Abstract
Recent ultrastructural studies have suggested that Glycoprotein Ib (GPIb) has a different distribution on external (surface) versus internal (open canalicular system) membranes in resting discoid platelets. The differential distribution proposed for GPIb differs from that reported for the fibrinogen receptor, GPIIb-IIIa, and could have profound physiological significance when platelets are activated by surfaces. The present study explored the distribution of GPIb on external and internal membranes of resting platelets. Immunogold cytochemical techniques were applied to ultrathin cryosections of washed platelets. Polyclonal antibodies or mixtures of monoclonal antibodies (AP1 and 6D1) were used for labeling. To avoid the technical problem posed by limited accessibility of antigens located in very narrow portions of the open canalicular system (OCS) to antibodies, the same methods were applied to patients with giant platelets syndromes. The OCS of normal resting platelets was also dilated by exposure of platelets to hypertonic conditions or to cytochalasin-B, an agent that prevents assembly of actin, and, reportedly, movement of GPIb. Morphometric analysis revealed that rates of labeling on internal versus external membranes of giant platelets does not differ significantly (0.93 ± 0.20), provided the OCS is sufficiently dilated. Platelets exposed to cytochalasin B (1.01 ± 0.31) or to hypertonic conditions (0.96 ± 0.20) revealed similar ratios for immunogold particles on external and internal membranes. Results of our study indicate that membranes of the exposed surface and lining OCS channels of resting platelets are continuous, identical structures and GPIb is homogeneously distributed on external and internal membranes.
Platelet membrane glycoproteins play a critical role in the regulation of adhesive and cohesive platelet functions. The platelet surface membrane and the contiguous surface-connected open canalicular system (OCS) contain the major glycoproteins (GP), GPIb and GPIIb-IIIa. GPIb plays a key role in hemostasis by mediating the adhesion of platelets to von Willebrand factor (VWF) bound to the subendothelium. 1,2 Interaction of GPIIb-IIIa with the arginine-glycine-aspartic acid (RGD) sequence in VWF facilitates platelet spreading onto subendothelium. 3 Subsequent binding of fibrinogen to GPIIb-IIIa promotes platelet aggregation and formation of an effective hemostatic plug at intermediate shear rates. 4
Ultrastructural studies using immunocytochemical techniques have demonstrated that GPIIb-IIIa receptors are distributed on external and internal platelet membranes and on the membranes of α-granules. 5 Although detailed morphometric studies are not available, it seems that GPIIb-IIIa receptors are homogeneously distributed on external versus internal membranes of platelets. 6 The overall impression from ultrastructural and flow cytometric studies is that the internal pools of GPIIb-IIIa located in the OCS and α-granules could supply additional receptors during the process of activation. 7-9 The amount of GPIIb-IIIa stored in internal pools could account for about 100% of the surface levels. 10
Immunocytochemical techniques have also demonstrated that the GPIb complexes are distributed on external and internal platelet membranes, 11,12 membranes of α-granules, 13 and dense bodies. 14 Differences in opinion exist in the literature regarding the quantitative distribution of GPIb on external and internal membranes in resting platelets. Some authors have found that the density of labeling for GPIb within the OCS is less than that observed on the platelet surface. 15,16 Other workers have failed to note significant differences, 12,17 whereas some using enzyme-linked immunsorbent assay techniques with monoclonal antibodies have predicted a large internal pool of GPIb that could replenish any amount of GPIb lost from the external membrane. 18 The latter authors estimated the number of internal copies of GPIb was 3 to 4 times greater than the number of GPIb molecules present on the platelet surface, figures relatively higher than those estimated for GPIIb-IIIa.
Immunocytochemical techniques have inherent limitations to access antigens located in external or internal membranes. 19,20 The difficulty for monoclonal antibodies to gain access to GPIIb-IIIa located on internal membranes of the platelet OCS has been known for a long time. 21 Better access to these antigens can be gained when Fab fragments of the antibodies or smaller probes are used. 10,21
In the present study, we have explored the distribution of GPIb on external and internal membranes of platelets in their resting state. Several approaches were used to facilitate the accessibility of antibodies to antigens located in very narrow portions of the OCS. Ultrathin cryosections of platelets from patients with giant platelet disorders, including the May-Hegglin anomaly and Epstein’s syndrome, were exposed to polyclonal antibodies or to mixtures of monoclonal antibodies (AP1 and 6D1). The same techniques were applied to normal platelets where OCS of normal platelets was artificially dilated by exposure to cytochalasin-B or to hypertonic buffers. Differences in labeling were morphometrically quantified.
Materials and Methods
Antibodies
Polyclonal antibodies to glycocalicin or a mixture of monoclonal antibodies specific for GPIb (AP1 and 6D1) were used for immunolabeling. The polyclonal antibody against GPIb, generously provided by Dr. Kenneth Clemetson (Bern, Switzerland), recognizes the glycocalicin portion of the glycoprotein and inhibits ristocetin-induced agglutination of platelets. 22 Monoclonal antibodies AP1 and AP2, kindly provided by Dr. T. J. Kunicki (La Jolla, CA) and 6D1, kindly provided by Dr. Barry Coller, Mount Sinai Medical Center (New York, NY), have been characterized and used in previous studies. 12,22,23
Preparation of Platelets
Blood for the present study was obtained after informed consent from normal donors and from previously characterized patients with diagnosed bleeding and giant platelet disorders. None of the individuals studied had taken aspirin for at least 10 days. Following venipuncture, the samples were mixed immediately with citrate-citric acid-dextrose (CCD), pH 6.5 (93.0 mmol/L sodium citrate, 7.0 mmol/L citric acid, 140 mmol/L dextrose), in a ratio of 9 parts blood to 1 part anticoagulant. Platelets from platelet-rich plasma (PRP) were separated by centrifugation at room temperature for 20 minutes at 100 × g. Platelets were washed twice with equal volumes of CCD containing 5 mmol/L adenosine and 3 mmol/L theophylline, 24 centrifuged to pellets at 900 × g for 15 minutes, and suspended in Hanks’ balanced salt solution (HBSS).
Depending on experimental purposes HBSS prepared from a ×10 concentration was diluted to normal (×1) or ×2 strength. For other experiments platelets in cytochalasin B (10−5 M final concentration) was added to HBSS. Platelets were always incubated in the corresponding HBSS media for 1 hour at 37°C before fixation in 4% paraformaldehyde in PBS, pH 7.2.
Preparation and Immunolabeling of Cryosections from Platelets Suspensions
Aliquots of washed platelets fixed in 4% paraformaldehyde were concentrated to a pellet by centrifugation. The supernatant was removed, fresh fixative added, and fixation of the pellet continued for 24 hours at 4°C. After this period, small portions of the pellets were washed and further infiltrated for 2 hours in a mixture of polyvinylpyrrolidone and sucrose as recommended by Tokuyasu. 25 Pyramid-shaped blocks of the infiltrated pellets were cut, mounted on metal stubs, and frozen in liquid nitrogen. Cryosections were obtained from these blocks according to ultracryomicrotomy techniques described elsewhere. 26 Sections were cut at −90°C with a MT6000-XL ultracryotome provided with a CR2000 cryounit (RMC, Tucson, AZ) and stored on formvar-coated copper grids.
An indirect immunocytochemical technique was used for the localization of GPIb. Sections were first incubated in 1:100 dilution of the antibody to GPIb for 45 minutes. After repeated washing with PBS, the location of the primary antibody was visualized by incubation with protein A-colloidal gold or goat anti-mouse IgG-colloidal gold (Amersham International, Poole, UK). The excess of gold particles was removed by washing in PBS. Finally, labeled grids were stained and embedded in a mixture of 2% polyvinyl-alcohol (PVA) and 0.3% uranyl acetate 27 before examination in a Philips 301 electron microscope.
Morphometric Quantification
The distribution of colloidal gold particles on the surface of immunolabeled platelets was analyzed in en face electron micrographs printed at final magnifications around ×60,000. The morphometric analysis was performed only on platelets with clearly distinguishable OCS. At least 10 photomicrographs were analyzed from each of the two patients with giant platelet disorders, normal platelets exposed to hypertonic conditions and cells incubated with cytochalasin B. The density of labeling on external and internal membranes was determined and expressed per micron. 28 A ratio of internal versus external density was calculated for each platelet micrograph analyzed. First, the number of gold particles related to internal membranes was determined on cross-sectioned membranes of the OCS and the total perimeter screened was recorded (usually 4–6 microns). Clusters of 2 or more particles were always considered as one event. Immediately after, an equivalent perimeter was randomly screened on the external surface of the same platelet. Ratios close to 1 indicate that the labeling was equally distributed on external and internal membranes. Ratios below 1 indicate a more frequent distribution of receptors on external membranes.
Statistics
Average values and standard deviations derived from morphometric data were calculated. Student’s t-test was used for statistical comparisons.
Results
Morphology
Normal Resting Platelets
The distribution of GPIb/IX receptors on external and internal membranes of resting platelets has been described in previous reports from our laboratory. The work has shown that immunogold particles detecting GPIb/IX are randomly dispersed on exposed surfaces of discoid platelets and membranes lining channels of the OCS. However, narrow segments of the OCS channels were often less well labeled with immunogold particles than more dilated elements. Rates of labeling on internal versus external membranes in normal resting platelets averaged 0.76 ± 0.14 (mean ± SD). The variability may account in part for differences in opinion regarding the frequency of GPIb/IX on external membrane of resting cells. Therefore, the experiments described below were carried out to resolve the problem.
Giant Resting Platelets
Platelets from patients with the May-Hegglin anomaly and Epstein’s syndrome contain extensive amounts of dilated internal membrane frequently associated with elements of the dense tubular system (DTS) in membrane complexes (Figures 1 and 2) ▶ ▶ . Frozen thin sections of giant platelets incubated with AP1 and 6D1 to detect GPIb/IX and post stained with anti-mouse Ig G coupled to 5 nm gold reveal generous labeling of internal and external membranes (Figures 3 and 4) ▶ ▶ . No difference in the frequency of GPIb on the outer and inner membranes could be detected with rates of labeling internal versus external averaging 0.93 ± 0.17.
Figure 1.
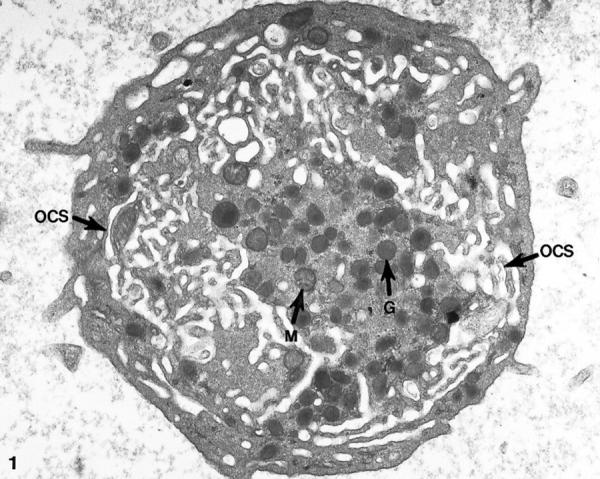
Thin section of a giant platelet from a patient with the May-Hegglin anomaly. An abundance of dilated channels making up the open canalicular system (OCS) fill the cytoplasm of the cell. Alpha granules (G) and occasional mitochondria (M) are prominent in the central region. Original magnification, ×18,800.
Figure 2.
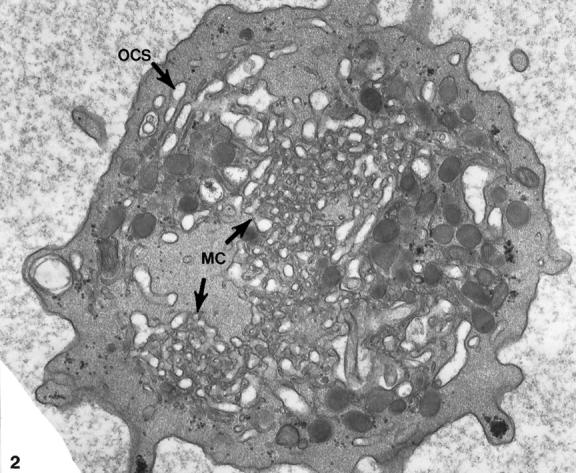
Thin section of a giant platelet from the peripheral blood of a patient with Epstein’s syndrome. An extensive system of dilated OCS channels fills the cell matrix. In some areas the OCS channels are in close association with canaliculi of the dense tubular system forming membrane complexes (MC). Original magnification, ×22,000.
Figure 3.
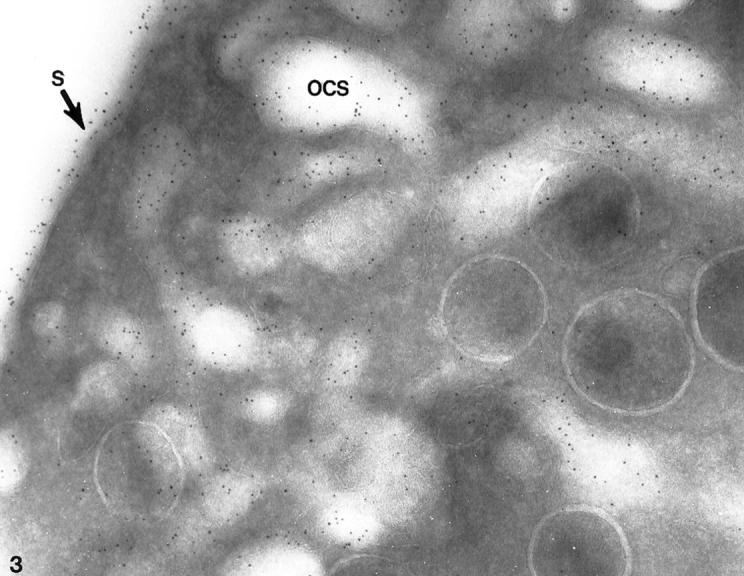
Giant platelet from patient with May-Hegglin anomaly. The frozen thin section has been stained with a polyclonal antibody to the glycocalicin portion of GPIb and protein A coupled to 5-nm gold particles. Immunogold particles indicating sites of GPIb cover the exterior surface (S) of the large cell and line channels of the dilated OCS. Densities of labeling for immunogold detecting GPIb are comparable on the external surface or on membranes lining channels of the OCS. Original magnification, ×80,000.
Figure 4.
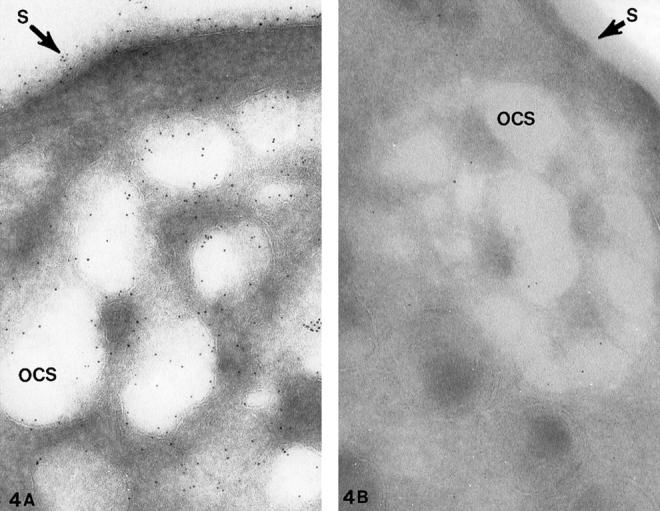
Cryosections of giant platelets from (A) a patient with Epstein’s syndrome (ES) and (B) a patient with Bernard Soulier syndrome (BSS). The cells were stained with the anti-glycocalicin antibody and protein A gold in the same manner as the cell in Figure 3 ▶ for the presence of GPIb. Gold particles indicating sites of GPIb cover the exposed surface (S) and membranes lining channels of the dilated OCS. There is no difference in the frequency of gold beads detecting GPIb on external or internal membranes of the giant ES cell. The specificity of the antiglycocalicin antibody for GPIb is indicated by the virtual absence of immunogold particles on and in the BSS platelet in B. Original magnifications, ×100,000 (A) and ×70,000 (B).
Cytochalasin-B-Treated Resting Platelets
Cytochalasin-B (CB) inhibits assembly of new actin filaments in stimulated platelets. It also has been reported to block clearance of GPIb/IX receptors from exposed surfaces to interior membranes of activated platelets and causes dilatation of the OCS in resting cells. 11,29 Frozen thin sections of platelets incubated with 10−5 M CB revealed preservation of discoid form despite dilatation of channels making up the OCS. Staining CB-treated cells for GPIb/IX revealed immunogold particles randomly dispersed on exposed surfaces and membranes lining channels of the OCS (Figures 5–7) ▶ ▶ ▶ . Analysis of particle frequencies on the internal and external membrane revealed no significant differences (1.01 ± 0.31).
Figure 5.
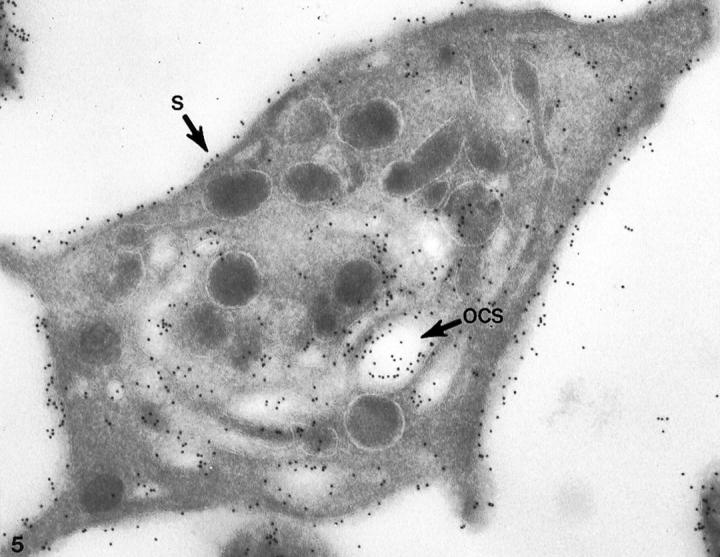
Cryosection of normal platelet prepared after incubation with cytochalasin B (CB). The thin frozen section was incubated first with the monoclonal antibodies AP1 and 6D1 against GPIb, and then to anti-mouse IgG coupled to 10 nm gold. Immunogold particles indicating sites of GPIb cover the exterior surface (S) and membranes lining channels of the OCS. Tangential sections of the exposed surface may appear to have more gold particles, but there is no significant difference in the frequency of gold particles indicating GPIb on cross sections of external and internal membranes. Original magnification, ×46,000.
Figure 6.
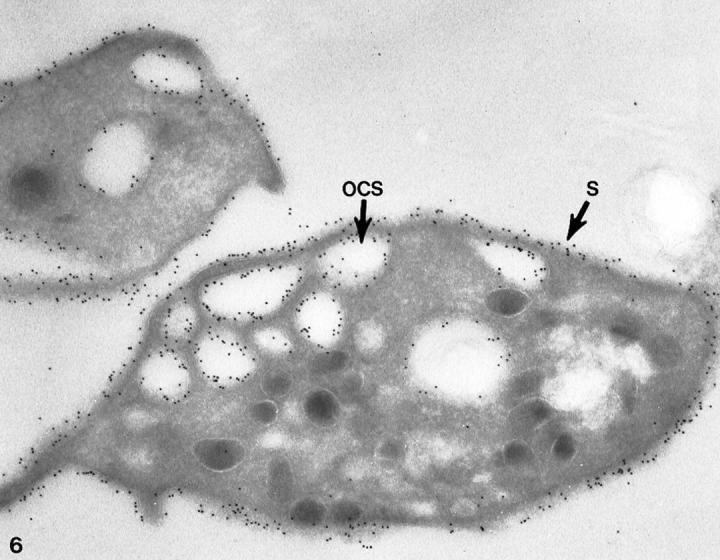
Cryosection of a normal platelet incubated with cytochalasin B stained for GPIb in the same manner as the cell in Figure 5 ▶ . Immunogold beads detecting sites of GPIb are prominent on membranes of the cell surface (S) and lining the OCS. The receptors appear to have the same density on internal and external membranes. Original magnification, ×42,000.
Figure 7.
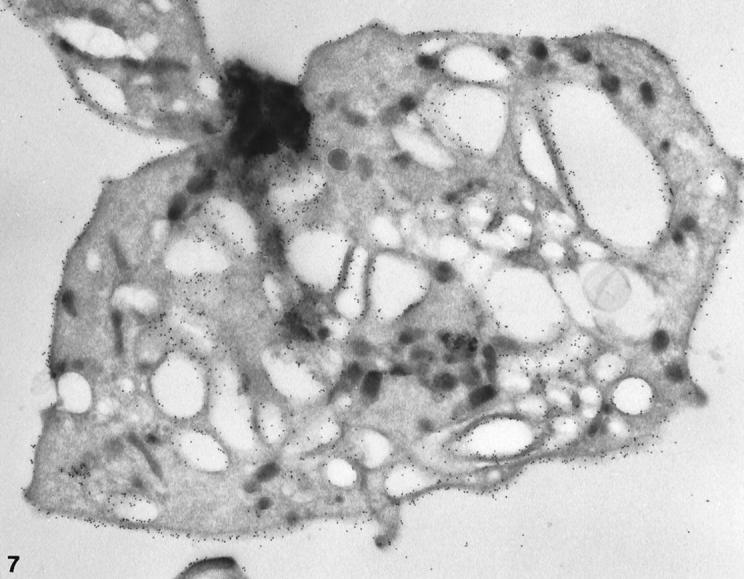
Cryosection of a larger normal platelet with a dilated OCS prepared after exposure to CB. The frozen thin section was stained with AP1, 6D1, and anti-mouse IgG coupled to 10 nm gold in the same manner as the cell in Figure 5 ▶ . Immunogold beads indicating sites of GPIb cover the external and internal membranes. The frequency of gold particles is essentially the same on the exposed surface (S) and membranes lining OCS channels. Original magnification, ×42,000.
Hypertonically Stressed Resting Platelets
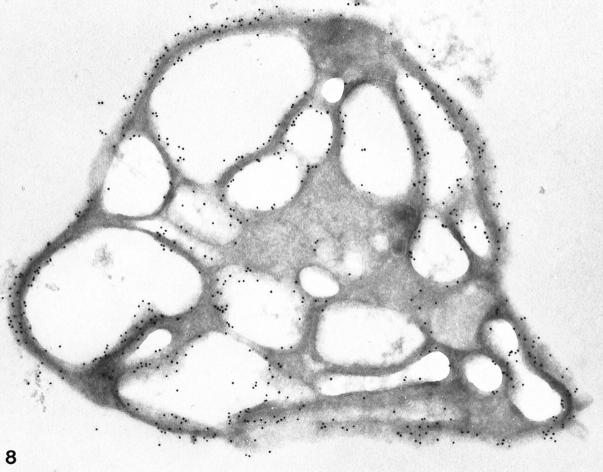
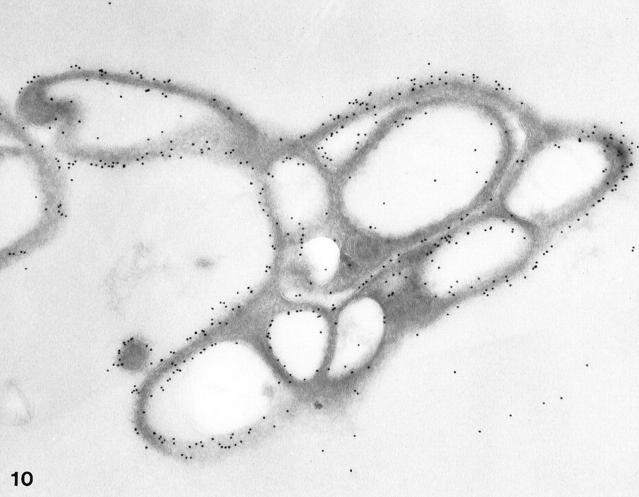
Exposure of resting platelets to a twice concentrated salt solution causes dehydration of the matrix and dilatation of OCS channels. 30 Frozen thin sections of platelets exposed to hypertonic stress stained for GPIb/IX revealed uniform deposition of immunogold particles detecting the receptor on internal and external surfaces (Figures 8–10) ▶ ▶ ▶ . No significant differences in frequency were observed (0.96 ± 0.20).
Figure 8.
Cryosection of a normal platelet prepared for study after incubation under hypertonic conditions (see text). The frozen section was incubated first with API and 6DI and then with anti-mouse IgG coupled to 10 nm gold. Hypertonic salt solutions have caused dilatation of the OCS. Gold particles detecting sites of GPIb are evenly dispersed on the exposed surface (S) and membranes lining channels of the OCS. There is no significant variation in the frequency of GPIb on external and internal membranes. Original magnification, ×39,000.
Figure 9.
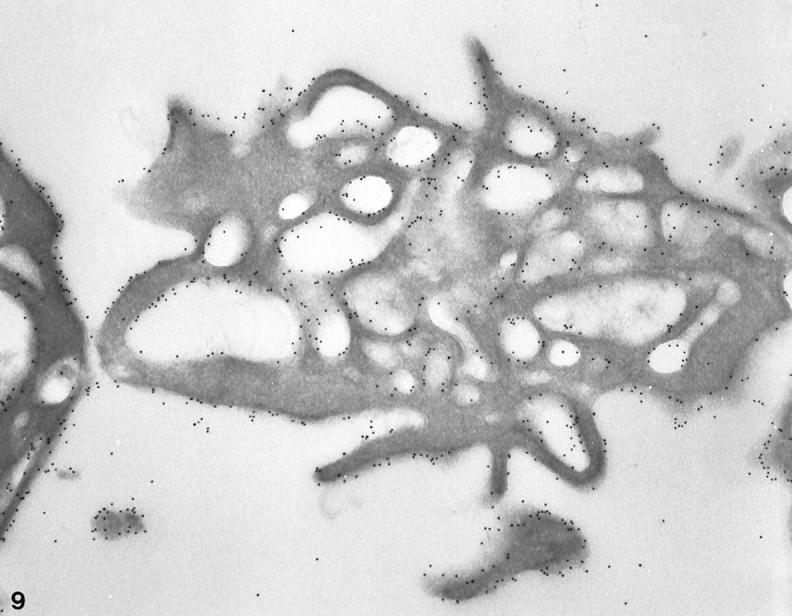
Frozen thin sections of a normal platelet exposed to hypertonic conditions before freezing. The cryosection has been stained for GPIb in the same manner as the cell in Figure 8 ▶ . Gold beads detecting sites of GPIb are evenly dispersed on the surface (S) membrane and linings of OCS channels. The variation in frequency of GPIb on the outer and interior membranes is not significant. Original magnification, ×33,000.
Figure 10.
Cyrosection of another normal cell prepared after exposure to hypertonic conditions. The frozen thin section was stained for GPIb in the same manner as the cells in Figures 8 and 9 ▶ ▶ . Gold particles indicating the presence of GPIb are dispersed evenly on the exposed surface (S) and in the OCS. Differences in gold particle frequency on outer and inner membranes are not apparent. Original magnification, ×42,000.
Comparative Morphometric Studies
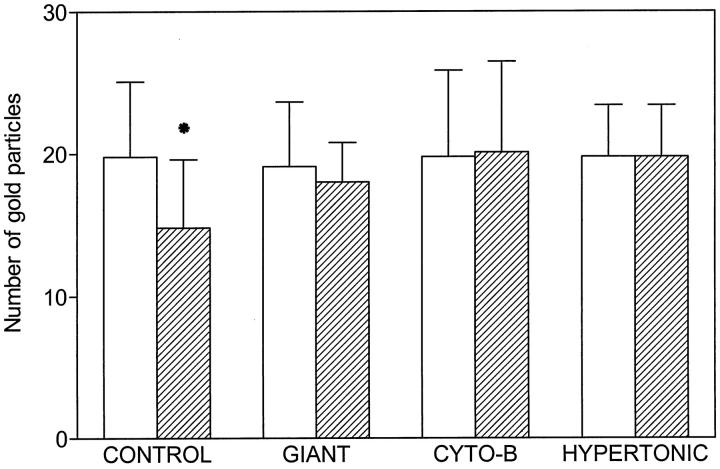
Figure 11 ▶ summarizes morphometric data obtained under the different conditions of our studies. Morphometric analysis of labeling on membranes of control platelets revealed a statistically significant reduction in the density of labeling of internal membranes (P < 0.05 versus external membranes).
Figure 11.
Comparison of the number of gold particles on external (white bars) and internal membranes (dashed bars) of control, giant, cytochalasin B-treated, and hypertonically treated platelets. Densities of labeling are expressed in particles/μm. Statistical differences were observed for labeling on external versus internal membranes in control platelets (*P < 0.05). Differences were not observed under conditions where the open canalicular system was naturally or artificially dilated.
Densities of labeling on internal membranes were essentially similar to those observed on external membranes when morphometric analysis were performed on giant platelets, platelets exposed to cytochalasin B, or hypertonically dilated platelets.
Discussion
Results of the present investigation have shown that GPIb receptors are homogeneously distributed on external and internal membranes of resting platelets. A similar concept was suggested by early studies, 12-17 but the difficulty in labeling GPIb on membranes in narrow channels of the OCS limited a final conclusion. The extensive system of dilated OCS channels and large membrane complexes in giant platelets from patients with the May-Hegglin anomaly 31,32 and Epstein’s syndrome 33 made them suitable candidates for evaluating the distribution of GPIb on resting platelet membranes. Our findings using immunogold labeling techniques revealed no significant differences in the density of labeling on internal and external membranes of giant platelets.
The mechanism responsible for formation of giant platelets is unknown. 34 As a result the membranes of the OCS may be abnormal, and it seemed reasonable to try to dilate channels of the OCS in normal platelets under conditions that should not cause translocation of GPIb/IX. CB, an agent that inhibits assembly of cytoplasmic actin filaments, was found in previous studies to make platelets more deformable to aspiration into micropipettes 35 and to prevent cold-induced shape change. 36 Examination in the electron microscope revealed that chilled, CB-treated platelets retained a relatively discoidal appearance, even though circumferential coils of microtubules had disappeared. The cells, however, were somewhat irregular due to dilation of OCS channels.
CB-treated platelets with a dilated OCS appeared to be an excellent model for our studies, particularly because other workers had proposed that CB prevented movement of GPIb on platelets and translocation from the exposed surface to interior membranes. 11,29 The present study has shown that CB-treated platelets are excellent subjects for studying the distribution of GPIb receptors. The density of immunogold particles detecting GPIb on internal and external membranes of CB-treated platelets was not significantly different.
Exposure of normal platelets to hypertonic salt solutions causes dehydration of the cell matrix, resulting in dilatation of the OCS. 30,37 The observation suggested that normal platelets prepared under hypertonic conditions might be useful for determining the distribution of GPIb on internal and external membranes. Results of the present study confirm that dilatation of the OCS occurs under hypertonic conditions, and that GPIb is homogeneously distributed on internal and external membranes of platelets exposed to these conditions.
The network of interconnecting surface membrane invaginations that make up the platelet OCS is unique. It is not found in any other type of blood cell. Sequestration of the tortuous, tube-like channels making up the OCS within the cytoplasm of resting platelets has made it difficult to appreciate that it is surface membrane, not some specialized compartment originating from another membrane system in the megakaryocyte precursor. Behnke’s 38 early studies and subsequent investigations 39 have shown that channels of the OCS are continuous with the exposed surface of the platelet and develop by invagination of the megakaryocyte membrane during sequestration of its cytoplasm into subunits.
However, internalization of surface membrane in the form of narrow channels does make it difficult to access or isolate them for definitive biochemical studies. As a result, some investigators consider the OCS as a membrane entity different from the exposed cell surface, even though their work supports the concept that exposed and internal membranes are continuous. 11,18 This opinion has resulted in different interpretations of the distribution of receptors on exposed versus internal membranes of resting platelets and their fate after platelet activation in suspension.
Several immunocytochemical studies indicate that, in resting platelets, GPIb is found at a higher frequency on external membranes than on the membranes lining the OCS. 14,15,40,41 Nevertheless, other methodological approaches have predicted a larger concentration of GPIb located in internal pools than is available on the exposed surface. 18 In contrast, preliminary studies with polyclonal antibodies suggested that GPIIb-IIIa was observed on the plasma membrane, the OCS, and the α-granule membrane 6 without emphasizing possible differences in density. Yet when micrographs of published work on immunocytochemical labeling of GPIIb-IIIa in cryosections are studied critically, 14,41 the impression gained is that GPIIb-IIIa has a distribution similar to that claimed for GPIb, with more intense labeling on the surface membrane and weaker or no labeling on many elements of the OCS.
Data from the present work suggest that receptors located on narrow channels of the OCS in resting platelets are often less efficiently labeled than those present on external membranes. In our study, a decreased labeling for GPIb was also frequently observed at points where membranes of two platelets or a platelet and a red blood cell were juxtaposed. The observation is in agreement with that reported in a previous publication detecting GPIb on resting and activated platelets. 12
Problems in labeling of this type can also be found in published material from other authors who have used a similar technical approach. 16 It is intriguing that the density of labeling at intersections between membranes of two colliding platelets is inferior to that seen on noncolliding membranes, where one would expect it to be doubled. It is also remarkable that an immunocytochemical approach, which fails to proportionally label membranes of juxtaposed platelets, could be used with confidence to quantify receptors located in narrow portions of the OCS. The increased labeling observed for GPIb in dilated OCS channels suggests that access of probes to this antigen is improved when the OCS is clinically or experimentally dilated.
The limitations of immunocytochemical probes to reach their targets have a variety of origins. Antibodies to receptors have limited access to internal portions of the OCS when used on platelets in suspension. 10,21 The size of the probes and the absence of a flow gradient between the external and internal membranes may explain these limitations. In the case of cryosections, the efficiency of labeling is also limited, not only by the accessibility of the antigens but also by the size of the primary antibody and the secondary probe. Usually, the primary marker is a single mouse IgG (monoclonal antibody) or a mixture of rabbit IgG (polyclonal). The size of the immunoglobulin is usually around 150 kd. The marker for this primary antibody can either be IgG from another species or protein-A, generally coupled to gold. 42 Protein A is a smaller molecule (40 kd), which facilitates its penetration into sections. 19 The efficiency of labeling improves with smaller gold particles. 43 It is remarkable that in the present study labeling was successfully achieved when a combination of polyclonal antibodies and protein A-gold (5 nm) was applied to the dilated OCS of resting giant platelets.
Griffiths and Hoppeler 44 found large differences in the efficiency of immunogold techniques to label antigens located in the Golgi zone or endoplasmic reticulum. According to these authors, the cross-linking effect of fixatives, steric hindrance, and valency of both ligand and target molecules are additional factors that limit labeling efficiencies. 45 In a recent article Wagner et al, 46 using a humanized receptor specific for GPIIb-IIIa, found that the number of antigens available to the antibody increased up to twofold. In the opinion of these authors the increased efficiency of the humanized antibody was the critical component determining the binding of antibody molecules. It is likely that all of the previous factors, together with the elevated presence of glycocalicin in the external expansions of GPIb, 47 could interfere with the labeling of GPIb pools located in deeper portions of the OCS. Moreover, despite careful and repeated washing, residual plasma proteins may remain in the OCS of isolated resting platelets and become deposited during the fixation procedure, thereby masking some glycoprotein antigens. Thus, in view of the previous considerations, we suggest that antigens located in narrow membranes are naturally less accessible to probes than those located on more exposed membranes.
According to some authors, GPIb becomes internalized during platelet activation as assessed by a decrease in labeling on the platelet surface and a parallel increase in the labeling related to the OCS. 11,48,49 Interestingly, the substantial modifications predicted in these studies were not observed when polyclonal antibodies were used in combination with protein A-gold (5 nm) probes for immunolabeling. 12 Based on the findings of the present study, dilation of the OCS in thrombin-activated platelets would facilitate the expression of antigenic sites recognized by antibodies, giving the impression of more intense labeling.
In a recently published article by Van Zanten et al, 16 the authors detected a 50% decrease in the presence of GPIb on the surface of platelets activated with specific thrombin receptor agonist, thrombin receptor activation peptide (TRAP). Interestingly, the morphometric analysis performed by the authors showed that the density of labeling on the internal membranes of the activated platelets was still slightly below that observed on external membranes of resting platelets. Clearly, the number does not exceed the frequency observed on exterior surfaces of resting platelets, as would be required if there were no differences in the frequency of GPIb on the internal and external membranes of resting cells, as our work has shown.
In summary, our data indicate that GPIb is homogeneously distributed on external and internal membranes of resting platelets, confirming that membranes of the OCS are continuous with the external surface. Our data may help to explain apparent discrepancies among studies published from different groups on the labeling of glycoproteins located on internal membranes of resting platelets.
Footnotes
Address reprint requests to Dr. James G. White, University of Minnesota Medical School, Department of Laboratory Medicine and Pathology and Pediatrics, Box 490, 420 Delaware Street S.E., Minneapolis, MN 55455. E-mail: white003@maroon.tc.umn.edu.
A Letter to the Editor based in part on this investigation was recently published (Blood 1998, 92:4874–4877).
References
- 1.Tschopp TB, Weiss HJ, Baumgartner HR: Decreased adhesion of platelets to subendothelium in von Willebrand’s disease. J Lab Clin Med 1974, 83:296-300 [PubMed] [Google Scholar]
- 2.Bolhuis PA, Sakariassen KS, Sander HJ, Bouma BN, Sixma JJ: Binding of factor VIII-von Willebrand factor to human arterial subendothelium precedes increased platelet adhesion and enhances platelet spreading. J Lab Clin Med 1981, 97:568-576 [PubMed] [Google Scholar]
- 3.Turitto VT, Weiss HJ, Baumgartner HR: Decreased platelet adhesion on vessel segments in von Willebrand’s disease: a defect in initial platelet attachment. J Lab Clin Med 1983, 102:551-564 [PubMed] [Google Scholar]
- 4.Weiss HJ, Turitto VT, Baumgartner HR: Platelet adhesion and thrombus formation on subendothelium in platelets deficient in glycoproteins IIb-IIIa, Ib, and storage granules. Blood 1986, 67:322-330 [PubMed] [Google Scholar]
- 5.Wencel-Drake JD, Plow EF, Kunicki TJ, Woods JL, Keller DM, Ginsberg MH: Localization of internal pools of membrane glycoproteins involved in platelet adhesive responses. Am J Pathol 1986, 124:324-334 [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer EM, Savidge GF, Vainchenker W, Berndt MC, Pidard D, Caen JP, Masse JM, Breton-Gorius J: Alpha-granule pool of glycoprotein IIb-IIIa in normal and pathologic platelets and megakaryocytes. Blood 1990, 75:1220-1227 [PubMed] [Google Scholar]
- 7.Sixma JJ: The role of platelet membrane glycoproteins in the adhesion of blood platelets. Agents Actions Suppl 1986, 20:15-26 [PubMed] [Google Scholar]
- 8.Escolar G, Leistikow E, White JG: The fate of the open canalicular system in surface and suspension-activated platelets. Blood 1989, 74:1983-1988 [PubMed] [Google Scholar]
- 9.Shattil SJ, Cunningham M, Hoxie JA: Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood 1987, 70:307-315 [PubMed] [Google Scholar]
- 10.Woods VL, Wolff LE, Keller DM: Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not to other extracellular proteins. J Biol Chem 1986, 261:15242-15251 [PubMed] [Google Scholar]
- 11.Hourdille P, Heilmann E, Combrie R, Winckler J, Clemetson KJ, Nurden AT: Thrombin induces a rapid redistribution of glycoprotein-Ib-IX complexes within the membrane systems of activated human platelets. Blood 1990, 76:1503-1513 [PubMed] [Google Scholar]
- 12.Escolar G, Clemetson K, White JG: Persistence of mobile receptors on surface-and suspension-activated platelets. J Lab Clin Med 1994, 123:536-546 [PubMed] [Google Scholar]
- 13.Berger G, Masse JM, Cramer EM: Alpha-granule membrane mirrors the platelet plasma membrane and contains the glycoproteins Ib, IX, and V. Blood 1996, 87:1385-1395 [PubMed] [Google Scholar]
- 14.Youssefian T, Masse JM, Rendu F, Guichard J, Cramer EM: Platelet and megakaryocyte dense granules contain glycoproteins Ib and IIb-IIIa. Blood 1997, 89:4047-4057 [PubMed] [Google Scholar]
- 15.Nurden A, Cazes E, Bihour C, Humbert M, Combrie R, Paponneau A: Confirmation that GP Ib-IX complexes have a reduced surface distribution on platelets activated by thrombin and TRAP-14-mer peptide. Br J Haematol 1995, 90:645-654 [DOI] [PubMed] [Google Scholar]
- 16.van Zanten GH, Heijnen HFG, Hu Y, Schut-Hese KM, Slotweg PJ, de Groot PG, Sixma JJ, Nieuwland R: A fifty percent reduction of platelet surface glycoprotein IB does not affect platelet adhesion under flow conditions. Blood 1998, 91:2353-2359 [PubMed] [Google Scholar]
- 17.White JG, Krumwiede MD, Cocking-Johnson D, Rao GHR, Escolar G: Retention of glycoprotein Ib/IX receptors on external surfaces of thrombin-activated platelets in suspension. Blood 1995, 86:3468-3478 [PubMed] [Google Scholar]
- 18.Michelson AD, Adelman B, Barnard MR, Carroll E, Handin RI: Platelet storage results in a redistribution of glycoprotein Ib molecules: evidence for a large intraplatelet pool of glycoprotein Ib. J Clin Invest 1988, 81:1734-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendayan M: Protein A-gold electron microscopic immunocytochemistry: methods, applications, and limitations. J Electron Microsc 1984, 1:243-270 [Google Scholar]
- 20.Cramer EM, Caen JP, Soria C, Berndt MC, Tenza D: Differential redistribution of platelet glycoproteins Ib and IIb-IIIa after plasmin stimulation. Blood 1991, 77:694-699 [PubMed] [Google Scholar]
- 21.Hourdille P, Benabdallah S, Belloc P, Nurden AT: Distribution of glycoprotein IIb-IIIa complexes in the surface membranes of human platelets and megakaryocytes. Br J Haematol 1985, 59:171-182 [DOI] [PubMed] [Google Scholar]
- 22.Wicki AN, Clementson KJ: Structure and function of platelet membrane glycoproteins Ib and V: effect of leukocyte elastase and other proteases on platelets response to von Willebrand factor and thrombin. Eur J Biochem 1985, 153:1-11 [DOI] [PubMed] [Google Scholar]
- 23.Sakariassen KS, Nievelstein PF, Coller BS, Sixma JJ: The role of platelet membrane glycoproteins Ib and IIb-IIIa in platelet adherence to human artery subendothelium. Br J Haematol 1986, 63:681-691 [DOI] [PubMed] [Google Scholar]
- 24.Gerrard JM, Phillips DR, Rao GH, Plow EF, Walz DA, Ross R, Harker LA, White JG: Biochemical studies of two patients with the gray platelet syndrome: selective deficiency of platelet α granules. J Clin Invest 1980, 66:102-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokuyasu KT: Use of polyvinylpyrrolidone and polyvinyl alcohol for cryoultramicrotomy. Histochem J 1989, 21:163-171 [DOI] [PubMed] [Google Scholar]
- 26.Griffiths G, McDowall A, Back R, Dubochet J: On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res 1984, 89:65-78 [DOI] [PubMed] [Google Scholar]
- 27.Webster P, Grab DJ: Intracellular colocalization of variant surface glycoprotein and transferrin-gold in Trypanosoma brucei. J Cell Biol 1988, 106:279-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escolar G, Monteagudo J, Castillo R, Cases A, Garrido M, Ordinas A: Ultrastructural immunolocalization and morphometric quantification of platelet membrane GPIb and GPIIb-IIIa in uremic patients. Prog Clin Biol Res 1988, 283:197-201 [PubMed] [Google Scholar]
- 29.Michelson AD, Benoit SE, Kroll MH, Ming Wi J, Roher MJ, Kestin AS, Barnard MR: The activation-induced decrease in the platelet surface expression of the glycoprotein Ib-X complex is reversible. Blood 1994, 83:3562-3563 [PubMed] [Google Scholar]
- 30.Frojmovic MM, Milton JG: Physical, chemical, and functional changes following platelet activation in normal, and giant platelets. Blood Cells 1983, 9:359-382 [PubMed] [Google Scholar]
- 31.May R: Leukocyteneinschlusse. Dtsch Arch Klin Med 1909, 96:1-6 [Google Scholar]
- 32.Hegglin R: Gleichzertge Konstitutionelle Veranderungen on Neutrophilen und Thrombocyten. Helv Med Acta 1945, 12:439-440 [PubMed] [Google Scholar]
- 33.Epstein CJ, Sahud MA, Piel CA, Goodman JR, Bernfield MR, Kusner JH, Albin AR: Hereditary macrothrombocytopathia, nephritis and deafness. Am J Med 1972, 52:299-310 [DOI] [PubMed] [Google Scholar]
- 34.White JG: Structural defects in inherited and giant platelet disorders. Harris H Hirschorn K eds. Advances in Human Genetics, 1991, vol 19.:pp 133-234 Plenum Press, New York [DOI] [PubMed] [Google Scholar]
- 35.White JG, Burris SM, Tukey D, Smith CM, II, Clawson CC: Micropipette aspiration of human platelets: influence of microtubules and actin filaments on deformability. Blood 1984, 64:210-214 [PubMed] [Google Scholar]
- 36.White JG, Krumwiede M: Influence of cytochalasin B on the shape change induced in platelets by cold. Blood 1973, 41:823-832 [PubMed] [Google Scholar]
- 37.Frojmovic MM, Wong T, White JG: Platelet plasma membrane is equally distributed between surface and osmotically evaginable surface-connecting membrane, independent of size subpopulation or species. Nouv Rev Fr Hematol 1992, 34:99-110 [PubMed] [Google Scholar]
- 38.Behnke O: The morphology of blood platelet membrane systems. Ser Haematol 1970, 3:3-16 [PubMed] [Google Scholar]
- 39.White JG, Clawson CC: A surface-connected canalicular system of blood platelets: a fenestrated membrane system. Am J Pathol 1980, 101:353-364 [PMC free article] [PubMed] [Google Scholar]
- 40.Kawakami H, Hirano H: Distribution of glycoconjugates on the plasma membrane and on membranes of the open-canalicular system in human platelets: a cytochemical study. Histochemistry 1989, 91:1-4 [DOI] [PubMed] [Google Scholar]
- 41.Nurden P: Bidirectional trafficking of membrane glycoproteins following platelet activation in suspension. Thromb Haemost 1997, 78:1305-1315 [PubMed] [Google Scholar]
- 42.Ghitescu L, Bendayan M: Immunolabeling efficiency of protein-A-gold complexes. J Histochem Cytochem 1990, 38:1523-1530 [DOI] [PubMed] [Google Scholar]
- 43.Slot JW, Geuze HJ: Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol 1981, 90:533-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths G, Hoppler H: Quantitation in immunocytochemistry: correlation of immunogold labelling to absolute number of membrane antigens. J Histochem Cytochem 1986, 34:1389-1398 [DOI] [PubMed] [Google Scholar]
- 45.Hodges GM, Smolira MA, Livingston DC: Scanning electron microscope immunocytochemistry in practice. Polak JM Varndell IM eds. Immunolabelling for Electron Microscopy. 1981, :pp 189-233 Elsevier, Amsterdam [Google Scholar]
- 46.Wagner CL, Mascelli Md, Neblock DS, Weisman HF, Coller BS, Jordan RE: Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 1996, 88:907-914 [PubMed] [Google Scholar]
- 47.Lopez JA: The platelet glycoprotein Ib-IX complex. Blood Coagul Fibrinolysis 1994, 5:97-119 [PubMed] [Google Scholar]
- 48.Michelson AD, Barnard MR: Thrombin-induced changes in platelet membrane glycoproteins Ib, IX, and IIb-IIIa complex. Blood 1987, 70:1673-1678 [PubMed] [Google Scholar]
- 49.Hourdille P, Gralnick HR, Heilman E, Derlon A, Ferrer A, Verzon G, Nurden AT: Von Willebrand factor bound to glycoprotein Ib is cleared from the platelet surface after platelet activation with thrombin. Blood 1992, 79:2011-2021 [PubMed] [Google Scholar]