Abstract
Hepatocellular carcinoma (HCC) is one of the most common fatal cancers worldwide. Hepatitis B virus and hepatitis C virus infections, exposure to aflatoxin, and excessive intake of alcohol have been identified as major risk factors. However, the molecular mechanisms underlying their development are still poorly understood. Recently, β-catenin, one of the key components of the Wnt signaling pathway, has been found to be mutated in about 20% of HCCs, suggesting a role of the Wnt pathway in their development. In this study, we examined β-catenin and APC mutations in 22 HCCs associated with HCV infection, using single-strand conformation polymorphism (SSCP) followed by direct DNA sequencing. β-Catenin mutations were found in nine (41%) cases, but no APC mutations were found. β-Catenin immunohistochemistry revealed nuclear accumulation of β-catenin protein in all nine tumors with a β-catenin mutation and two additional tumors without a mutation. These results suggest that activation of the Wnt signaling pathway by β-catenin mutation contributes significantly to the hepatocellular carcinogenesis associated with HCV infection.
β-Catenin protein, originally identified as a submembrane component of the cadherin-mediated cell-cell adhesion system, functions as a downstream transcriptional activator of the Wnt signaling pathway, forming complexes with the DNA-binding proteins Tcf and Lef-1. 1,2 The APC protein regulates the level of β-catenin protein, in cooperation with GSK-3β, via phosphorylation of serine/threonine residues encoded on exon 3 of the β-catenin gene. 1,3,4 This phosphorylation is followed by degradation of β-catenin through the ubiquitin-proteasome pathway. 5,6 Many APC mutations lead to truncated proteins with loss of β-catenin regulatory activity, causing accumulation of β-catenin protein. 4 Most β-catenin mutations detected in human neoplasms were located at any of four serine/theronine residues (codons 33, 37, 41, and 45) of exon 3 or at the contiguous residues of serine 33 and resulted in stabilization and accumulation of β-catenin protein. 7 About 80% of colorectal carcinomas have inactivating mutations in the APC gene, 8 whereas about 50% of colorectal cancers lacking an APC mutation contained a β-catenin mutation, substituting for APC mutations and activating the Wnt signaling pathway. 9
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, representing 4% of all malignant tumors, and is the seventh most frequent carcinoma in males and the ninth most frequent carcinoma in females. 10-12 Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, exposure to aflatoxin B1, and excessive intake of alcohol have been identified as major risk factors. 10,13,14 HCCs associated with HBV infection are most frequent in Southeast Asia and sub-Saharan Africa, 15 whereas HCCs associated with HCV are most prevalent in southern Europe and Japan. In Italy, Spain, and Japan, 50–75% of cases of HCCs are associated with HCV infection. 16
Mechanisms underlying hepatocarcinogenesis associated with HBV and HCV infection are not fully understood, except that both HBV and HCV virus infections can lead to chronic infection, causing cirrhosis, which is a well-known precursor of hepatic malignancy. 13,17 HBV is a double-stranded DNA virus and has been shown to be integrated into the HCC genome of most patients with serological evidence of HBV infection, 10,17 suggesting a direct carcinogenic effect through interaction with transformation-associated genes. Furthermore, the X protein of HBV appears to be a potent transactivator and to interact with p53, possibly interfering with its tumor suppressor activity. 18 In contrast, HCV is a single-stranded, positive-sense RNA virus and appears to be a nonintegrating virus. 17,19 However, the HCV core protein has some potential direct carcinogenic effects in vitro. HCV core protein transforms rat embryo fibroblasts into a malignant phenotype and suppresses apoptotic cell death in culture. 20,21 It may also play an important role in the promotion of cell growth by repressing the transcriptional activity of p53. 22 Furthermore, the HCV core protein induces HCCs in transgenic mice. 23
Molecular mechanisms underlying the development of HCCs are still poorly understood. p53 mutations have been found in about 25–45% of HCCs (see the review by Montesano et al). 14 HCCs that develop in patients highly exposed to aflatoxin B1 frequently contain a specific G→T transversion mutation in codon 249, whereas HCCs from areas with little exposure to this carcinogen did not contain specific p53 mutations. 14 Cyclin D1 gene was amplified 3–16-fold in five of 45 (11%) HCCs associated with HBV or HCV infection. 24 Activation of c-myc by DNA amplification was observed in 28 of 77 (36%) HCCs, and this occurred more frequently in the patients with HBV infection than in those with HCV infection. 25 Reduced expression of E-cadherin due to methylation at CpG sites around the promoter region and allelic deletions of the E-cadherin gene itself have been recognized in about 60% of HCCs. 26,27 Recently, β-catenin mutations were found in 19–23% of etiologically unspecified HCCs, 28,29 suggesting a role for the Wnt signaling pathway in the evolution of HCCs.
The objective of this study was to assess involvement of the Wnt signaling pathway in HCCs associated with HCV infection. We have screened mutations of the β-catenin and APC genes by SSCP followed by DNA sequencing in 22 HCCs associated with HCV infection.
Materials and Methods
Tumor Samples
Sixteen HCCs associated with HCV infection were obtained from the First Department of Surgery, Yamanashi Medical University (Yamanashi, Japan), and six were from the Department of Pathology, Institute of Clinical Pathology, University Hospital (Zürich, Switzerland). HCV serology was performed by second- or third-generation enzymed-linked immunosorbent assay and tested positive in all cases. HCV RNA was also tested by standard reverse transcriptase-polymerase chain reaction (RT-PCR) methods and was positive in all cases. All of the patients were negative for HBs antigen. The mean age of the patients was 62.7 ± 6.3 years (range 49–75 years). Seventeen patients were males and five were females (Table 1) ▶ . HCCs were diagnosed as well, moderately, or poorly differentiated, according to the WHO classification 30 (Table 1) ▶ . Tumors were fixed in buffered formalin and embedded in paraffin. Tumor tissues were manually microdissected, after microscopic identification and labeling. Care was taken to avoid contamination with normal or cirrhotic liver tissue. DNA was extracted as described previously. 31
Table 1.
β-Catenin Mutations in Hepatocellular Carcinomas Associated with Hepatitis C Virus Infection
| Case no. | Age (years) | Sex | Histology | β-Catenin mutation | Nuclear accumulation of β-catenin protein | APC codon 1493 polymorphism |
|---|---|---|---|---|---|---|
| 1 | 61 | M | Mod | — | − | G/A |
| 2 | 63 | M | Mod | Codon 37, TCT→TAT, Ser→Tyr | + | A/A |
| 3 | 69 | F | Well | — | − | A/A |
| 4 | 65 | M | Mod | — | + | G/A |
| 5 | 68 | M | Poor | — | − | A/A |
| 6 | 61 | M | Mod | — | − | A/A |
| 7 | 61 | M | Mod | Codon 45, TCT→TTT, Ser→Phe | + | G/A |
| 8 | 64 | M | Well | — | − | A/A |
| 9 | 62 | M | Mod | Codon 33, TCT→TAT, Ser→Tyr | + | G/A |
| 10 | 63 | M | Mod | Codon 37, TCT→CCT, Ser→Pro | + | A/A |
| 11 | 68 | M | Mod | — | + | G/A |
| 12 | 65 | M | Poor | — | − | A/A |
| 13 | 70 | F | Mod | — | − | A/A |
| 14 | 75 | F | Mod | — | − | A/A |
| 15 | 64 | F | Mod | Area 1: codon 45, TCT→CCT, Ser→Pro | + | A/A |
| Area 2: codon 45, TCT→CCT, Ser→Pro | + | |||||
| Area 3: codon 45, TCT→CCT, Ser→Pro | + | |||||
| 16 | 65 | M | Mod | Area 1: codon 32, GAC→TAC, Asp→Tyr | + | A/A |
| Area 2: codon 32, GAC→GCC, Asp→Ala | + | |||||
| 17 | 56 | M | Mod | — | − | G/A |
| 18 | 67 | F | Mod | Area 1: codon 41, ACC→GCC, Thr→Ala | + | G/G |
| Area 2: codon 37, TCT→TAT, Ser→Tyr | − | |||||
| 19 | 63 | M | Mod | — | − | G/G |
| 20 | 49 | M | Mod | Codon 32, GAC→GGC, Asp→Gly | + | A/A |
| 21 | 51 | M | Well | Codon 32, GAC→AAC, Asp→Asn | + | G/A |
| 22 | 50 | M | Mod | — | − | G/A |
SSCP Analysis and Direct DNA Sequencing for β-Catenin Mutations
PCR-SSCP analysis was carried out for exon 3 of the β-catenin gene, which contains the four potential GSK-3β phosphorylation sites, 29 using the following primers: 5′-ATGGAACCAGACAGAAAAG-3′ (nt 254–272, sense) and 5′-TACAGGACTTGGGAGGTATC-3′ (nt 386–405, antisense). PCR was carried out in a total volume of 10 μl, consisting of 2 μl of DNA solution, 0.5 U of Taq DNA polymerase (Sigma, St. Louis, MO), 0.5 μCi of [α-33P]dCTP (ICN Biomedicals, Costa Mesa, CA; specific activity, 3000 Ci/mmol), 1.5 mmol/L MgCl2, 0.2 mmol/L of each deoxynucleoside triphosphate (dNTP), 0.4 μmol/L of both sense and antisense primers, 10 mmol/L Tris-HCl (pH 8.3), and 50 mmol/L KCl in the RoboCycler Gradient 96 (Stratagene, La Jolla, CA) with an initial step of denaturation at 95°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 1 minute, annealing at 53°C for 1 minute, polymerization at 72°C for 1 minute, and then a final extension of 5 minutes at 72°C. Five microliters of PCR products was mixed with 12.5 μl loading buffer (95% formamide, 20 mmol/L EDTA, 0.05% xylene cyanol and bromophenol blue), denatured at 95°C for 10 minutes, and quenched on ice. Four microliters of the above mixture was run on a 6% polyacrylamide nondenaturing gel containing 8% glycerol, at 4 W for 14 hours at room temperature, and/or on a 6% polyacrylamide nondenaturing gel containing 6% glycerol, at 40 W for 3.5 hours, with cooling by fan. Gels were dried at 80°C and autoradiographed for 12–48 hours.
Samples that showed mobility shifts in SSCP analysis were further analyzed by direct DNA sequencing. Two samples that did not show mobility shifts but showed nuclear accumulation of β-catenin protein by immunohistochemistry were also sequenced. PCR amplification was carried out as described above in the absence of [α-33P]dCTP. Five microliters of PCR products was digested with 1 U of shrimp alkaline phosphatase and 5 U of exonuclease I at 37°C for 15 minutes. After inactivation of these enzymes at 80°C for 15 minutes, 15 pmol primer (the same primers for PCR) and 2 μl of 5× sequenase buffer (200 mmol/L Tris-HCl, pH 7.5, 100 mmol/L MgCl2, 250 mmol/L NaCl) were added. The template-primer mixture was heated at 100°C for 5 minutes and then placed in ice-cold water. Dithiothreitol (0.1 mol/L), 3 U Sequenase, version 2.0 (USB, Cleveland, OH), and 0.5 μCi [α-33P]dATP or [α-33P]dCTP were added to samples, which were then divided into four wells containing each termination mixture. Samples were incubated at 42°C for 6 minutes and mixed with 5 μl stop solution (USB). After being heated at 80°C for 3 minutes, samples were loaded onto a 6% polyacrylamide/7 mol/L urea gel and run at 70 W for 1.5–3 hours. Gels were dried at 80°C and autoradiographed for 12–48 hours.
SSCP Analysis for APC Mutations
Mutations in codons 1255–1513 in exon 15 of the APC gene, corresponding to the mutation cluster region (MCR), were screened by SSCP analyses in 22 HCCs. This region covers about two-thirds of all APC somatic mutations in colon tumors in FAP and non-FAP patients. 32-34 Three pairs of primers were used to amplify the following overlapping fragments: for codons 1255–1363 (fragment 15A), 5′-AACCAAGAAACAATACAGA-3′ and 5′-CACTTTTGGAGGGAGATTT-3′; for codons 1342–1433 (fragment 15B), 5′-AGAATCAGCCAGGCACAAAG-3′ and 5′-GCTTGGTGGCATGGTTTGT-3′; for codons 1410–1513 (fragment 15C), 5′-GCAGTGGAATGGTAAGTGG-3′ and 5′-TCATCGAGGCTCAGAGCA-3′. Prescreening for mutations by PCR-SSCP analysis in this region was performed in a total volume of 10 μl, consisting of 1 μl of DNA solution, 0.5 U of Taq DNA polymerase (Sigma), 0.5 μCi of [α-33P]dCTP (ICN Biomedicals; specific activity, 3000 Ci/mmol), 2.0 mmol/L (for fragment 15A) or 1.5 mmol/L (for fragments 15B and C) MgCl2, 0.2 mmol/L of each dNTP, 0.4 μmol/L of both sense and antisense primers, 10 mmol/L Tris-HCl (pH 8.3), and 50 mmol/L KCl in the RoboCycler Gradient 96 (Stratagene, La Jolla, CA), with an initial step of denaturation at 95°C for 5 minutes, followed by 40 cycles of denaturation at 95°C for 60 seconds; annealing at 47°C (for fragment 15A), 57°C (for segment 15B), or 55°C (for segment 15C) for 70 seconds; polymerization at 72°C for 70 seconds; and a final extension of 5 minutes at 72°C. After amplification, 5 μl of PCR products was mixed with 12.5 μl loading buffer (95% formamide, 20 mmol/L EDTA, 0.05% xylene cyanol, and bromophenol blue), denatured at 95°C for 10 minutes, and quenched on ice. Four microliters of this mixture was run on a 6% polyacrylamide nondenaturing gel containing 6% glycerol at 40 W for 5 hours with cooling by fan. Gels were dried at 80°C and autoradiographed for 12–48 hours.
Samples that showed mobility shifts in the SSCP analysis were further analyzed by direct DNA sequencing. The PCR products were sequenced using the same primers for PCR and with the same protocol as described above for β-catenin mutations.
β-Catenin Immunohistochemistry
Twenty-two cases of HCCs and their respective nontumorous liver tissue adjacent to carcinomas were subjected to immunohistochemical analysis of β-catenin expression. The sections were deparaffinized in xylene and rehydrated in graded ethanol. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxidase in methanol for 30 minutes at room temperature. For antigen retrieval, the sections were microwaved in antigen unmasking solution (Vector Laboratories, Burlingame, CA) three times for 5 minutes. After incubation with 5% skimmed milk for 1 hour at room temperature, the sections were incubated with the primary antibody against β-catenin (Transduction Laboratories, Lexington, KY; 1:1000–2000) overnight at 4°C. The reaction was visualized with a Vectastein Elite ABC kit (Vector Laboratories) and 3,3′-diaminobenzidine solution (Vector Laboratories). The sections were then counterstained with hematoxylin. Formalin-fixed, paraffin-embedded sections of human colon were used as positive controls. Sections without primary antibody served as negative controls.
Results
β-Catenin Mutations
SSCP followed by direct DNA sequencing revealed that nine of 22 (41%) HCCs contained a β-catenin mutation. All mutations were single nucleotide substitutions occurring at different putative phosphorylation sites of serine/theronine or their contiguous residues in exon 3: three at codon 32, one at codon 33, three at codon 37, one at codon 41, and two at codon 45 (Table 1 ▶ , Figure 1 ▶ ). In all cases, a wild-type base was also detectable. Except for two cases (cases 18 and 20), we also analyzed adjacent cirrhotic, nontumorous liver. None of the adjacent nontumorous tissues analyzed contained a β-catenin mutation.
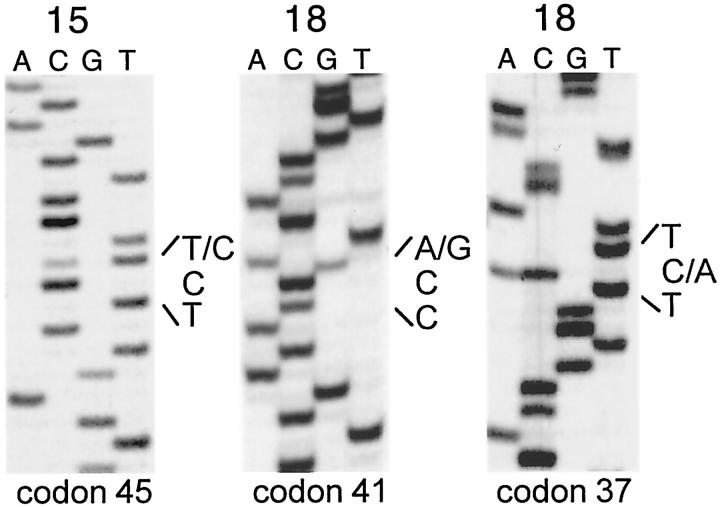
Figure 1.
Representative DNA sequencing autoradiographs of β-catenin mutations. Left: TCT→CCT mutation at codon 45, leading to Ser→Pro (case 15). Center: ACC→GCC mutation at codon 41, leading to Thr→Ala (case 18, area 1). Right: TCT→TAT mutation at codon 37, leading to Ser→Tyr (case 18, area 2).
In three cases (cases 15, 16, and 18), two or three different tumor areas were separately analyzed. Two different mutations were detected in two tumor areas from the same patients (cases 16 and 18), whereas identical mutations in three different tumor areas were found in another case (cases 15, Table 1 ▶ , Figure 1 ▶ ).
APC Mutation and Polymorphism
SSCP followed by direct sequencing showed no miscoding mutations but a polymorphism at codon 1493 (ACG→ACA, Thr→Thr) in HCCs analyzed. A/A homozygote, G/G homozygote, and G/A heterozygote were observed in 12 (55%), two (9%), and eight (36%) HCCs, respectively. These frequencies were not significantly different from those in blood DNAs from 50 healthy individuals from USA (A/A homozygote, 40%; G/G homozygote, 6%; G/A heterozygote, 54%, P = 0.5615, Fisher’s exact test).
β-Catenin Immunohistochemistry
In the nontumorous liver tissue examined, the cytoplasmic membrane of bile duct epithelial cells showed strong β-catenin protein expression, whereas the cytoplasmic membrane of hepatocytes showed weak or moderate expression (Figure 2A) ▶ .
Figure 2.

β-Catenin immunohistochemistry. A, left: Nontumorous liver showing weak membrane staining. A, right: Proliferating bile ducts showing strong membrane staining. B, C: Nuclear accumulation of β-catenin protein in neoplastic hepatocytes in hepatocellular carcinoma associated with HCV infection. Magnifications: ×75 (A and B), ×150 (C).
In all HCCs, the cytoplasmic membrane of neoplastic hepatocytes showed a weak to moderate expression of β-catenin, which was not different from that of hepatocytes in surrounding nontumorous tissues. Focal accumulation of β-catenin protein in the nuclei of neoplastic cells was observed in 11 of 22 (50%) HCCs. Nine of these contained a β-catenin mutation (Table 1) ▶ . In most cases (10 of 11, 91%), neoplastic cells with nuclear β-catenin accumulation were restricted to the periphery of tumor nodules (Figure 2, B and C) ▶ or were in small tumor nests detached from large nodules. In one case (case 20), strong β-catenin protein expression was observed in the nuclei and cytoplasm of neoplastic hepatocytes, diffusely throughout the tumor. Most neoplastic cells with nuclear β-catenin protein accumulation also showed β-catenin protein expression in the cytoplasmic membrane.
Paraffin sections of normal human colon epithelial cells (positive control) showed strong immunoreactivity to β-catenin. Sections without primary antibody showed no immunoreactivity.
Discussion
β-Catenin mutations have been identified in a variety of human tumors at low frequencies. These include colon cancer (7–15%), 9,35,36 endometrial carcinoma (13%), 37 ovarian cancer (8%), 38 medulloblastoma (4%), 39 prostate cancer (5%), 40 and bone and soft-tissue tumors (3%). 41 Recently, a higher frequency of β-catenin mutation has been reported in skin tumors (75%), 42 thyroid carcinoma (61%), 43 and childhood hepatoblastoma (48%). 44
The present study shows that β-catenin mutations are frequent (41%) in HCCs associated with HCV infection. This frequency is higher than in previous studies of nonspecified HCCs (19–23%). 28,29 It remains to be clarified whether HCCs associated with HCV more frequently contain β-catenin mutations than HCCs associated with other etiological factors. There is evidence that the frequency of β-catenin mutations may differ in HCCs induced by different chemical carcinogens in mice. β-Catenin mutations were found in nine of 24 (37.5%) methylene chloride-induced and 17 of 42 (41%) oxazepam-induced liver tumors, but only in three of 18 (17%) vinyl carbamate-induced liver tumors, one of 18 (6%) TCDD-induced liver tumors, and one of 22 (5%) spontaneous liver tumors in B6C3F1 mice. 45
Consistent with previous studies, 7 seven of 10 (70%) β-catenin mutations detected in this study were located at specific serine/threonine residues (codons 33, 37, 41, and 45) of exon 3; this is also consistent with the role of these residues as putative phosphorylation targets of GSK-3β. Mutations at the contiguous residue of serine 33 may alter the protein structure and limit the access of GSK-3β, leading to inhibition of phosphorylation of β-catenin and its degradation through the ubiquitin-proteasome pathway. 28
The clonality of HCCs has been studied by several investigators, but this issue is still controversial. 46-49 Based on X-linked RFLP analysis of the phosphoglycerokinase gene, Aihara et al 48 found that all seven HCCs induced by HCV were monoclonal. By using the X-chromosome inactivation method, Kawai et al 47 reported that nine of 10 HCCs analyzed were monoclonal in origin, whereas another one was polyclonal in origin. By DNA fingerprinting and hepatitis B virus DNA integration pattern analysis, Sheu 46 found that 12 of 18 hepatitis B surface antigen-positive or negative patients were different in clonality, suggesting that the majority of HCCs are polyclonal. Of the cases in this study, two different mutations were detected in two cases (cases 16 and 18), whereas another case (cases 15) showed identical mutations in three different areas of HCCs (Table 1) ▶ . Taken together, these results suggest that development of HCC may be polyclonal in some cases, but intrahepatic metastases may also occur. 50 Alternatively, different mutations may be due to genetic heterogeneity within the tumor.
Mutations of β-catenin or APC genes result in stabilization of β-catenin and a significant increase in this protein within the cell. Accumulated β-catenin may be translocated into the nucleus, where it serves as a transcriptional factor through binding with the Tcf-Lef family. 4,7,51 In this study, we showed a correlation between the presence of β-catenin mutations and nuclear accumulation of β-catenin protein in HCCs. Nuclear accumulation of β-catenin was observed in 11 cases; nine of these carried a β-catenin mutation. The absence of β-catenin mutations in the other two cases with β-catenin protein accumulation suggests that there may be other mechanisms leading to β-catenin accumulation, such as alterations in GSK3β, axin, or other components of the Wnt signaling pathway. It is of interest to note that, except for one case (case 20), in which tumor cells with nuclear accumulation of β-catenin were observed diffusely throughout the tumor, the nuclear accumulation of β-catenin was largely restricted to the periphery of the tumor nests. The mechanism and biological significance of the specific localization of neoplastic cells with nuclear accumulation of β-catenin remain to be elucidated.
Miscoding APC mutation was not found in any of the HCCs analyzed. Several previous studies suggested that APC may not be alterated in HCCs because the APC locus on chromosome 5q did not show loss of heterozygosity. 52-54 Another study 55 screened five HCCs by RNase protection assay and revealed no mutations. The present study examined the region that covers the sequence where about 70% of APC somatic mutations have been found in human colon cancers. 33,34 Although the possibility of mutations in other regions of the APC gene cannot be excluded, our data suggest that mutation in the β-catenin, but not the APC gene, is a major causal event in the activation of the Wnt signaling pathway in HCCs associated with HCV infection.
It has been suggested that some polymorphism in the APC gene, rather than altering the function of the encoded protein, may create an unstable tract that is hypermutable, leading to somatic, truncating mutations occurring at adjacent sequences and indirectly causing cancer predisposition. 56 Of more than 20 polymorphisms identified to date in the APC gene, 57 the I1307K polymorphism has been studied in detail and has been related to a predisposition to colorectal cancer in Ashkenazi Jewish populations. 56,58 Little is known about the significance of other APC polymorphisms. In the present study, we did not find the I1307K polymorphism but found the ACG/ACA polymorphism in codon 1493, as previously reported. 32 There was no difference in the frequencies of A/A, G/A, and G/G genotypes in APC codon 1493 between HCCs analyzed in this study and blood DNA from healthy individuals.
In summary, we show a high frequency of β-catenin mutations and focal nuclear accumulation of β-catenin protein in neoplastic hepatocytes in HCCs associated with HCV infection. These results indicate that activation of the Wnt signaling pathway by β-catenin mutations is frequently involved in the development of HCCs associated with HCV infection.
Acknowledgments
The authors are grateful to Mireille Laval and Nicole Lyandrat for their excellent technical assistance.
Footnotes
Address reprint requests to Dr. Hiroko Ohgaki, Unit of Molecular Pathology, International Agency for Research on Cancer, 150 Cours Albert Thomas, 69372 Lyon Cedex 08, France. E-mail: ohgaki@iarc.fr.
Supported by grants from the Academy of Finland and the Conselho National de Desenvolvimento Científico e Tecnológico, Brazil.
References
- 1.Barth AI, Nathke IS, Nelson WJ: Cadherins, catenins, and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol 1997, 9:683-690 [DOI] [PubMed] [Google Scholar]
- 2.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 3.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P: Binding of GSK3b to the APC-β-catenin complex and regulation of complex assembly. Science 1996, 272:1023-1026 [DOI] [PubMed] [Google Scholar]
- 4.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P: Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 1995, 92:3046-3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R: β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997, 16:3797-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW: Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J Biol Chem 1997, 272:24735-24738 [DOI] [PubMed] [Google Scholar]
- 7.Polakis P: The oncogenic activation of β-catenin. Curr Opin Genet Dev 1999, 9:15-21 [DOI] [PubMed] [Google Scholar]
- 8.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 9.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW: Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res 1998, 58:1130-1134 [PubMed] [Google Scholar]
- 10.Buendia MA: Hepatitis B viruses and hepatocellular carcinoma. Adv Cancer Res 1992, 59:167-226 [DOI] [PubMed] [Google Scholar]
- 11.Ng IO, Chung LP, Tsang SW, Lam CL, Lai EC, Fan ST, Ng M: p53 gene mutation spectrum in hepatocellular carcinomas in Hong Kong Chinese. Oncogene 1994, 9:985-990 [PubMed] [Google Scholar]
- 12.Caselmann WH, Alt M: Hepatitis C virus infection as a major risk factor for hepatocellular carcinoma. J Hepatol 1996, 24:61-66 [PubMed] [Google Scholar]
- 13.Idilman R, De Maria N, Colantoni A, Van Thiel DH: Pathogenesis of hepatitis B and C-induced hepatocellular carcinoma. J Viral Hepatol 1998, 5:285-299 [DOI] [PubMed] [Google Scholar]
- 14.Montesano R, Hainaut P, Wild CP: Hepatocellular carcinoma: from gene to public health. J Natl Cancer Inst 1997, 89:1844-1851 [DOI] [PubMed] [Google Scholar]
- 15.Brechot C: Hepatitis B, C viruses, and primary liver cancer. Baillieres Clin Gastroenterol 1996, 10:335-373 [DOI] [PubMed] [Google Scholar]
- 16.Bisceglie AM: Hepatitis C and hepatocellular carcinoma. Hepatology 1997, 26:34S-38S [DOI] [PubMed] [Google Scholar]
- 17.Tabor E: Viral hepatitis and liver cancer. Goldin RD Thomas HC Gerber MA eds. Pathology of Viral Hepatitis. 1998, :pp 161-177 Arnold, London [Google Scholar]
- 18.Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC: Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci USA 1994, 91:2230-2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okuda K: Hepatitis C virus and hepatocellular carcinoma. Tabor E Di Bisceglie AM Purcell RH eds. Etiology, Pathology, and Treatment of Hepatocellular Carcinoma in North America. 1991, :p 119 Gulf Publishing Company, Houston, London, Paris, Zurich, Tokyo, [Google Scholar]
- 20.Ray RB, Lagging LM, Meyer K, Ray R: Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J Virol 1996, 70:4438-4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray RB, Meyer K, Ray R: Suppression of apoptotic cell death by hepatitis C virus core protein. Virology 1996, 226:176-182 [DOI] [PubMed] [Google Scholar]
- 22.Ray RB, Steele R, Meyer K, Ray R: Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem 1997, 272:10983-10986 [DOI] [PubMed] [Google Scholar]
- 23.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K: The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nature Med 1998, 4:1065-1067 [DOI] [PubMed] [Google Scholar]
- 24.Nishida N, Fukuda Y, Komeda T, Kita R, Sando T, Furukawa M, Amenomori M, Shibagaki I, Nakao K, Ikenaga M: Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer Res 1994, 54:3107-3110 [PubMed] [Google Scholar]
- 25.Peng SY, Lai PL, Hsu HC: Amplification of the c-myc gene in human hepatocellular carcinoma: biologic significance. J Formos Med Assoc 1993, 92:866-870 [PubMed] [Google Scholar]
- 26.Kanai Y, Ushijima S, Hui AM, Ochiai A, Tsuda H, Sakamoto M, Hirohashi S: The E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomas. Int J Cancer 1997, 71:355-359 [DOI] [PubMed] [Google Scholar]
- 27.Hirohashi S: Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 1998, 153:333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y: Activation of the β-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 1998, 58:2524-2527 [PubMed] [Google Scholar]
- 29.De La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C: Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 1998, 95:8847-8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishak KG, Anthony PP, Sobin LH: Histological typing of tumors of the liver. International Histological Classification of Tumors. World Health Organization 1994. Berlin, Heidelberg, Springer-Verlag.
- 31.Watanabe K, Tachibana O, Sato K, Yonekawa Y, Kleihues P, Ohgaki H: Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol 1996, 6:217-224 [DOI] [PubMed] [Google Scholar]
- 32.Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y, Mori T, Utsunomiya J, Baba S, Petersen G, Hamilton SR, Kinzler KW, Vogelstein B, Nakamura Y: Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA 1992, 89:4452-4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y: Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1992, 1:229-233 [DOI] [PubMed] [Google Scholar]
- 34.Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, Muraoka M, Takahashi H, Amada Y, Fukayama M, Maeda Y, Iwama T, Mishima Y, Mori T, Koike M: Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res 1994, 54:3011-3020 [PubMed] [Google Scholar]
- 35.Iwao K, Nakamori S, Kameyama M, Imaoka S, Kinoshita M, Fukui T, Ishiguro S, Nakamura Y, Miyoshi Y: Activation of the β-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res 1998, 58:1021-1026 [PubMed] [Google Scholar]
- 36.Samowitz WS, Powers MD, Spirio LN, Nollet F, Van Roy F, Slattery ML: β-Catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res 1999, 59:1442-1444 [PubMed] [Google Scholar]
- 37.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S: β-Catenin mutation in carcinoma of the uterine endometrium. Cancer Res 1998, 58:3526-3528 [PubMed] [Google Scholar]
- 38.Palacios J, Gamallo C: Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 1998, 58:1344-1347 [PubMed] [Google Scholar]
- 39.Zurawel RH, Chiappa SA, Allen C, Raffel C: Sporadic medulloblastomas contain oncogenic β-catenin mutations. Cancer Res 1998, 58:896-899 [PubMed] [Google Scholar]
- 40.Voeller HJ, Truica CI, Gelmann EP: Beta-catenin mutations in human prostate cancer. Cancer Res 1998, 58:2520-2523 [PubMed] [Google Scholar]
- 41.Iwao K, Miyoshi Y, Nawa G, Yoshikawa H, Ochi T, Nakamura Y: Frequent β-catenin abnormalities in bone and soft-tissue tumors. Jpn J Cancer Res 1999, 90:205-20910189891 [Google Scholar]
- 42.Chan EF, Gat U, McNiff JM, Fuchs E: A common human skin tumour is caused by activating mutations in β-catenin. Nature Genet 1999, 21:410-413 [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Rostan G, Tallini G, Herrero A, D’Aquila TG, Carcangiu ML, Rimm DL: Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Cancer Res 1999, 59:1811-1815 [PubMed] [Google Scholar]
- 44.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T: Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the β-catenin gene. Cancer Res 1999, 59:269-273 [PubMed] [Google Scholar]
- 45.Anna CH, Foley JF, White CM, Sills RC, Barrett JC, Devereux TR: β-Catenin mutations in chemically induced mouse hepatocellular adenomas and carcinomas. Proc Am Assoc Cancer Res 1999, 40:505(Abstract) [Google Scholar]
- 46.Sheu JC: Molecular mechanism of hepatocarcinogenesis. J Gastroenterol Hepatol 1997, 12:S309-S313 [DOI] [PubMed] [Google Scholar]
- 47.Kawai S, Imazeki F, Yokosuka O, Ohto M, Shiina S, Kato N, Omata M: Clonality in hepatocellular carcinoma: analysis of methylation pattern of polymorphic X-chromosome-linked phosphoglycerate kinase gene in females. Hepatology 1995, 22:112-117 [PubMed] [Google Scholar]
- 48.Aihara T, Noguchi S, Sasaki Y, Nakano H, Imaoka S: Clonal analysis of regenerative nodules in hepatitis C virus-induced liver cirrhosis. Gastroenterology 1994, 107:1805-1811 [DOI] [PubMed] [Google Scholar]
- 49.Sheu JC, Huang GT, Chou HC, Lee PH, Wang JT, Lee HS, Chen DS: Multiple hepatocellular carcinomas at the early stage have different clonality. Gastroenterology 1993, 105:1471-1476 [DOI] [PubMed] [Google Scholar]
- 50.Blum HE, Offensperger WB, Walter E, Offensperger S, Wahl A, Zeschnigk C, Gerok W: Hepatocellular carcinoma and hepatitis B virus infection: molecular evidence for monoclonal origin and expansion of malignantly transformed hepatocytes. J Cancer Res Clin Oncol 1987, 113:466-472 [DOI] [PubMed] [Google Scholar]
- 51.Willert K, Nusse R: β-Catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 1998, 8:95-102 [DOI] [PubMed] [Google Scholar]
- 52.Ding SF, Habib NA, Dooley J, Wood C, Bowles L, Delhanty JD: Loss of constitutional heterozygosity on chromosome 5q in hepatocellular carcinoma without cirrhosis. Br J Cancer 1991, 64:1083-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding SF, Delhanty JD, Dooley JS, Bowles L, Wood CB, Habib NA: The putative tumor suppressor gene on chromosome 5q for hepatocellular carcinoma is distinct from the MCC and APC genes. Cancer Detect Prev 1993, 17:405-409 [PubMed] [Google Scholar]
- 54.Fujimori M, Tokino T, Hino O, Kitagawa T, Imamura T, Okamoto E, Mitsunobu M, Ishikawa T, Nakagama H, Harada H: Allelotype study of primary hepatocellular carcinoma. Cancer Res 1991, 51:89-93 [PubMed] [Google Scholar]
- 55.Horii A, Nakatsuru S, Miyoshi Y, Ichii S, Nagase H, Ando H, Yanagisawa A, Tsuchiya E, Kato Y, Nakamura Y: Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res 1992, 52:6696-6698 [PubMed] [Google Scholar]
- 56.Laken SJ, Petersen GM, Gruber SB, Oddoux C, Ostrer H, Giardiello FM, Hamilton SR, Hampel H, Markowitz A, Klimstra D, Jhanwar S, Winawer S, Offit K, Luce MC, Kinzler KW, Vogelstein B: Familial colorectal cancer in Ashkenazim due to a hypermutable tract in APC. Nature Genet 1997, 17:79-83 [DOI] [PubMed] [Google Scholar]
- 57.Laurent-Puig P, Beroud C, Soussi T: APC gene: database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res 1998, 26:269-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White RL: Excess risk of colon cancer associated with a polymorphism of the APC gene? Cancer Res 1998, 58:4038-4039 [PubMed] [Google Scholar]