Abstract
Yolk sac tumors (YSTs) are malignant tumors that occur in the gonads of children and young adults, and at extragonadal sites in young children. The histological features of YSTs are variable and can be superimposed on other germ cell tumor histologies. Malignant endodermal cells within YSTs express α-fetoprotein, which can be detected in tumor tissue or serum. However, additional markers of endoderm differentiation would be beneficial for the classification of these tumors. Transcription factor GATA-4 regulates the differentiation and function of murine yolk sac endoderm, and its expression correlates with proliferation and cell survival in certain tissues. To see whether GATA-4 plays a role in human YSTs, we surveyed its expression in human germ cell tumors and cell lines. Northern analysis demonstrated expression of GATA-4 mRNA in four human germ cell tumor lines exhibiting yolk sac endoderm differentiation. GATA-4 protein was detected in eight of nine pediatric YSTs by immunohistochemistry. Three of five immature teratomas exhibited GATA-4 in neural blastematous cells and in cylindrical epithelium, whereas all 16 mature teratomas were devoid of GATA-4. We conclude that GATA-4 is a clinically useful marker of human YSTs and speculate that it may play a role in the maintenance of the malignant phenotype.
Pediatric yolk sac tumors (YSTs) are rare, highly malignant tumors occurring in the gonads of children and young adults, and at extragonadal sites in young children. 1 These tumors are also termed endodermal sinus tumors because of their resemblance to the endoderm sinus of the rodent placenta. 2 Indeed the morphological appearance of these neoplasms recapitulates the structure of the mammalian yolk sac. Pure YSTs exhibit a labyrinthine histology with papillary processes and endoderm-lined cavities, termed Schiller-Duval bodies, supported by a loose stroma. 1 Malignant endoderm cells within YSTs express α-fetoprotein (AFP), and the serum AFP level is frequently elevated in patients with these tumors. 3 However, the histological appearance of YSTs can vary, and small YST foci can be found mixed with other germ-cell tumor histologies, such as teratomas or embryonal carcinomas, creating diagnostic challenges. 1
The molecular mechanisms involved in normal yolk sac development and malignant transformation are not fully understood. Recent studies have implicated transcription factor GATA-4 in the differentiation and function of murine yolk sac endoderm. 4-7 GATA-4 belongs to a family of related zinc finger proteins that regulate gene expression in a variety of organs (reviewed in ref. 8 ). In addition to its role in yolk sac endoderm, GATA-4 appears to regulate transcription in cardiomyocytes, 7,9 enterocytes, 10 and hepatocytes. 11 Within somatic cells of the mouse gonad (ie, granulosa, theca, Sertoli, and Leydig cells), expression of GATA-4 correlates with cell proliferation and survival. 12-14 Given the established role of GATA-4 in murine endoderm differentiation and cellular proliferation, we have now examined the expression of GATA-4 in human YSTs and cell lines. We find that GATA-4 protein is expressed in YSTs originating from either gonadal or extragonadal sites, but not in mature teratomas. We suggest that GATA-4 may serve as a useful nuclear marker of malignant endoderm cells within YSTs.
Materials and Methods
Pediatric Yolk Sac Tumors and Teratomas
Paraffin-embedded, paraformaldehyde-fixed human tissue was originally collected for diagnostic purposes. These samples included nine yolk sac tumors (three gonadal and six extragonadal), one embryonal carcinoma (vaginal), 16 benign teratomas (12 gonadal and four sacrococcygeal), and five immature teratomas (two gonadal and three extragonadal) (see Table 1 ▶ ). Serial sections (5 μm) were stained with hematoxylin-eosin or subjected to immunohistochemistry for GATA-4 or AFP. All tumor histology was reviewed by a designated pathologist (P. H.). The study was approved by the ethical committees of both institutions.
Table 1.
Characteristics of Patients with Teratomas and Composition of These Tumors
| Patient | Tumor | Composition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age at diagnosis and sex | Localization | Histology | Skin + appendages | Cartilage | Bone | Resp. epith. | Muscle | Fat | Conn. tissue | Gut | Neural | Other |
| 1 | 2.7 yrs M | Testis | Ben. | X | X | X | X | ||||||
| 2 | 5.4 yrs M | Testis | Ben. | X | †‡ | ||||||||
| 3 | 4.45 yrs M | Testis | Ben. | X | |||||||||
| 4 | 4.7 yrs F | Ovary | Ben. | X | X | X | |||||||
| 5 | 12.4 yrs F | Ovary | Ben. | X | X | ||||||||
| 6 | 11 yrs F | Ovary | Ben. | X | X | X | |||||||
| 7 | 9.8 yrs F | Ovary | Ben. | X | X | X | X | X | X | X | |||
| 8 | 7.3 yrs F | Ovary | Ben. | X | X | X | |||||||
| 9 | 9.6 yrs F | Ovary | Ben. | X | X | X | X | ||||||
| 10 | 9.75 yrs F | Ovary | Ben. | X | X | X | |||||||
| 11 | 6.4 yrs F | Ovary | Ben. | X | X | X | |||||||
| 12 | 9.8 yrs F | Ovary | Ben. | X | |||||||||
| 13 | 66 d F | SCT | Ben. | X | X | X | † | ||||||
| 14 | 1 d F | SCT | Ben. | X | X | X | X | X | X | †‡ | |||
| 15* | 26 d F | SCT | Ben. | X | X | X | † | ||||||
| 16 | 6 d F | SCT | Ben. | X | X | X | X | X | X | § | |||
| 17 | 11.6 yrs F | Ovary | Imm. | X | X | X | |||||||
| 18 | 3 d F | SCT | Imm. | X | X | X | ‡ | ||||||
| 19 | 1 d F | SCT | Imm. | X | X | X | † | ||||||
| 20 | 1 d F | Abd. cavity | Imm. | X | X | X | † | ||||||
| 21 | 66 d M | Testis | Imm. | X | X | †§ | |||||||
| Total | 14 | 7 | 3 | 5 | 3 | 3 | 16 | 2 | 14 |
SCT, sacrococcygeal teratoma; Abd. cavity, abdominal cavity; Ben., benign; Imm., immature; Resp. epith., respiratory epithelium; Conn. tissue, connective tissue.
*Residual tumor; same patient as no. 14.
†Columnar epithelium.
‡Nonspecific glands.
§Transitional epithelium.
Normal Mouse Yolk Sac Samples
Mouse embryos were obtained by mating male and female B6DJLF1/J mice. For estimating embryonal age, noon of the day on which the copulation plug was found was considered as 0.5 days p.c. Precise staging of dissected embryos was performed using the Atlas of Mouse Development. 15 The yolk sacs an placentas from 6 to 14 days p.c. were collected together with the embryos, fixed with 4% paraformaldehyde, and embedded in paraffin. All experiments on animals were performed according to the National Research Council’s guide.
Human Germ Cell Tumor Lines
Two human germ cell tumor lines, originally derived from testicular yolk sac tumors, 16 were kindly provided by Dr. Pera (Institute of Cancer Research, Surrey, UK). The GCT 72 cell line exhibits properties of visceral endoderm, whereas the GCT 44, GCT 46, and GCT 85 line resemble parietal endoderm.
In Situ Hybridization
Mouse embryos along with the extraembyonic membranes and placenta were washed briefly in phosphate-buffered saline, fixed in 4% paraformaldehyde in PBS, and embedded in paraffin. The sections were subjected to in situ hybridization as described. 17 Tissue sections were incubated with 1 × 10 6 cpm of 33P-labeled (1000–3000 Ci/mmol; Amersham, Life Technologies, Arlington Heights, IL) antisense or sense riboprobe in a total volume of 80 μl. Antisense and sense riboprobes for GATA-4 were prepared as described elsewhere. 4,17
Northern Hybridization
PolyA RNA was isolated from the lysed cells and analyzed for expression of GATA-4 and cyclophilin (used as a loading control) message, using Northern hybridization. 4 One microgram of polyA RNA was subjected to electrophoresis on a 1% denaturing agarose gel and then transferred onto nylon membranes (Hybond N; Amersham, Arlington Heights, IL). To prepare the GATA-4 probe, a partial length human GATA-4 cDNA was used. 18 The cDNA inserts were labeled with [32P]deoxy-CTP (3000 Ci/mmol; Amersham) and Prime-a-gene kit (Promega, Madison, WI). Hybridization and washing of the membranes were performed as previously described. 4 A Fujifilm IP-Reader Bio-Imaging Analyzer BAS 1500 (Fuji Photo Co. Ltd., Tokyo, Japan) was used to analyze the hybridization signal with the MacBas software supplied by the manufacturer, using a Macintosh personal computer (Apple Computer, Cupertino, CA).
Immunohistochemistry
Tissue sections from paraffin-embedded human tissue samples were fixed in 4% paraformaldehyde and subjected to immunohistochemistry, using either a commercial polyclonal anti-mouse GATA-4 IgG (Santa Cruz Biotechnology, Santa Cruz, CA) or affinity-purified polyclonal rabbit anti-mouse GATA-4 4,17 or nonimmune IgG as the primary antibody. Earlier studies by us and others have demonstrated that these antibodies have no cross-reactivity with other GATA-binding proteins. 4,13,17 Immunohistochemistry for AFP was performed using a commercial polyclonal antibody raised against human AFP (Dako, Glostrup, Denmark). A commercially available avidin-biotin immunoperoxidase system was used to visualize bound antibody (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA). 3-Amino-9-ethylcarbazole (Sigma Chemicals, St. Louis, MO) was used as the chromogen, and the development reaction occurred in the presence of 0.03% H202.
Results
Murine Yolk Sac Endoderm, a Tissue with Histological Features Reminiscent of Human YSTs, Expresses GATA-4
The histological similarities between human YSTs and the rodent yolk sac/placenta have been appreciated for decades. 2 In light of these similarities, we explored whether GATA-4, a known marker of yolk sac endoderm in the mouse, plays a role in human YSTs. To illustrate the selective expression pattern of GATA-4 in yolk sac endoderm, we first performed in situ hybridization studies on the developing mouse embryo (Figure 1) ▶ . Consistent with earlier reports, 4 abundant GATA-4 mRNA was observed in the visceral and parietal endoderm of the postimplantation mouse embryo (Figure 1, A–F) ▶ . These two layers of endoderm, derivatives of a transient cell layer termed primitive endoderm, contribute to extraembryonic tissues but not to the embryo proper. 19 In contrast, no GATA-4 mRNA was seen in embryonic ectoderm (Figure 1, A–F) ▶ . After gastrulation, GATA-4 mRNA was detected in nascent mesoderm, including progenitors of the heart (Figure 1, B, C, E, F) ▶ . We found that expression of GATA-4 in the visceral endoderm layer persists throughout gestation. Examination of the chorionic plate of a late gestation mouse embryo (Figure 1, G and H) ▶ demonstrated GATA-4 mRNA in yolk sac endoderm cells within the crypts of Duval, structures analogous to the Schiller-Duval bodies found in human YSTs; in contrast, trophectoderm derivatives within the placenta were devoid of this transcript.
Figure 1.
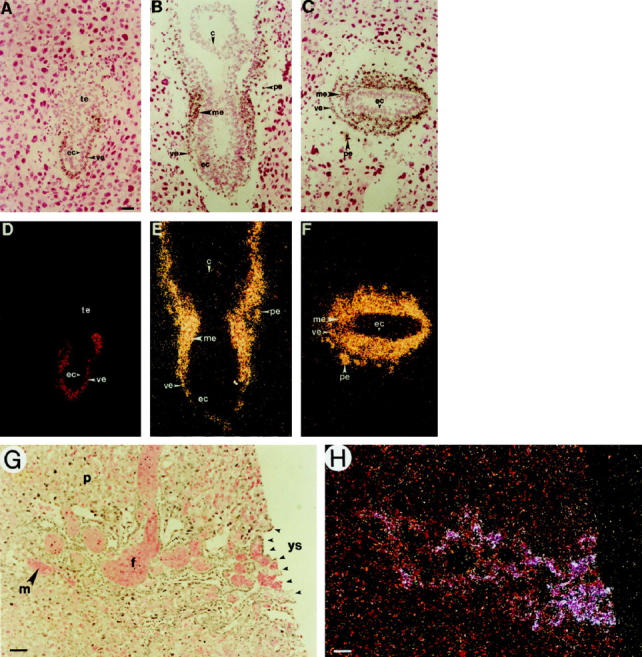
In situ hybridization of GATA-4 mRNA in mouse embryos. Corresponding bright-field (A–C, G) and dark-field (D–F, H) views are shown. A and C: Longitudinal section through a 6-day p.c. embryo. B and E: Longitudinal section through a 7-day p.c. embryo. C and F: Transverse section through a 7-day p.c. embryo. G and H: Section through the chorionic plate of a 14-day p.c. embryo. Note that GATA-4 mRNA is abundantly expressed in the visceral and parietal endoderm of the postimplantation embryo. Later, some expression is seen in the nascent embryonic mesoderm, but not in ectoderm or trophectoderm. In the late gestation embryo, GATA-4 mRNA is expressed by yolk sac endoderm cells within the “Crypts of Duval.” c, chorion; ec, ectoderm; f, fetal blood sinus; m, maternal blood sinus; me, mesoderm (nascent); p, placental cells (decidua); pe, parietal endoderm; te, trophectoderm; ve, visceral endoderm; ys, yolk sac. Original magnification: A–H, ×200. Scale bar = 20 μm.
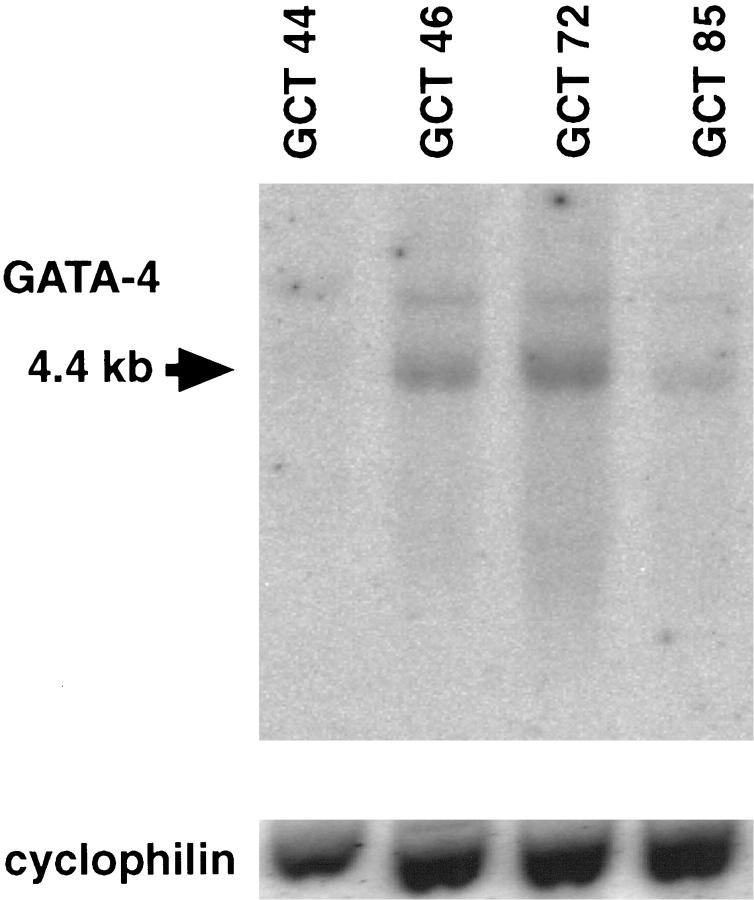
GATA-4 mRNA Is Expressed in Human Germ Cell Tumor Lines Exhibiting Yolk Sac Endoderm Differentiation
Previous studies have shown that murine cell lines exhibiting visceral or parietal endoderm differentiation express GATA-4. 4,5 To see whether human tumor cell lines exhibiting yolk sac endoderm differentiation also express this transcription factor, we performed Northern analysis on RNA from a series of human germ cell tumor lines. The cell line GCT 72 displays properties of visceral endoderm, whereas the GCT 44, GCT 46, and GCT 85 cell lines resemble parietal endoderm. 16 As shown in Figure 2, a ▶ 4-kb GATA-4 transcript comigrating with the transcript found in normal ovary RNA was detected in each of these lines. In contrast, GATA-4 mRNA was not detected in a variety of human tumor cell lines lacking endodermal differentiation, such as HeLa (cervical carcinoma), K562 (erythroleukemia), or HL-60 (promyelocytic leukemia) (data not shown). We conclude that GATA-4 expression is evident in human tumor cell lines exhibiting yolk sac endoderm differentiation.
Figure 2.
Expression of GATA-4 mRNA in cell lines derived from human germ cell tumors. PolyA RNA (1.0 μg) from different cell lines (GCT 44, 46, 72, and 85) was subjected to Northern hybridization analysis with 32P-labeled human GATA-4 and cyclophilin cDNA probes. The arrow indicates the location for GATA-4 (ovarian RNA was used as a control; data not shown).
Human YSTs but Not Mature Teratomas Express GATA-4
To explore the role of GATA-4 in human YSTs, we used immunohistochemistry to compare the expression of GATA-4 and the established marker, AFP, in a series of nine pediatric patients with YSTs, including both gonadal and extragonadal tumors. The clinical, laboratory, and pathological findings in these patients are summarized in Table 2 ▶ . To detect GATA-4 antigen, we used either a commercially available goat polyclonal anti-mouse GATA-4 IgG or, in selected cases, a rabbit polyclonal antibody produced in our laboratory. Identical results were obtained with these antibodies. Previous studies have shown that these antibodies react selectively with GATA-4 and stain the nucleus of murine yolk sac endoderm, cardiomyocytes, granulosa, Sertoli, and Leydig cells. 12,13,17 GATA-4 protein was detected in the nucleus of malignant endoderm cells within the human YST specimens, but not in fibrotic stroma or necrotic areas (Table 2 ▶ , Figure 3 ▶ ). The majority of YSTs examined (eight of nine) expressed GATA-4, and this antigen was detected in both extragonadal (Figure 3B) ▶ and gonadal (Figure 3E) ▶ YSTs. Tumor regions immunoreactive for GATA-4 often expressed cytoplasmic AFP (Figure 3C) ▶ , but the proportion of viable tumors cells staining for GATA-4 and AFP varied among the specimens (Table 2) ▶ . It should be noted that GATA-4 antigen was detected in malignant cells in one YST specimen with negative AFP immunohistochemistry but abundant AFP in the serum (Table 2 ▶ , Patient 3), suggesting that GATA-4 complements other markers. As expected, 13,14 GATA-4 antigen was detected in seminiferous tubules of normal testicular tissue adjacent to foci of YST (Figure 3E) ▶ .
Table 2.
Characteristics of the Patients with Germ Cell Tumors and Results of Immunohistochemistry for AFP and GATA-4
| Patient | Tumor | Immunohisto- chemistry | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Age at diagnosis (years) and sex | Outcome (years after diagnosis) | Localization | Other | Histology | Necrosis/hemorrhage (% of total tumor tissue) | Serum-AFP (mg/L) | AFP GATA-4 positivity (% of viable tumor tissue) | |
| 1 | 11 F | NED (1) | Vagina | EC | 85 /5 | 25,500 | 0 | 0 | |
| 2 | 2.5 F | NED (7) | Pelvis | YST | 5 /5 | 92,700 | 30 | 90 | |
| 3 | 1.3 M | Died (5) | Pelvis | Lung met. | YST | 5 /10 | 48,500 | 0 | 10 |
| 4 | 1.6 M | NED (8) | Pelvis | YST | − /− | 53,200 | 5 | 80 | |
| 5 | 1.0 M | NED (7) | Testis | YST | − /− | 430 | 1–2 | 5 | |
| 6 | 14.5 F | NED (14) | Ovary | Omn. met. | YST | 90 /− | <25 | 1–2 | 5 |
| 7 | 1.5 F | NED (2) | Pelvis | YST | <5 /10 | 16,500 | 1–2 | 0 | |
| 8 | 1.6 F | NED (16) | Pelvis | YST | <5 /− | 9,300 | 15 | 95 | |
| 9 | 2.1 F | NED (1) | Pelvis | Lung met. | YST | 50 /− | 19,954 | 5 | 40 |
| 10 | 4.0 M | NED (2) | Testis | YST | <5 /− | 2,173 | 1–2 | 10 |
NED, no evidence of disease; Lung met., lung metastasis; Omn. met., omentum metastasis; EC, embryonal carcinoma; YST, yolk sac tumor.
Figure 3.
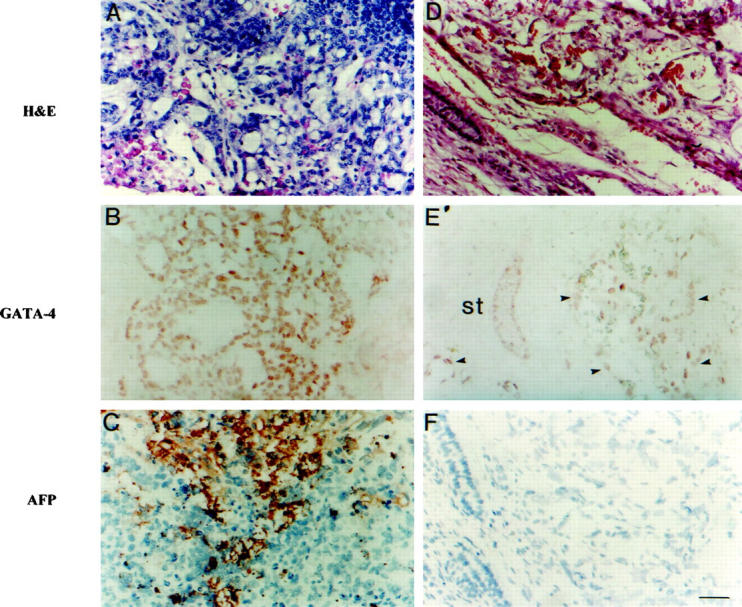
Immunohistochemistry for GATA-4 and AFP in pediatric yolk sac tumors. H&E (A and D), GATA-4 (B and E), and AFP (C and F) stainings from consecutive sections through two separate yolk sac tumors are shown; note the nuclear localization of GATA-4 and cytoplasmic staining for AFP. In addition, a section stained for GATA-4 from a testicular YST demonstrates the presence of GATA-4 protein in normal seminiferous tubules (st) and in the tumor cells (arrowheads). Original magnification, ×200. Scale bar = 50 μm.
To further assess the selectivity of GATA-4 as a marker of malignant yolk sac endoderm differentiation, we performed immunohistochemistry for this antigen on a series of other human germ cell tumor specimens, including benign mature teratomas (n = 16) and immature teratomas (n = 5). The pathological characteristics of these tumors are summarized in Table 1 ▶ . By definition, 1 none of these tumors contained malignant yolk sac endoderm. No GATA-4 antigen was detected in any of the mature teratomas tested, although normal testicular and ovarian tissue adjacent to the tumors in these samples did stain for GATA-4. Within the immature teratomas, only small focal areas of cylindrical epithelium containing goblet cells (three different cases) expressed GATA-4 protein; these same areas were also positive for AFP. Ectodermal or mesodermal derivatives did not express GATA-4, whereas focal positivity was found in three of five samples in the blastematous neural cells. On the basis of these results, we propose that immunohistochemistry for GATA-4, in conjunction with histology and AFP immunochemistry, may be useful for distinguishing malignant YSTs from teratomas.
Discussion
We have shown that GATA-4, a marker of yolk sac endoderm differentiation in rodents, is also a marker of malignant endoderm differentiation in human YSTs and germ cell tumor lines with endodermal features. Immunohistochemical analysis demonstrated that the vast majority (eight of nine) human YSTs express GATA-4 antigen. In contrast, we found that mature teratomas are devoid of this antigen. In general, GATA-4 expression in YST specimens correlates with AFP expression, although in one instance we detected GATA-4 antigen in a bona fide YST in the face of negative AFP immunohistochemistry. Thus GATA-4 is a complementary marker for the detection of extraembryonic differentiation within pediatric germ cell tumors.
Although GATA-4 is a potentially useful marker for pathological classification of germ cell tumors, the presence of this antigen is not pathognomonic for YSTs. Some immature teratomas (three of five) were found to focally express GATA-4 within blastematous cells and cylindrical epithelium. In a similar fashion, AFP antigen, the traditional marker of malignant extraembryonic endoderm, can be detected in embryonal tissue within immature teratomas. 20 Therefore, proper classification of YSTs and other germ cell malignancies continues to require judicious interpretation of both histopathology and tumor markers. 20
GATA-4 has been implicated in the in vitro differentiation 5 and in vivo function 6 of visceral endoderm. Related GATA-binding proteins may also influence endoderm differentiation, based on studies in mouse 19 and lower organisms such as Drosophila and C. elegans. 21 It is tempting to speculate that GATA-binding proteins may have a role in the maintenance of the malignant phenotype in YSTs. It is of interest that recent data have linked GATA-binding proteins with antiapoptotic processes, which often operate in malignant cells. Specifically, down-regulation of GATA-1 is associated with apoptosis in proerythroblasts, 22 and decreased expression of GATA-4 is associated with cardiomyocyte death 9 and ovarian follicular atresia via apoptosis. 12
Although there are limited data on the molecular events leading to the formation of germ cell tumors, several molecules have been implicated in the pathophysiology of these tumors. For example, the tumor suppressor gene product p53 is detected in most adult testicular germ cell tumors and in one-third of pediatric cases, 23 and the overexpression of p53 has been suggested to be a favorable prognostic factor in testicular germ cell tumors. C-kit expression is common in pediatric testicular germ cell tumors, and the c-kit/stem cell factor signaling pathway was speculated to play a role in the initiation and progression of these tumors. 23 Likewise, the platelet-derived growth factor α-receptor has been demonstrated within in situ and occult testicular germ cell tumors, and it may serve as a molecular marker for these tumors. 24 The clinical usefulness of this molecule, however, remains unclear because of the low number of tumors studied. However, none of the above-mentioned molecules has been specifically linked to yolk sac tumors. The detection of GATA-4 mRNA and protein in human endoderm-derived cell lines and YSTs suggests that this transcription factor should be added to the list of molecules potentially involved in the pathogenesis of germ cell malignancies.
Taken together, our results demonstrate that GATA-4 is expressed throughout mammalian yolk sac development. This factor is also expressed in readily detectable amounts in pediatric gonadal and extragonadal yolk sac tumors, providing a new nuclear marker for extraembyonic differentiation in germ cell tumors. Our unpublished studies have revealed that GATA-4 is also expressed in certain other types of gonadal tumors, such as Sertoli and Leydig cell tumors of the testis, and granulosa and theca cell tumors of the ovary, representing transformed counterparts for the cells expressing this transcription factor during mammalian development. Based on the these data and the results presented herein, GATA-4 transcription factor also plays a role in the pathophysiology of gonadal and extragonadal tumors.
Acknowledgments
The authors thank Ms. Kristina von Boguslawski for help with the immunohistochemistry.
Footnotes
Address reprint requests to Dr. Markku Heikinheimo, Children’s Hospital, University of Helsinki, Stenbäckinkatu 11, 00290 Helsinki, Finland. E-mail: markku.heikinheimo@helsinki.fi.
Supported by the Sigrid Juselius Foundation (to D. B. W. and M. H.), the Finnish Pediatric Foundation (to S. S. and M. H.), the University Central Hospital in Helsinki (to S. S., M. A., M. L., O. R., and M. H.), Pediatric Cardiology SCOR HL61006 (to D. B. W.), the Washington University Monsanto-Searle Agreement (to D. B. W.), and an Established Investigator Award from the American Heart Association (to D. B. W.).
References
- 1.Perlman EJ, Hawkins EP: Pediatric germ cell tumors: protocol update for pathologists. Pediatr Dev Pathol 1998, 1:328-335 [DOI] [PubMed] [Google Scholar]
- 2.Teilum G: Classification of endodermal sinus tumour (mesoblastoma vitellinum) and so-called embryonal carcinoma of the ovary. Acta Pathol Microbiol Scand 1965, 64:407-429 [DOI] [PubMed] [Google Scholar]
- 3.Talerman A, Haije WG, Baggerman L: Serum α-fetoprotein (AFP) in patients with germ cell tumors of the gonads and extragonadal sites: correlation between endodermal sinus (yolk sac) tumor and raised serum AFP. Cancer 1980, 46:380-385 [DOI] [PubMed] [Google Scholar]
- 4.Arceci RJ, King AAJ, Simon MC, Orkin SH, Wilson DB: Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermal derivatives and heart. Mol Cell Biol 1993, 13:2235-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB: Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development 1995, 121:3877-3888 [DOI] [PubMed] [Google Scholar]
- 6.Narita N, Bielinska M, Wilson DB: Wild type visceral endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol 1997, 189:270-274 [DOI] [PubMed] [Google Scholar]
- 7.Molkentin JD, Olson EN: GATA4: a novel transcriptional regulator of cardiac hypertrophy? Circulation 1998, 96:3833-3835 [PubMed] [Google Scholar]
- 8.Evans T: Regulation of cardiac gene expression by GATA-4/5/6. Trends Cardiovasc Med 1997, 7:75-83 [DOI] [PubMed] [Google Scholar]
- 9.Grépin C, Nemer G, Nemer M: Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development 1997, 124:2387-2395 [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Sedgwick T, Shi YB, Evans T: Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol 1998, 18:2901-2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossard P, Zaret KS: GATA transcription factors as potentiators of gut endoderm differentiation. Development 1998, 125:4909-4917 [DOI] [PubMed] [Google Scholar]
- 12.Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS, Wilson DB: Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology 1997, 138:3505-3514 [DOI] [PubMed] [Google Scholar]
- 13.Viger RS, Mertineit C, Trasler JM, Nemer M: Transcription factor GATA-4 is expressed in asexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance. Development 1998, 125:2665-2675 [DOI] [PubMed] [Google Scholar]
- 14.Ketola I, Rahman N, Toppari J, Bielinska M, Tinge S, Tapanainen JS, Huhtaniemi IT, Wilson DB, Heikinheimo M: Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology 1999, 140:1470-1480 [DOI] [PubMed] [Google Scholar]
- 15.Kaufman MH: Atlas of Mouse Development. 1992. Academic Press, London
- 16.Pera MF, Blasco Lafita MJ, Mills J: Cultured stem-cells from human testicular teratomas: the nature of human embryonal carcinoma and its comparison with two types of yolk-sac carcinoma. Int J Cancer 1999, 40:334-343 [DOI] [PubMed] [Google Scholar]
- 17.Heikinheimo M, Scandrett J, Wilson DB: Localization of transcription factor GATA-4 to regions of mouse embryo involved in cardiac development. Dev Biol 1999, 164:361-373 [DOI] [PubMed] [Google Scholar]
- 18.White RA, Dowler LL, Gatason LL, Adkison LR, Angeloni SV, Wilson DB: Assignment of the transcription factor GATA4 gene to human chromosome 8 and mouse chromosome 14: Gata4 is a candidate gene for Ds (Disorganization). Genomics 1995, 27:20-26 [DOI] [PubMed] [Google Scholar]
- 19.Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS: GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev 1998, 12:3579-3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heifetz SA, Cushing B, Giller R, Shuster JJ, Stolar CJ, Vinocur CD, Hawkins EP: Immature teratomas in children: pathologic considerations: a report from the combined Pediatric Oncology Group/Children’s Cancer Group. Am J Surg Pathol 1998, 2:1115-1124 [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Hill RJ, Heid PJ, Fukuyama M, Sugimoto A, Priess JR, Rothman JH: end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev 1997, 11:2883-2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blobel GA, Orkin SH: Estrogen-induced apoptosis by inhibition of the erythroid transcription factor GATA-1. Mol Cell Biol 1996, 16:1687-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou PM, Barquin N, Guinan P, Ridaura Sanz C, Gonzalez-Crussi F: Differential expression of p53, c-kit, and CD34 in prepubertal and postpubertal testicular germ cell tumors. Cancer 1997, 79:2430-2434 [DOI] [PubMed] [Google Scholar]
- 24.Oosterhuis JW, Gillis AJ, van Roozendaal CE, van Zoelen EJ, Looijenga LH: The platelet-derived growth factor α-receptor 1.5 kb transcript: target for molecular detection of testicular germ cell tumours of adolescents and adults. APMIS 1998, 106:207-213 [DOI] [PubMed] [Google Scholar]