Abstract
Recently the stem cell-like regenerative potential of adult liver cells was demonstrated by serial transplantation. This repopulation capacity could be useful for the treatment of genetic liver diseases by cell transplantation and/or expansion of genetically manipulated cells. However, previous experiments used unfractionated populations of liver cells, and therefore it remained undetermined whether all hepatocytes or only a subpopulation (stem cells) possessed this high regenerative ability. To address this question we used centrifugal elutriation to separate hepatocytes by cell density. Unexpectedly, small hepatocytes (16 μm) had lower repopulation capacity during the first round of transplantation when compared with both the medium-sized (21 μm) and large (27 μm) cells. We also compared the repopulation capacity of hepatocytes that had undergone different degrees of in vivo expansion. Previous cell division neither reduced nor increased the repopulation capacity of transplanted liver cells. Finally, retroviral tagging experiments demonstrated that liver-repopulating cells occur at a frequency of >1:10,000. We conclude that short-term therapeutic liver repopulation does not require progenitor or stem cells.
Liver regeneration after partial hepatectomy and most chemical injuries occurs by the division of fully differentiated hepatocytes and does not require stem or progenitor cells. 1 However, if the ability of differentiated cells to divide is impaired, progenitor cell-dependent liver regeneration can be observed. 2,3 The physiological significance of these cells during normal liver turnover and response to injury remains controversial.
We have created a mouse model of the human disease hereditary tyrosinemia type I which is due to the lack of the enzyme fumarylacetoacetate hydrolase (FAH). 4 FAH catalyzes the last step of the tyrosine degradation pathway, and animals lacking this enzyme develop severe hepatic dysfunction due to the accumulation of toxic upstream metabolites. 5 It has been demonstrated in both humans and mice that positive selection for FAH-expressing hepatocytes occurs in an FAH-deficient liver. 6,7 The drug 2-(2-nitro-4-trifluoro-methylbenzoyl)1,3-cyclohexedione (NTBC) can prevent the neonatal lethality and liver dysfunction of FAH-deficient mice. 8 Mice treated with NTBC and then removed from treatment develop acute hepatocellular damage and die within 2 months. We have used this model to show the regenerative potential of hepatocytes by transplanting genetically marked wild-type cells into FAH-deficient mice. Wild-type cells transplanted into the spleen or portal vein repopulate the recipient animal to >90% within 6 to 8 weeks. 6 As few as 1000 donor cells were sufficient to rescue FAH-deficient animals and restore liver function. 6 Serial transplantation of limiting numbers of cells was performed and seven generations of animals were successfully repopulated. 9 This experiment demonstrated that the regenerative potential of the serially repopulating hepatocytes was similar to that of hematopoietic stem cells and exceeded 100 cell doublings. This high capacity for cell division raised the question whether liver progenitor or stem cells may be responsible for the serial repopulation. Interestingly however, the only donor-derived cells in the repopulated livers were hepatocytes. 9 No evidence for the emergence of biliary epithelium or any other hepatic cell type was found. Oval cells, on the other hand can give rise to both biliary epithelium and hepatocytes. 10 This finding therefore was more consistent with the view that serial repopulation was carried out by differentiated hepatocytes and not progenitor cells.
To date all liver repopulation experiments reported by us and others have used unfractionated suspensions of liver cells isolated by in situ collagenase perfusion. 11 These suspensions consist mostly of hepatocytes, but also contain various other hepatic cells including biliary epithelium, stellate cells, Kupffer cells, endothelial cells, and fibroblasts. Therefore two main hypotheses regarding liver repopulation can be formulated: 1) the majority of cells, ie, the differentiated hepatocytes themselves, are capable of repopulation; 2) a rarer subpopulation of cells (stem cells/progenitor cells) with high regenerative capacity is responsible. It is also possible that both hypotheses are correct, that differentiated hepatocytes are capable of short-term repopulation, and that a specific subpopulation is required for serial transplantation.
Several experimental approaches can be used to distinguish these possibilities, and three of these are reported here. First, centrifugal elutriation was used to isolate three distinct populations of adult murine hepatocytes that differed in size. The individual fractions were transplanted in competition with unfractionated hepatocytes of a genetically distinct mouse strain. Second, we wanted to test the effect of prior cell division on the ability to repopulate. The stem cell model of repopulation predicts aging and reduced capacity for cell division of differentiated hepatocytes. 12 Third, we used retroviral tagging to test the clonality of liver repopulation. 13
Materials and Methods
Mouse Strains and Animal Husbandry
The following mouse strains were used for the competitive transplantation experiments: the FAHΔexon5 strain of mice, 4 which has previously been described by this laboratory; ROSA-26 β-galactosidase transgenic animals obtained from and described by P. Soriano 14 ; the Fanconi anemia complementation group C (FANCC) knockout mice created by this lab and previously described 15 ; and the dopamine D2 receptor (DOPA) knockout mice kindly donated by M. Low. 16 All of these mice were of the inbred 129SvJ strain and congenic for the purposes of transplantation. All FAHΔexon5 breeders and mutant animals were treated with NTBC-containing water at a concentration of 7.5 mg/l (provided by S. Lindstedt, Gothenborg, Sweden). NTBC is a potent inhibitor of 4-OH-phenylpyruvate dioxygenase, the second enzyme of tyrosine catabolism. 17 Treatment with this drug prevents liver failure in FAH-mutant mice and is necessary for the animals to survive and breed. For genotyping of the FAHΔexon5 and the FANCC heterozygotes, a 3-primer polymerase chain reaction was carried out on 200 ng of tail-cut DNA as previously described. 4,15 Staining of a small portion of tail from the ROSA-26 animals for the presence of β-galactosidase activity was used to genotype these animals. For the DOPA mice, genomic DNA was digested with the restriction enzyme EcoRV (Pharmacia, Uppsala, Sweden), and then Southern blots were probed for the presence of the neomycin phosphotransferase (neo) gene used in creating the knockout construct. Animal care and experiments were all in accordance with the Guidelines of the Department of Animal Care at Oregon Health Sciences University.
Elutriation, Centrifugation, Separation, and Sizing
Elutriation was performed using a J2-M Beckman centrifuge with a JM6 rotor and large chamber (15 ml). After the initial isolation by in situ perfusion with collagenase, the cells were resuspended in 4 ml of elutriation media (5% Dulbecco’s minimum essential medium (DMEM) with 0.003% (w/v) DNase and 5 mmol/L ethylenediaminetetraacetic acid (EDTA)) at 4°C to avoid clumping. With the centrifuge spinning at 1000 rpm (4°C), flow rates were determined for separate pump settings, and then the cells were added at a flow rate of 7 ml/minute. Fractions (150 ml) were isolated from the following flow rates of 8 ml/minute to 30 ml/minute in 2-ml/min gradients. The fractions were then spun down and resuspended in 2 ml of DMEM with 10% fetal calf serum (FCS). One hundred microliters were diluted in 20 ml of Isoton fluid and sized using a Coulter Multisizer II (Hialeah, FL). Data were analyzed using MULTISIZER AccuComp 1.19 software. Isolated fractions of the appropriate size and purity were used for transplantation.
Cells were transplanted into the inbred FAHΔexon5 strain of SV129 mice. For transplantation all animals used were between 6 and 10 weeks old.
Transplantation Procedures
Parenchymal hepatocytes were isolated from congenic animals by a two-step collagenase perfusion. 18 Cell number and viability were determined by Trypan blue exclusion in a hemocytometer. The donor cells were resuspended in 100 μl of DMEM (GIBCO BRL, Gaithersburg, MD) with 15% FCS and injected intrasplenically 19 into FAHΔexon5 recipient animals. All mutant mice were kept on NTBC until the time of transplantation, when it was discontinued. The weights of experimental animals were measured weekly.
Retrovirus Tagging
The G1FSvNa retrovirus construct and methods used for in vivo and ex vivo hepatocyte transduction were as previously described. 6,20 Briefly, for ex vivo experiments 500,000 isolated mouse hepatocytes were plated per 100-mm dish containing DMEM with 10% FCS, 2 mmol/L glutamine, and the antibiotics penicillin and streptomycin. The cells were cultured for 36 hours, then the media were removed and replaced with 5 ml of retroviral supernatant containing 8 μg/ml polybrene. After 4 hours the supernatant was removed, and 10 ml of SUM3 media 21 was added to each plate. The cells were harvested 36 to 48 hours later by trypsin treatment and intrasplenically transplanted. For in vivo experiments, 0.5-ml aliquots of supernatant were directly injected via the portal vein as previously described. 6
Southern Blot Analysis
For Southern blots, liver DNA was isolated from liver that was freshly obtained or frozen at −80°C. Random 5- × 5-mm sections of tissue from the left lower lobe were used for the DNA isolation. Capillary transfer and hybridizations were performed according to standard protocols. 22 For detection of the neomycin phosphotransferase gene used in generating the transgenic animals, isolated DNA was digested with BamHI (Pharmacia) and probed with a 680-bp PstI (Pharmacia) fragment isolated from a neomycin phosphotransferase cDNA. A Beckman SI Phosphoimager was used to quantitate relative band intensities.
To quantitate the relative ratios of ROSA to genetically unmarked wild-type cells, Southern blots were performed as described above, and the intensity of the neo signal was quantitated with a Beckman SI Phosphoimager. This signal was compared with a Southern blot standard curve generated by mixing known ratios of pure wild-type and ROSA DNA. The control samples were derived by 1) harvesting liver DNA from FAH-mutant mice transplanted with known mixes (1:9, 1:1, and 9:1) of unfractionated cells from the two strains and b) mixing pure DNA from the two strains at known ratios. In addition to loading the gels with exactly the same amount of total DNA, the neo-signal derived from the nonparenchymal cells of the recipient mutant mouse was used as an internal standard. This was possible, because the overall degree of repopulation (∼95%) was similar in all experiments.
Histology and Immune Histology
For immunohistochemical analysis of isolated cells, approximately 20,000 cells were resuspended in 100 μl of DMEM and centrifuged onto poly l-lysine slides in a Cytospin III (Shandon, Pittsburgh, PA) at 500 × g for 5 minutes. The slides were fixed in 10% formalin for 10 minutes and then transferred to 80% ethanol.
The slides were incubated with a polyclonal rabbit antibody to rat FAH 23 and mouse keratin 19 (CK19). 24,25 The FAH antibody was diluted in phosphate-buffered saline (PBS), pH 7.4, and applied at concentrations of 1:300,000 at 37°C for 30 minutes. The CK19 antibody was used at a 1:5,000 dilution. Endogenous peroxidase activity was blocked with 3% H2O2 and methanol. Avidin and biotin pretreatment was used to prevent endogenous staining. The secondary antibody was biotinylated goat anti-rabbit IgG used at 1:250 dilution (Vector Laboratories, Burlingame, CA; BA-1000). Color development was performed with the aminoethylcarazole detection kit from Venatan Medical Systems (Tucson, AZ; catalog item 250–020).
β-Galactosidase tissue staining consisted of washing sections of freshly harvested liver twice in PBS for 5 minutes. The liver sections were fixed by soaking in cold PBS containing 2% glutaraldehyde and 1% formaldehyde for 10 minutes and then washed twice with PBS. The sections were then stained overnight in 1 mmol/L β-galactosidase, 3 mmol/L ferricyanide, 3 mmol/L ferrocyanide, 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1 mmol/L MgCl2, and 1 mmol/L NP40 at 37°C.
For β-galactosidase staining of cell suspensions, cells were fixed for 5 minutes in cold PBS with 2% glutaraldehyde and stained overnight in the same solution described above.
Results
Competitive Repopulation with Elutriated Cell Fractions
In many studies of liver regeneration, small, periportal zone 1 hepatocytes proliferate most readily. For example, after 15% partial hepatectomy, most thymidine-labeled cells are found in this location. 26 We therefore hypothesized that hepatocytes of different sizes may differ in their liver repopulation potential. To address this question hepatocytes isolated from congenic 129SvJ animals were sorted by density using centrifugal elutriation and transplanted into FAH-mutant recipients. Centrifugal elutriation allowed the collection of differently sized fractions of hepatocytes without any loss of viability. The cell size profile within each fraction was then determined by analyzing a small aliquot of each fraction in a Coulter Multisizer II instrument with Multisizer AccuComp 1.19 software. Analysis of unfractionated cells in 6 independent experiments revealed the existence of three distinct populations varying in size and contribution to the liver mass (Figure 1) ▶ . The majority of the hepatocytes were approximately 21 μm in diameter, with the other two fractions consisting of hepatocytes of approximately 16 and 27 μm in diameter. Further characterization of these fractions consistently showed that the 21-μm fraction provided 60 to 75% of all of the cells in the suspension. The other two fractions of isolated hepatocytes displayed yields of 5 to 15% for the 16-μm-diameter population and 15 to 25% for the 27-μm-diameter fraction. The use of different medium flow rates through the elutriator permitted the isolation of relatively pure populations of each of these three sizes (>80%, Table 1 ▶ ). The profiles from each enriched fraction used in one competitive transplantation and the profile of an unfractionated population of hepatocytes are depicted in Figure 1 ▶ . Quantitative data regarding the size distribution of elutriated cells are given in Table 1 ▶ .
Figure 1.
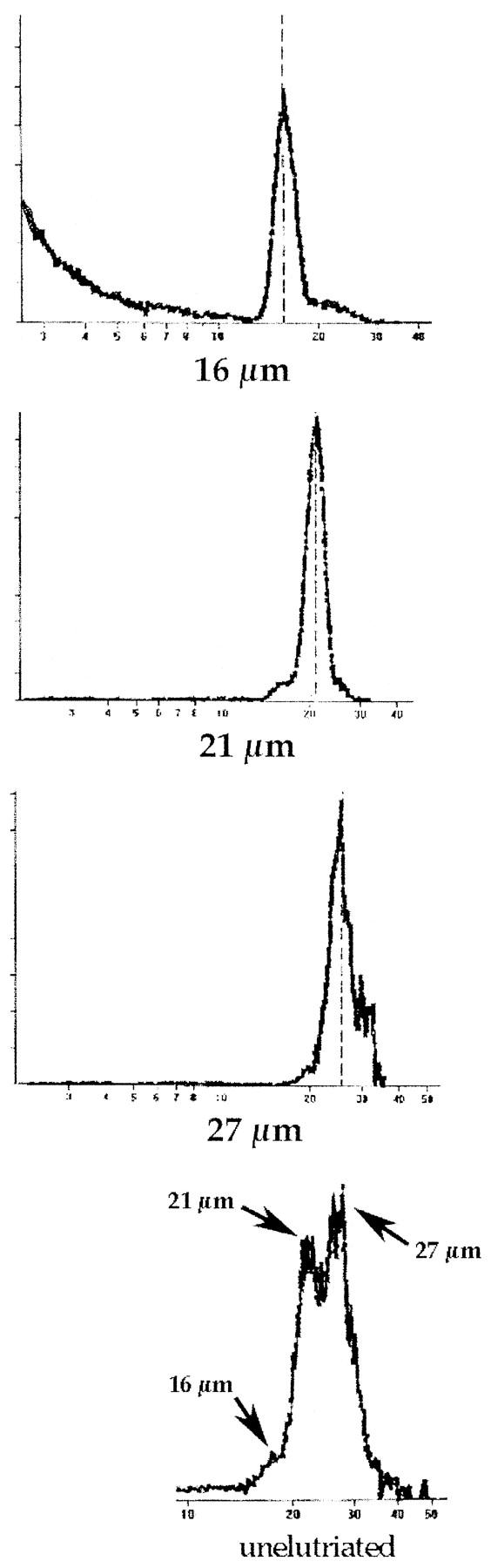
Size profiles of hepatocytes isolated by centrifugal elutriation. Size profiles of the elutriated fractions are shown beginning with the smallest cells at the top. The bottom panel depicts the profile of an unfractionated suspension of hepatocytes. The cell number is given by the y axis, and the x axis indicates the cell diameter in micrometers.
Table 1.
Size Distribution of Elutriated Cells
| Fraction | Mean size ± 2 SD | 13.5–18.5 μm* | 18–23 μm* | 25–30 μm* |
|---|---|---|---|---|
| Small | 15.84 ± 2.54 | 81 | 21 | 3 |
| Small | 16.08 ± 2.24 | 84 | 16 | 4 |
| Small | 16.22 ± 2.48 | 79 | 18 | 3 |
| Medium | 20.24 ± 3.12 | 19 | 82 | 2 |
| Medium | 21.6 ± 3.78 | 4 | 89 | 26 |
| Large | 26.76 ± 1.84 | 4 | 20 | 87 |
| Large | 26.6 ± 1.62 | 4 | 19 | 86 |
* These ranges represent the mean size ± 2 standard deviations (SD) of each of the three size fractions. The numbers represent percentage of cells in this size range.
Cell viability after elutriation was >80% in each group, as determined by Trypan blue exclusion. The majority of small cells (90–95%, data not shown) were mononucleated, whereas most of the 21-μm and 27-μm cells (70–80%, data not shown) were binucleated.
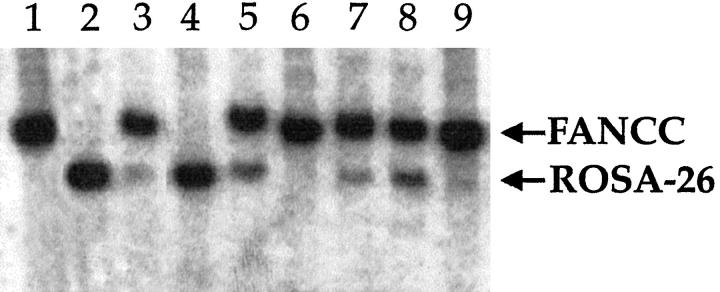
Elutriated cells were always transplanted in competition with unfractionated cells from a second animal carrying a neo marker in a different chromosomal location. Both populations were from donor animals of the same sex and age. The unfractionated cells were always isolated first, and therefore the time from isolation to transplantation was shorter for elutriated cells, providing them with a potential mild survival advantage. All mice used (recipients and donors) were from the same inbred mouse strain, 129SvJ, and thus differed only in their neo marker integration site. Only heterozygotes were used, and there is no evidence that any of the targeted gene disruptions (D2 receptor, FANCC, and ROSA-26) have an effect on hepatocyte division in the heterozygous state. Cells from the ROSA-26 strain were usually used for elutriation. However, to be certain that the marker insertions had no effect on repopulation capacity, FANCC hepatocytes were used for elutriation in some experiments and competed with unsorted ROSA-26 cells. The different neo marker integration sites permitted the accurate measurement of the contribution of the different populations to overall repopulation by quantitative Southern blot analysis (Figure 2) ▶ . In addition, the β-galactosidase-expressing ROSA-26 cells provided a rapid visual estimate of the degree of repopulation contributed by “blue cells” (Figure 3) ▶ . The size-enriched populations of cells were transplanted at ratios of 1:1, 1:4, or 1:5 to the unsorted cells to more easily determine their capacity for repopulation. In all experiments, the repopulation ability of the isolated fractions never exceeded 45% when transplanted at a 1:1 ratio (Table 2) ▶ . Therefore none of the enriched fractions had a repopulation capacity substantially superior to unfractionated cells. Overall, the 21-μm fraction appeared to compete the best of the three fractions, showing an average percent repopulation of ∼35% in 1:1 and 21% in 1:4 transplants. The extent of repopulation contributed by the 27-μm fraction was only slightly lower, averaging ∼33% in 1:1 and 15% in 1:4 transplants. However, the differences between these two groups were not significant when analyzed using the Student’s t-test (P = 0.85 for the 1:1 transplants). In contrast, the fraction containing the isolated 16-μm cells competed least well. Small cells never contributed more than 8% when transplanted at a 1:1 ratio. The differences in the 1:1 competitive repopulation assays were statistically significant (P < 0.0001 for the 16-μm:21-μm comparison; P = 0.01 for the 16-μm:27-μm comparison). The total number of cells transplanted was also varied. There was some variation in the measured degree of repopulation even in animals that received the exact identical mix of cells. For example one of the mice that received a 1:1 mix of 27-μm cells and unfractionated competitors had only 17% repopulation, whereas the other two had 40% and 42%, respectively (Table 2) ▶ . The most likely explanation for the observed variation is the area of tissue sampling. For the elutriation experiments, we isolated DNA from randomly selected small chunks of liver.
Figure 2.
Southern blot analysis of competitively repopulated livers. DNA from repopulated livers was probed with the neo gene, which produces differently sized fragments in the different strains of donor mice. The small (16 μm) hepatocytes competed poorly (lanes 3, 4, 6, and 9). lane 1, FANCC control; lane 2, ROSA-26 control; lane 3, 1:1 ratio of 16-μm ROSA-26 to unsorted FANCC cells (repopulation 8%); lane 4, 1:1 ratio of 16-μm FANCC cells to unsorted ROSA-26 cells (repopulation <5%); lane 5, 1:1 ratio of 27-μm ROSA-26 to unsorted FANCC cells (repopulation 42%); lane 6, 1:5 ratio of 16-μm ROSA-26 to unsorted FANCC cells (repopulation <5%); lane 7, 1:4 ratio of 27-μm ROSA-26 to unsorted FANCC cells (repopulation ∼15%); lane 8, 1:1 ratio of 21-μm ROSA-26 to unsorted FANCC cells (repopulation ∼35%); lane 9, 1:1 ratio of 16-μm ROSA-26 to unsorted FANCC cells (repopulation <5%).
Figure 3.
β-Galactosidase staining of competitively repopulated livers. Portions of 3 β-galactosidase-stained livers repopulated with 1:1 mixes of sorted and unsorted hepatocytes are shown. The sizes of the elutriated hepatocytes are given below. The 16-μm cells (right liver) competed least effectively.
Table 2.
Competitive Repopulation of Elutriated Size Fractions versus Unsorted Hepatocytes
| Cell size (μm) | Ratio* | Individual % repopulation† | Average % repopulation‡ |
|---|---|---|---|
| 16 | 1:1 | <5, <5, 8, <5 | <5 |
| 16 | 1:5 | <5, <5, <5, <5, <5 | <5 |
| 21 | 1:1 | 38, 30, 40, 30 | 35 ± 5 |
| 21 | 1:4 | 10, 20, 35, 13, 26 | 21 ± 9 |
| 27 | 1:1 | 42, 40, 17 | 33 ± 11 |
| 27 | 1:4 | 15, 15 | |
| 27 | 1:5 | 10, 10 |
*The ratio is of elutriated to nonfractionated cells.
† The percentage of repopulation measured in individual mice is given.
‡ Averages are given with standard deviation when three or more samples were analyzed.
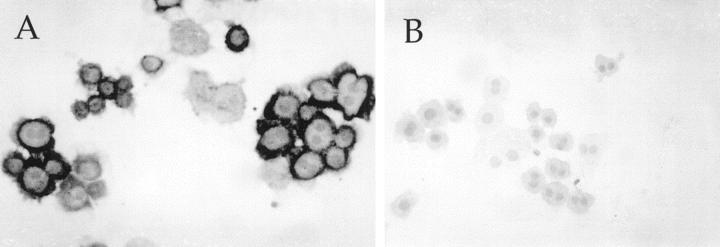
To determine whether the fractionated population of cells was indeed hepatocytes, cytospin slides prepared from different populations were analyzed for the presence of specific markers. It was determined that at least 90% of cells transplanted were indeed hepatocytes by FAH immunocytochemistry (Figure 4) ▶ . The cells were also negative for CK-19 staining, indicating the absence of duct cells in the preparations.
Figure 4.
FAH and CK19 staining of 21-μm hepatocytes. Elutriated hepatocytes were centrifuged onto glass slides and stained with antibodies of FAH (A) and cytokeratin 19 (B). As shown by the dark cytoplasmic granularity, 90% of the cells contained FAH, whereas none were labeled with the antibody to the biliary epithelial antigen.
Competitive Repopulation of Serially Transplanted Hepatocytes
Our previous serial transplantation experiments had generated animals whose livers were repopulated with liver cells that had undergone many rounds of cell division. 9 The vast majority of cells in these repopulated livers were fully differentiated hepatocytes of donor origin. We hypothesized that the repopulation ability of these “aged” hepatocytes may differ from cells derived from young donor animals, which had divided many fewer times.
We therefore performed competitive repopulation experiments with hepatocytes from FAHΔexon5 animals that had been repopulated with donor cells from heterozygote D2 receptor knockout mice. Animals from both round 1 (average number of cell divisions ∼15) and round 2 of serial transplantation (average number of cell divisions ∼30) were used. The cells were transplanted in competition with hepatocytes isolated from 2-month-old naive ROSA-26 mice. In other experiments we used serially transplanted ROSA-26 hepatocytes in competition with normal congenic wild-type hepatocytes. These experiments showed (Table 3) ▶ that hepatocytes from naïve animals and serially transplanted hepatocytes (from either one or two rounds) competed equally well. Impressively, cells from a round 7 serial transplantation recipient (at least 60 cell divisions) competed effectively with unexpanded cells. Therefore, neither fresh, previously untransplanted hepatocytes nor serially transplanted hepatocytes had a selective advantage in therapeutic repopulation in our model.
Table 3.
Competitive Repopulation between Freshly Isolated Hepatocytes versus Serially Transplanted Hepatocytes
| Rounds of transplantation | Donor cell genotypes* | Ratio | Individual % repopulation† | Average % repopulation‡ |
|---|---|---|---|---|
| 2 | ROSA/Dopa | 1:3 | 45, 48 | |
| 2 | ROSA/WT | 1:1 | 50 | |
| 2 | Dopa/ROSA | 3:1 | 71, 73 | |
| 3 | ROSA/Dopa | 1:3 | 38, 39, 42 | 40 ± 2 |
| 65, 62, 70, 60, 61, | ||||
| 3 | Dopa/ROSA | 3:1 | 65, 54, 58, 60, 69, | 63 ± 5 |
| 62, 71 | ||||
| 3 | ROSA/WT | 4:1 | 65, 65, 70, 70 | 68 ± 3 |
| 7 | ROSA/WT | 1:1 | 46 | |
| 7 | ROSA/WT | 1:4 | 28, 30 |
* The left genotype indicates the cells which had previously been serially transplanted.
† The percentage of repopulation measured in individual mice is given.
‡ Averages of the percentage of repopulation are given with standard deviation when three or more samples were analyzed.
Clonality of Repopulation in Retrovirally Marked Populations
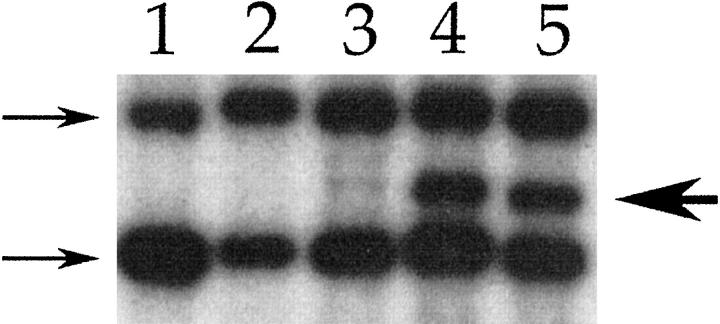
In the hematopoietic system, retroviral marking has been used extensively to ask questions about the clonality of bone marrow repopulation and the relative frequency of cells capable of long-term reconstitution of the organ. 13 We therefore decided to use this same approach to liver repopulation. When Moloney murine leukemia virus-based retroviral vectors transduce cells, their genome is integrated into the host chromosome, but the site of integration is random. 27 Therefore, each proviral integration site represents a clonal marker. Predominance of a single clone or a few clones in a population can be detected as an integration site-specific junction fragment on Southern blot analysis. We have previously shown that FAHΔexon5 hepatocytes corrected via in vivo or ex vivo gene therapy selectively repopulate mutant liver. 6,20 We applied the same viral vector and gene transfer techniques for retroviral marking. First, we transduced FAH-deficient hepatocytes for 5 hours in vitro with the previously described vector 36 hours after plating. These cells were then harvested 24 hours later, and 200,000 infected cells were intrasplenically transplanted into each recipient. After the selection and repopulation period of 8 weeks, hepatocytes were isolated from surviving recipients, and either 10,000 or 100,000 cells were then serially transplanted into secondary recipients. An aliquot of each cell suspension was processed for DNA isolation and subjected to Southern blot analysis. The G1FSvN proviral DNA could be detected at an average copy number of 1 per hepatocyte genome in all repopulated livers. In most of the independently performed experiments, no proviral junction fragments were detected when either 10,000 or 100,000 cells were serially transplanted (Table 4) ▶ . Monoclonal repopulation, however, was observed in one instance. In this experiment the primary recipient was transplanted with 200,000 ex vivo transduced cells, and 10,000 cells were serially transplanted. Southern blot analysis of the primary recipient failed to show predominance of a clonal population. It is interesting, however, that secondary recipients displayed a single proviral integration site, indicating monoclonal repopulation (Figure 5) ▶ . Continued serial transplantation of this clonal population for two additional rounds resulted in complete rescue of liver function and normal histology in the recipients (data not shown). This result, although obtained only in one instance, demonstrates that liver repopulation can be effected by a single cell derived from adult mouse liver.
Table 4.
Serial Transplantation of Hepatocytes Retrovirally Marked Either in Vivo or ex Vivo
| Transplant round | Infection | No. of cells | No. of animals analyzed | Clonality |
|---|---|---|---|---|
| 1 | In vivo | 200,000 | 3 | no |
| 1 | In vivo | 150,000 | 4 | no |
| 1 | In vivo | 100,000 | 2 | no |
| 2 | In vivo | 100,000 | 3 | no |
| 3 | In vivo | 10,000 | 3 | no |
| 2 | Ex vivo | 100,000 | 2 | no |
| 2 | Ex vivo | 10,000 | 2 | yes |
| 3 | Ex vivo | 10,000 | 1 | no |
| 3 | Ex vivo | 100,000 | 2 | no |
| 4 | Ex vivo | 3,000 | 1 | no |
| 4 | Ex vivo | 100,000 | 2 | no |
Figure 5.
Southern blot analysis of liver repopulated with retrovirally tagged cells. The neomycin phosphotransferase gene was used as a probe. The 2 arrows at left indicate the bands created by the neo expression cassette of the FAH knockout mouse. The arrow at right indicates a junction fragment created by proviral integration. lane 1: Control liver from FAH knockout mouse; lanes 2 and 3: liver repopulated with 100,000 retrovirally tagged cells during the first round of transplantation. lane 2 shows no junction fragments, but a faint junction fragment can be seen in lane 3; lanes 4 and 5 show liver repopulated in round 2 of transplantation with hepatocytes from the animal in lane 3. A dominant junction fragment is readily observed, indicating monoclonal repopulation.
We also performed in vivo retroviral marking by infusion of G1FSvN into the portal vein of hepatectomized FAH-deficient mice. After the selection period (8 weeks), hepatocytes were harvested from repopulated livers, analyzed by Southern blot, and serially transplanted for two additional rounds. Similar to the ex vivo marking experiments described above, monoclonal or oligoclonal repopulation was never observed in multiple independent experiments when 10,000 or 100,000 cells were serially transplanted. We therefore conclude that the serially transplantable population of liver cells occurs at a frequency substantially higher than 1:10,000.
Discussion
Recent work by us and others has provided evidence that transplanted liver cells from a healthy donor can replace >90% of hepatocytes of a recipient animal in a process we term “therapeutic liver repopulation.” 28-30 This procedure is similar to reconstitution of the hematopoietic system by bone marrow transplantation, and it therefore holds great promise for the treatment of hereditary and acquired liver diseases in humans. 28 Although the principle of therapeutic liver repopulation is firmly established, the exact nature of the repopulating cell(s) has not been defined. What fraction of liver cells is capable of therapeutic liver repopulation? Can subpopulations be isolated that have a higher repopulation capacity? Can some populations give rise to both hepatocytes and bile duct epithelium? Are different populations required for short-term repopulation (rapid growth for a limited number of cell divisions) and long-term repopulation (capacity for >100 cell doublings)? The answers to these questions will be important in using liver cell transplantation for treatment of human patients. In contrast to bone marrow transplantation, the number of liver cells that can be injected into a patient is limited by the occurrence of cell emboli and portal hypertension. 31,32 It therefore would be highly advantageous to remove nonrepopulating cells and only transplant cells with a high capacity for repopulation and cell division. In the current study we have addressed some of these issues in the tyrosinemic mouse model of therapeutic liver repopulation.
Competitive repopulation with sorted fractions of liver cells has not been previously reported. We chose size sorting because small, periportal hepatocytes had been previously reported to proliferate preferentially in some experimental settings. 26 Our results clearly show that, contrary to our expectations, small, mononucleated hepatocytes repopulated significantly less well than larger hepatocytes. The elutriated 16-μm population contained ∼15% of larger cells. It is therefore even possible that small hepatocytes don’t repopulate at all and that the entire repopulation achieved by this fraction was due to contaminating larger cells. The reason for the lower repopulation capacity of this fraction currently is not known. Liver repopulation is a complex process that involves homing to the liver, entry through the sinusoids, migration and integration into the parenchyma, and subsequent cell division. Therefore, the data presented here cannot simply be interpreted to indicate a lower capacity for cell division in the small cells. They may home less efficiently or not possess the ability to integrate into the parenchyma. Additional experiments directed at measuring these parameters will be needed to determine the precise mechanism of the lower repopulation efficiency. Although the largest cells (27 μm) repopulated slightly less well than 21-μm hepatocytes, the difference between the two groups was small and not significant statistically. The most abundant size fraction constituting approximately two-thirds of all cells isolated by collagenase perfusion had the best repopulation properties. Thus, cell sorting before transplantation is not required at least for short-term repopulation.
It is important, however, to make some qualifications regarding the interpretation of these results. First, our experiments addressed only one round of repopulation, representing approximately 15 cell divisions. It is possible that serial transplantation of sorted populations that addresses the issue of long-term regenerative capacity may yield different results. Second, it is possible that size fractionation alone is not capable of enriching for the most regenerative liver cells and that a subpopulation with this property exists within the 21-μm fraction. Third, we excluded nonparenchymal cells from the current experiments. It is conceivable that liver progenitor cells reside within this fraction.
The results obtained with competitive repopulation between naïve and serially transplanted liver cells were surprising. Before the experiments, we had considered two possible scenarios: first, serial transplantation may enrich for more highly regenerative cells and these populations would therefore outcompete “normal” hepatocytes; second, multiple cell divisions may age the cells so that naïve hepatocytes would dominate. Our data supported neither hypothesis. Serial transplantation neither enhanced nor diminished the repopulation capacity of the cells to any significant degree. One interpretation is that virtually all cells in the transplanted fraction (ie, all hepatocytes) have stem cell-like regenerative capacity. Alternatively, the ratio between stem/progenitor cells and differentiated cells may be kept very constant by a regulatory mechanism and may not be altered by serial transplantation. Repopulated livers would reestablish the same ratio as naïve livers. In either case the data support the notion that therapeutic repopulation by unsorted adult liver cell suspensions does not impair the regenerative capacity of the repopulated liver. This is promising for the use of this procedure in a clinical setting. In addition, this result indicates that cell division itself does not limit the regenerative capacity of hepatocytes and that it may be possible to expand hepatocytes in vitro manyfold before use in transplantation. In this regard, however, it is important to caution that mouse cells have telomeres which are longer than those of human cells, so our results may not be directly applicable to humans. 33 We have not yet measured the telomere length in serially transplanted cells or determined whether hepatocytes express telomerase activity in some settings. The lack of age effect on repopulation ability observed here appears inconsistent with the delayed response to partial hepatectomy observed in older animals. 34,35 It is possible that the altered response in these studies was due to age effects on nonhepatocytes involved in liver regeneration (stellate cells for example). Alternatively, serial transplantation may not accurately mimic in situ aging of cells. Competitive repopulation with hepatocytes harvested from old and young animals will be needed to address this question directly.
The retroviral marking studies reported here were performed with relatively high numbers of marked transplanted cells. Most experiments produced the consistent result that only polyclonal repopulation was observed when 10,000 or 100,000 marked cells were serially transplanted. This indicates that the serially transplantable population of liver cells is considerably more common than 1:10,000. This applies to cells retrovirally transduced in tissue culture as well as to those marked in vivo. Interestingly, we observed one example of initial polyclonal repopulation becoming monoclonal on the second round of serial transplantation. Although this observation cannot be interpreted in terms of the frequency of serially repopulating cells, it illustrates the fact that a single adult liver cell has the regenerative capacity to serially reconstitute several generations of mouse livers. The retroviral marking studies reported here need to be refined in the future by reducing the number of marked cells to 100 to 1,000 until clonality can be detected. Because we used a Moloney murine leukemia virus-based vector, it can be argued that we preferentially labeled cells that divide rapidly after partial hepatectomy or after plating in tissue culture. Future experiments will therefore have to include marking of quiescent cells with lentiviral vectors.
Taken together the results reported here indicate that the ability to produce one round of liver repopulation resides in the majority of adult mouse hepatocytes and not a rare stem cell population.
Acknowledgments
We thank Angela Major and Billie Smith for excellent technical support. The antibody for FAH was a generous gift from Dr. Robert Tanguay, Universite Laval, Quebec City, Canada. The CK-19 antibody was provided by Dr. Lucie Germain, Universite Laval, Quebec City, Canada. The transgenic D2 receptor knockout mice were a gift from Malcolm Low, Oregon Health Sciences University, Portland, OR.
Footnotes
Address reprint requests to Dr. Markus Grompe, Medical and Molecular Genetics L103, Oregon Health Sciences University, 3181 SW Sam Jackson Park Road, Portland, OR 97201. E-mail: grompem@ohsu.edu.
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK 51592 (to M. G. and M. F.) and NIDDK National Research Service Award DK009429 (to K. O.).
References
- 1.Michalopoulos GK, DeFrances MC: Liver regeneration. Science 1997, 276:60-66 [DOI] [PubMed] [Google Scholar]
- 2.Alison MR, Golding M, Sarraf CE: Liver stem cells: when the going gets tough they get going. Int J Exp Pathol 1997, 78:365-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C: Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol 1997, 26:343-352 [DOI] [PubMed] [Google Scholar]
- 4.Grompe M, Al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P: Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 1993, 7:2298-2307 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GA, Grompe M, Lambert M, Tanguay RM: Hypertyrosinemia. Scriver CR Beaudet AL Sly W Valle D eds. The Metabolic Basis of Inherited Disease, 1999, vol. 1.:pp 1077-1106 McGraw-Hill, New York [Google Scholar]
- 6.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M: Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 7.Kvittingen EA, Rootwelt H, Berger R, Brandtzaeg P: Self-induced correction of the genetic defect in tyrosinemia type I. J Clin Invest 1994, 94:1657-1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M: Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet 1995, 10:453-460 [DOI] [PubMed] [Google Scholar]
- 9.Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M: Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol 1997, 151:1273-1280 [PMC free article] [PubMed] [Google Scholar]
- 10.Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T: Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology 1997, 25:329-334 [DOI] [PubMed] [Google Scholar]
- 11.Alpini G, Phillips JO, Vroman B, LaRusso NF: Recent advances in the isolation of liver cells. Hepatology 1994, 20:494-514 [PubMed] [Google Scholar]
- 12.Harrison DE, Astle CM: Loss of stem cell repopulating ability upon transplantation: effects of donor age, cell number, and transplantation procedure. J Exp Med 1982, 156:1767-1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cepko CL, Ryder EF, Austin CP, Walsh C, Fekete DM: Lineage analysis using retrovirus vectors. Methods Enzymol 1993, 225:933-960 [DOI] [PubMed] [Google Scholar]
- 14.Friedrich G, Soriano P: Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 1991, 5:1513-1523 [DOI] [PubMed] [Google Scholar]
- 15.Whitney MA, Royle G, Low MJ, Kelly MA, Axthelm MK, Reifsteck C, Olson S, Braun RE, Heinrich MC, Rathbun RK, Bagby GC, Grompe M: Germ cell defects and hematopoietic hypersensitivity to γ-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood 1996, 88:49-58 [PubMed] [Google Scholar]
- 16.Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ: Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 1997, 19:103-113 [DOI] [PubMed] [Google Scholar]
- 17.Schulz A, Ort O, Beyer P, Kleinig H: SC-0051, a 2-benzoyl-cyclohexane-1,3-dione bleaching herbicide, is a potent inhibitor of the enzyme p-hydroxyphenylpyruvate dioxygenase. FEBS Lett 1993, 318:162-166 [DOI] [PubMed] [Google Scholar]
- 18.Grompe M, Jones SN, Loulseged H, Caskey CT: Retroviral-mediated gene transfer of human ornithine transcarbamylase into primary hepatocytes of spf and spf-ash mice. Hum Gene Ther 1992, 3:35-44 [DOI] [PubMed] [Google Scholar]
- 19.Ponder KP, Gupta S, Leland F, Darlington G, Finegold M, DeMayo J, Ledley FD, Chowdhury JR, Woo SL: Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci USA 1991, 88:1217-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overturf K, Al-Dhalimy M, Manning K, Ou CN, Finegold M, Grompe M: Ex vivo hepatic gene therapy of a mouse model of hereditary tyrosinemia type I. Hum Gene Ther 1998, 9:295-304 [DOI] [PubMed] [Google Scholar]
- 21.Darlington GJ, Kelley JH, Buffone GJ: Growth and hepatospecific gene expression of human hepatoma cells in a defined medium. In Vitro Cell Dev Biol 1987, 23:349-354 [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T (editors): Molecular Cloning: A Laboratory Manual., Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press, 1989
- 23.Labelle Y, Puymirat J, Tanguay RM: Localization of cells in the rat brain expressing fumarylacetoacetate hydrolase, the deficient enzyme in hereditary tyrosinemia type 1. Biochim Biophys Acta 1993, 1180:250-256 [DOI] [PubMed] [Google Scholar]
- 24.Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L: Keratin 19 as a biochemical marker of skin stem cells in vivo, and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci 1996, 109:1017-1028 [DOI] [PubMed] [Google Scholar]
- 25.Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, Tanguay RM, Germain L: Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem 1998, 46:1291-1301 [DOI] [PubMed] [Google Scholar]
- 26.Bucher NLR, Swaffield MN: The rate of incorporation of labeled thymidine into the deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res 1964, 240:1611-1625 [PubMed] [Google Scholar]
- 27.Goff SP: Genetics of retroviral integration. Annu Rev Genet 1992, 26:527-544 [DOI] [PubMed] [Google Scholar]
- 28.Grompe M, Laconi E, Shafritz DA: Principles of therapeutic liver repopulation. Semin Liver Dis 1999, 19:7-14 [DOI] [PubMed] [Google Scholar]
- 29.Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL: Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 1991, 66:245-256 [DOI] [PubMed] [Google Scholar]
- 30.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA: Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol 1998, 153:319-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC: Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 1998, 338:1422-1426 [DOI] [PubMed] [Google Scholar]
- 32.Grossman M, Raper SE, Kozarsky K, Stein EA, Engelhardt JF, Muller D, Lupien PJ, Wilson JM: Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolemia. Nat Genet 1994, 6:335-341 [DOI] [PubMed] [Google Scholar]
- 33.Prowse KR, Greider CW: Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci USA 1995, 92:4818-4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucher N, Swaffield M, DiTroia J: The influence of age upon the incorporation of thymidine-2-C14 into the DNA of regenerating rat liver. Cancer Res 1964, 24:509-512 [PubMed] [Google Scholar]
- 35.Beyer HS, Sherman R, Zieve L: Aging is associated with reduced liver regeneration and diminished thymidine kinase mRNA content and enzyme activity in the rat. J Lab Clin Med 1991, 117:101-108 [PubMed] [Google Scholar]