Abstract
Synaptophysin is a protein involved in neurotransmitter exocytosis and is a neuroendocrine marker. We studied synaptophysin immunohistochemical expression in 35 human liver specimens (normal and different pathological conditions), in rat models of galactosamine hepatitis and carbon tetrachloride-induced cirrhosis, and in freshly isolated rat stellate cells. Synaptophysin reactivity was present in perisinusoidal stellate cells in both human and rat normal liver biopsies. The number of synaptophysin-reactive perisinusoidal cells increased in pathological conditions. Double staining for α-smooth muscle actin and synaptophysin, detected by confocal laser scanning microscopy, unequivocally demonstrated colocalization of both markers in lobular stellate cells. In addition, freshly isolated rat stellate cells expressed synaptophysin mRNA (detected by polymerase chain reaction) and protein. Finally, electron microscopy showed the presence of small electron translucent vesicles, comparable to the synaptophysin-reactive synaptic vesicles in neurons, in stellate cell projections. We conclude that synaptophysin is a novel marker for quiescent as well as activated hepatic stellate cells. Together with the stellate cell’s expression of neural cell adhesion molecule, glial fibrillary acidic protein, and nestin, this finding raises questions about its embryonic origin and its differentiation. In addition, the presence of synaptic vesicles in stellate cell processes suggests a hitherto unknown mechanism of interaction with neighboring cells.
Hepatic stellate cells have been demonstrated to be involved in conditions ranging from acute necrotizing and regenerating liver disorders 1-3 to chronic and fibrosing hepatic diseases. 4-6 Essential functions exerted in these conditions are the production of extracellular matrix components, 7-9 the production of transforming growth factor β1 (TGF-β1) 10,11 and hepatocyte growth factor (HGF), 12-16 and the role of effector cell responsible for microvascular tone regulation in the liver. 17,18
Based on their expression of vimentin, desmin, and α-smooth muscle actin (α-SMA), 19-24 stellate cells have been considered to be of mesenchymal origin. In addition, several markers of neural/neuroectodermal differentiation have been found. Stellate cells express the intermediate filament glial fibrillary acidic protein (GFAP), 25-27 and activated rat stellate cells express the intermediate filament nestin. 27 Human stellate cells are immunoreactive with neural cell adhesion molecule (N-CAM) antibody, 28 whereas rat stellate cells become positive for N-CAM on activation. 29
Synaptophysin (SYN) is a major transmembrane glycoprotein of small (30–80 nm) electron-translucent (SET) vesicles. This class of vesicles includes the presynaptic vesicles in neuronal cells and the somewhat larger synaptic-like microvesicles in neuroendocrine cells. 30 The SYN protein is implicated in the control of exocytosis 30 and neurotransmitter release, eg, in the neuromuscular synapse. 31 Immunohistochemical detection of SYN is commonly used in conjunction with chromogranin-A, neuron-specific enolase, and Leu-7 immunohistochemistry to determine neuroendocrine origin or differentiation in tissues and tumors throughout the body. 32,33 To the best of our knowledge, only neural and neuroendocrine cell types have been shown to express SYN, 34 with the exception of rabbit thrombocytes. 35
In the present study we demonstrated expression of SYN in quiescent as well as activated human and rat hepatic stellate cells.
Materials and Methods
Animals
Male Wistar-Kyoto rats (Harlan, The Netherlands), 250 to 300 g, were used in D-galactosamine (Gal) intoxication experiments and for isolation of stellate cells. Male Wistar rats, 250 to 300 g body weight, were used for carbon tetrachloride (CCl4) fibrogenic regimens. All animals were fed ad libitum and received care in accordance with the institution’s guidelines for the care and use of laboratory animals in research. Gal hepatitis was induced by a single intraperitoneal (i.p.) injection of 500 mg/kg Gal (Sigma-Aldrich, Steinheim, Germany). Animals (14 intoxicated, 2 controls) were killed at 12, 24, 36, 48, and 72 hours and 7, 14, and 21 days after i.p. injection. CCl4 cirrhosis was induced by gastric gavage of CCl4, together with administration of phenobarbital (350 mg/l) in the drinking water, starting 7 days before CCl4 administration. CCl4was mixed 1:1 with corn oil and started at a dose of 80 μl CCl4. Subsequent doses were adjusted to percentage of weight loss, as described by Proctor and Chatamra, 36 with modifications according to Fischer-Nielsen et al. 37 A total of 5 rats were killed, one animal each at 2, 4, 6, 8, and 10 weeks. Liver specimens were fixed in B5 fixative and further specimens were snap-frozen in liquid nitrogen-cooled isopentane. The frozen specimens were stored at −70°C until use.
Human Liver Specimens
A series of 35 human liver specimens, taken for diagnostic purposes or resected before transplantation, was used for this study: 5 near-normal liver biopsies, 5 specimens showing regeneration after submassive necrosis, 15 specimens with chronic fibrotic disease in a noncirrhotic stage (hepatitis B, n = 3; hepatitis C, n = 5; autoimmune hepatitis, n = 2; chronic biliary subobstruction, n = 2; alcoholic hepatitis, n = 3) and 10 specimens with cirrhosis (posthepatitis C, n = 5; alcoholic, n = 3; primary biliary cirrhosis, n = 2). The diagnoses were based on histopathological examination of routinely processed tissue and on clinical and laboratory data. Each specimen was received freshly. One part was fixed in formalin or B5 fixative for diagnostic purposes, one part was snap-frozen in liquid nitrogen-cooled isopentane and stored at −70°C until use, and one small part was immediately fixed in 2.5% glutaraldehyde 0.1 mol/L phosphate buffer and further treated for routine electron microscopy.
Isolation of Stellate Cells
Rat stellate cells were isolated by collagenase/pronase digestion as described by De Bleser et al, 38 followed by density gradient centrifugation with Optiprep (Nycomed, Sweden). Collagenase type IV was purchased from Sigma (St. Louis, MO), and applied in a concentration of 0.05% (w/v) for reperfusion and afterward for digestion in a shaking suspension. Pronase E was purchased from Merck (Darmstadt, Germany) and was applied in a concentration of 0.2% (w/v) for reperfusion and 0.05% (w/v) for digestion. Cells were harvested at densities <1.053 (9% Optiprep), according to Alpini et al. 39 Purity as determined by desmin, vimentin, α-SMA, and oil red O staining on cytospins of freshly isolated cells was 75 to 85%, and viability was systematically over 95%.
Cytospins
Freshly isolated rat stellate cells (1 × 10 5 cells per slide) were spun onto glass slides for 10 minutes at a speed of 500 rpm. The glass slides were washed in PBS, left to dry, and subsequently fixed in acetone.
Immunohistochemistry
Immunohistochemistry was performed on cryosections of rat and human liver biopsies and on cytospins of rat stellate cells fixed in acetone. The polyclonal synaptophysin antibody was purchased from Dako (Copenhagen, Denmark) and applied in a 1/50 dilution. The monoclonal anti-α-SMA antibody was purchased from Sigma-Aldrich (Steinheim, Germany) and applied in a 1/400 dilution. Desmin antibody from Boehringer Mannheim (Mannheim, Germany) and vimentin monoclonal antibody from Amersham (Roosendaal, The Netherlands) were applied in a 1/20 dilution. Polyclonal TGF-β1 antibody from Ciba (Groot-Bijgaarden, Belgium) was applied in a 1/200 dilution. For polyclonal antibodies, a three-step unlabeled peroxidase-anti-peroxidase method was used, as described previously. 40 For monoclonal antibodies, a three-step indirect immunoperoxidase procedure was used, as described previously. 41
Double-staining was performed with a sequential fluorescent method on 20- to 60-μm-thick cryostat sections on 3-aminopropyltriethoxysilan-coated slides. The cryostat sections were dried overnight, fixed in acetone for 10 minutes, and washed in PBS. Then, the sections were incubated with a mixture of normal rabbit serum and normal swine serum diluted 1/5 in PBS for 45 minutes. The polyclonal SYN antibody was incubated at room temperature for 1 hour, followed by fluoresceine isothiocyanate (FITC) or tetramethylrhodamine isothiocyanate (TRITC) labeled swine-anti-rabbit antibodies (Dakopatts, Copenhagen, Denmark). Subsequently, the monoclonal antibody anti-α-SMA was incubated for 1 hour at room temperature, followed by FITC- or TRITC-labeled rabbit-anti-mouse antibodies (Dakopatts). The antibodies were diluted in PBS containing 0.3% Triton X100. All incubation steps were followed by a wash in three changes of PBS, pH 7.4, for 5 minutes. Sections were mounted with glycerol/PBS with para-phenylene-diamine. Controls consisted of omission of the first step (polyclonal SYN antibody) and/or omission of the anti-α-SMA monoclonal antibody and were consistently negative. Additional controls consisted of incubation of the polyclonal antibody with the rabbit-anti-mouse secondary antibody and incubation of the monoclonal antibodies with the swine-anti-rabbit antibody. No nonspecific labeling could be detected. Double staining was detected using confocal laser scanning microscopy (Zeiss 410 inverted laser scan microscope).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
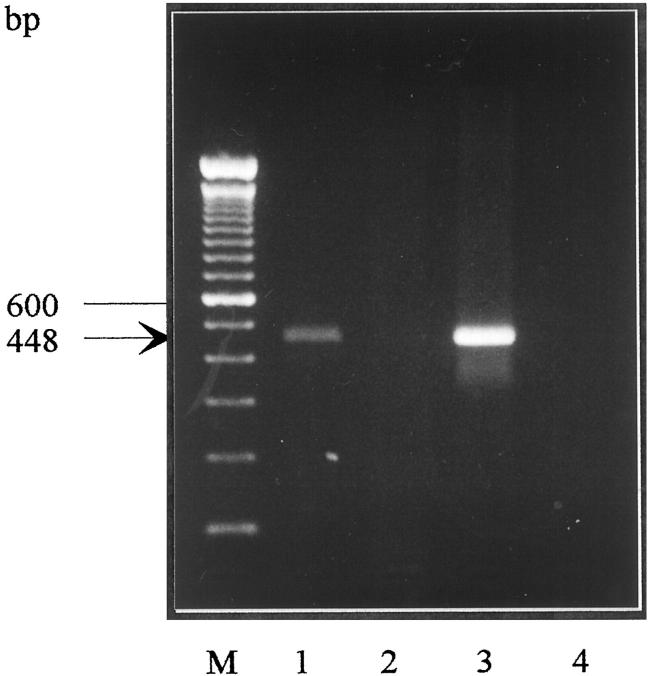
Total RNA was extracted according to Chomczynski and Sacchi. 42 RT-PCR was performed using Promega Taq polymerase, 0.5 U per 50 μl reaction. Cycling was performed in a Biometra Trio cycler (Westburg, The Netherlands). Total RNA was reverse transcribed in a reaction volume of 50 μl, using murine leukemia virus reverse transcriptase at 37°C. Thirty-four cycles of PCR (1 minute at 94°C, 1 minute at 68°C, and 1 minute at 72°C), followed by 10 minutes at 72°C, were performed, using a forward primer 5′-TGTACTTTGATGCACCCWCCTGCS-3′ (SYNP5) and a reverse primer 5′-CAGCCTGTCTCCTTRAACACGAACC-3′ (SYNM2). These primers are complementary to the published cDNA sequence of rat SYN 43 (accession number XO6388), positions 262 to 285 and 685 to 709, respectively. The PCR product was calculated to a length of 448 bp, as was confirmed on agarose gel electrophoresis stained with ethidium bromide. Identification of the PCR product as the rat SYN cDNA was achieved by sequencing.
FTO-2B rat hepatoma cell line was used as negative control; rat brain was used as positive control. RT-PCR for detection of G3PDH mRNA (accession number XO2231) was performed on the cDNA samples, to control cDNA quality. Primers used were 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. The cycling program was 5 minutes at 94°C, followed by 28 cycles (1 minute at 94°C, 30 seconds at 65°C, 2 minutes at 72°C) and 10 minutes at 72°C (adapted from Clontech, Palo Alto, CA). Expected length of the amplified region was 452 bp.
Results
In Vivo Studies
Human Liver Biopsies/Immunohistochemistry
In normal human liver, perisinusoidal cells were immunoreactive to SYN in a discontinuous pattern. Immunoreactive cells were evenly distributed throughout the lobule. Sometimes fat vacuoles indenting the nucleus were recognized, suggesting the immunoreactive cells were stellate cells. In portal tracts, nerve bundles were immunoreactive as described, 44 serving as an internal control.
In submassive necrosis, SYN reactivity was abundant in spindle cells in and around necrotic areas. In addition, SYN-positive perisinusoidal cells with stellate projections were seen, scattered in the surviving parenchyma. The number of SYN-reactive cells was markedly higher in submassive necrosis specimens than in normal human liver. Sparse immunoreactive cells were seen in portal tracts. Portal and lobular nerve bundles and nerve fibers were immunoreactive.
In all chronic liver diseases, a higher number of SYN-reactive perisinusoidal cells were seen compared to the number seen in normal biopsies. Immunoreactive cells were found mainly throughout the lobule and near fibrotic septa (Figure 1, a and c) ▶ . α-SMA immunoreactivity parallelled the lobular staining pattern of SYN (Figure 1b) ▶ ; septa showed only occasional SYN-reactive cells, whereas myofibroblasts in fibrous septa and portal tracts showed abundant α-SMA reactivity (Figure 1b) ▶ . Nerve fibers were strongly immunoreactive for SYN and negative for α-SMA; smooth muscle cells in vessel walls were immunoreactive for α-SMA and negative for SYN staining.
Figure 1.
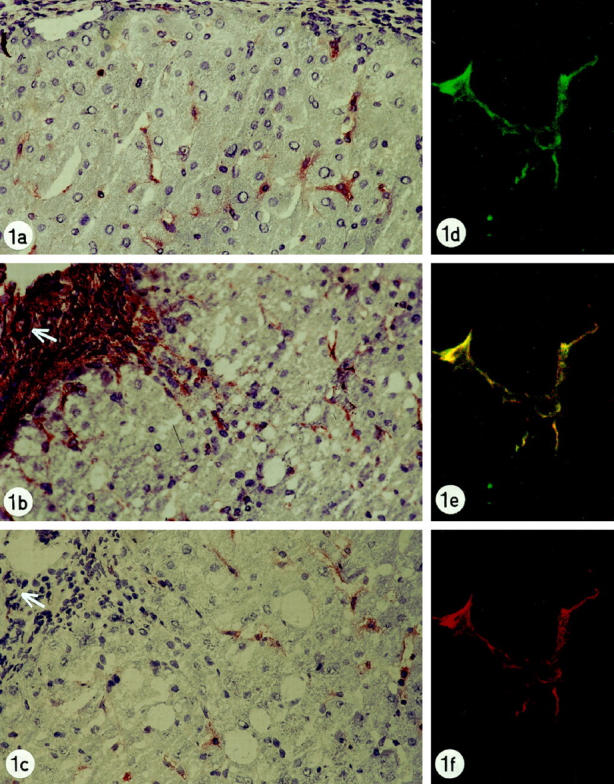
a: Liver biopsy of patient with chronic biliary disease showing synaptophysin reactivity in perisinusoidal cells with stellate projections. Original magnification, ×250. b: Liver biopsy of patient with chronic hepatitis C showing α-smooth muscle actin-reactivity in lobular perisinusoidal stellate cells, in septal myofibroblasts (left upper side of figure) and in smooth vessel cells of an arterial wall (arrow) Original magnification, ×250. c: Liver biopsy of patient with chronic hepatitis C showing synaptophysin-immunoreactivity in lobular perisinusoidal cells with stellate projections. In the fibrotic septum (upper left side of figure), no reactivity is present. Arterial wall, arrow. Original magnification, ×250. d-f: Liver biopsy of patient with chronic hepatitis C: double staining for synaptophysin and α-smooth muscle antigen, detected by the confocal laser scanning microscope: Lobular stellate cell showing reactivity for synaptophysin (green labeling, d), α-smooth muscle actin (red labeling, f) and colocalization for synaptophysin and α-smooth muscle actin (yellow double-labeling, e). Original magnification, ×1000.
Double staining for α-SMA and SYN detected by confocal laser scanning microscopy proved colocalization for both markers in lobular stellate cells. Lobular staining showed complete overlap, confirming that SYN-immunoreactive cells were hepatic stellate cells (Figure 1, d–f) ▶ . Septal myofibroblasts, vessel walls, and nerve fibers showed no overlap.
Electron microscopy showed scattered SET vesicles in the cytoplasm of hepatic stellate cells of normal (Figure 2a) ▶ as well as diseased human liver (Figure 2b) ▶ . In the cytoplasmic processes of stellate cells many SET vesicles, comparable to the synaptic vesicles found in neurons, were observed (Figure 2) ▶ . The processes of stellate cells could be distinguished from neural endings because of the presence of submembranous dense plaques and the absence of neurotubules (Figure 2) ▶ .
Figure 2.
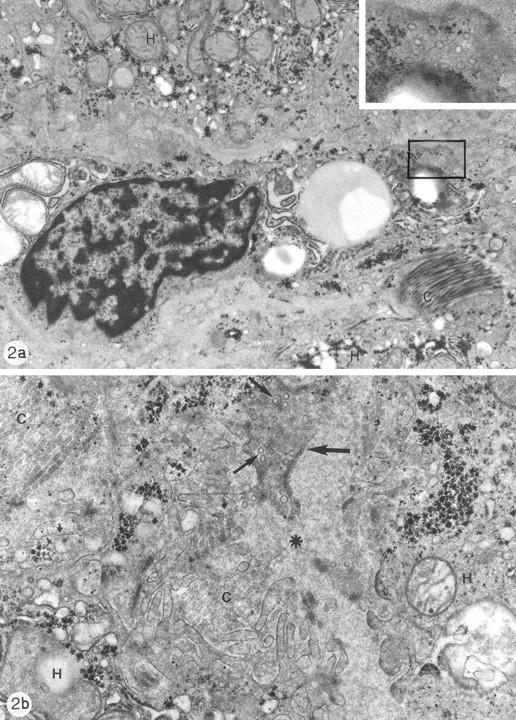
a: Electron micrograph of normal human liver biopsy showing a stellate cell in the Disse space, flanked by part of two hepatocytes (H). The stellate cell contains fat droplets. Collagen bundles (C). Original magnification, ×14,500. Inset: Detail of a cytoplasmic processus containing many small clear vesicles, comparable to the synaptic vesicles found in neurons. Original magnification, ×46,000. b: Electron micrograph of human liver biopsy with chronic subobstruction. Disse space (*) and sinusoidal recess between two hepatocytes (H). Part of a cytoplasmic process of a stellate cell (arrow) containing many small clear vesicles (small arrows). Collagen bundles (C). Original magnification, ×36,800.
Rat Liver Biopsies/Immunohistochemistry
Biopsies from normal rats showed immunoreactivity for SYN in perisinusoidal cells in a discontinuous pattern (Figure 3a) ▶ . The reactive cells were evenly distributed and scattered throughout the lobule. Cells stained by SYN antibody contained a nucleus, differentiating them from nerve endings; this nucleus was often indented by fat vacuoles (Figure 3a) ▶ .
Figure 3.
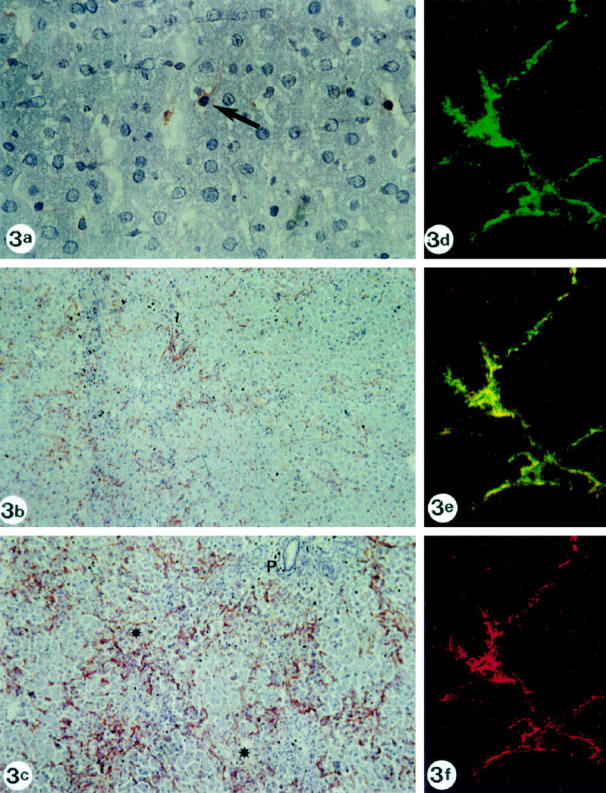
a: Normal rat biopsy immunostained for synaptophysin, showing reactivity in perisinusoidal stellate cells. Fat droplets can be recognized in the cytoplasm of some immunoreactive cells (arrow; original magnification, ×400). b: Rat liver biopsy 36 hours after galactosamine intoxication. Synaptophysin reactivity is seen in a higher number of stellate cells, compared with the normal rat liver shown in a. Stellate cells are clustered around necrotic areas. Original magnification, ×100. c: Rat liver biopsy 48 hours after galactosamine intoxication. Synaptophysin reactivity is seen in stellate cells. The number of stellate cells reaches its maximum at 48 hours. Stellate cells are clustered around necrotic areas. P, portal tract; *, necrotic areas. Original magnification, ×100. d-f: Rat liver biopsy 36 hours after galactosamine intoxication. Double staining for synaptophysin and α-smooth muscle antigen, detected by the confocal laser scanning microscope: lobular stellate cell showing reactivity for synaptophysin (green labeling, d), α-smooth muscle actin (red labeling, f) and colocalization for synaptophysin and α-smooth muscle actin (yellow double-labeling, e). Original magnification, ×1000.
Rats with Gal hepatitis showed SYN reactivity in cells along the sinusoids, periportally, and in and around necrotic areas. The number of SYN-positive stellate cells increased parallel to the severity of necrosis, attaining a maximum around 48 hours after intoxication (Figure 3, b and c) ▶ and decreasing to normal intensity during the following weeks. The SYN immunoreactivity paralleled the staining pattern and the increased number of α-SMA-, TGF-β1- and desmin-reactive cells.
Biopsies from CCl4-intoxicated rats showed SYN reactivity in cells along the sinusoids and in occasional cells in fibrotic septa. The overall number of SYN-positive cells did not reach levels similar to the number of immunoreactive cells in Gal hepatitis. Repeating the dose of CCl4 induced formation of fibrotic septa and development of cirrhosis after 6 weeks, as described previously. 37
Double staining for α-SMA and SYN detected by confocal laser scanning microscope proved colocalization in rat lobular stellate cells, confirming their stellate cell nature (Figure 3, d–f) ▶ .
In Vitro Studies
Cytospins of freshly isolated rat stellate cells, isolated from normal rat livers as well as livers from rats intoxicated with Gal, were reactive on SYN immunohistochemical staining. SYN reactivity of varying intensity was found in the majority of cells; the number of positive cells was compatible with purity of 75 to 85%.
RT-PCR
Transcription of the SYN gene in freshly isolated rat liver stellate cells was demonstrated by RT-PCR. FTO-2B rat hepatocyte cell line showed no transcription; rat brain showed abundant transcription, as expected (Figure 4) ▶ . Control RT-PCR detecting G3PDH mRNA showed satisfying quality of synthesized cDNA (results not shown).
Figure 4.
Transcription of the SYN gene in freshly isolated rat liver stellate cells was demonstrated by RT-PCR. The PCR product was calculated to a length of 448 bp, as was confirmed on agarose gel electrophoresis stained with ethidium bromide. Rat hepatic stellate cells (lane 1) show transcription; FTO-2B rat hepatocyte cell line (lane 2) showed no transcription; rat brain (lane 3) showed abundant transcription. Lane M: 100-bp ladder. Lane 4: negative control (no sample). Identification of the PRC product as rat synaptophysin cDNA was achieved by sequencing.
Discussion
In this study we have shown that quiescent as well as activated human and rat hepatic stellate cells express SYN. The SYN protein is known to be present in membranes of neuronal synaptic vesicles containing neurotransmitters; SYN-positive secretory granules in pancreatic tissue have been demonstrated to contain glucagon or insulin 45 ; tumors of bronchial epithelium and gastrointestinal mucosa can also demonstrate SYN reactivity. 46,47 Expression of SYN is advocated as a sign of neuroendocrine differentiation or subdifferentiation. 32,33 Functionally, SYN is most probably involved in membrane fusion leading to exocytosis. 30
Based on the expression of vimentin, desmin, and α-SMA, 19-21,23,24 hepatic stellate cells have been considered to be of mesenchymal origin. Since Kupffer’s first description of Sternzellen (hepatic stellate cells) in liver, using gold chloride staining in search of nerve fibers, 48 indications of neural/neuroendocrine differentiation of hepatic stellate cells have been accumulating. Stellate cells show GFAP reactivity, 25-27 N-CAM expression, 28,29 nestin reactivity, 27 and SYN expression. All these can be considered as arguments in favor of a neural/neuroendocrine differentiation or origin. Neural tissue originates from the ectodermal layer. Neuroendocrine tissue is thought to originate from either the neural crest, migrating out of the ectodermal layer, or from the endodermal layer. 49 Expression of so-called mesenchymal intermediate filaments (vimentin, desmin, α-SMA) appears not to be contradictory to the suggested neural/neuroendocrine origin, because, for example, in the aorticopulmonary septum, smooth muscle cells supposed to originate from the neural crest also express α-SMA 50 and desmin. 51 In view of the evidence mentioned above, unavoidable questions arise about the mesenchymal origin and functional differentiation of hepatic stellate cells. As a consequence, new studies focusing on the expression of neural and neuroendocrine markers in embryonic as well as adult liver become necessary.
Close contacts between hepatic stellate cells and bare nerve endings have been demonstrated ultrastructurally. 52-54 Functional complexes of myofibroblasts, mast cells, and cholinergic nerve terminals have been suggested to play a role in the development of cirrhosis. 55 Vagotomy was reported to significantly slow down hepatic regeneration. 56-59 Stimulation of sympathetic nerves was described as having a deteriorating effect on Gal hepatitis in rats. 60 Taken together with the evidence on the role of hepatic stellate cells in regeneration 1-3 and fibrosis, 4-6 it is tempting to suggest that the central nervous system influences regeneration and fibrosis through hepatic stellate cells. The central nervous system could, for instance, influence the stellate cells’ production of extracellular matrix components or the secretion of growth factors from stellate cells, with stellate cells functioning as the end organ effector cells of the central nervous system.
Similar interactions of hepatic stellate cells with liver innervation have been postulated in other settings. Glycogenolysis in hepatocytes was proposed to be modulated in a paracrine way through adrenergic stimulation of hepatic stellate cells. 61 Hepatic stellate cells were hypothesized to influence sinusoidal microcirculation, controlled by endocrine and paracrine substances (eg, endothelins and nitric oxide 18,54 ) as well as under central nervous control. 52-54 The demonstration of SYN expression in hepatic stellate cells (Figures 1 and 3) ▶ ▶ adds credibility to these hypotheses, SYN being a synaptic vesicle protein. In addition, we were able to demonstrate the ultrastructural presence of SET vesicles in cytoplasmic processes of human stellate cells, comparable to the SYN-reactive synaptic vesicles found in neurons (Figure 2) ▶ .
Recently, Knittel et al demonstrated two morphologically and functionally different cell populations of the fibroblast lineage in fibrotic rat liver. 62 They describe, on the one hand, hepatic stellate cells (P100-, desmin-, and GFAP-positive) located at the scar-parenchymal interface, and on the other hand myofibroblasts (P100-, desmin-, and GFAP-negative) located within the septa. The two distinct populations were suggested to play similar, but not identical, roles in matrix production. Our finding that α-SMA is strongly reactive in septa as well as in lobular (parenchymal) stellate cells, but that SYN marks only the lobular stellate cells, is an additional argument in favor of two distinct (portal-septal and parenchymal) types of myofibroblasts (Figure 1, b and c) ▶ .
The demonstration of SYN reactivity in hepatic stellate cells may represent a useful immunohistochemical marker for human hepatic stellate cells (Figure 1) ▶ . Desmin staining has been demonstrated to be positive in only a minor fraction of human hepatic stellate cells. Vimentin staining is too nonspecific, because endothelial cells as well as Kupffer cells are positive. α-SMA staining only becomes positive on switching of the hepatic stellate cells to the so-called myofibroblast cell phenotype. 24 GFAP was demonstrated to be positive in only a periportal subpopulation of human stellate cells. 27 N-CAM is rather nonspecific in that it also stains reactive bile ductules 41 and nerve endings. 28 Nestin, finally, stains bile duct cells and vascular endothelium in rat livers and has not been tested on human liver biopsies yet. In vitro, however, nestin staining of human stellate cells was negative. 27 SYN is immunoreactive in human stellate cells (Figure 1) ▶ and nerve bundles. In vivo, nerve endings and stellate cells can be distinguished by their morphology: stellate cells contain a nucleus that in the resting state is indented by lipid droplets; parenchymal nerve endings as a rule do not contain a nucleus.
Whether SYN stains every single hepatic stellate cell in human and rat specimens is a question that cannot be solved. There is no marker for quiescent human stellate cells. As a consequence, double labeling cannot be performed. The number of SYN-positive cells in normal human liver is concordant with the number of stellate cells that was previously described, based on electron microscopic studies. 52 In addition, SYN and α-SMA double staining showed overlap in all lobular stellate cells, in pathological specimens.
Because SYN is immunoreactive in quiescent as well as activated stellate cells and in rat as well as human stellate cells, it is a potentially useful marker for future diagnostic use.
In conclusion, SYN staining is a promising tool for diagnostic practice and fundamental research in the field of hepatology and hepatic stellate cells. Experimental evidence on the interaction between stellate cells and hepatic innervation and accumulating evidence of neural/neuroendocrine differentiation of stellate cells awaits further research. The data presented here challenge current views on the origin and function of hepatic stellate cells.
Acknowledgments
We thank Paula Aertsen for her excellent technical support.
Footnotes
Address reprint requests to Tania Roskams, M.D., Ph.D., Leuven University, Laboratory for Histo- and Cytochemistry, Minderbroederstraat 12, B-3000 Leuven, Belgium. E-mail: tania.roskams@uz.kuleuven.ac.be.
Supported by a grant from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
References
- 1.Dabeva MD, Shafritz DA: Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol 1993, 143:1606-1620 [PMC free article] [PubMed] [Google Scholar]
- 2.Jonker AM, Dijkhuis FW, Kroese FG, Hardonk MJ, Grond J: Immunopathology of acute galactosamine hepatitis in rats. Hepatology 1990, 11:622-627 [DOI] [PubMed] [Google Scholar]
- 3.Johnson SJ, Hines JE, Burt AD: Macrophage and perisinusoidal cell kinetics in acute liver injury. J Pathol 1992, 166:351-358 [DOI] [PubMed] [Google Scholar]
- 4.Yokoi Y, Namihisa T, Matsuzaki K, Miyazaki A, Yamaguchi Y: Distribution of Ito cells in experimental hepatic fibrosis. Liver 1988, 8:48-52 [DOI] [PubMed] [Google Scholar]
- 5.Geerts A, Lazou JM, De Bleser P, Wisse E: Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology 1991, 13:1193-1202 [PubMed] [Google Scholar]
- 6.Hautekeete ML, Geerts A: The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch 1997, 430:195-207 [DOI] [PubMed] [Google Scholar]
- 7.Gressner AM: Perisinusoidal lipocytes and fibrogenesis. Gut 1994, 35:1331-1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geerts A, Greenwel P, Cunningham M, De Bleser P, Rogiers V, Wisse E, Rojkind M: Identification of connective tissue gene transcripts in freshly isolated parenchymal, endothelial, Kupffer and fat-storing cells by northern hybridization analysis. J Hepatol 1993, 19:148-158 [DOI] [PubMed] [Google Scholar]
- 9.Friedman SL: Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 1993, 328:1828-1835 [DOI] [PubMed] [Google Scholar]
- 10.Fausto N, Laird AD, Webber EM: Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J 1995, 9:1527-1536 [DOI] [PubMed] [Google Scholar]
- 11.Date M, Matsuzaki K, Matsushita M, Sakitani K, Shibano K, Okajima A, Yamamoto C, Ogata N, Okumura T, Seki T, Kubota Y, Kan M, McKeehan WL, Inoue K: Differential expression of transforming growth factor-β and its receptors in hepatocytes and nonparenchymal cells of rat liver after CCl4 administration. J Hepatol 1998, 28:572-581 [DOI] [PubMed] [Google Scholar]
- 12.Alison MR, Poulsom R, Jeffery R, Anilkumar TV, Jagoe R, Sarraf CE: Expression of hepatocyte growth factor mRNA during oval cell activation in the rat liver. J Pathol 1993, 171:291-299 [DOI] [PubMed] [Google Scholar]
- 13.Hu Z, Evarts RP, Fujio K, Marsden ER, Thorgeirsson SS: Expression of hepatocyte growth factor and c-met genes during hepatic differentiation and liver development in the rat. Am J Pathol 1993, 142:1823-1830 [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita T, Tashiro K, Nakamura T: Marked increase of HGF mRNA in non-parenchymal liver cells of rats treated with hepatotoxins. Biochem Biophys Res Commun 1989, 165:1229-1234 [DOI] [PubMed] [Google Scholar]
- 15.Ramadori G, Neubauer K, Odenthal M, Nakamura T, Knittel T, Schwogler S, Meyer zum Buschenfelde KH: The gene of hepatocyte growth factor is expressed in fat-storing cells of rat liver and is downregulated during cell growth and by transforming growth factor-β. Biochem Biophys Res Commun 1992, 183:739-742 [DOI] [PubMed] [Google Scholar]
- 16.Schirmacher P, Geerts A, Pietrangelo A, Dienes HP, Rogler CE: Hepatocyte growth factor/hepatopoietin A is expressed in fat-storing cells from rat liver but not myofibroblast-like cells derived from fat-storing cells. Hepatology 1992, 15:5-11 [DOI] [PubMed] [Google Scholar]
- 17.Rockey DC, Weisiger RA: Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology 1996, 24:233-240 [DOI] [PubMed] [Google Scholar]
- 18.Rockey D: The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology 1997, 25:2-5 [DOI] [PubMed] [Google Scholar]
- 19.Burt AD, Robertson JL, Heir J, MacSween RN: Desmin-containing stellate cells in rat liver: distribution in normal animals and response to experimental acute liver injury. J Pathol 1986, 150:29-35 [DOI] [PubMed] [Google Scholar]
- 20.Takase S, Leo MA, Nouchi T, Lieber CS: Desmin distinguishes cultured fat-storing cells from myofibroblasts, smooth muscle cells and fibroblasts in the rat. J Hepatol 1988, 6:267-276 [DOI] [PubMed] [Google Scholar]
- 21.Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, Usui K: Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology 1984, 4:709-714 [DOI] [PubMed] [Google Scholar]
- 22.Ballardini G, Groff P, Badiali de Giorgi L, Schuppan D, Bianchi FB: Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology 1994, 19:440-446 [PubMed] [Google Scholar]
- 23.Ballardini G, Fallani M, Biagini G, Bianchi FB, Pisi E: Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch B Cell Pathol 1988, 56:45-49 [DOI] [PubMed] [Google Scholar]
- 24.Schmitt Graff A, Kruger S, Bochard F, Gabbiani G, Denk H: Modulation of α smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol 1991, 138:1233-1242 [PMC free article] [PubMed] [Google Scholar]
- 25.Neubauer K, Knittel T, Aurisch S, Fellmer P, Ramadori G: Glial fibrillary acidic protein-a cell type specific marker for Ito cells in vivo and in vitro. J Hepatol 1996, 24:719-730 [DOI] [PubMed] [Google Scholar]
- 26.Niki T, De Bleser PJ, Xu G, Van Den Berg K, Wisse E, Geerts A: Comparison of glial fibrillary acidic protein and desmin staining in normal and CCl4-induced fibrotic rat livers. Hepatology 1996, 23:1538-1545 [DOI] [PubMed] [Google Scholar]
- 27.Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A: Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology 1999, 29:520-527 [DOI] [PubMed] [Google Scholar]
- 28.Nakatani K, Seki S, Kawada N, Kobayashi K, Kaneda K: Expression of neural cell adhesion molecule (N-CAM) in perisinusoidal stellate cells of the human liver. Cell Tissue Res 1996, 283:159-165 [DOI] [PubMed] [Google Scholar]
- 29.Knittel T, Aurisch S, Neubauer K, Eichhorst S, Ramadori G: Cell-type-specific expression of neural cell adhesion molecule (N-CAM) in Ito cells of rat liver. Up-regulation during in vitro activation and in hepatic tissue repair. Am J Pathol 1996, 149:449-462 [PMC free article] [PubMed] [Google Scholar]
- 30.Edelmann L, Hanson PI, Chapman ER, Jahn R: Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J 1995, 14:224-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alder J, Xie ZP, Valtorta F, Greengard P, Poo M: Antibodies to synaptophysin interfere with transmitter secretion at neuromuscular synapses. Neuron 1992, 9:759-768 [DOI] [PubMed] [Google Scholar]
- 32.Poola I, Graziano SL: Expression of neuron-specific enolase, chromogranin A, synaptophysin and Leu-7 in lung cancer cell lines. J Exp Clin Cancer Res 1998, 17:165-173 [PubMed] [Google Scholar]
- 33.Schurmann G, Betzler M, Buhr HJ: Chromogranin A, neuron-specific enolase, and synaptophysin as neuroendocrine cell markers in the diagnosis of tumours of the gastro-entero-pancreatic system. Eur J Surg Oncol 1990, 16:298-303 [PubMed] [Google Scholar]
- 34.Bargou RC, Leube RE: The synaptophysin-encoding gene in rat and man is specifically transcribed in neuroendocrine cells. Gene 1991, 99:197-204 [DOI] [PubMed] [Google Scholar]
- 35.Bahler M, Cesura AM, Fischer G, Kuhn H, Klein RL, Da Prada M: Serotonin organelles of rabbit platelets contain synaptophysin. Eur J Biochem 1990, 194:825-829 [DOI] [PubMed] [Google Scholar]
- 36.Proctor E, Chatamra K: High yield micronodular cirrhosis in the rat. Gastroenterology 1982, 83:1183-1190 [PubMed] [Google Scholar]
- 37.Fischer Nielsen A, Poulsen HE, Hansen BA, Hage E, Keiding S: CCl4 cirrhosis in rats: irreversible histological changes and differentiated functional impairment. J Hepatol 1991, 12:110-117 [DOI] [PubMed] [Google Scholar]
- 38.De Bleser P, Geerts A, Van Eyken P, Vrijsen R, Lazou J-M, Desmet V, Wisse E: Tenascin synthesis in cultured rat liver fat storing cells. Wisse E Knook D McCuskey R eds. Cells of the Hepatic Sinusoid. 1991, :pp 218-221 Kupffer Cell Foundation, Rijswijk, The Netherlands, [Google Scholar]
- 39.Alpini G, Phillips JO, Vroman B, LaRusso NF: Recent advances in the isolation of liver cells. Hepatology 1994, 20:494-514 [PubMed] [Google Scholar]
- 40.Roskams T, Willems M, Campos RV, Drucker DJ, Yap SH, Desmet VJ: Parathyroid hormone-related peptide expression in primary and metastatic liver tumours. Histopathology 1993, 23:519-525 [DOI] [PubMed] [Google Scholar]
- 41.Roskams T, van den Oord JJ, De Vos R, Desmet VJ: Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol 1990, 137:1019-1025 [PMC free article] [PubMed] [Google Scholar]
- 42.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 43.Sudhof TC, Lottspeich F, Greengard P, Mehl E, Jahn R: The cDNA and derived amino acid sequences for rat and human synaptophysin. Nucleic Acids Res 1987, 15:9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanda N, Fukuda Y, Imoto M, Koyama Y, Nakano I, Urano F: Localization of synaptophysin immunoreactivity in the human liver. Scand J Gastroenterol 1994, 29:275-279 [DOI] [PubMed] [Google Scholar]
- 45.Kalina M, Lukinius A, Grimelius L, Hoog A, Falkmer S: Ultrastructural localization of synaptophysin to the secretory granules of normal glucagon and insulin cells in human islets of Langerhans. Ultrastruct Pathol 1991, 15:215-219 [DOI] [PubMed] [Google Scholar]
- 46.Gould VE, Lee I, Warren WH: Immunohistochemical evaluation of neuroendocrine cells and neoplasms of the lung. Pathol Res Pract 1988, 183:200. [DOI] [PubMed] [Google Scholar]
- 47.Oberg K: Neuroendocrine gastrointestinal tumours. Ann Oncol 1996, 7:453-463 [DOI] [PubMed] [Google Scholar]
- 48.Kupffer K. ueber Sternzellen der Leber: Briefliche Mitteilung an Professor Waldleyer. Archiv fur mikroskopische Anatomie und Entwicklungsmechanik 1876, 12:353–358
- 49.Larsen WJ: Human Embryology. 1993:p 215 Churchill Livingston, New York
- 50.Beall AC, Rosenquist T: Smooth muscle cells of neural crest origin form the aorticopulmonary septum in the avian embryo. Anat Rec 1990, 226:360-366 [DOI] [PubMed] [Google Scholar]
- 51.Sumida H, Nakamura H, Akimoto N, Okamota N, Satow Y: Desmin distribution in the cardiac outflow tract of the chick embryo during aortico-pulmonary septation. Arch Histol Jpn 1987, 50:525-531 [DOI] [PubMed] [Google Scholar]
- 52.Lafon M, Bioulac-Sage P, Le Bail N: Nerves and perisinusoidal cells in human liver. Wisse E Knook D Decker K eds. Cells of the Hepatic Sinusoid. 1989, :pp 230-234 Kupffer Cell Foundation, Rijswijk, The Netherlands, [Google Scholar]
- 53.Bioulac Sage P, Lafon ME, Saric J, Balabaud C: Nerves and perisinusoidal cells in human liver. J Hepatol 1990, 10:105-112 [DOI] [PubMed] [Google Scholar]
- 54.Ueno T, Sata M, Sakata R, Torimura T, Sakamoto M, Sugawara H, Tanikawa K: Hepatic stellate cells and intralobular innervation in human liver cirrhosis. Hum Pathol 1997, 28:953-959 [DOI] [PubMed] [Google Scholar]
- 55.Akiyoshi H, Terada T: Mast cell, myofibroblast and nerve terminal complexes in carbon tetrachloride-induced cirrhotic rat livers. J Hepatol 1998, 29:112-119 [DOI] [PubMed] [Google Scholar]
- 56.Tanaka K, Ohkawa S, Nishino T, Niijima A, Inoue S: Role of the hepatic branch of the vagus nerve in liver regeneration in rats. Am J Physiol 1987, 253:G439-G444 [DOI] [PubMed] [Google Scholar]
- 57.Lamar C, Jr, Holloway LSJ: The effect of vagotomy on hepatic regeneration in rats. Acta Hepatogastroenterol (Stuttgart) 1977, 24:7-10 [PubMed] [Google Scholar]
- 58.Kato H, Shimazu T: Effect of autonomic denervation on DNA synthesis during liver regeneration after partial hepatectomy. Eur J Biochem 1983, 134:473-478 [DOI] [PubMed] [Google Scholar]
- 59.Michalopoulos GK: Liver regeneration: molecular mechanisms of growth control. FASEB J 1990, 4:176-187 [PubMed] [Google Scholar]
- 60.Iwai M, Shimazu T: Exaggeration of acute liver damage by hepatic sympathetic nerves and circulating catecholamines in perfused liver of rats treated with D-galactosamine. Hepatology 1996, 23:524-529 [DOI] [PubMed] [Google Scholar]
- 61.Jungermann K, Stümpel F: Role of hepatic, intrahepatic and hepatoenteral nerves in the regulation of carbohydrate metabolism and hemodynamics of the liver and intestine. Hepatogastroenterology 1999, 46:1414-1417 [PubMed] [Google Scholar]
- 62.Knittel T, Kobold D, Saile B, Mehde M, Ramadori G: Rat liver myofibroblasts and activated hepatic stellate cells: two different cell populations of the fibroblast lineage involved in hepatic tisue repair. J Hepatol 1998, 28(suppl):72 [Google Scholar]