Abstract
Extranodal mucosa-associated lymphoid tissue (MALT)-type lymphomas and nodal and splenic marginal zone B cell lymphomas (MZBL) share morphological and immunophenotypic features with marginal zone B cells of reactive lymphoid tissues. Although displaying a similar immunophenotype, recent investigations suggest fundamental genetic differences among these subgroups. To determine the prevalence of the t(11;18) in a larger series of MALT-type lymphomas and to investigate a possible occurrence in other lymphomas, we screened 106 non-Hodgkin’s lymphomas (NHL) by interphase cytogenetics using yeast artificial chromosome (YAC) probes flanking the breakpoint at 11q21. A signal constellation indicating a disruption in 11q21 and thus pointing to the presence of the t(11;18) was observed in 9 of 33 (27%) low-grade lymphomas of MALT type. The complete absence of t(11;18)-positive cells in 32 primary and secondary extranodal high-grade lymphomas suggests that low-grade lymphomas of MALT type characterized by the t(11;18) are unlikely to transform into high-grade tumors. The absence of tumor cells carrying the t(11;18) in nodal MZBL challenges the assumption that most, if not all, of these tumors represent the nodal manifestation of a so far undetected low-grade lymphoma of MALT type. The t(11;18) was not detected in a single case of 29 splenic MZBL investigated. This observation strengthens the view that splenic MZBL are biologically different from extranodal MZBL of MALT type.
Marginal zone B cell lymphomas (MZBL) as defined in the REAL classification system 1 comprise a spectrum of nodal and extranodal neoplasms including monocytoid B cell lymphomas, splenic lymphomas, and mucosa-associated lymphoid tissue (MALT)-type lymphomas. These tumors share morphological and immunophenotypic features with marginal zone B cells of reactive lymphoid tissues and have also been suggested to exhibit similar genetic abnormalities and, hence, a common biological background. 2 Recent investigations, however, also revealed fundamental differences, at least on the genetic level. The t(11;18)(q21;q21) is the most frequent structural chromosome aberration in extranodal MALT-type lymphomas, 3,4 whereas it was not encountered in rare classical cytogenetic reports of other types of MZBL. 2,5,6 In contrast to MZBL of MALT type, splenic MZBL frequently display complex karyotypic abnormalities, 2,7 structural alterations, or deletions, in 7q 8,9 and mutations of p53. 10,11 However, no unifying cytogenetic alteration has been identified so far in this subgroup. This holds also true for nodal MZBL, for which even fewer cytogenetic data are available. Moreover, the relationship of nodal MZBL to its extranodal counterparts and even its acceptance as a separate entity is still controversial. 12
The t(11;18)(q21;q21) previously described in single cases of lymphomas termed small lymphocytic lymphoma, diffuse small B cell lymphoma with features of MALT or MALT-type lymphoma 13-16 could be clearly associated with extranodal MZBL of MALT type in two recent cytogenetic series. 3,4 The finding that in all reported cases the t(11;18)(q21;q21) constitutes the sole cytogenetic aberration detected in chromosome banding analysis implies a possible role of this genetic event for lymphomagenesis. Recently, the breakpoint region in 18q21 was delineated by molecular cytogenetics 17 and the translocation was found to involve an inhibitor of apoptosis gene, API2 in 11q21 and a previously unknown gene, MLT, in 18q21. 18 Based on a yeast artificial chromosome (YAC) contig covering the distal long arm of chromosome 11 19 we identified YAC DNA clones flanking the breakpoint region in 11q21. An interphase FISH probe set, reliably indicating a disruption in 11q21, was established and subsequently applied to a total of 106 non-Hodgkin’s lymphomas (NHL), including 33 extranodal low-grade lymphomas of MALT type, 32 extranodal high-grade B cell lymphomas, 29 splenic, and 12 nodal MZBL.
Our study was designed to determine the prevalence of the t(11;18)(q21;q21) in extranodal MZBL of MALT type in a larger series and to investigate a possible occurrence also in related MZBL. The screening of extranodal high-grade lymphomas also contributes to the question whether these cases might represent a transformation of previously detected or undetected t(11;18)-positive low-grade cases.
Materials and Methods
Case Selection
One hundred six cases of non-Hodgkin’s lymphoma (NHL) were selected from the archives of the Institute of Pathology, University of Würzburg, Germany, on the basis of the availability of cytogenetic preparations or frozen tumor tissues. All cases were carefully reviewed and diagnoses were made according to the criteria of the REAL classification system. 1 Briefly, extranodal low-grade MZBL of MALT type (n = 33) were composed of small- to medium-sized tumor cells with a characteristic diffuse and/or marginal zone based infiltration pattern and the formation of lymphoepithelial lesions. Twenty one cases were of gastric origin; five cases involved the salivary glands, two the thyroid gland, three the orbit, and one case each the lung and the tonsil. The immunophenotype of the tumor cells was CD20+, CD22+, IgM+, IgD−, CD5−, CD10−, and CD23−. Thirty-two extranodal high-grade B cell lymphomas (gastric origin, 28; thyroid, 3; lung, 1) contained sheets of blasts with a cytologic spectrum ranging from centroblasts to immunoblasts and plasmablasts. Ten of 28 gastric cases simultaneously exhibited a low-grade component. In all extranodal high-grade lymphomas, morphological and clinical data were consistent with a primary extranodal origin. 4
Splenic MZBL (n = 29) were composed of small- to intermediate-sized lymphocytes mainly surrounding and/or replacing reactive follicle centers and included cases with and without IgD expression. Lymphomas classified as nodal MZBL (n = 12) failed to show histological or clinical evidence for splenic or extranodal involvement.
Fluorescence in Situ Hybridization (FISH)
The interphase FISH assay was established by selecting YAC DNA clones flanking the breakpoint region in 11q21. 19 In particular, YAC clone 805c4 was chosen on the telomeric side; for the region centromeric to the breakpoint, YAC clones 963c8 and 966e4 were pooled to enhance signal intensity. All YAC clones were obtained from CEPH (Paris, France). After amplification of human sequences by Alu-PCR, 20 probes were generated by nick translation with biotin-16-dUTP or digoxigenin-11-dUTP (Roche Diagnostics, Mannheim, Germany). FISH was performed on cytogenetic preparations or tumor cells isolated from frozen tumor tissue according to standard protocols. 21 In normal interphase cells, hybridization resulted in a close spatial relation of the differentially labeled YAC clones leading to two red/green signal pairs per cell. In tumors carrying the t(11;18), a derivative signal constellation with one signal pair and one separate red and green signal per nucleus was observed. To determine the cutoff level in normal interphase nuclei, cytogenetic preparations of five reactive lymph node specimens served as a negative control.
At least 100 (in most cases 200) intact nuclei per slide were evaluated on a Zeiss Axiophot fluorescence microscope (Zeiss, Jena, Germany). Illustrations were performed using the ISIS imaging system (MetaSystems, Altlussheim, Germany).
Conventional Cytogenetic Analysis
In 69 of 106 cases investigated by FISH (17/33 extranodal low-grade MZBL of MALT type, 21/29 splenic MZBL, 9/12 nodal MZBL, 22/32 extranodal high-grade B cell lymphomas) results could be compared with data from conventional G-banding analysis 4 (and unpublished data).
Results
Control Cases
In normal controls a signal constellation indicative of a disruption in 11q21 was observed in 0 to 1.5% of 200 nuclei evaluated per case (mean, 0.8%; standard deviation, 0.51%). The cutoff value for the diagnosis of a breakage in 11q21 and, hence, a probable presence of a t(11;18), was therefore set at 3%, which is above the mean percentage of cells with a false positive signal constellation plus three standard deviations. 22
Tumor Cases
The FISH results of the tumor cases are summarized in Table 1 ▶ . Nine of 33 extranodal low-grade MZBL of MALT type (27.3%) exhibited a disruption in 11q21 with a percentage of cells carrying the respective signal constellation ranging from 15% to 71% (Figure 1) ▶ . No chromosomal breakage in 11q21 could be detected in 22 extranodal high-grade lymphomas without and in 10 extranodal high-grade lymphomas with a concomitant low-grade component. No deletions of one or more hybridization signals were observed in any case with the exception of one MZBL of MALT type which exhibited a del(11)(q21q23) in G-banding analysis 4 and displayed only one signal pair in 22% of cells.
Table 1.
Detection of a Chromosomal Breakage in 11q21 Pointing to the Presence of the t(11;18) in 106 NHL Investigated by FISH
| Diagnosis | No. of cases | Breakage in 11q21 |
|---|---|---|
| Extranodal MZBL of MALT type | ||
| (n = 33) | ||
| Stomach | 21 | 7 |
| Salivary gland | 5 | — |
| Thyroid gland | 2 | 1 |
| Orbit | 3 | — |
| Lung | 1 | 1 |
| Tonsil | 1 | — |
| Extranodal diffuse large B cell NHL | 32 | — |
| Splenic MZBL | 29 | — |
| Nodal MZBL | 12 | — |
Figure 1.
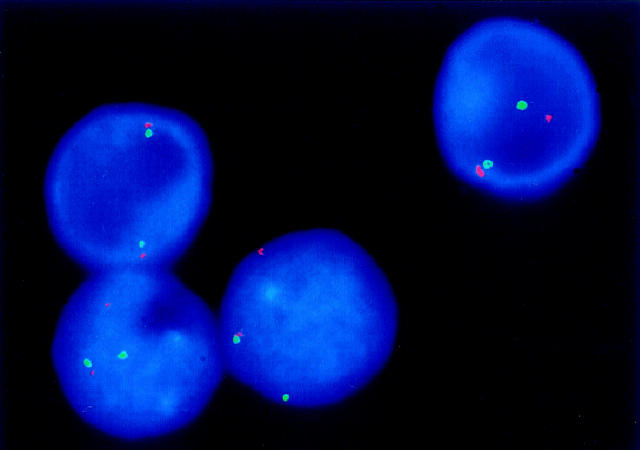
FISH in a t(11;18)-positive extranodal lymphoma of MALT-type with YAC DNA probes flanking the breakpoint in 11q21. One interphase nucleus shows a close spatial relationship of differentially labeled YAC clones indicating two normal chromosomes 11, whereas the other three nuclei exhibit a dissociation of one red/green signal pair, pointing to the presence of the t(11;18).
Correlation between FISH and G-Banding Analysis
By conventional karyotyping, the t(11;18)(q21;q21) had been detected in 5 of 17 cases of extranodal low-grade MZBL of MALT type, 4 whereas it was not encountered in the other subgroups. All five positive cases showed a derivative signal constellation in 16 to 71% of cells evaluated by FISH. In addition, one tumor with only normal karyotypes in G-banding analysis, was found to harbor a chromosomal breakage in 11q21 in 15% of cells. All cases with aberrant chromosome clones, but negativity for the t(11;18) by karyotyping, displayed a normal signal constellation also in the FISH assay.
Discussion
Extranodal low-grade lymphomas of MALT-type have been strongly associated with the translocation t(11;18)(q21;q21) by conventional karyotyping in two recent series. 3,4
In a series of 33 extranodal low-grade lymphomas of MALT-type, 17 of which had been subjected to conventional banding analysis in our previous report, 4 we found a chromosomal breakage in 11q21, pointing to a t(11;18) in nine cases. Thus, the frequency of the t(11;18) in the largest series available to date for this type of lymphoma could be determined at 27%. Overall, FISH data and results obtained in G-banding analysis were in accord. In addition, one case without clonal chromosome aberrations in classical cytogenetics was found to harbor a disruption in 11q21 in 15% of cells as detected in our FISH assay.
The most striking finding from our study is the complete absence of der(11)-positive cells in 32 extranodal high-grade lymphomas, including 10 cases with a concomitant low-grade component (secondary high-grade lymphomas). These results are in accordance with our previous findings obtained by conventional karyotyping and, by using interphase cytogenetics on the cell-by-cell level, exclude the presence of t(11;18)-positive cells in our high-grade cases investigated.
Primary extranodal high-grade lymphomas are characterized on the genetic level by complex karyotypic alterations, 4 frequent rearrangements of the c-myc locus in 8q24 23 and obvious absence of the t(11;18) and have been suggested to exhibit a biological background distinct from secondary extranodal high-grade lymphomas. 24,25 None of the 10 secondary high-grade lymphomas investigated showed evidence of the presence of the t(11;18). The clonal relationship between low-grade and simultaneously present high-grade lymphomas arising in extranodal sites is still a matter of debate. The same immunoglobulin (Ig) light chain restriction in 8 of 9 cases with low- and high-grade components 26 and the reports of identical immunoglobulin heavy chain gene rearrangements in five tumors 27,28 suggest that secondary high-grade lymphomas may result from transformation of the low-grade tumor. On the other hand, a multifocal and multiclonal development of lymphoma could also be demonstrated in different infiltrates of the gastric mucosa. 29,30
Although it cannot be ruled out that the t(11;18) disappeared during tumor progression from low-grade to high-grade lymphoma, one would expect to find the t(11;18), der(11), or der(18) marker chromosomes in at least small subclones in some of the cases. Moreover, loss of a primary chromosomal translocation would represent a rather uncommon finding during tumor progression, because high-grade transformation of low-grade tumors is generally accompanied by the acquisition of additional, secondary genetic alterations, eg, of tumor suppressor genes such as p53 in follicular lymphomas characterized by the primary chromosomal translocation t(14;18)(q32;q21). 31 We conclude, therefore, that extranodal lymphomas of MALT type characterized by the t(11;18) are unlikely to transform into high-grade lymphomas, although they may clinically present with early dissemination or advanced tumor stages. 3,13,16
The t(11;18) was not present in the 12 nodal MZBL investigated. However, there is no general agreement that primary nodal lymphomas with a marginal zone growth pattern comprise a separate entity. It has been suggested that a substantial proportion, if not all of the cases may represent the nodal manifestation of a primary extranodal lymphoma. 32-34 In addition, a primary nodal counterpart of splenic MZBL characterized by absent or attenuated mantle cuffs and negativity of the tumor cells for IgD was recently described. 12 In our cases, after careful re-evaluation, there was no clinical or histological evidence for a primary extranodal origin. Moreover, our group recently observed a high rate of complex karyotypic alterations among nodal MZBL (Ott MM, Rosenwald A, Katzenberger T, Dreyling M, Krumdiek A, Kalla J, Ott G, Müller-Hermelink HK, unpublished manuscript). The negativity of our nodal MZBL for the t(11;18) challenge the assumption that most of these tumors represent the nodal manifestation of a possibly undetected low-grade lymphoma of MALT type. However, data available so far are still too scarce to permit a definite conclusion about whether these lymphomas include cases carrying the t(11;18).
None of 29 cases of splenic MZBL showed a signal constellation indicating a t(11;18). This finding is not unexpected, because splenic MZBL, although related to other marginal zone lymphomas, are classified as a separate entity in the REAL and upcoming WHO classification 35 and differ from MZBL of MALT type with regard to some morphological and immunophenotypical aspects. In splenic MZBL, the tumor infiltrate often occupies a broad area around naked follicle centers in addition to the marginal zone and the tumor cells express IgD, but only rarely CD43. 36,37 In addition to our findings, other alterations on the genetic level, eg, occurrence of complex karyotypes, structural aberrations, or deletions in 7q and 10q 8,9 and alterations of p53 10,11 have been reported that clearly separate splenic MZBL from MZBL of MALT type. Taken together, these observations strengthen the view that splenic MZBL are biologically different from extranodal lymphomas of MALT type.
In summary, we conclude from our study that the t(11;18)(q21;q21) is exclusively found in a subset of extra- nodal low-grade MZBL of MALT-type and that these tumors are unlikely to show transition to high-grade lymphomas. Future studies will have to integrate the biological meaning of the development of t(11;18)-positive cell clones in the evolution of MALT-type lymphomas. In a given tumor the presence of the t(11;18), which can now be detected routinely in interphase cytogenetics, may have prognostic implications. Of particular interest, the significance of the t(11;18) with respect to lymphoma eradication by antibiotic treatment directed against Helicobacter pylori will have to be determined.
Acknowledgments
We thank Mrs. Heike Brückner, Mrs. Andrea Trumpfheller, Mrs. Maria Reichert, and Mrs. Katrin Wildenberger for expert technical assistance, and Mr. Erwin Schmitt for the photographic work.
Footnotes
Address reprint requests to German Ott, M.D., Institute of Pathology, University of Würzburg, Josef-Schneider-Str. 2, 97080 Würzburg, Germany. E-mail: path042@mail.uni-wuerzburg.de.
Supported by the Deutsche Forschungsgemeinschaft (DFG), SFB 172, Teilprojekt C8 to G. O. and H. K. M.-H., DFG grant Ka 1449/1-1 to J. K., and DFG grant Ot 168/1-1 to M. M. O.
References
- 1.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Diebold J, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Müller-Hermelink HK, Pileri SA, Piris MA, Ralfkier E, Warnke RA: A revised American-European classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 2.Dierlamm J, Pittaluga S, Wlodarska I, Thomas J, Moogaerts M, Michaux L, Driessen A, Mecucci C, Cassiman J-J, De Wolf-Peeters C, Van den Berghe H: Marginal zone B-cell lymphomas of different sites share similar cytogenetic and morphologic features. Blood 1996, 87:299-307 [PubMed] [Google Scholar]
- 3.Auer IA, Gascoyne RD, Connors JM, Cotter FE, Greiner TC, Sanger WG, Horsman DE: t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997, 8:979-985 [DOI] [PubMed] [Google Scholar]
- 4.Ott G, Katzenberger T, Greiner A, Kalla J, Rosenwald A, Heinrich U, Ott MM, Müller-Hermelink HK: The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res 1997, 57:3944-3948 [PubMed] [Google Scholar]
- 5.Oscier DG, Matutes E, Gardiner A, Glide S, Mould S, Brito-Babapulle V, Ellis J, Catovsky D: Cytogenetic studies in splenic lymphomas with villous lymphocytes. Br J Haematol 1993, 85:487-491 [DOI] [PubMed] [Google Scholar]
- 6.Slovak ML, Weiss LM, Nathwani BN, Bernstein L, Levine AM: Cytogenetic studies of composite lymphomas: Monocytoid B-cell lymphoma and other B-cell non-Hodgkin’s lymphomas. Hum Pathol 1993, 24:1086-1094 [DOI] [PubMed] [Google Scholar]
- 7.Sole F, Woessner S, Florensa L, Espinet B, Mollejo M, Martin P, Piris MA: Frequent involvement of chromosomes 1, 3, 7 and 8 in splenic marginal zone B-cell lymphoma. Br J Haematol 1997, 98:446-449 [DOI] [PubMed] [Google Scholar]
- 8.Hernandez JM, Mecucci C, Criel A, Meeus P, Van Hoof A, Louwagie A, Scheiff J-M, Michaux L, Boogaerts M, Van den Berghe H: Cytogenetic analysis of B-cell chronic lymphoid leukemias classified according to morphologic and immunophenotypic (FAB) criteria. Leukemia 1995, 9:2140-2146 [PubMed] [Google Scholar]
- 9.Oscier DG, Gardiner A, Mould S: Structural abnormalities of chromosome 7q in chronic lymphoproliferative disorders. Cancer Genet Cytogenet 1996, 92:24-27 [DOI] [PubMed] [Google Scholar]
- 10.Baldini L, Fracchiolla NS, Cro LM, Trecca D, Romitti L, Polli E, Maiolo AT, Neri A: Frequent p53 gene involvement in splenic B-cell leukemia/lymphomas of possible marginal zone origin. Blood 1994, 84:270-278 [PubMed] [Google Scholar]
- 11.Baldini L, Guffanti A, Cro LM, Fracchiolla NS, Colombi M, Motta M, Maiolo AT, Neri A: Poor prognosis in non-villous splenic marginal zone cell lymphoma is associated with p53 mutations. Br J Haematol 1997, 99:375-378 [DOI] [PubMed] [Google Scholar]
- 12.Campo E, Miquel R, Krenacs L, Sorbara L, Raffeld M, Jaffe ES: Primary nodal marginal zone lymphomas of splenic and MALT type. Am J Surg Pathol 1999, 23:59-68 [DOI] [PubMed] [Google Scholar]
- 13.Levine EG, Arthur DC, Machnicki J, Frizzera G, Hurd D, Peterson B, Gajl-Peczalska KJ, Bloomfield CD: Four new recurring translocations in non-Hodgkin lymphoma. Blood 1989, 74:1796-1800 [PubMed] [Google Scholar]
- 14.Griffin CA, Zehnbauer BA, Beschorner WE, Ambinder R, Mann R: t(11;18)(q21;q21) is a recurrent chromosome abnormality in small lymphocytic lymphoma. Genes Chromosomes Cancer 1992, 4:153-157 [DOI] [PubMed] [Google Scholar]
- 15.Horsman D, Gascoyne R, Klasa R, Coupland R: t(11;18)(q21;q21.1): a recurring translocation in lymphomas of mucosa-associated lymphoid tissue (MALT)? Genes Chromosomes Cancer 1992, 4:183-187 [DOI] [PubMed] [Google Scholar]
- 16.Leroux D, Seite P, Hillion J, Le Marc’hadour F, Pegourie-Bandelier B, Jacob MC, Larsen CJ, Sotto JJ: t(11;18)(q21;q21) may delineate a spectrum of diffuse small B-cell lymphoma with extranodal involvement. Genes Chromosomes Cancer 1993, 7:54-56 [DOI] [PubMed] [Google Scholar]
- 17.Stoffel A, Rao PH, Louie DC, Krauter K, Liebowitz DN, Koeppen H, Le Beau MM, Chaganti RSK: Chromosome 18 breakpoint in t(11;18)(q21;q21) translocation associated with MALT lymphoma is proximal to BCL2 and distal to DCC. Genes Chromosomes Cancer 1999, 24:156-159 [PubMed] [Google Scholar]
- 18.Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, De Wolf-Peeters C, Hagemeijer A, Van den Berghe H, Marynen P: The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 1999, 93:3601-3609 [PubMed] [Google Scholar]
- 19.Tunnacliffe A, Jones C, Le Paslier D, Todd R, Cherif D, Birdsall M, Devenish L, Yousry C, Cotter FE, James MR: Localization of Jacobson syndrome breakpoints on a 40-Mb physical map of distal chromosome 11q. Genome Res 1999, 9:44-52 [PMC free article] [PubMed] [Google Scholar]
- 20.Lengauer C, Green ED, Cremer T: Fluorescence in situ hybridization of YAC clones after Alu-PCR amplification. Genomics 1992, 13:826-828 [DOI] [PubMed] [Google Scholar]
- 21.Lichter P, Bentz M, Joos S: Detection of chromosomal aberrations by means of molecular cytogenetics: Painting of chromosomes and chromosomal subregions and comparative genomic hybridization. Meth Enzymol 1995, 254:334-359 [DOI] [PubMed] [Google Scholar]
- 22.Klinger K, Landes G, Shook D, Harvey R, Lopez L, Locke P, Lerner T, Osathanondh R, Leverone B, Houseal T, Pavelka K, Dackowski W: Rapid detection of chromosome aneuploidies in uncultured amniocytes by using fluorescence in situ hybridization (FISH). Am J Hum Genet 1992, 51:55-65 [PMC free article] [PubMed] [Google Scholar]
- 23.Van Krieken JHJM, Raffeld M, Raghoebier S, Jaffe ES, van Ommen GJB, Kluin PM: Molecular genetics of gastrointestinal non-Hodgkin’s lymphomas: unusual prevalence and pattern of c-myc rearrangements in aggressive lymphomas. Blood 1990, 76:797-800 [PubMed] [Google Scholar]
- 24.Chan WY, Wong N, Chan AB, Chow JH, Lee JC: Consistent copy number gain in chromosome 12 in primary diffuse large cell lymphomas of the stomach. Am J Pathol 1998, 152:11-16 [PMC free article] [PubMed] [Google Scholar]
- 25.Ott G, Kalla J, Steinhoff A, Rosenwald A, Katzenberger T, Roblick U, Ott MM, Müller-Hermelink HK: Trisomy 3 is not a common feature in malignant lymphomas of mucosa-associated lymphoid tissue type. Am J Pathol 1998, 153:689-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JKC, Ng CS, Isaacson PG: Relationship between high-grade lymphoma and low-grade B-cell mucosa-associated lymphoid tissue lymphoma (MALToma) of the stomach. Am J Pathol 1990, 136:1153-1164 [PMC free article] [PubMed] [Google Scholar]
- 27.Montalban C, Manzanal A, Castrillo JM, Escribano L, Bellas C: Low-grade gastric B-cell MALT lymphoma progressing into high grade lymphoma. Clonal identity of the two stages of the tumour, unusual bone involvement and leukaemic dissemination. Histopathology 1995, 27:89-91 [DOI] [PubMed] [Google Scholar]
- 28.Peng H, Du M, Diss TC, Isaacson PG, Pan L: Genetic evidence for a clonal link between low and high-grade components in gastric MALT B-cell lymphoma. Histopathology 1997, 30:425-429 [DOI] [PubMed] [Google Scholar]
- 29.Ott MM, Linke B, Gerhard N, Kneba M, Greiner A, Ott G, Müller-Hermelink HK: Characterization of clonal B-cell populations in gastric MALT lymphomas and chronic gastritis by means of the polymerase chain reaction. Verh Dtsch Ges Pathol 1994, 78:302-304 [PubMed] [Google Scholar]
- 30.Greiner A, Müller-Hermelink HK: Recent advances in gastric extranodal B-cell lymphoma. Curr Diagn Pathol 1996, 3:91-98 [Google Scholar]
- 31.Sander CA, Yano T, Clark HM, Harris C, Longo D, Jaffe ES, Raffeld M: p53 mutation is associated with progression in follicular lymphomas. Blood 1993, 82:1994-2004 [PubMed] [Google Scholar]
- 32.Nizze H, Cogliatti SB, von Schilling C, Feller AC, Lennert K: Monocytoid B-cell lymphoma: morphological variants and relationship to low-grade B-cell lymphoma of the mucosa associated lymphoid tissue. Histopathology 1991, 18:403-414 [DOI] [PubMed] [Google Scholar]
- 33.Ortiz-Hidalgo C, Wright DH: The morphological spectrum of monocytoid B-cell lymphoma and its relationship to lymphomas of mucosa-associated lymphoid tissue. Histopathology 1992, 21:555-561 [DOI] [PubMed] [Google Scholar]
- 34.Mollejo M, Menarguez J, Cristobal E, Algara P, Sanchez-Diaz E, Fraga M, Piris MA: Monocytoid B-cells: a comparative clinical pathological study of their distribution in different types of low-grade lymphomas. Am J Surg Pathol 1994, 18:1131-1139 [PubMed] [Google Scholar]
- 35.Jaffe ES, Harris NL, Diebold J, Müller-Hermelink HK: World Health Organization classification of lymphomas: a work in progress. Ann Oncol 1998, 9:25-30 [DOI] [PubMed] [Google Scholar]
- 36.Isaacson PG, Matutes E, Burke M, Catovsky D: The histopathology of splenic lymphoma with villous lymphocytes. Blood 1994, 84:3828-3834 [PubMed] [Google Scholar]
- 37.Mollejo M, Menarguez J, Lloret E, Sanchez A, Campo E, Algara P, Cristobal E, Sanchez E, Piris MA: Splenic marginal zone lymphoma: A distinctive type of low-grade B-cell lymphoma. Am J Surg Pathol 1995, 19:1146-1157 [PubMed] [Google Scholar]