. MCF7 cells were grown as previously described under normal conditions in the presence of 5% fetal bovine serum, to provide a negative control. 20 Alternatively MCF7 cells were subjected to estrogen-deprived conditions in the presence of charcoal-stripped serum before stimulation by estradiol (10−8 mol/L) for 48 hours before harvesting to induce psoriasin expression and provide a positive control. As an additional positive control MDA-MB-231 human breast cancer cells were transfected with a plasmid containing the cytomegalovirus (CMV) promotor adjacent to the psoriasin cDNA (Hiller-Hitchcock T, Leygue E, Cummins-Leygue C, Murphy LC, Watson PH, manuscript in preparation), and stable transfectants (CL7FD3 cell clone) expressing psoriasin mRNA were also used.
Antibody Reagents
A psoriasin-specific chicken IgY polyclonal antibody was generated by immunization of chickens with a 14-amino acid peptide corresponding to the carboxy terminus of psoriasin (KQSHGAAPCSGGSQ; Bionostics, Toronto, and Aves Labs). A >90% pure IgY fraction from chicken egg yolk was obtained in phosphate-buffered saline (PBS) and then further purified by passing it over a psoriasin peptide affinity column made by binding the synthetic peptide to N-hydroxy-succinimide-activated Sepharose 4B (Pharmacia Biotech), according to the manufacturer’s instructions. The bound IgY was then eluted with 5.0 mol/L sodium thiocyanate, followed by dialysis against PBS. Additional antibodies used included a commercial anti-S100 antibody (Sigma, St. Louis, MO) as well as a rabbit polyclonal antibody, raised against the recombinant protein (kindly provided by Prof J. Celis, University of Aarhus, Aarhus, Denmark).
Western Blot Analysis
For tumors, multiple sections (10–20 × 20 μm) were cut from the face of frozen tissue blocks immediately adjacent to the face of the matching paraffin block. 17 For cell lines, trypsinized cell pellets were obtained from breast cancer cell lines (grown to ∼80% confluence). Total protein lysates were extracted from both the cell line pellets and frozen tissue sections, using Tri-reagent (Sigma), as described by the manufacturer. The recovered protein was dissolved in SDS isolation buffer (50 mmol/L Tris, pH 6.8, 20 mmol/L EDTA, 5% sodium dodecyl sulfate (SDS), 5 mmol/L β-glycerophosphate) and a cocktail of protease inhibitors (Boehringer Mannheim, Laval, PQ). Protein concentrations were determined using the Micro-BCA protein assay kit (Pierce, Rockford, IL). Sixty micrograms of total protein lysates were run on a 16.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) mini gel, using Tricine SDS-PAGE to separate the proteins, 21 and then transferred to 0.2-μm Nitrocellulose (Bio-Rad, Mississauga, ON). After blocking in 10% skimmed milk powder in Tris-buffered saline-0.05% Tween (TBST buffer), blots were incubated with chicken IgY anti-psoriasin antibody (∼15 μg/ml in TBST), followed by incubation with secondary antibody, rabbit IgG anti-chicken IgY conjugated to horseradish peroxidase (1:5000 dilution in TBST; Jackson ImmunoResearch Laboratories), and visualization by incubation with Supersignal (Pierce), per the manufacturer’s instructions. Exposed x-ray films were photographed, and the band intensities were determined by video image analysis, using MCID M4 software (Imaging Research, ST. Catherine’s, ON). All signals were adjusted with reference to the psoriasin-transfected MDA-MB-231 cell control (CL7FD3), run on each blot.
Immunohistochemistry
Immunohistochemistry was performed on 5-μm paraffin-embedded breast tumor tissue sections from tissue blocks fixed in 10% neutral buffered formalin for 18–24 hours. After deparaffinizing, clearing, and hydrating to PBS buffer (pH 7.4) containing 0.05% Tween 20 (Mallinckrodt), the sections were pretreated with hydrogen peroxide (3%) for 10 minutes to remove endogenous peroxidases, and nonspecific binding was blocked with normal rabbit serum (1:50; Sigma). Primary chicken IgY anti-psoriasin antibody (1:500 dilution in PBS) was applied for 1 hour at 37°C followed by washing and incubation with the secondary antibody, peroxidase-conjugated affinity purified rabbit anti-chicken (1:200 dilution), for 1 hour at room temperature. Detection was performed with 3,3′-diaminobenzidine tetrahydrochloride peroxidase substrate (Sigma) and counterstaining with methyl green (2%), followed by dehydration, clearing, and mounting. A positive tissue control and a negative reagent control (normal rabbit serum only/no primary antibody) were run in parallel in all experiments. Immunostaining was scored semiquantitatively by assessing the average signal intensity (on a scale of 0 to 3) and the proportion of tumor cells showing a positive nuclear signal (0, none; 0.1, less than one-tenth; 0.5, less than one-half; 1.0 greater than one-half). The intensity and proportion scores were then multiplied to give an overall score, and tumors with a score equal to or higher than 1.0 were deemed positive.
In Situ Hybridization
In situ hybridization was performed on paraffin sections (5 μm) according to a previously described protocol. 1 Linearized psoriasin plasmid cDNA (1.0 μg/μl) was used to generate UTPS35-labeled sense and antisense RNA probes with the Riboprobe System (Promega, Madison, WI) according to the manufacturer’s instructions. Sense and antisense probes were equalized by diluting 1 × 10 6 cpm/μl in hybridization solution. These were then applied to paraffin sections (approximately 30 μl of probe per section) that had undergone postfixation with 4% paraformaldehyde (pH 7.4) in PBS and further pretreatments with triethanolamine/acetic anhydride and proteinase K before hybridization. Sections were then coverslipped, sealed, and incubated overnight in a humid chamber at 42°C. After coverslip removal, sections underwent incubation in posthybridization solution and buffered RNase A (20 μg/ul), followed by several washes in descending dilutions of standard saline citrate buffer to remove weakly bound nonspecific label. After dehydration in ethanol containing 300 mmol/L ammonium acetate, the sections were coated in NTB-2 Kodak emulsion, subsequently developed after various time intervals from 2 to 5 weeks, and counterstained with Lee’s methylene blue and basic fuchsin. Psoriasin expression was assessed by bright-field microscopic examination at low power (10× objective) magnification with reference to the negative sense and positive control tumor sections run with each batch. Levels were scored semiquantitatively as previously described 22 by assessing the average signal intensity (on a scale of 0 to 3) and the proportion of tumor cells showing a positive signal (0, none; 0.1, less than one-tenth; 0.5, less than one-half; 1.0 greater than one-half). The intensity and proportion scores were then multiplied to give an overall score, and tumors with a score equal to or higher than 1.0 were deemed positive.
Reverse Transcriptase-Polymerase Chain Reaction Analysis
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed based on extracted RNA (600 ng) that was reverse transcribed in a total volume of 20 μl as described previously. 1 Briefly, reverse transcription was completed with the following reaction mixture: for each sample, 200 ng (2 μl of 0.1 μg/μl) of total RNA was added to 16 μl of RT mix (4 μl of 5× RT buffer; 1 μl of each of dATP, dCTP, dGTP, and dTTP, all at 2.5 mmol/L; 2 μl of 0.1% bovine serum albumin; 2 μl of 0.1 mol/L dithiothreitol; 1 μl of 0.25 mol/L random hexamer primer; 2 μl of dimethyl sulfoxide (DMSO), and 1 μl of 200 units/μl of Moloney murine leukemia virus reverse transcriptase) and incubated at 37°C for 1.5 hours. Each PCR was performed in 50-μl volume, using 1 μl of the completed RT reaction (cDNA); 30.8 μl of sterile water; 5 μl of 10× PCR buffer; 5 μl of 25 mmol/L MgCl2; 200 mmol/L each of dATP, dCTP, dGTP, and dTTP; 1 μl of DMSO; 1 unit of Taq DNA polymerase; and 0.5 μl of 50 mmol/L PCR primers. The psoriasin primers were sense (5′-AAG AAA GAT GAG CAA CAC-3′) and antisense (5′-CCA GCA AGG ACA GAA ACT-3′) corresponding to the cDNA sequence, 13 or alternatively, PCR was performed with GAPDH primers, sense (5′-ACC CAC TCC TCC ACC TTT G-3′) and antisense (5′-CTC TTG TGC TCT TGC TGG G-3′). 23 For PCR amplification the reaction comprised an initial step of 5 minutes at 94°C, and then 45 cycles (30 seconds at 94°C, 30 seconds at 56°C, 30 seconds at 72°C) for psoriasin or 35 cycles (45 seconds at 93°C, 45 seconds at 58°C, 30 seconds at 72°C) for GAPDH. PCR products of the two genes amplified from the same RT reaction were loaded into the same wells onto a 1.5% agarose gel before electrophoresis and ethidium bromide staining to visualize psoriasin (246 bp) and GAPDH (198 bp) cDNAs under UV illumination.
Preliminary experiments were performed with cell line and tumor RNA samples to establish the appropriate RNA input and PCR cycle number conditions to achieve amplification with both psoriasin and GAPDH primers in the linear range in a typical sample. Tumors from each cohort were processed as a batch, from frozen sectioning to RNA extraction, reverse transcription in triplicate, and then duplicate PCRs from each RT reaction. For each batch controls included RT-negative and RNA-negative controls and both psoriasin-positive (estradiol-stimulated MCF7) and psoriasin-negative (untransfected, wild-type MDA-MB-231 cells) RNA controls. All primary tumor PCR signals were assessed in gels and autoradiographs by video image capture and with a MCID-M4 image analysis program. Psoriasin expression was standardized to GAPDH expression assessed from the same RT reaction in separate PCR reactions and run in parallel on the same gel, and the mean of each duplicate PCR was then expressed relative to the levels in the MCF7 cell line standard. The invasive tumor component within each section was also assessed in the adjacent mirror image paraffin section, and the percentage area occupied by tumor was used to correct for differences in epithelial cell content of the tumor sections used for RNA extraction.
Statistical Analysis
For analysis of associations, standardized psoriasin mRNA levels were used either as a continuous variable or transformed into low- or high-expression categories, using a level of one relative density unit. This cutpoint was selected to correspond to the lowest level at which protein could be detected by Western blot. Correlations with estrogen (ER) and progesterone (PR) receptor levels and inflammation were tested using Spearman’s test. Associations with categorical variables were tested by either Mann-Whitney or analysis of variance tests for selected dependent variables, or unpaired t-test for independent variables, or a χ 2 test.
Results
Characterization of Psoriasin-Specific Antibody
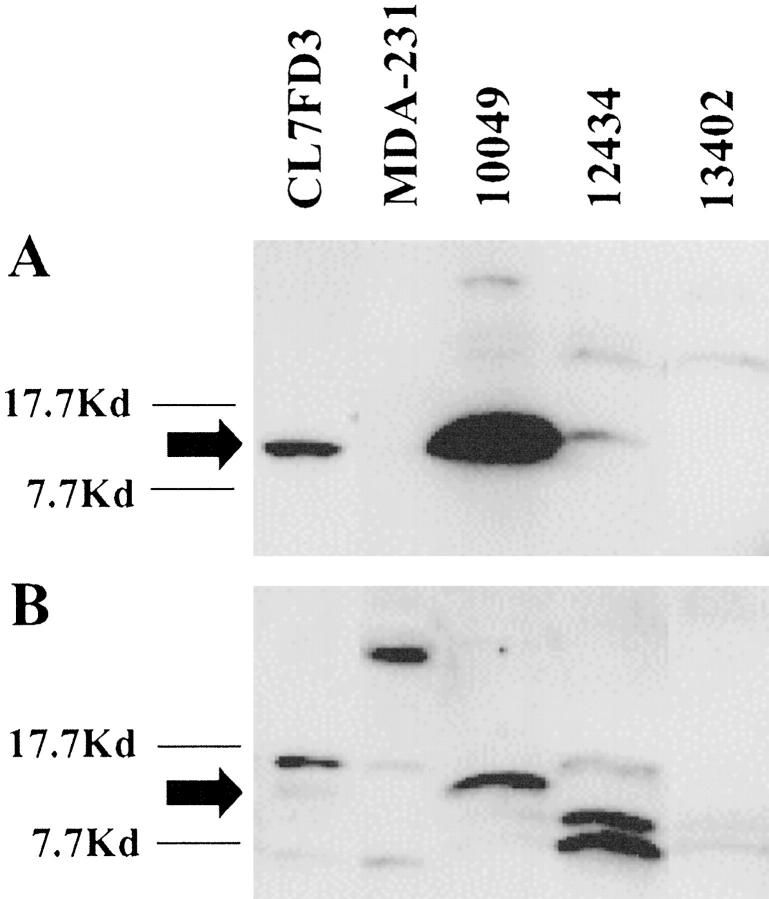
Multiple S100 proteins are expressed in individual tissues and cells. To specifically distinguish psoriasin expression within archival formalin-fixed and paraffin-embedded tissues we raised a polyclonal antibody in chicken against a synthetic peptide that corresponded to the COOH terminus of psoriasin. This 14-amino acid region was selected on the basis of very low homology to other S100 proteins. Western blot analysis of an MDA-MB-231 breast cell line transfected with a plasmid incorporating psoriasin cDNA under the control of a CMV promotor (and known to express psoriasin mRNA by Northern blot; unpublished data) and breast tumors showed a single band corresponding to a protein of approx 11.7 kd with the chicken IgY antibody (Figure 1A) ▶ . This signal could be completely inhibited by preincubation of the primary antibody with psoriasin synthetic peptide (data not shown) and was absent from the wild-type and vector-alone transfected MDA-MB-231 control cells. By comparison, a commercial anti-S100 antibody (Sigma), known to detect several S100 proteins in MDA-MB-231 cells, 24 weakly recognized the same 11.7-kd protein in transfected cells as well as several other S100 proteins in most samples (Figure 1B) ▶ . Both antibodies reacted with additional higher molecular mass bands in tumor samples. However, specificity of the 11.7-kd psoriasin signal was further confirmed by Western blot using another anti-psoriasin polyclonal rabbit antibody previously raised against a recombinant psoriasin protein (data not shown).
Figure 1.
Western blot analysis of cell lines and tumors to demonstrate anti-psoriasin IgY antibody specificity. A: A protein band (approx 11.7 kd) detected using a chicken IgY anti-psoriasin antibody in a psoriasin-transfected MDA-MB-231 breast cell line and two tumors (10049, 12434), but absent in tumor 13402 and wild-type MDA-MB-231 cells. B: Detection of several S100-like proteins, using a commercial polyclonal S100 antibody applied to the same samples, in addition to weak detection of the same (approx 11.7 kd) protein band seen in A.
Localization of Cellular Expression of Psoriasin
To assess cellular localization of psoriasin we studied paraffin-embedded tissue blocks from breast, skin, and larynx by immunohistochemistry. The breast tumors studied possessed either high (six cases) or low (seven cases) levels of psoriasin mRNA and total protein expression (determined by Western blot and RT-PCR analysis of protein and RNA extracted from sections cut from the adjacent mirror-image frozen tissue blocks). Skin biopsies from the margins of two psoriatic lesions and a squamous carcinoma of larynx were also studied, as psoriasin was originally identified as a highly expressed protein in psoriatic skin and has also been identified as an expressed sequence tag in a cDNA library from laryngeal squamous carcinoma (http://www.ncbi.nlm.nih.gov/UniGene/Hs.112408). All cases were subjected to both immunohistochemistry and in situ hybridization on adjacent paraffin sections, and both signals were assessed independently, using a semiquantitative scoring system as described in Materials and Methods.
In breast tumors psoriasin protein was detected predominantly within epithelial tumor cells and was localized within both tumor cell nuclei as well as cytoplasm. Psoriasin was also present within some stromal cells and in some cases also on the luminal aspects of endothelial cells within small vessels (Figure 2) ▶ . However, in situ hybridization demonstrated that mRNA expression was limited to epithelial tumor cells in all cases (Figure 2) ▶ . The nuclear immunohistochemical staining was completely abolished by competition with the immunizing peptide and was not present in tumors that were negative for psoriasin but showed additional immunoreactive bands on Western blot (eg, see case 13402, Figure 1 ▶ , and case 8840, Figure 4 ▶ ). Immunohistochemistry and Western blot were concordant in 12/13 cases. In one case Western blot analysis was negative and weak focal staining was seen by immunohistochemistry. Specificity of the nuclear signal was further confirmed by the fact that the presence of immunohistochemically detected protein expression, assessed on the basis of nuclear staining, was highly concordant (92%) with expression detected by in situ hybridization mRNA.
Figure 2.
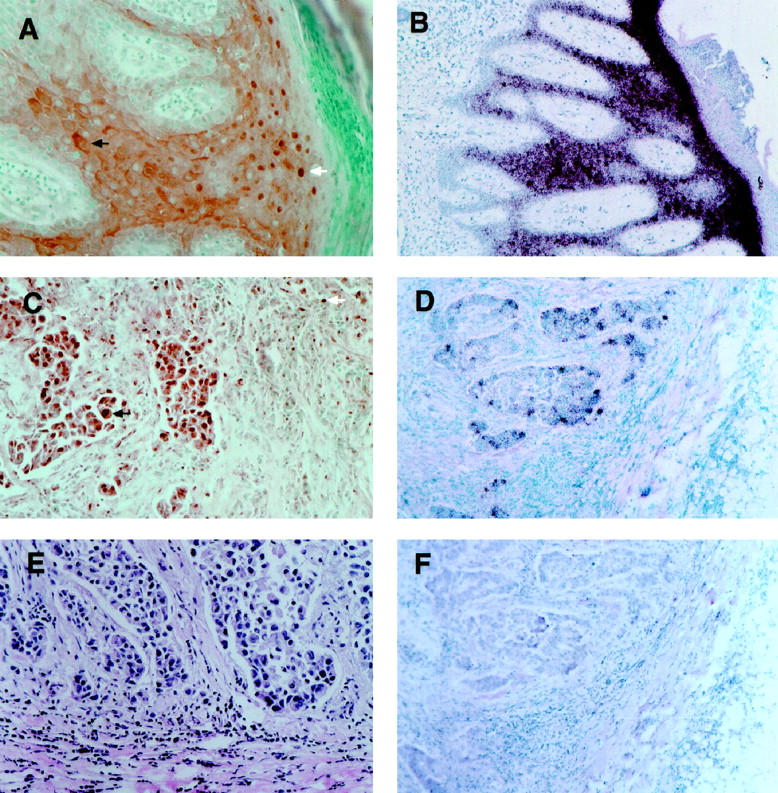
Immunohistochemical and in situ hybridization analysis of the cellular distribution and patterns of expression of psoriasin within psoriatic skin and breast carcinoma. Psoriasin protein is localized in hyperplastic epidermis of skin to both nuclei (A, white arrow) and cytoplasm (A, black arrow) of keratinocytes. Similar nuclear and cytoplasmic staining is seen in breast epithelial tumor cells (C, black arrow; case 8965). Psoriasin protein is also detected within occasional stromal inflammatory cells (C, white arrow). E: H&E-stained section from the same region of the tumor shown in C. Psoriasin mRNA expression in skin is restricted to epithelial cells in suprabasal layers of epidermis (B) and scattered invasive epithelial tumor cells in breast tumors (D), detected using antisense probe (B and D) compared to sense probe (F). Original magnification for all panels at the microscope, ×200.
Figure 4.
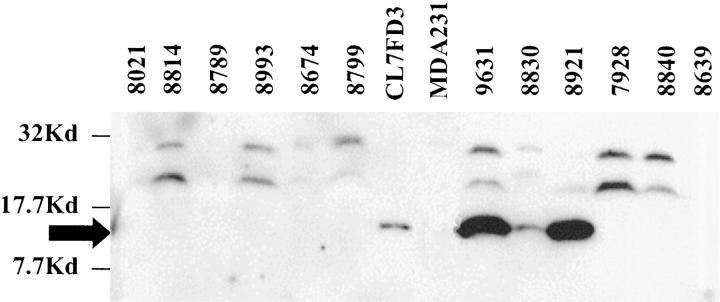
Western blot analysis of psoriasin protein expression in invasive breast tumors. Psoriasin (black arrow) is detected in 3/12 representative tumors and within the positive control (CL7FD3).
In skin, immunohistochemical staining was localized to keratinocytes within the mid to upper zones of the epidermis of skin showing psoriasiform hyperplasia. These keratinocytes corresponded to the cells that also showed mRNA expression by in situ hybridization in adjacent sections (Figure 2) ▶ . The adjoining normal skin was negative. Occasional positive immunohistochemical staining, but no mRNA signal, was also observed in stromal cells in the dermis underlying the psoriatic lesion. As seen in breast tumor cells, psoriasin protein was localized both to the nucleus and cytoplasm within keratinocytes (Figure 2) ▶ . The same nuclear and cytoplasmic localization was also detected in a squamous laryngeal carcinoma (data not shown). However, the polyclonal rabbit anti-psoriasin antibody previously shown to provide immunofluorescent staining in frozen skin sections 13,25 did not detect any signal on paraffin sections from skin or breast. Additional experiments were performed with the chicken IgY anti-psoriasin antibody on skin and breast tumor sections in which immunohistochemical conditions (microwave versus protease antigen retrieval) and tissue treatment/fixation conditions (formalin versus alcohol versus paraformaldehyde versus frozen) were varied, and nuclear localization persisted under all conditions (data not shown).
Expression of Psoriasin mRNA in Invasive Breast Tumors
The changes in psoriasin expression previously observed in association with the transition from in situ to invasive carcinoma suggested a functional role in the early stages of progression. However, alteration of psoriasin expression in normal skin has also been associated with abnormal keratinocyte differentiation. To examine further the relationship of psoriasin with differentiation and invasiveness, we used RT-PCR and Western blot to examine psoriasin mRNA and protein levels in a cohort of invasive tumors. These tumors included several different tumor types and a range of differentiation, as determined by tumor grade and estrogen receptor status (Table 1) ▶ .
Table 1.
Clinicopathological Parameters, Histological Composition of the Tumor Section, and Psoriasin Expression in 57 Invasive Breast Carcinomas Assessed by RT-PCR and Western Blot
| TB# | Clinicopathological parameters | Psoriasin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | ER | PR | GrSc | Size | NS | Inf | RT-PCR | RT-PCR/Inv% | WB | |
| 11549 | muc | 194 | 133 | 3 | − | 2 | 0.06 | 0.15 | − | |
| 10515 | muc | 341 | 176 | 3 | − | 1 | 0.08 | 0.14 | − | |
| 9948 | muc | 46 | 22 | 6.5 | − | 1 | 0.10 | 0.16 | − | |
| 10582 | muc | 109 | 62 | 2.3 | na | 1 | 0.14 | 0.34 | − | |
| 8832 | muc | 295 | 177 | 4 | − | 2 | 1.94 | 2.77 | − | |
| 8021 | muc | 331 | 328 | 2.3 | − | 2 | 0.11 | 0.15 | − | |
| 11387 | tub | 105 | 35 | 3.5 | na | 2 | 0.09 | 0.29 | − | |
| 9483 | tub | 56 | 0 | 1.2 | − | 2 | 0.09 | 0.91 | − | |
| 11651 | tub | 67 | 24 | 2.2 | − | 3 | 0.23 | 0.77 | − | |
| 8814 | tub | 232 | 103 | 2 | − | 2 | 0.44 | 1.45 | − | |
| 8720 | tub | 29 | 73 | 2 | − | 1 | 0.52 | 5.21 | − | |
| 12072 | tub | 8.3 | 5 | 2.3 | + | 3 | 0.67 | 1.34 | − | |
| 13041 | med | 3.4 | 9 | 2 | − | 5 | 0.40 | 0.49 | − | |
| 13153 | med | 4.9 | 2.4 | 3 | na | 5 | 0.61 | 0.76 | − | |
| 11867 | med | 1.4 | 9 | 1.6 | + | 5 | 1.60 | 2.67 | + | |
| 13058 | med | 4.6 | 12 | 2.8 | − | 5 | 1.63 | 2.04 | − | |
| 12434 | med | 1 | 1.3 | 1.2 | − | 5 | 1.63 | 3.27 | + | |
| 8639 | ilc | 52 | 83 | na | − | 1 | 0.20 | 0.67 | − | |
| 8799 | ilc | 111 | 139 | 6 | + | 2 | 0.31 | 3.15 | − | |
| 8993 | ilc | 142 | 528 | 8 | + | 1 | 0.52 | 0.86 | − | |
| 9801 | ilc | 2.1 | 9.8 | na | − | 3 | 0.56 | 1.60 | − | |
| 8921 | ilc | 2.3 | 8.9 | 8 | − | 2 | 2.07 | 3.77 | + | |
| 8961 | ilc | 0.7 | 3.4 | 2.5 | − | 3 | 2.34 | 5.84 | + | |
| 9000 | idc | 392 | 596 | 7 | 2.5 | − | 1 | 0.07 | 0.09 | − |
| 13402 | idc | 49 | 35 | 4 | 2.8 | − | 2 | 0.07 | 0.17 | − |
| 11971 | idc | 97 | 25 | 4 | 1.5 | − | 2 | 0.13 | 0.42 | − |
| 8684 | idc | 74 | 43 | 7 | 5 | + | 1 | 0.14 | 0.35 | − |
| 12853 | idc | 17.3 | 83 | 9 | 4.8 | + | 4 | 0.15 | 0.22 | − |
| 8840 | idc | 74 | 68 | 7 | 1.8 | + | 3 | 0.17 | 0.37 | − |
| 8834 | idc | 10 | 147 | 5 | 2 | − | 2 | 0.17 | 0.34 | − |
| 8674 | idc | 16.7 | 4.5 | 9 | na | − | 2 | 0.19 | 0.35 | − |
| 12037 | idc | 225 | 144 | 4 | 3.5 | + | 2 | 0.20 | 0.40 | − |
| 12868 | idc | 93 | 141 | 9 | 3.5 | na | 1 | 0.21 | 0.28 | − |
| 8599 | idc | 58 | 81 | 4 | 3.5 | − | 1 | 0.24 | 0.79 | − |
| 10105 | idc | 0.9 | 3.8 | 9 | 3 | + | 4 | 0.24 | 0.40 | − |
| 7928 | idc | 33 | 72 | 5 | 3 | + | 2 | 0.27 | 0.67 | − |
| 13414 | idc | 15.5 | 59 | 5 | 4.1 | − | 2 | 0.28 | 0.56 | − |
| 11343 | idc | 78 | 44 | 4 | na | − | 3 | 0.29 | 0.73 | − |
| 10644 | idc | 130 | 4.7 | 9 | 3.2 | + | 2 | 0.32 | 0.81 | − |
| 10137 | idc | 42 | 26 | 7 | 1.8 | − | 1 | 0.44 | 0.89 | − |
| 10064 | idc | 0.8 | 4.6 | 9 | 2.5 | na | 2 | 0.53 | 0.88 | − |
| 11769 | idc | 1.1 | 3.5 | 7 | na | − | 3 | 0.56 | 0.80 | − |
| 8932 | idc | 114 | 27 | 4 | 2 | − | 1 | 0.56 | 1.13 | − |
| 10906 | idc | 46 | 6.6 | 9 | 4.5 | na | 5 | 0.58 | 0.64 | − |
| 8789 | idc | 0.8 | 0.4 | 7 | na | na | 3 | 0.66 | 1.64 | − |
| 10150 | idc | 70 | 42 | 7 | na | − | 1 | 0.67 | 1.68 | − |
| 11459 | idc | 3.6 | 98 | 5 | 4.6 | + | 3 | 0.67 | 0.96 | − |
| 13191 | idc | 17.2 | 9.2 | 9 | 3.2 | − | 2 | 0.69 | 0.87 | − |
| 10124 | idc | 1.9 | 12.9 | 9 | 3 | − | 4 | 1.00 | 1.42 | − |
| 8830 | idc | 0.7 | 8 | 9 | 6 | + | 4 | 1.06 | 1.32 | + |
| 8790 | idc | 6 | 50 | 5 | 1.5 | + | 2 | 1.07 | 3.58 | − |
| 11118 | idc | 6.6 | 11.8 | 5 | 8.5 | + | 2 | 1.10 | 2.20 | − |
| 12715 | idc | 1.5 | 16 | 7 | 3 | na | 3 | 1.24 | 2.06 | + |
| 9631 | idc | 0.7 | 4.5 | 9 | na | + | 4 | 1.32 | 3.10 | + |
| 8965 | idc | 0.4 | 9.9 | 7 | na | + | 4 | 1.85 | 2.64 | + |
| 10049 | idc | 0.8 | 14 | 9 | 3.7 | + | 4 | 2.01 | 5.04 | + |
| 8704 | idc | 0.7 | 3.5 | 7 | 3.5 | + | 2 | 2.60 | 6.50 | + |
TB, tumor bank case number; type, mucinous (muc), tubular (tub), medullary (med), lobular (ilc), ductal (idc); ER, PR, estrogen/progesterone receptor levels (fmol/mg protein); GrSc, Nottingham grade score; Size, tumor size (cms); NS, nodal status, positive (+), negative (−), not available (na); Inf, estimate of inflammatory infiltrate, low (1) to high (5). RT-PCR, psoriasin mRNA level determined by RT-PCR; RT-PCR/Inv%, psoriasin mRNA level determined by RT-PCR and adjusted for the percentage tumor cell content of the tissue section (as described in Materials and Methods); WB, psoriasin protein level determined by Western blot.
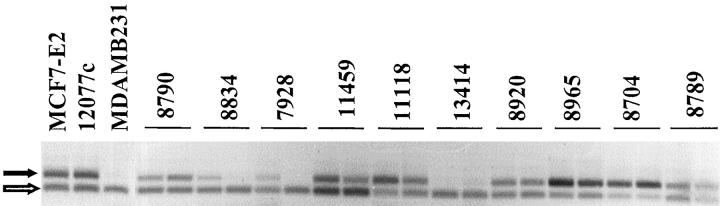
Psoriasin mRNA was detected in all tumors by RT-PCR, but the levels varied considerably and were mostly low (Figure 3) ▶ . Within the invasive ductal subgroup there was no significant difference in psoriasin expression with tumor grade. There was also no significant difference between tumor size or type, although there was a trend toward lower levels of expression in both well-differentiated tumor types, tubular and mucinous carcinomas, whereas lobular and medullary carcinomas showed a trend toward higher expression than invasive ductal tumors. However, higher levels of psoriasin mRNA expression showed a significant inverse correlation with both ER and PR levels (r = −0.66, P = 0.0001; r = −0.47, P = 0.0003, Spearman) and with ER and PR negative status (ER-ve vs. ER+ve; n = 28 vs. 29, mean (SD) 1.032 (0.7) vs. 0.32 (0.36), P < 0.0001 Mann-Whitney; PR-ve vs. PR+ve, n = 25 vs. 32, 1.05 (0.72) vs. 0.37 (0.40), P < 0.0001) in all tumors and within the invasive ductal subgroup. Psoriasin expression was also higher in axillary node-positive cases in all tumors (mean (SD) = 0.86 (0.73) vs. 0.59 (0.66), and the difference was statistically significant for the invasive ductal subgroup (mean (SD) = 0.88 (0.79) vs. 0.38 (0.28), P = 0.035, t-test). These relationships with ER, PR, and nodal status (Table 2) ▶ were also evident and remained statistically significant after correction of psoriasin levels for the relative tumor cell content, assessed as a percentage within the paraffin sections adjacent to the frozen tissue sections studied.
Figure 3.
RT-PCR analysis of psoriasin mRNA expression in invasive breast tumors. Psoriasin (upper black arrow) and GAPDH (lower open arrow) from duplicate PCRs of 10 representative tumors. Control lanes include estradiol-treated MCF7-E2 cells, a tumor control 12077c, and wild-type MDA-MB-231 cells.
Table 2.
Relationship between Psoriasin Expression and Prognostic and Tissue Factors
| All | IDC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Low Ps | High Ps | n | Low Ps | High Ps | ||||
| ER | − | 28 | 14 | 4 | P = 0.0001 | 19 | 10 | 9 | P = 0.0019 |
| + | 29 | 28 | 1 | 15 | 15 | 0 | |||
| PR | − | 25 | 13 | 12 | P = 0.001 | 15 | 8 | 7 | P = 0.018 |
| + | 32 | 29 | 3 | 19 | 17 | 2 | |||
| NS | − | 30 | 24 | 6 | ns (P = 0.095) | 14 | 13 | 1 | P = 0.0002 |
| + | 19 | 11 | 8 | 15 | 8 | 7 | |||
| INFL | Low | 34 | 29 | 5 | P = 0.049 | 20 | 17 | 3 | ns (P = 0.07) |
| High | 18 | 11 | 7 | 14 | 8 | 6 | |||
| Size | <2 | 12 | 9 | 3 | 6 | 5 | 1 | ||
| 2–5 | 29 | 22 | 7 | ns | 18 | 14 | 4 | ns | |
| ≥5 | 7 | 4 | 3 | 3 | 1 | 2 | |||
| Grade | Low | 12 | 10 | 2 | |||||
| Mod | 10 | 7 | 3 | ns | |||||
| High | 12 | 8 | 4 | ||||||
| Type | idc | 34 | 25 | 9 | |||||
| ilc | 6 | 4 | 2 | ||||||
| med | 5 | 2 | 3 | ns | |||||
| muc | 6 | 5 | 1 | ||||||
| tub | 6 | 6 | 0 |
ER, PR, estrogen/progesterone receptor status; NS, nodal status; INFL, inflammatory infiltrate; Size, tumor size (cms); Grade, Nottingham grade; Type, mucinous (muc), tubular (tub), medullary (med), lobular (ilc), ductal (idc); Low Ps/High Ps, low/high psoriasin mRNA level determined by RT-PCR (cutpoint values used as described in Materials and Methods). P values determined by χ2 or ANOVA tests. ns, not significant.
Psoriasin protein was detected by Western blot analysis in 10 tumors (Table 1 ▶ and Figure 4 ▶ ). These tumors (six ductal, two lobular, two medullary) corresponded to those with the highest mRNA levels observed by RT-PCR (above 1.0 arbitrary expression units). Also consistent with RT-PCR analysis, Western blot-positive invasive ductal tumors were also significantly associated with ER-negative (P < 0.0001) and PR-negative (P < 0.0012) and node-positive (P = 0.0143) status (Table 2) ▶ .
The relationship between psoriasin mRNA and protein expression and host inflammatory response was also examined (Table 2) ▶ . Psoriasin mRNA showed a significant positive correlation in the entire cohort (n = 57, r = 0.47, P = 0.0002), in the entire cohort excluding the medullary carcinoma subgroup, which includes inflammatory infiltrates as a diagnostic criterion (n = 52, r = 0.42, P = 0.0022), and within the invasive ductal subgroup alone (n = 34, r = 0.39, P = 0.023). Cases with Western blot-detectable psoriasin protein also showed increased inflammatory infiltrates, both in the entire cohort (mean (SD) = 3.6 (1.1) vs. 2.3 (1.2), P = 0.004) and in the entire cohort excluding the medullary subgroup (mean (SD) = 3.3 (0.89) vs. 2.1 (0.98), P = 0.007).
Discussion
We have developed a psoriasin-specific antibody and confirmed its specificity as well as its ability to detect the psoriasin protein in formalin-fixed and paraffin-embedded specimens. We have shown that there is a high concordance between psoriasin mRNA and protein levels in invasive tumors, and persistance of psoriasin expression at higher levels is significantly associated with poor prognostic markers, including ER- and PR-negative and lymph node-positive status. Psoriasin expression within breast tumor cells is also associated with inflammatory infiltrates.
Indirect support for a role for S100 genes in breast tumor progression is provided by several observations. Disruption of calcium signaling pathways has been implicated as a central mechanism in tumorigenesis and specifically in the process of invasion and metastasis. 26 Moreover, the chromosomal location of the S100 gene family lies in a region of chromosome 1 that frequently (>50%) shows loss of heterozygosity in invasive tumors. 27 Furthermore, several S100 genes are expressed in breast cell lines and tumors and are known to manifest alteration of their expression in association with tumorigenesis and progression. 11,24 In particular, S100A2 and S100A4 have been identified to be differentially expressed between normal and neoplastic cells 3,28,29 and up-regulated in metastatic as compared to nonmetastatic cells in both mouse and rat mammary tumor cell lines. 5,30 In vivo studies of breast tumors have also shown a correlation between high levels of S100A4 expression, nodal metastasis, and ER-negative status. 31 More direct evidence has emerged from modulation of S100A4 expression in transfected cell lines that have shown that overexpression of S100A4 can also induce the metastatic phenotype in mouse, rat, and human cells. 4,6,32 Furthermore, there is evidence that S100A4 may exert its effect on cell cytoskeleton 8,9 and motility, 7 and it has also been demonstrated that up-regulation of S100A4 in mouse tumor cell lines can down-regulate expression of E- cadherin and disturb the intracellular distribution of B-catenin. 10
A possible role for psoriasin (S100A7) in breast cancer first emerged when it was also identified as a cDNA down-regulated in a nodal metastasis relative to a primary breast tumor. 33 Nevertheless, the significance of the initial observation was unclear because of the fact that expression was only detectable in a small proportion of cells within invasive primary tumors studied by in situ hybridization and overall could be detected in only 18% of primary tumor specimens assessed by Northern analysis. An explanation for this paradox became apparent when psoriasin was also identified by us as a gene that is particularly highly expressed in the ductal epithelial cells of preinvasive ductal carcinoma in situ, 1 which can be present as a significant component with invasive tumor specimens. We have now shown that when higher levels of psoriasin expression persist within invasive tumors, this correlates with indicators of increased metastatic potential. It should be noted that the strong relationship with ER status is compatible with studies of S100A4 31 and the in vitro observation 33 (and our unpublished data) that psoriasin is regulated by estradiol in MCF7 cells. Although it is interesting that the nature of this correlation is different between the in vitro and in vivo situations, similar differences have been found with other genes in breast tumors, 34 suggesting that additional external factors may influence psoriasin regulation in vivo.
Although the biological effect of alteration of psoriasin in breast tumors is currently unknown, it is interesting to speculate from this pattern of expression that psoriasin may be important in the invasive phenotype. 16 This role might be mediated through an indirect influence on the effector cells of the host immune response or perhaps through a more direct influence on the epithelial tumor cell. The first hypothesis is supported by the correlation seen here with the degree of host inflammatory cell response within breast tumors and the previous evidence that implicates psoriasin as a chemotactic factor. 14 However, psoriasin protein was only detected in approximately 50% of medullary and ductal tumors with marked inflammatory responses. The second hypothesis is supported by our observation that psoriasin may not only be secreted 13,15 but also can be localized in both nuclear and cytoplasmic compartments in normal skin and breast tumors. Although further studies beyond immunohistochemistry are necessary to confirm this observation, the pattern of expression is consistent between cells in two closely related epithelia, epidermis and breast ductal epithelium, and the detection of nuclear and cytoplasmic signal was unrelated to tissue fixation or immunohistochemistry protocol, which may effect staining with some antibodies. 35,36 Dual localization and alteration of the subcellular localization with disease has also been observed with another S100 related keratinocyte protein, profilaggrin, expressed in the epidermis. 2,37 Similarly, altered cellular distribution of proteins such as BRCA1 and B-catenin are also recognized to be an important aspect of tumor progression. 38-40 Furthermore, other S100 proteins have previously been associated with both extracellular and intracellular actions, 41 and previous studies have also indicated potential interactions for S100A4 with both cytoskeletal 8,9 and nuclear 42 proteins. It has also recently been shown that other secreted S100 proteins can be localized to cytoplasm and nucleus, 43,44 and specifically S100A2 has been found in breast cell nuclei, whereas S100A6 localizes to the cytoplasm; 24 however, the functional significance of these findings remains unknown.
In conclusion, we have shown that expression of psoriasin (S100A7) mRNA and protein correlates with indicators of poor prognosis in invasive breast tumors, including ER, PR, and nodal status, but is not related to differentiation, as manifested by invasive tumor type or grade. The relationship observed between psoriasin and the inflammatory response is also compatible with a role as a chemotactic factor; however, the possibility of additional intracellular functions is raised by the presence of its nuclear localization in both skin and breast tumors. Further studies will be necessary to confirm the latter observation and pursue the biological functions of psoriasin in relation to breast tumor progression.
Acknowledgments
The authors thank Prof. J. E. Celis (University of Aarhus, Aarhus, Denmark) for kindly providing anti-psoriasin antibody and Helmut Dotzlaw and Caroline Cummins-Leygue for assistance with cell transfections. We also thank Bionostics, North York, for assistance with antibody production. The tissues used in this study were provided by the Manitoba Breast Tumor Bank, which is funded by the National Cancer Institute of Canada.
Footnotes
Address reprint requests to Dr. Peter Watson, Department of Pathology, D212-770 Bannatyne Ave., University of Manitoba, Winnipeg, MB R3E OW3, Canada. E-mail: pwatson@cc.umanitoba.ca.
Supported by grants from the Medical Research Council of Canada (MRC) and the U.S. Army Medical Research and Materiel Command (USAMRMC). The Manitoba Breast Tumor Bank is supported by funding from the National Cancer Institute of Canada (NCIC). P. H. W. is an MRC Clinician-Scientist; L. C. M. is an MRC Scientist; E. L. is a recipient of a USAMRMC Postdoctoral Fellowship. T. H.-H. is a recipient of an MRC studentship award.
References
- 1.Leygue E, Snell L, Hiller T, Dotzlaw H, Hole K, Murphy LC, Watson PH: Differential expression of psoriasin messenger RNA between in situ and invasive human breast carcinoma. Cancer Res 1996, 56:4606-4609 [PubMed] [Google Scholar]
- 2.Schafer BW, Heizmann CW: The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci 1996, 21:134-140 [DOI] [PubMed] [Google Scholar]
- 3.Lee SW, Tomasetto C, Swisshelm K, Keyomarsi K, Sager R: Down-regulation of a member of the S100 gene family in mammary carcinoma cells and reexpression by azadeoxycytidine treatment. Proc Natl Acad Sci USA 1992, 89:2504-2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd BH, Platt-Higgins A, Rudland PS, Barraclough R: Human S100A4 (p9Ka) induces the metastatic phenotype upon benign tumour cells. Oncogene 1998, 17:465-473 [DOI] [PubMed] [Google Scholar]
- 5.Sherbet GV, Lakshmi MS: S100A4 (MTS1) calcium binding protein in cancer growth, invasion and metastasis. Anticancer Res 1998, 18:2415-2421 [PubMed] [Google Scholar]
- 6.Grigorian M, Ambartsumian N, Lykkesfeldt AE, Bastholm L, Elling F, Georgiev G, Lukanidin E: Effect of mts1 (S100A4) expression on the progression of human breast cancer cells. Int J Cancer 1996, 67:831-841 [DOI] [PubMed] [Google Scholar]
- 7.Ford HL, Salim MM, Chakravarty R, Aluiddin V, Zain SB: Expression of Mts1, a metastasis-associated gene, increases motility but not invasion of a nonmetastatic mouse mammary adenocarcinoma cell line. Oncogene 1995, 11:2067-2075 [PubMed] [Google Scholar]
- 8.Bastholm L, Elling F, Georgiev G, Lukanidin EKM, Tarabykina S, Bronstein I, Maitland N, Lomonosov M, Hansen K, Georgiev G, Lukanidin E: Metastasis-associated Mts1 (S100A4) protein modulates protein kinase C phosphorylation of the heavy chain of nonmuscle myosin. J Biol Chem 1998, 273:9852-9856 [DOI] [PubMed] [Google Scholar]
- 9.Ford HL, Zain SB: Interaction of metastasis associated Mts1 protein with nonmuscle myosin. Oncogene 1995, 10:1597-1605 [PubMed] [Google Scholar]
- 10.Keirsebilck A, Bonne S, Bruyneel E, Vermassen P, Lukanidin E, Mareel M, Van Roy F: E-cadherin and metastasin (mts-1/S100A4) expression levels are inversely regulated in two tumor cell families. Cancer Res 1998, 58:4587-4591 [PubMed] [Google Scholar]
- 11.Borglum AD, Flint T, Madsen P, Celis JE, Kruse TA: Refined mapping of the psoriasin gene S100A7 to chromosome 1cen-q21. Hum Genet 1995, 96:592-596 [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann HJ, Olsen E, Etzerodt M, Madsen P, Thogersen HC, Kruse T, Celis JE: Psoriasin binds calcium and is upregulated by calcium to levels that resemble those observed in normal skin. J Invest Dermatol 1994, 103:370-375 [DOI] [PubMed] [Google Scholar]
- 13.Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B, et al: Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol 1991, 97:701-712 [DOI] [PubMed] [Google Scholar]
- 14.Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B, Etzerodt M, Honore B, Celis JE, Thestrup-Pedersen K: Psoriasin: a novel chemotactic protein. J Invest Dermatol 1996, 107:5-10 [DOI] [PubMed] [Google Scholar]
- 15.Celis JE, Rasmussen HH, Vorum H, Madsen P, Honore B, Wolf H, Orntoft TF: Bladder squamous cell carcinomas express psoriasin and externalize it to the urine. J Urol 1996, 155:2105-2112 [PubMed] [Google Scholar]
- 16.Watson PH, Leygue ER, Murphy LC: Psoriasin (S100A7). Int J Biochem Cell Biol 1998, 30:567-571 [DOI] [PubMed] [Google Scholar]
- 17.Hiller T, Snell L, Watson PH: Microdissection RT-PCR analysis of gene expression in pathologically defined frozen tissue sections. Biotechniques 1996, 21:38-40 [DOI] [PubMed] [Google Scholar]
- 18.Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW: Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 1992, 20:479-489 [DOI] [PubMed] [Google Scholar]
- 19.Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410 [DOI] [PubMed] [Google Scholar]
- 20.Leygue ER, Watson PH, Murphy LC: Estrogen receptor variants in normal human mammary tissue. J Natl Cancer Inst 1996, 88:284-290 [DOI] [PubMed] [Google Scholar]
- 21.Schagger H, von Jagow G: Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 1987, 166:368-379 [DOI] [PubMed] [Google Scholar]
- 22.Leygue E, Snell L, Dotzlaw H, Hole K, Hiller-Hitchcock T, Roughley PJ, Watson PH, Murphy LC: Expression of lumican in human breast carcinoma. Cancer Res 1998, 58:1348-1352 [PubMed] [Google Scholar]
- 23.Ercolani L, Florence B, Denaro M, Alexander M: Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem 1988, 263:15335-15341 [PubMed] [Google Scholar]
- 24.Ilg EC, Schafer BW, Heizmann CW: Expression pattern of S100 calcium-binding proteins in human tumors. Int J Cancer 1996, 68:325-332 [DOI] [PubMed] [Google Scholar]
- 25.Ostergaard M, Rasmussen HH, Nielsen HV, Vorum H, Orntoft TF, Wolf H, Celis JE: Proteome profiling of bladder squamous cell carcinomas: identification of markers that define their degree of differentiation. Cancer Res 1997, 57:4111-4117 [PubMed] [Google Scholar]
- 26.Kohn EC, Liotta LA: Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res 1995, 55:1856-1862 [PubMed] [Google Scholar]
- 27.Munn KE, Walker RA, Varley JM: Frequent alterations of chromosome 1 in ductal carcinoma in situ of the breast. Oncogene 1995, 10:1653-1657 [PubMed] [Google Scholar]
- 28.Wicki R, Franz C, Scholl FA, Heizmann CW, Schafer BW: Repression of the candidate tumor suppressor gene S100A2 in breast cancer is mediated by site-specific hypermethylation. Cell Calcium 1997, 22:243-254 [DOI] [PubMed] [Google Scholar]
- 29.Ebralidze A, Tulchinsky E, Grigorian M, Afanasyeva A, Senin V, Revazova E, Lukanidin E: Isolation and characterization of a gene specifically expressed in different metastatic cells and whose deduced gene product has a high degree of homology to a Ca2+-binding protein family. Genes Dev 1989, 3:1086-1093 [DOI] [PubMed] [Google Scholar]
- 30.Barraclough R, Rudland PS: The S-100-related calcium-binding protein, p9Ka, and metastasis in rodent and human mammary cells. Eur J Cancer 1994, 30A:1570-1576 [DOI] [PubMed] [Google Scholar]
- 31.Albertazzi E, Cajone F, Leone BE, Naguib RN, Lakshmi MS, Sherbet GV: Expression of metastasis-associated genes h-mts1 (S100A4) and nm23 in carcinoma of breast is related to disease progression. DNA Cell Biol 1998, 17:335-342 [DOI] [PubMed] [Google Scholar]
- 32.Grigorian MS, Tulchinsky EM, Zain S, Ebralidze AK, Kramerov DA, Kriajevska MV, Georgiev GP, Lukanidin EM: The mts1 gene and control of tumor metastasis. Gene 1993, 135:229-238 [DOI] [PubMed] [Google Scholar]
- 33.Moog-Lutz C, Bouillet P, Regnier CH, Tomasetto C, Mattei MG, Chenard MP, Anglard P, Rio MC, Basset P: Comparative expression of the psoriasin (S100A7) and S100C genes in breast carcinoma and co-localization to human chromosome 1q21–q22. Int J Cancer 1995, 63:297-303 [DOI] [PubMed] [Google Scholar]
- 34.Yarden RI, Lauber AH, El Ashry D, Chrysogelos SA: Bimodal regulation of epidermal growth factor receptor by estrogen in breast cancer cells. Endocrinology 1996, 137:2739-2747 [DOI] [PubMed] [Google Scholar]
- 35.Scully R, Ganesan S, Brown M, De Caprio JA, Cannistra SA, Feunteun J, Schnitt S, Livingston DM: Location of BRCA1 in human breast and ovarian cancer cells (technical comments). Science 1996, 272:123-124 [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Chen P-L, Riley DJ, Lee W-H, Allred DC, Osborne CK: Location of BRCA1 in human breast and ovarian cancer cells (technical comments). Science 1996, 272:125-126 [DOI] [PubMed] [Google Scholar]
- 37.Ishida-Yamamoto A, Takahashi H, Presland RB, Dale BA, Iizuka H: Translocation of profilaggrin N-terminal domain into keratinocyte nuclei with fragmented DNA in normal human skin and loricrin keratoderma. Lab Invest 1998, 78:1245-1253 [PubMed] [Google Scholar]
- 38.Wilson CA, Ramos L, Villasenor MR, Anders KH, Press MF, Clarke K, Karlan B, Chen JJ, Scully R, Livingston D, Zuch RH, Kanter MH, Cohen S, Calzone FJ, Slamon DJ: Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nature Genet 1999, 21:236-240 [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Chen CF, Riley DJ, Allred DC, Chen PL, Von Hoff D, Osborne CK, Lee WH: Aberrant subcellular localization of BRCA1 in breast cancer. Science 1995, 270:789-791 [DOI] [PubMed] [Google Scholar]
- 40.Sheng H, Shao J, Williams CS, Pereira MA, Taketo MM, Oshima M, Reynolds AB, Washington MK, DuBois RN, Beauchamp RD: Nuclear translocation of beta-catenin in hereditary and carcinogen-induced intestinal adenomas. Carcinogenesis 1998, 19:543-549 [DOI] [PubMed] [Google Scholar]
- 41.Hessian PA, Edgeworth J, Hogg N: MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol 1993, 53:197-204 [PubMed] [Google Scholar]
- 42.Albertazzi E, Cajone F, Lakshmi MS, Sherbet GV: Heat shock modulates the expression of the metastasis associated gene MTS1 and proliferation of murine and human cancer cells. DNA Cell Biol 1998, 17:1-7 [DOI] [PubMed] [Google Scholar]
- 43.Yang Q, O’Hanlon D, Heizmann CW, Marks A: Demonstration of heterodimer formation between S100B and S100A6 in the yeast two-hybrid system and human melanoma. Exp Cell Res 1999, 246:501-509 [DOI] [PubMed] [Google Scholar]
- 44.Mandinova A, Atar D, Schafer BW, Spiess M, Aebi U, Heizmann CW: Distinct subcellular localization of calcium binding S100 proteins in human smooth muscle cells and their relocation in response to rises in intracellular calcium. J Cell Sci 1998, 111:2043-2054 [DOI] [PubMed] [Google Scholar]