Abstract
Recently it has been shown that epithelial cell expression of the estrogen receptor (ER) and that of the proliferation-associated marker Ki-67 are almost mutually exclusive in the normal premenopausal human breast but that coexpression frequently occurs in estrogen receptor-positive (ER+) breast cancers. This coexpression may indicate disordered expression of ER in the cell cycle or failure to suppress division of ER+ cells and could be important in neoplastic transformation. The purpose of this study was to determine whether in situ proliferations known to be associated with different levels of risk for developing breast cancer contain these coexpressing cells and, if so, the stage at which they occur. We found that ER+ proliferating cells were rare in premenopausal lobules but increased with age in the normal breast. There was no difference in nonlesional tissue between cancerous and noncancerous breasts. The percentage of dual-expressing cells was significantly increased, however, in all of the in situ proliferations and correlated positively with the level of risk of developing breast cancer. We suggest that development of at least some human breast cancers is associated with increasing failure to down-regulate ER as cells enter the cycle or to suppress division of ER+ cells. The mechanism may involve the loss of a tumor suppresser gene.
Estrogen is thought to be important in the pathogenesis of breast cancer and is associated with most of the epidemiological risk factors associated with the disease. 1 Furthermore, the antiestrogen tamoxifen decreases proliferation in breast cancers, 2,3 although its role in preventing the disease is in dispute at present. 4 Estrogen is also associated with epithelial proliferation in the noncancerous breast during the menstrual cycle 5,6 and in pregnancy 7 and acts on cells via the estrogen receptor (ER). Recently Clarke 8 has shown that there is almost mutual exclusion of steroid receptor expression and cell proliferation in normal human breast tissue, judged by the lack of dual immunostaining for ER and the human Ki-67 proliferation-associated nuclear antigen, which is usually demonstrable in the late G1, S, G2, and M phases of the cell cycle. This relationship, however, is lost in some breast cancers, in which a variable percentage of proliferating cells are estrogen receptor positive (ER+). This coexpression may therefore indicate disordered control of cell division in ER+ cells or disordered regulation of ER in dividing cells and could represent an important pathogenetic step in the development of breast cancer. We have previously shown that the percentage of ER+ cells within precancerous lesions correlates with the risk attributed to them in prospective studies. 9 Furthermore, a fundamental change appears to occur between hyperplasia of usual type (without atypia, HUT) and atypical ductal hyperplasia (ADH). The percentage of ER+ cells in the former type of lesion increases with age, as it does in the normal breast, whereas in ADH it is high at all ages, as it is in ER+ ductal carcinoma in situ (DCIS). This suggests that regulation of ER expression or control of numbers of ER+ cells escapes from the normal age-related regulatory mechanisms at the ADH stage. 9 The purpose of the present study was to determine whether ER+/Ki-67+ cells occur in precancerous lesions and, if so, whether the proportion relates to the stage of the lesion in the putative pathway from normal to cancer via HUT, ADH, and DCIS.
Materials and Methods
Patients
Blocks and slides of 100 cases spanning a 10-year period were retrieved from the files of the Department of Pathology at the Royal Liverpool University Hospital. Normal breast lobules were assessed in 14 pre- and 14 postmenopausal breasts. Twenty-five cases with lobules showing postmenopausal involution and extensive ER positivity were also studied. The proliferative lesions included 15 HUT, 10 ADH, 21 lobular in situ neoplasia (LIN), 11 DCIS of low nuclear grade (LNG), 12 intermediate nuclear grade (ING), and 10 of high nuclear grade (HNG). Only ER+ cases of HNG DCIS were selected; cases of LNG and ING DCIS were selected solely on morphological criteria, but all proved to be ER+ on subsequent immunostaining. All of the diagnoses were made following the Pathology Guidelines of the UK National Breast Screening Program 10 and were made by two pathologists (B. S. S., J. P. S.). The criteria for diagnosing ADH were those of Page and Rogers. 11 Initially, the lobular in situ proliferations were subclassified into lobular carcinoma in situ and atypical lobular hyperplasia, but there were often significant difficulties in distinguishing the two processes, which frequently merged in the same sections. Furthermore, the results eventually obtained were similar in the two lesions. Some of the blocks studied contained more than one lesion.
Immunostaining Procedure
The method has been described previously. 8 All of the tissue samples were fixed in 10% formalin before processing. The processing schedule included a further 4 hours of fixation in methacarn (a mixture of methanol, chloroform, and glacial acetic acid used to aid fixation and cutting of breast sections) and then routine processing in paraffin wax. The antibody-binding epitopes of the antigens were retrieved by microwave treatment for 15–20 minutes in boiling citric acid (10 mM, pH 6), and the slides were allowed to cool for 20 minutes in the citric acid. The tissue sections were then incubated for 1 hour with the anti-ER antibody 1D5 (Dako Ltd. Ely, Cambridge, UK) diluted 1:75, for 45 minutes with a 1:200 diluted biotinylated horse anti-mouse antibody (Dako Ltd.), and for 30 minutes with a 1:100 diluted streptavidin-Cy3 conjugate (Sigma, Poole, UK). The slides were then incubated for 1 hour with 1:125 diluted polyclonal rabbit anti-human Ki-67 antibodies (Ki-67p; Novocastra, Newcastle upon Tyne, UK) and for 1 hour with a 1:25 diluted fluorescein-conjugated goat anti-rabbit antibody (Sigma) to visualize proliferating cells. All incubations were at room temperature, and washes in phosphate-buffered saline were performed in between. DNA was stained by immersion in a solution of 4′,6-diamidino-2-phenylindole (Sigma) at a concentration of 250 ng/ml in phosphate-buffered saline for 10 minutes, and coverslips were mounted on the tissue sections in an antifading medium (Vectashield; Vector Laboratories). Control slides were included in each analysis by performing the same procedures and substituting nonimmune serum for primary antibodies and secondary antibodies individually.
Assessment of Immunostaining
Quantification of the fluorochrome-labeled cells was performed by either scoring the entire lesion or between 1000 and 4000 cells across several representative fields (chosen using a 4′,6-diamidino-2-phenylindole filter), depending on the size of the lesion. Each field was examined under a high-power lens for the red (Cy3), green (fluorescein), and blue (4′,6-diamidino-2-phenylindole) fluorochromes, using the appropriate filters in succession to assess the presence or absence of double-labeled cells. A triple-band filter in which all three fluorochromes could be seen simultaneously was used for confirmation of dual staining. If normal breast tissue was present around the lesions studied, then the total number of Ki-67+ cells and the number of Ki-67/ER+ cells were also recorded within the normal areas.
The data were analyzed by the Pearson product moment correlation coefficient and the nonparametric Mann-Whitney and Kruskal-Wallis tests, using SPSS software for Windows (release 6.1).
Results
Normal Female Breast Tissue
In breast lobules with a normal premenopausal appearance the percentages of ER+ (6.8%) and Ki-67+ (2.6%) cells were similar to those found in previous studies. 5,6,8 The results are summarized in Table 1 ▶ , where for all normal and pathological categories the percentages of cells staining for each marker and for both are given. Also given are the percentages of double-labeled cells that would be expected if the two variables were independent. This was calculated by multiplying the percentage of ER+ and Ki-67+ cells and then dividing by 100 for each lesion. The actual number of dual-positive cells and the number expected were then compared using the Mann-Whitney test. The observed/expected (O/E) ratio gives an indication of whether, in any of the lesions studied, the two markers were positively or negatively associated with each other and of the strength of the association. In the former case, values greater than 1 would be expected and in the latter case, values less than 1. In Table 1 ▶ , the significant P values are all found in cases with a negative association between the two markers.
Table 1.
Coexpression of ER and Ki-67 in Normal Breast and Precancerous Breast Lesions
| Morphology | Mean age (SD) | No. of cases | Mean No. cells counted (SD) | Mean % ER positive (SD) | Mean % Ki-67 positive (SD) | Mean % dual positive (SD) | Mean % dual positive expected (SD) | P values for observed vs expected (Mann-Whitney test) | Mean observed/expected (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Premenopausal lobule* | 42 (10) | 14 | 1475 (885) | 6.8 (5.4) | 2.6 (3.1) | 0.01 (0.02) | 0.17 (0.37) | 0.0003 | 0.05 (0.14) |
| Postmenopausal lobule† | 65 (7.5) | 14 | 1099 (328) | 42 (23) | 0.34 (0.33) | 0.04 (0.05) | 0.11 (0.12) | 0.044 | 0.39 (0.52) |
| 90% ER+ lobule‡ | 54 (12) | 25 | 686 (525) | 92 (2.4) | 0.43 (0.92) | 0.20 (0.61) | 0.39 (0.81) | 0.050 | 0.28 (0.37) |
| Hyperplasia of usual type | 56 (5.3) | 15 | 1141 (579) | 45 (27) | 2.4 (2.7) | 0.42 (0.68) | 0.74 (0.63) | 0.036 | 0.46 (0.48) |
| Atypical ductal hyperplasia | 53 (6.0) | 10 | 1407 (757) | 91 (4.3) | 3.6 (2.8) | 2.8 (2.5) | 3.3 (2.7) | 0.52 | 0.75 (0.29) |
| Ductal carcinoma in situ (LNG) | 60 (11) | 11 | 1671 (719) | 92 (6.3) | 6.4 (5.9) | 5.4 (5.8) | 5.9 (5.6) | 0.55 | 0.83 (0.24) |
| Ductal carcinoma in situ (ING) | 55 (8.7) | 12 | 1767 (981) | 85 (9.5) | 7.7 (9.1) | 5.8 (8.0) | 6.4 (8.1) | 0.54 | 0.89 (0.15) |
| Ductal carcinoma in situ (HNG) | 55 (6.5) | 10 | 1385 (311) | 49 (33) | 14 (9.1) | 4.3 (4.0) | 5.7 (4.4) | 0.34 | 0.66 (0.25) |
| Lobular in situ neoplasia | 53 (8.3) | 21 | 1504 (737) | 78 (8) | 2.1 (2.5) | 1.21 (1.44) | 1.57 (1.84) | 0.51 | 0.76 (0.34) |
*Excludes ducts and lobules exhibiting minor morphological deviations from normal.
†Lobules from postmenopausal women exhibiting morphological evidence of involution.
‡Lobules from postmenopausal women exhibiting involutional changes and more than 90% ER+ cells (see ref. 9 ).
In premenopausal lobules with a completely normal appearance, dual-labeled cells formed 0.01% of all cells counted. The O/E was 0.05, revealing a strong negative association (P = 0.0003) (Figure 1A) ▶ . Of the 20,650 cells counted, only two showed coexpression of ER and Ki-67. Normal postmenopausal lobules showed an increased number of ER+ cells, as we have reported previously, 9 and some exhibiting involutional change were composed almost entirely of ER+ cells. The percentages of Ki-67+ cells in these two types of postmenopausal lobule were significantly lower than those seen in premenopausal lobules (P = 0.004). The percentages of dual-labeled cells were significantly higher at 0.04% and 0.20% of all cells (highest value for P = 0.009); however, the negative association between ER and Ki-67 was still maintained (highest value for P = 0.05 versus 90% ER+ postmenopausal lobules). The mean O/E ratios were also higher at 0.39 and 0.28 (see Table 1 ▶ ).
Figure 1.
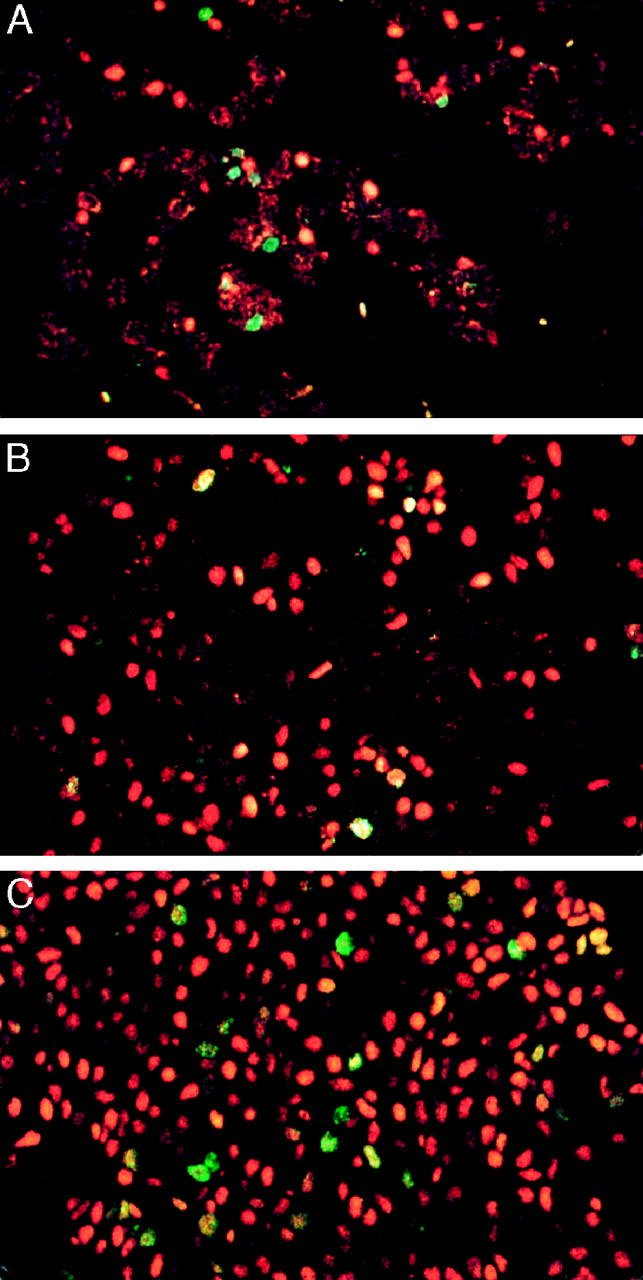
The distribution of estrogen receptor (ER) and Ki-67 antigen in normal breast and in situ proliferations visualized by indirect immunofluorescence, using the fluorochromes Cy3 (orange/red) for ER and fluorescein (green) for Ki-67 antigen. Dual-labeled cells have a speckled orange/green or yellow appearance. A: A normal breast lobule showing mutual exclusion between ER and Ki-67. B: Hyperplasia of the usual type containing occasional dual-labeled cells. C: Low nuclear grade ductal carcinoma in situ containing numerous dual labeled cells. Magnification, ×400.
The percentage of dual-labeled cells in nonlesional tissue was then related to the pathological changes present elsewhere in the breast. A more rapid method of assessment was used for this part of the investigation, in which whole sections were screened for Ki-67+ cells. The percentage of Ki-67+ cells that were also positive for ER was then determined. For normal breast tissue surrounding HUT and benign breast lesions, ADH, and in situ neoplasias (LIN, DCIS), 5.5%, 6.7%, and 9.1%, respectively, of Ki-67+ cells were ER+, but the differences were not statistically significant (P = 0.86).
The values obtained for the percentages of Ki-67+ that were ER+ were also related to patient age, regardless of pathological changes observed. Ducts and lobules showing minor (nonlesional) deviations from normality were included to maximize the number of cells. A significant correlation was observed (r = 0.26, P = 0.016).
Proliferative Lesions
The mean percentage of ER+ cells varied from 45% for HUT to 92% for LNG DCIS and was significantly greater for all in situ proliferations when compared with normal premenopausal lobules. The proportion of Ki-67+ cells varied from 2.1% for LIN to 14% for HNG DCIS, with only DCIS (all nuclear grades) having a statistically higher percentage than normal premenopausal lobules. Dual-labeled cells were significantly more numerous in all proliferative lesions than normal pre- and postmenopausal lobules (highest value for P = 0.015 for >90% ER+ postmenopausal lobule versus HUT) (Figure 1, B and C) ▶ . HUT had a significantly lower percentage of dual-positive cells than all of the other proliferations (HUT versus ADH, P = 0.002), and also unlike them HUT maintained the negative association between ER and Ki-67 (P = 0.035) (data shown in Table 1 ▶ ).
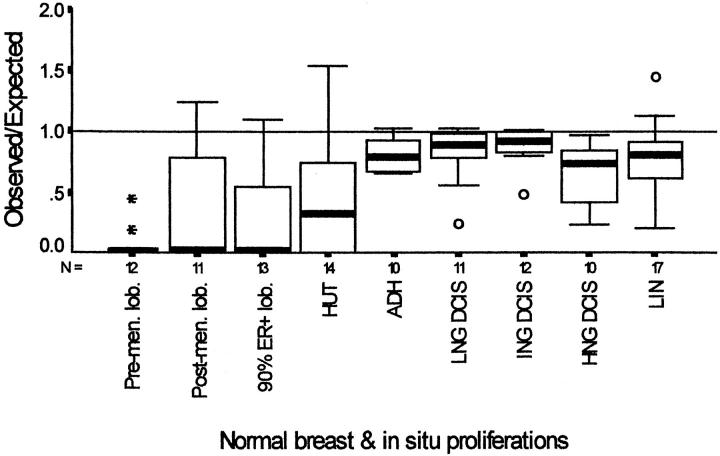
O/E ratios are compared in Figure 2 ▶ , where the box plots show an increase with the degree of atypia, except for HNG DCIS, for which the value declined. The O/E values were significantly lower for premenopausal lobules in comparison with the different in situ proliferations (highest value for P = 0.005 for premenopausal lobules versus HUT). However, statistical significance was not achieved when the in situ proliferations were compared with normal postmenoapusal lobules. In no case was the median value greater than 1.
Figure 2.
Boxplot graphs of observed/expected values plotted for ER+ Ki-67+ cells in normal breast and in situ proliferations. The boxes contain the values between the 25th and 75th percentiles; the lines across the boxes indicate the medians; the whiskers extend to the highest and lowest values excluding outliers. ° and * identify outliers and extreme values. Pre-men. lob, premenopausal lobules excluding ducts and lobules exhibiting minor morphological deviations from normal. Postmen. lob, lobules from postmenopausal women exhibiting morphological evidence of involution; 90% ER+lob, lobules from postmenopausal women exhibiting involutional changes and more than 90% ER+ cells; HUT, hyperplasia of usual type; ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ; LNG, low nuclear grade; ING, intermediate nuclear grade; HNG, high nuclear grade; LIN, lobular in situ neoplasia.
Discussion
In this study we have confirmed that estrogen receptor-expressing proliferating (ER+/Ki-67+) cells are very rare in morphologically normal premenopausal breast lobules. 8 We have also confirmed that ER+ cells increase and Ki-67+ cells decline with age. Despite this inverse relationship, however, the proportion of dual-expressing cells in morphologically normal breast tissue increases, reaching an average of about 11% of Ki-67+ cells after menopause. The percentage of dual-positive cells shows a statistically significant increase in all of the in situ proliferations studied in comparison to normal lobules. The proportion of dual-labeled cells correlates with the risk attributed to such lesions in prospective studies. 12,13 Of these lesions HUT (low-risk lesion) contains the smallest percentage of dual-positive cells, whereas DCIS-ING (high-risk lesion) contains the highest. This suggests that dual expression of ER and Ki-67 may be the manifestation of an important early molecular change in the development of malignant breast neoplasia and that the severity of the change is a major determinant of risk.
Expressing the results as O/E ratios gives further insight. Although the ratio increased in proportion to the risk associated with the lesion on which it was calculated, the median values never exceeded unity. The negative association between ER and Ki-67 seen in normal lobules was lost, but coexpression was not seen with greater frequency than would be expected by chance if the two markers were expressed independently of each other. Put another way, ER+ cells were no more likely to be dividing than ER negative cells, even in high-risk lesions. These in situ proliferations are thus characterized by a lack of suppression of ER expression as cells enter the cycle or of ER+ cells entering the cycle rather than a predisposition for ER+ cells to divide. This would be consistent with the loss of a tumor suppresser gene. Loss of heterozygosity (LOH) at many loci is common in ADH, DCIS, and LIN 14-16 and infrequent in HUT, 17 where the negative association between the two markers is generally maintained. In addition, there are in vitro data which show that in normal ER-negative breast cells transfected with ER, estrogen suppresses the proliferation of these cells, 18 and indeed a putative tumor suppresser gene that may have this function has been suggested. 19
A second point of interest is that the O/E ratio was less abnormal in HNG DCIS than in non-HNG DCIS, suggesting that the latter is unlikely to evolve into the former. This contention is supported by other molecular evidence. High-grade in situ and invasive breast carcinomas show lower incidences of ER expression and a greater tendency to express type I growth factor receptors, such as EGFR and c-erbB-2. Recently, it has been shown, using comparative genomic hybridization, that there is a higher frequency of 16q losses in non-HNG DCIS than in high-grade cases, arguing strongly in favor of separate histogenetic pathways. 20
The percentage (although not absolute numbers) of ER+ proliferating cells was found to increase with age, despite the reduced number of Ki-67+ cells. The negative association between ER and Ki-67 was maintained but was weaker. This does not exclude disproportionate coexpression of these markers as an early premalignant change. First, the incidence of breast cancer in the population of women studied is high and increases dramatically with age. Second, most of these postmenopausal tumors are ER+. Third, the material we studied comprised surgical biopsy and resection specimens containing screen-detected abnormalities or specimens from symptomatic patients. Normal autopsy tissue from women with no history of breast disease was not included. It seems unlikely, however, that the percentage of ER+/Ki-67+ in nonlesional breast tissue could be used to assess cancer risk, as significant differences were not detected between breasts with and without DCIS. Characterization of the cell signaling pathways maintaining the normal relationship between ER and cell proliferation could lead to the identification of molecules whose abnormal expression may help in the recognition of early breast neoplasia and better assessment of cancer risk.
Acknowledgments
We are especially grateful to the Cancer and Polio Research Fund, which provided a grant to B. S. S., and for the support and facilities provided by the Clatterbridge Cancer Research Trust.
Footnotes
Address reprint requests to Prof. John P. Sloane, Pathology Department, The Duncan Building, Daulby Street, Liverpool L69 3GA, UK. E-mail: j.p.sloane@Liverpool.ac.uk.
Supported by a grant from the Cancer Research and Polio Fund.
References
- 1.Kelsey JL, Gammon MD, John EM: Reproductive and hormonal risk factors: reproductive factors and breast cancer. Epidemiol Rev 1993, 15:36-47 [DOI] [PubMed] [Google Scholar]
- 2.Johnston SR, MacLennan KA, Sacks NP, Salter J, Smith IE, Dowsett M: Modulation of Bcl-2 and Ki67 expression in estrogen receptor positive human breast cancer by tamoxifen. Eur J Cancer 1994, 30A:1663-1669 [DOI] [PubMed] [Google Scholar]
- 3.Howell A: Clinical evidence for the involvement of estrogen in the development and progression of breast cancer. Proc R Soc Edinb 1989, 95B:49-57 [Google Scholar]
- 4.Pritchard KI: Is tamoxifen effective in prevention of breast cancer? Lancet 1998, 252:80-81 [DOI] [PubMed] [Google Scholar]
- 5.Williams G, Anderson E, Howell A, Watson R, Coyne J, Roberts SA, Potten CS: Oral contraceptive (OCP) use increases proliferation and decreases estrogen receptor content of epithelial cells in the normal human breast. Int J Cancer 1991, 48:206-210 [DOI] [PubMed] [Google Scholar]
- 6.Battersby S, Robertson BJ, Anderson TJ, King RJB, McPherson K: Influence of menstrual cycle, parity and oral contraceptive use on steroid hormone receptors in normal breast. Br J Cancer 1992, 65:601-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson TJ, Battersby S: The involvement of estrogen in the development and function of the normal breast: histological evidence. Proc R Soc Edinb 1989, 95B:23-32 [Google Scholar]
- 8.Clarke RB, Howell A, Potten CS, Anderson E: Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 1997, 57:4987-4991 [PubMed] [Google Scholar]
- 9.Shoker BS, Jarvis C, Sibson R, Walker C, Sloane JP: Estrogen receptor expression in the normal and pre-cancerous breast. J Pathol 1999, 188:237-244 [DOI] [PubMed] [Google Scholar]
- 10.National Co-ordinating Group for Breast Screening Pathology: Pathology Reporting in Breast Cancer Screening, ed 2, NHSBSP publication no. 3. Revised February 1997
- 11.Page DL, Rogers LW: Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol 1992, 23:1095-1097 [DOI] [PubMed] [Google Scholar]
- 12.Dupont WD, Page DL: Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985, 312:146-151 [DOI] [PubMed] [Google Scholar]
- 13.London SJ, Connolly JL, Schnitt SJ, Colditz GA: A prospective study of benign breast disease and the risk of breast cancer. JAMA 1992, 267:941-944 [PubMed] [Google Scholar]
- 14.Stratton MR, Collins N, Lakhani SR, Sloane JP: Loss of heterozygosity in ductal carcinoma in situ of the breast. J Pathol 1995, 175:195-201 [DOI] [PubMed] [Google Scholar]
- 15.Lakhani SR, Collins N, Stratton MR, Sloane JP: Aypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol 1995, 48:611-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakhani SR, Collins N, Sloane JP, Stratton MR: Loss of heterozygosity in lobular carcinoma in situ of the breast. J Clin Pathol 1995, 48:M74-M78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakhani SR, Slack DN, Hamoudi RA, Collins N, Stratton MR, Sloane JP: Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab Invest 1996, 74:129-135 [PubMed] [Google Scholar]
- 18.Zajchowski DA, Sager R, Webster L: Estrogen inhibits the growth of estrogen receptor-negative, but not estrogen receptor positive human mammary epithelial cells expressing a recombinant estrogen receptor. Cancer Res 1993, 53:5004-5011 [PubMed] [Google Scholar]
- 19.Liu HC, Golder-Novoselsky E, Seto MH, Webster L, McClary J, Zajchowski DA: The novel estrogen-responsive B box protein (EBBP) gene is tamoxifen regulated in cells expressing an estrogen receptor DNA-binding domain mutant. Mol Endocrinol 1998, 12:1733-1748 [DOI] [PubMed] [Google Scholar]
- 20.Buerger H, Boecker W, Otterbach F, Simon R, Poremba C, Diallo R, Decker T, Riethdorf L, Dockhorn-Dworniczak B: Comparative genomic hybridization (CGH) of ductal carcinoma in situ of the breast—evidence of multiple genetic pathways. J Pathol 1999, 187:396-402 [DOI] [PubMed] [Google Scholar]