Abstract
B-cell lymphomas of mucosa-associated lymphoid tissue (MALT) type develop against a background of chronic inflammation and have functional autoantigen receptors. Because they respond to environmental factors in vivo, the expression of costimulatory molecules, which play a key role in the differentiation of normal B-lymphocytes and in T-/B-cell interaction, may be critical in early MALT-type lymphoma pathogenesis until further chromosomal aberration leads to progression. We found a high number of tumor-infiltrating T-lymphocytes (TITLs) in all low-grade MALT-type lymphomas. The TITLs in low-grade lymphomas were activated and expressed a memory and immunocompetent phenotype. Reverse transcriptase-polymerase chain reaction analyses and immunohistochemistry confirmed the presence of CD40-ligand and Fas-ligand in 80% of low-grade lymphomas. In contrast to the TITLs, the tumor B cells did not express CD40-ligand or Fas-ligand in vivo or in vitro. Moreover, the cytokine profile in vivo suggested a Th2/Th3-weighted profile (interleukin-10, interleukin-13, transforming growth factor β1) rather than Th1-weighted (interferon-γ, interleukin-2). By interphase fluorescence in situ hybridization analysis the translocation t(11;18)(q21;q21) was found in four of nine (44%) cases studied. Interestingly, there was a four times higher proliferation and survival rate of purified t(11;18)-positive tumor B cells in vitro, although there were no significant profile differences from the TITLs in vivo. The finding of essential costimulating molecules in low-grade MALT-type lymphomas in vivo indicates a locally directed cognate T-/B-cell interaction. Consequently, a potentially equipped inflammatory background may not only determine the fate of autoreactive B-cells, but is also crucial to lymphoma maintenance and progression.
B-cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) arise in sites primarily devoid of lymphoid tissue and against a background of long-lasting chronic inflammation. 1 Their propensity to remain localized may be dependent on continuous antigen stimulation and/or help provided by the residual lymphoid tissue. 2 Both histopathological and clinical features of MALT-type lymphomas suggest that lymphomagenesis is, at least in part, T-cell dependent. This idea has gained considerable support from clinical observations that most human low-grade gastric MALT-type lymphomas can regress at early stages on Helicobacter pylori eradication 3,4 and that some low-grade MALT-type lymphomas respond to autologous T-cell help, resulting in tumor cell proliferation in vitro. 5
Recently, in vitro and molecular studies suggested that MALT-type lymphoma B cells are antigen sensitive but autoreactive 6-8 and thus have escaped a tolerance mechanism that is normally checked by various T-cell subsets through interactions of cell surface molecules and cytokines. Experimental observations suggest that antigen and Fas-ligand/CD40-ligand expressed on activated T-cells act during cognate T-/B-cell interaction and are crucial checkpoints for germinal center B-cell maturation, generating marginal zone B cells, but act to delete harmful B cells 9,10 . The maturation of B cells to effective producers of high-affinity and high-specificity antibodies is controlled by defined T-cell subsets through cell surface molecules and soluble cytokines. 11-13 In particular, whereas Th1-type cytokines (ie, interleukin-2 (IL2), interferon-γ (INF-γ)) are important in cell-mediated immune responses and the eradication of microbial pathogens, 14 Th2- and Th3-type cytokines (IL4, IL6, IL10, and transforming growth factor β1 (TGF-β1)) have been demonstrated to be crucial for B-cell maturation 15,16 but may also support the growth of otherwise uncontrolled B cells in autoimmune disorders 17 and suppress cell-mediated autoimmunity (ie, TGF-β1). Therefore, the discovery of functionally heterogeneous CD4+ T-cell subsets that secrete different cytokines may determine not only the type of B-cell immune response but may also provide key molecules in B-cell lymphoma initiation and progression.
Recently, in a larger series a t(11;18)(q21;q21) chromosome translocation was found to be the characteristic aberration in low-grade MALT-type lymphoma 18 involving a novel gene MLT and the HIAP1 gene, encoding an inhibitor of apoptosis. 19 Although its biological and clinical significance is so far unknown, it is suggested that the rearranged genes may support increased B-cell survival and decrease T-cell-dependent help. 20
We present herein a detailed characterization of the microenvironment of low-grade MALT-type lymphomas, which is different from chronic gastritis, and show that essential costimulatory molecules are present in all cases irrespective of the t(11;18) alteration, which may provide an important background for tumor progression.
Materials and Methods
Patients and Specimens
Fresh tissues were received within 2–6 hours after surgery for the preparation of cell suspensions, snap-freezing in liquid nitrogen, and routine fixation for histological examination. Nonneoplastic tonsillar tissues with reactive tonsillitis and resection samples from patients with primary low-grade MALT-type lymphoma of the stomach were selected from the lymph node registry in Würzburg. The tumors were always confined to the stomach and regional lymph nodes without clinical evidence of generalized disease (Table 1) ▶ . Additionally, one low-grade MALT-type lymphoma of the thyroid gland and one case of gastritis (no. 11; as a control) were investigated (Table 1) ▶ . For diagnostic purposes, the morphological and immunophenotypical analyses of paraffin-embedded and freshly frozen sections were performed by standard methods as recently described. 1 In all cases the diagnosis was confirmed according to the criteria of the Revised European American Lymphoma (REAL) Classification. 21
Table 1.
Specimen Data
| Patient | Site of origin | Diagnosis | t(11;18) (q21;q21) | Stage* |
|---|---|---|---|---|
| 1 | Stomach | Low-grade MALT | No | EI2 |
| 2 | Stomach | Low-grade MALT | n.d. | EII1 |
| 3 | Stomach | Low-grade MALT | Yes | EI2 |
| 4 | Stomach | Low-grade MALT | No | EI2 |
| 5 | Stomach | Low-grade MALT | No | EI1 |
| 6 | Stomach | Low-grade MALT | No | EI2 |
| 7 | Stomach | Low-grade MALT | Yes | EII1 |
| 8 | Thyroid | Low-grade MALT | Yes | n.d. |
| 9 | Stomach | Low-grade MALT | Yes | EII1 |
| 10 | Stomach | Low-grade MALT | No | EI2 |
| 11 | Stomach | Gastritis | No | — |
* Stage according to modified classification of Musshoff. 53
n.d., not determined.
Immunostaining
Immunohistochemical staining was performed on 4-μm cryostat sections of fresh frozen surgical specimens. Biopsy tissues were kept at −70°C as snap-frozen blocks until sections were prepared at the time of the experiments. The immunoperoxidase method was applied by a three-step incubation procedure with diluted affinity-purified anti-rabbit, anti-rat, anti-mouse antibodies (all from Dako, Hamburg, Germany) and serum from each species as a control, as described in detail elsewhere. 22 APAAP staining was used to visualize mouse anti-human CD40-ligand (kind gift from RA Krozcek, Berlin, Germany) as described. 23 The following primary antibodies were used in this study: rabbit anti-human Fas-ligand (SC-834-G; Santa Cruz Biotechnology, Santa Cruz, CA), rat anti-human IL-2 (MQ1–17H12; Pharmingen, Heidelberg, Germany), mouse anti-human IL-10 (mAb 2,17; R&D Systems, Wiesbaden, Germany), rat anti-human IL-13 (JES10-5A2; Pharmingen), and TGF-β1 (BMS4435; Boehringer Mannheim, Mannheim, Germany). CD40-ligand and Fas-ligand served in immunostained cytospins as positive controls for cytokines from different cell lines (Jukat, Raji, THP-1) stimulated with phorbol myristic acetate/ionomycin or lipopolysaccharide over 24 hours. 24,25
Flow Cytometry
Flow cytometric analysis was performed on a FACScan (Becton Dickinson) with an argon ion laser tuned at 488 nm, using LYSIS II for data acquisition and analysis and triple immunostaining with directly conjugated antibodies (anti-CD19 (HD 37; Sigma); anti-κ (KP-53; Sigma), anti-λ (HP6054; Sigma), anti-CD3 (UCHT-1; Sigma), anti-CD14 (Leu-M3; Becton Dickinson), anti-CD40 (mAb89), 26 anti-CD4 (R-8886; Sigma), anti-CD8 (R-806; Dako), anti-CD38 (MHCD3804; MEDAC), anti-IgD (I-2768; Sigma), anti-IgM (P-9295; Sigma), anti-CD25 (R-811; Dako), anti-CD69 (BD 347823; Becton Dickinson)). Each measurement contained 20,000 cells. As a specificity control and for instrument setup isotype controls were used in parallel for each directly conjugated antibody regarding species, isotype, and type of fluorochrome (all from Sigma, Deisenhofen, Germany).
B-Cell Purification, Cell Culture, Stimulation, and Cytogenetic Analysis
Viable lymphocyte single-cell suspensions were isolated by density-gradient centrifugation and negative depletion using magnetic beads coupled with anti-CD2 and anti-CD14 (Dynal, Hamburg, Germany) to remove macrophages and T cells. Thereafter, as a control, nonneoplastic memory B cells from tonsils were depleted of CD38+ (clone ACT 13.5; Serotec, Wiesbaden, Germany) and IgD+ (clone HJ9; Sigma) cells to remove germinal center cells, plasma cells, and mantle zone cells as described elsewhere. 27,28 Lymphoma cells were further purified by negative depletion of nonneoplastic bystander B cells, using beads coated with antibodies to immunoglobulin heavy and light chains not expressed by the lymphoma, including IgD. Tumor-infiltrating T cells were isolated from primary cell suspensions, using anti-CD4+- and anti-CD8+-coupled magnetic beads and Detach-a-beads (all from Dynal). If the purity of cell isolates was >95%, CD19+/IgD−/CD38−/sIg+ cell suspensions were subjected to further analyses. Purified B cells were stimulated for reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analyses with 500 ng/ml ionomycin and 10 ng/ml 12-O-tetradecanoylphorbol-13-acetate for 6 or 24 hours. To measure the proliferation the B cells were stimulated in the CD40-system as described previously. 6
Cytogenetic analyses were performed as described. 18 The presence of the t(11;18)(q21;q21) was determined either by conventional karyotyping or by applying a fluorescence in situ hybridization (FISH) assay that was recently developed to detect the chromosomal breakage in 11q21 in interphase cells (A. Rosenwald, manuscript submitted for publication). Karyotypes were described according to the International System for human cytogenetic nomenclature.
Western Blot
For each sample total protein extraction was prepared from 1 × 10 6 cells from cell culture. The cells were resuspended in 100 μl of Laemmli buffer with 1 μmol/L leupeptin (Sigma), 1 μmol/L pepstatin (Sigma), and 1 mmol/L pefabloc (Boehringer Mannheim) 29 and boiled for 5 minutes, and the DNA was shared with a cannula. The solution was spun down, and the protein supernatant was stored at −20°C. The protein extractions were monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. 29 In each lane 30 μl of protein extract mixed with bromphenol blue was applied. Proteins were transferred to a nitrocellulose membrane, which was probed with anti-CD40-ligand (TRAP cell culture supernatant; dilution 1:5) or anti-Fas-ligand (clone G247-4; Pharmingen) as the primary antibody, respectively. For detection the ECL system (Amersham, Freiburg, Germany) was used according to the manufacturer’s instructions.
RNA Extraction
All tumor tissue blocks were snap-frozen in liquid nitrogen and stored at −70°C until extraction of RNA. Total RNA was prepared (TRIzol reagent; Life Technology, Paisley, UK) from 20 sections of about 10 μm from the frozen tumor tissue blocks. The integrity of the RNA was checked by electrophoresis on a 2% formaldehyde-agarose gel, and the yield of RNA was quantitated by measuring the optical density. To check for carry-over material during the isolation step, extraction buffer without tissue was used as a negative control.
cDNA Synthesis
First-strand cDNA was synthesized with 1 μg of total cellular RNA. RNA, 2 μg of dT-15 primer, and diethylpyro-carbonate (DEPC)-treated water at a final volume of 8 μl were incubated for 10 minutes at 65°C. After chilling on ice, a master mix consisting of deoxynucleoside triphosphates and dithioreitol (final concentrations of 1 mmol/L each and 10 mmol/L, respectively), 25 U of recombinant RNase inhibitor (Promega, Heidelberg, Germany), RT-buffer, and 200 U of Moloney-murine reverse transcriptase (GIBCO BRL, Eggenstein, Germany) were added to a final reaction volume of 25 μl. After 70 minutes of incubation at 37°C, the samples were heated to 98°C for 4 minutes. The efficiency of cDNA synthesis was assessed by PCR with glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers (Table 2) ▶ . The GAPDH-PCR was performed in a final volume of 25 μl, using 2.5 μl 10× PCR-buffer (USB, Cleveland, OH), 2.0 μl 25 mmol/L MgCl2, 0.5 μl 10 mmol/L dNTPs, 0.5 μl 20 pmol of each primer, 0.5 U Taq-polymerase (USB), 18.4 μl PCR-water, and 1 μl cDNA.
Table 2.
Primer Sequences Used for RT-PCR
| mRNA | Sense primer (5′ → 3′) | Antisense primer (5′ → 3′) | Annealing temperature (°C) | MgCl2 (mmol/L) |
|---|---|---|---|---|
| GAPDH | TGAAGGTCGGAGTCAACGGATTTGGT | CATGTGGGCCATGAGGTCCACCAC | 65 | 2.0 |
| CD40-L | CCTCAAATTGCGGCACATGT | GACAAACACCGAAGCACCTGG | 60 | 1.5 |
| Fas-L | AACTCAAGGTCCATGCCTCTG | GGTGAGTTGAGGAGCTACAGACA | 60 | 1.5 |
| IFN-γ-RE | GCCGTCCTCAGTGCCTACACCA | TTATACTGGATCTCACTTCCGTTCA | 65 | 1.5 |
| IFN-γ | TTGGAAAGAGGAGAGTGACAG | TACTGGGATGCTCTTCGACCTC | 60 | 1.5 |
| IL2 | ATGTACAGGATGCAACTCCTGT | GTTTCAGATCCCTTTAGTTCCAG | 60 | 1.5 |
| IL4 | ATGGGTCTCACCTCCCAACTGCT | CGAAACACTTTGAATATTTCTCTCTCAT | 65 | 1.5 |
| IL10 | AGACTTTCTTTCAAATGAAGGATC | TCAGTTTCGTATCTTCATTGTCA | 60 | 1.5 |
| IL13 | TGGCGCTTTTGTTGACCACGGT | AAGCTGGAAAACCCAGCTGAGA | 60 | 1.5 |
| TGF-β1 | AACCCACAACGAAATCTATGAC | AACCCACAACGAAATCTATGAC | 60 | 1.5 |
Semiquantitative Polymerase Chain Reaction
For gross quantitation, we used expression of GAPDH as an external standard, which was amplified in separate reactions. Twenty-five cycles of amplification for each sample were carried out in a DNA thermal cycler 2600 (Perkin-Elmer Centus, Emeryville, CA), and aliquots were taken after 21, 23, and 25 cycles. Each cycle of amplification consisted of 30 seconds of denaturation at 94°C, 30 seconds of annealing at 60°C, and 1 minute of elongation at 72°C. The aliquots were subjected to electrophoresis, and the amount of amplification was assessed by comparing signal intensites. After the cDNA amount in each sample was adjusted to generate an equal result in GAPDH amplification, specific RT-PCRs for CD40-ligand, Fas-ligand, and cytokines were carried out. Specific oligonucleotides were selected in such a manner that they were only able to hybridize with cDNA and not with genomic DNA (Table 2) ▶ . The master-mix conditions for the specific PCRs were the same as already described for GAPDH, except for the MgCl2 concentration (Table 2) ▶ . PCR fragments were sequenced to ascertain specificity. Ionomycin-stimulated peripheral blood lymphocyte (PBL) cDNA was used as a positive control. The specificity and sensitivity for each primer pair of the cytokine PCRs were seperately tested as described. 30 Two microliters of cDNA from stimulated PBLs was taken as the control cDNA in each test reaction. The primer pairs were tested with different MgCl2 concentrations (1.0, 1.5, 2.0, 2.5, 3.0, 3.5 mmol/L) and different annealing temperatures. Under consideration of the intensity of the PCR product and the purity of the backround analyzed on an agarose gel, we selected the most sensitive combination of MgCl2 concentration and annealing temperature. The control PCRs in Figure 5 ▶ showed different intensities due to the different concentrations of cytokine mRNAs in the control PBLs.
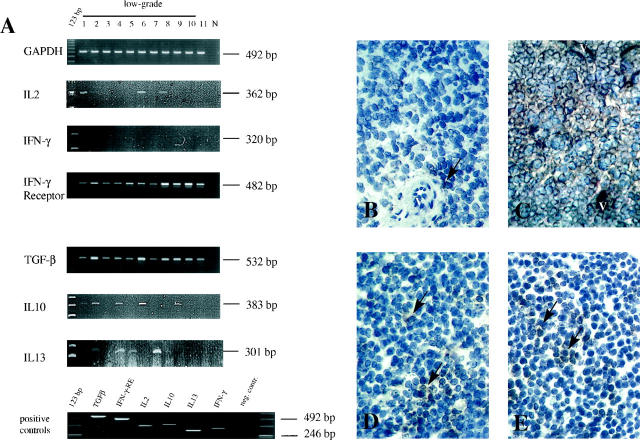
Figure 5.
A: Semiquantitative RT-PCR analysis of cytokine expression in MALT-type lymphoma tissues. N, negative control. Th1-weighted cytokines were normally absent, despite weak expression of IL2 in cases 1, 6, and 8. In contrast, Th2/Th3-weighted cytokines (TGF-β1, IL10, and IL13) were present in low-grade lymphomas. Ionomycin-stimulated normal PBLs from healthy donors were taken as positive controls. Protein expression in immunohistochemistry paralleled mRNA expression. B: Only a few IL2-positive cells (arrow) (V, vessels) with a faint cytoplasmatic staining were found perivascularly and within the tumor tissue (case 1; PAP, cryostat section, ×400). C: Strong cytoplasmic staining for TGF-β1 in tumor cells as well as in small blood vessels (case 6; PAP, cryostat section, ×200). D and E: IL10 (case 6; PAP, cryostat section, ×250; D) and IL13 (case 7; PAP, cryostat section, ×200; E). Numerous positive brown staining cells (arrows) were found loosely distributed within the tumor tissues.
Results
Tumor-Infiltrating T-Cells in MALT-Type B-Cell Lymphoma Are CD28 Positive and Express CD69 Irrespective of the Presence of t(11;18)
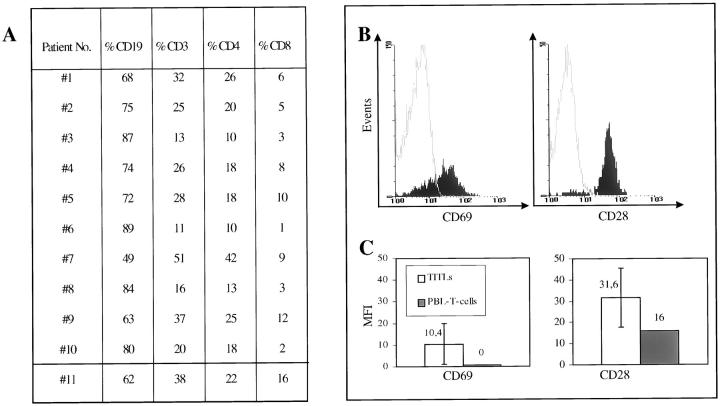
As determined by FACScan analysis, low-grade lymphomas contained high numbers of tumor-infiltrating T-lymphocytes (TITLs) (for details see Figure 1A ▶ ). The CD4/CD8 ratio was 3.3 in t(11;18)-positive cases and 3.3 in t(11;18)-negative cases. To investigate whether TITLs were activated (CD69+) and immunocompetent (CD28+), three-color fluorescence-activated cell sorter (FACS) analysis was used. As shown in Figure 1, B and C ▶ , low-grade TITLs expressed significantly more CD69 than the T cells from PBLs of the same patient. Moreover, in contrast to PBL T cells, all lymphoma TITLs expressed CD28, with an even higher average mean fluorescence intensity (MFI). Although CD69 and CD28 MFI expression was higher in t(11;18)-negative low-grade lymphoma TITLs (MFI CD69/28: 12/33) as compared with t(11;18)-positive TITLs (MFI CD69/28: 9/31), the differences were statistically insignificant.
Figure 1.
Characterization of tumor infiltrating T cells in low-grade MALT-type lymphomas, using multicolor FACS analysis of freshly isolated tumor cell suspensions. A: Summary of the percentage of B and T cells in tumor tissues (1–10, low-grade MALT-type lymphomas; 11, follicular gastritis, H. pylori-associated). B: Characteristic FACS histograms of TITLs of low-grade MALT-type lymphoma (no. 5) with high expression of CD69 and CD28 (solid line, isotype control; dashed lines, CD69 and CD28) of TITLs. C: Summary of FACS data of TITLs mean fluorescence intensity (MFI) of CD69 and CD28 expression as compared with T cells from PBLs of the same patients.
Activated TITLs but Not Lymphoma B-Cells Express CD40-Ligand and Fas-Ligand in Vivo and in Vitro
To investigate whether CD40-ligand and Fas-ligand mRNA were present in lymphoma tissues in vivo, a semiquantitative RT-PCR was used. Within the low-grade lymphomas 80% were positive for CD40-ligand and Fas-ligand mRNA, whereas immunohistochemistry found protein expression that paralleled mRNA expression in all cases. Notably, CD40-ligand-positive as well as Fas-ligand-positive cells were found in considerable numbers outside the reactive residual germinal centers within the tumor mass (Figure 2,B and C) ▶ .
Figure 2.
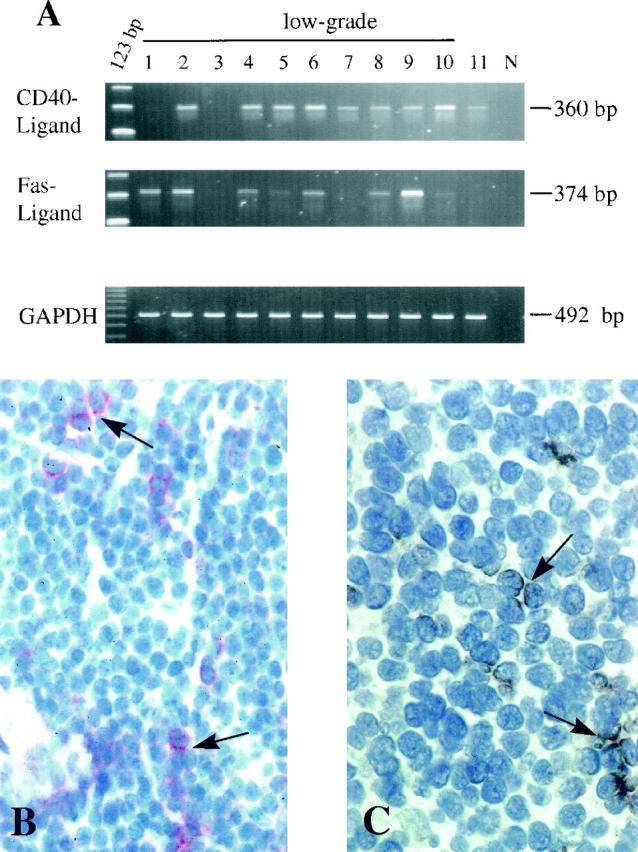
CD40-ligand and Fas-ligand expression in MALT tumor tissues in vivo. A: A semiquantitative RT-PCR with GAPDH as standard. N, negative control. B: Immunohistochemical detection of scattered CD40-Ligand expressing cells within the low-grade tumor infiltrate (no. 6; arrow; APAAP, cryostat section, ×250). C: Fas-ligand expression in the same tissue, giving brown spotted signals (no. 6; arrow; PAP, peroxidase-anti-peroxidase; cryostat section, ×400).
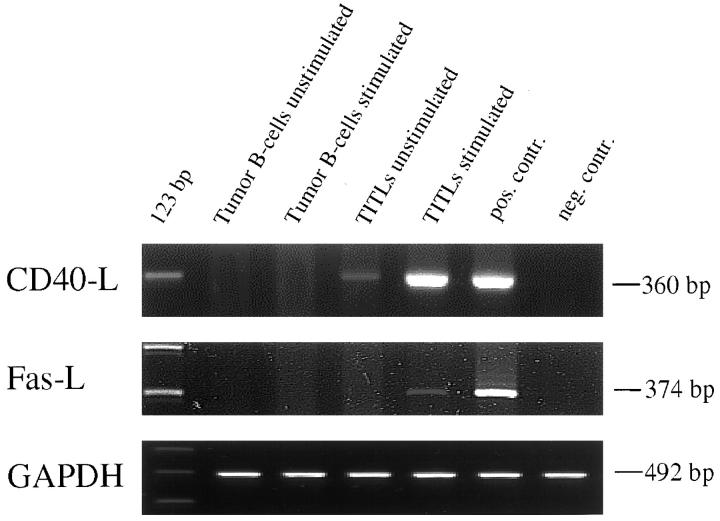
To prove that the TITLs and not the tumor B cells were the source of CD40-ligand or Fas-ligand, TITLs and lymphoma B cells were purified and investigated seperatly in vitro. After ionomycin/12-O-tetradecanoylphorbol-13-acetate stimulation, purified TITLs were induced to express CD40-ligand mRNA as well as Fas-ligand mRNA, whereas tumor B cells could not be triggered, even after stimulation to express Fas-ligand and CD40-ligand (Figure 3) ▶ . Because B cells were recently found to be able to express low basic levels of CD40-ligand mRNA, 31 we also measured the protein expression by Western blot assay and could not detect any CD40-ligand protein in normal memory B cells and tumor B cells as compared with the positive control (Figure 4A) ▶ . In addition, neither Fas-ligand mRNA nor protein was found in MALT tumor B cells (Figure 4B) ▶ .
Figure 3.
CD40-ligand mRNA is expressed by unstimulated and stimulated TITLs but not by malignant B cells. Fas-ligand mRNA is expressed by stimulated TITLs. Lymphocytes were purified and cultured for 6 hours with or without stimulation, as described in Materials and Methods. GAPDH RT-PCR is given as the control.
Figure 4.
There is neither CD40-ligand nor Fas-ligand protein expression in tumor B cells, as demonstrated by Western blot analyses. CD40-ligand (A) and Fas-ligand protein (B) was detectable in a stimulated C8166 T-cell line (lane 2) as the positive control, and CD40-ligand protein was detectable in a Jurkat T-cell line (lanes 7 and 8). Neither protein expression was found in unstimulated nor in stimulated normal and tumor B cells (lanes 3–6). The cells were stimulated for 24 hours as described in Materials and Methods. Lanes 1 and 2: Cell line C8166 unstimulated and stimulated. Lanes 3 and 4: Purified memory B cells unstimulated and stimulated. Lanes 5 and 6: Tumor B cells (no. 8) unstimulated and stimulated. Lanes 7 and 8: Jurkat cell line, unstimulated and stimulated, respectively.
Cytokines Expressed in Vivo Suggest a TH2/3-Weighted but Not a TH1-Weighted Profile
The most prominent cytokine found in all lymphoma tissues by RT-PCR and immunohistochemistry was TGF-β1, which was expressed in the cytoplasm of tumor cells and by endothelial cells of small vessels (Figure 5C) ▶ . IL10 mRNA- and protein-expressing cells were present in 60% of low-grade lymphomas. IL13 mRNA/protein was detected in 40% of the cases. Cytokine-expressing cells were found to be loosely distributed within the tumor tissue (Figure 5, D and E) ▶ .
IL2 was found in three low-grade cases (nos. 1, 6, and 8 (weak)), with some scattered positive cells surrounding small blood vessels (Figure 5B) ▶ . IFN-γ was found at a very low level in one low-grade case (no. 1), whereas no protein expression was found by immunohistochemistry (data not shown). IFN-γ receptor mRNA (as a control) was always detecable.
t(11;18) May Affect Tumor B-Cell Proliferation and Survival in Vivo
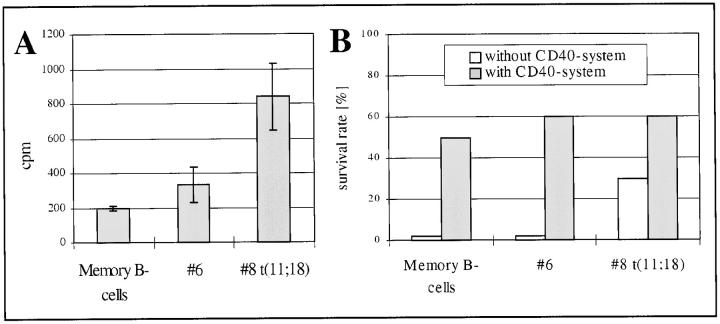
In one t(11;18)-negative and t(11;18)-positive case, cell culture material was compared with normal memory B cells in vitro. Strikingly, the basal proliferation (without any stimulation) and the survival rate were significantly higher in the t(11;18)-positive case as compared with the t(11;18)-negative case or the control memory B cells (Figure 6A) ▶ . Notably, the differences in survival could be reversed by triggering the lymphocytes in the CD40 system (Figure 6B) ▶ .
Figure 6.
There is a higher proliferation of purified tumor B cells in case 8 with t(11;18) as compared with case 6 (no translocation) or normal memory B cells without any stimuli after 3 days of cell culture (A). Data are expressed as mean of triplicate [3H]thymidine cpm uptake values, with SD as an error bar. The different proliferation in each case was paralleled by the survival rate as determined by trypan blue staining and lowest in normal memory B cells and case 6 and highest in case 8 after 7 days of cell culture without any stimuli (B, open bars). However, these differences disappeared by triggering with anti-CD40 (B, closed bars) and resulted in increased survival of tumor and normal B cells.
Discussion
Tumor regression after eradication of a chronic stimulus is unique to MALT-type lymphomas 3,4,32 and indicates an intricate relationship between the tumors and their hosts. However, the presence of TITLs alone, even with a high CD4/CD8 ratio, is not sufficient to explain the symbiotic interaction between MALT-type lymphoma B cells and the TITLs. Because in vitro activated T cells can promote MALT-type lymphoma growth, 33 our in vivo findings that TITLs of low-grade lymphomas consisted of activated T cells (CD69+), which coexpress immunocompetence markers (CD28+), became significant.
Beyond the ability of CD4+ T cells to provide help in the priming of tumor-specific CD8+ CTLs, evidence from mouse models and cell lines indicates that tumor-specific CD4+ T cells can orchestrate additional effector functions in tumor immunity. 34-37 Therefore, the observation that CD40-ligand was expressed within the MALT tumor tissues of at least low-grade lymphomas is important in three ways. First, as shown recently, 2,6 CD40-ligand is the most potent stimulus for proliferation of MALT-type lymphoma B cells in vitro and may also promote tumor growth in vivo. Second, CD40-ligand is able to inhibit drug-induced apoptosis in lymphoma cell lines in vitro, 38,39 suggesting that it may induce the resistance of tumor cells to proapoptotic and antiproliferative effects of cytotoxic drugs in vivo. Third, CD40-ligand is known to be expressed on T cells after triggering by specific antigens. 40 Its detection in tumor tissues thus favors the idea of antigen-mediated tumor promotion by activated T cells. This notion is further strengthened by the observation that the TITLs but not the tumor B cells expressed CD40-ligand, in contrast to the recently described aberrant CD40-ligand expression of tumor-B cells in some low-grade non-Hodgkin’s lymphoma (eg, chronic lymphocytic leukemia 41,42 ). Therefore, the presence of CD40-ligand in tumor tissues points to the possibility of nonautonomous tumor growth supported by activated CD40-ligand-positive T cells and is consistent with tumor regression after antibiotic treatment.
In addition to CD40-ligand, we also found Fas-ligand to be expressed in vivo. During normal T-/B-cell interaction Fas-ligand expression is commonly detectable on highly activated but not on resting T cells and is important to the deletion of harmful autoreactive B cells.
Given that most MALT-type B-cell lymphomas were found to express antigen receptors with specificity for autoantigens, 7,8,29,43,44 the presence of Fas-ligand in the tumor tissues was surprising. A possible explanation for this result could be either an aberrant expression of Fas-ligand by the tumor cells as described recently for some carcinomas 45 or a so far unknown escape mechanism in which the tumor B cells become resistant to Fas triggering. Our results favor the latter idea, because we could not detect any Fas-ligand mRNA or protein expression in normal or tumor B cells, even under rigorous stimulation in vitro. Furthermore, it is possible that impaired Fas-mediated apoptosis or Fas mutations as described recently in some B-cell lymphomas 46 are operative in MALT-type lymphomas as well and may lead to a functional Fas resistance. 20,47
The finding of Th2- and Th3-type cytokines but only low levels of Th1-type cytokines in MALT tumor tissues in vivo is in contrast to a recent report on four cases in which TITLs secreted a Th1 cytokine (INF-γ) after H. pylori stimulation in vitro. 48 This discrepancy may be explained either by TGF-β1 expression in the tumor cells in vivo, which may cause a deviation of the T-cell response to a Th2 phenotype, as shown recently in mice, 49 or by in vitro selection (due to culture conditions) of an otherwise (eg, major histocompatibility complex-restricted) controlled Th1 population, although it cannot be ruled out that a few cells in the tumor microenvironment may be functionally significant. However, the existence of Th1 cytokines would argue against tumor promotion and favor antitumor immunity, 37 whereas Th2-type (IL10, IL13) and Th3-type (TGF-β1) cytokines were shown to be potent promoters for B-cell proliferation and differentiation. 40,50,51
The observation that activated T cells secrete Th2-type cytokines in MALT-type lymphomas in vivo may attribute to them a function that goes beyond solely translating H. pylori-specific signals into growth signals for MALT lymphoma B cells. Thus Blanckenstein et al 52 recently proposed that presentation of tumor antigens by B cells to CD4+ T cells diverts antitumor immunity toward a “nonproductive” Th2-type humoral immune response and away from a “productive” Th1-type cellular response. Therefore, it is possible that a Th1-weighted immunity established during H. pylori-associated chronic inflammation as described recently 53,54 may support antitumor immunity, whereas a Th2-weighted profile in vivo favors progression of lymphoproliferative disease and aberrant B-cell clones. If this indeed turns out to be a general phenomenon it will be important to design strategies that will break Th2-mediated T-cell tolerance and/or polarization to promote the development of an effective tumor immunotherapy, even in low-grade MALT-type lymphomas progressively growing after H. pylori eradication. In this context, it may be important that the negative and the positive t(11;18) cases did not differ regarding their expression profiles and appear to be dependent on the same environment. A reason for this could be that the t(11;18) translocation, which represents the most frequent structural abnormality in MALT-type lymphomas, 18 involves genes that do not impair the T-/B-cell interaction. This may explain why purified low-grade tumor B cells respond uniformly in the context of CD40 triggering and cytokine stimulation 2 but behave differently without exogenous stimuli in vitro. Therefore, it will be interesting to compare tumor regression in vivo with respect to the presence of t(11;18) because tumor B cells may still benefit from the immunological setting but have developed sophisticated survival strategies, even without a need for continuous T-cell collaboration.
Acknowledgments
We thank M. Reichert and C. Kohaut for expert technical assistance and A. Schimpl and A. Schultz for critically reading the manuscript.
Footnotes
Address reprint requests to Dr. Axel Greiner, Institute of Pathology, University of Würzburg, Josef-Schneider Strasse 2, 97080 Würzburg. E-mail: path040@mail.uni-wuerzburg.de.
Supported by the Wilhelm-Sander Stiftung (grant 94.025.2, SFB 172 (B4) and grant 98.086.1 to S. S.) and the 1ZKF2000 (B3 to A. G.)
References
- 1.Greiner A, Müller Hermelink HK: Recent advances in gastric extranodal B-cell lymphoma. Curr Diagn Pathol 1996, 3:91-98 [Google Scholar]
- 2.Greiner A, Knörr C, Qin Y, Sebald W, Schimpl A, Banchereau J, Müller Hermelink HK: Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am J Pathol 1997, 150:1583-1593 [PMC free article] [PubMed] [Google Scholar]
- 3.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG: Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342:575-577 [DOI] [PubMed] [Google Scholar]
- 4.Bayerdorffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M: Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995, 345:1591-1594 [DOI] [PubMed] [Google Scholar]
- 5.Hussell T, Isaacson PG, Crabtree JE, Spencer J: The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993, 342:571-574 [DOI] [PubMed] [Google Scholar]
- 6.Greiner A, Knörr C, Qin Y, Schultz A, Marx A, Kroczek RA, Müller Hermelink HK: CD40 ligand and autoantigen are involved in the pathogenesis of low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Dev Immunol 1998, 6:187-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin Y, Greiner A, Hallas C, Haedicke W, Müller Hermelink HK: Intraclonal offspring expansion of gastric low-grade MALT-type lymphoma: evidence for the role of antigen-driven high-affinity mutation in lymphomagenesis. Lab Invest 1997, 76:477-485 [PubMed] [Google Scholar]
- 8.Hussell T, Isaacson PG, Crabtree JE, Dogan A, Spencer J: Immunoglobulin specificity of low grade B cell gastrointestinal lymphoma of mucosa-associated lymphoid tissue (MALT) type. Am J Pathol 1993, 142:285-292 [PMC free article] [PubMed] [Google Scholar]
- 9.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC: Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell 1996, 87:319-329 [DOI] [PubMed] [Google Scholar]
- 10.Goodnow CC: Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA 1996, 93:2264-2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, Elson CO, Pillai S, McGhee JR: Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med 1993, 178:1309-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armitage RJ, Macduff BM, Spriggs MK, Fanslow WC: Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol 1993, 150:3671-3680 [PubMed] [Google Scholar]
- 13.Liu YJ, Arpin C: Germinal center development. Immunol Rev 1997, 156:111-126 [DOI] [PubMed] [Google Scholar]
- 14.Sher A, Coffman RL: Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol 1992, 10:385-409 [DOI] [PubMed] [Google Scholar]
- 15.Pound JD, Gordon J: Maintenance of human germinal center B cells in vitro. Blood 1997, 89:919-928 [PubMed] [Google Scholar]
- 16.Burdin N, van Kooten C, Galibert L, Abrams JS, Wijdenes J, Banchereau J, Rousset F: Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J Immunol 1995, 154:2533-2544 [PubMed] [Google Scholar]
- 17.De Vita S, Dolcetti R, Ferraccioli G, Pivetta B, De Re V, Gloghini A, D’Agosto A, Bartoli E, Carbone A, Boiocchi M: Local cytokine expression in the progression toward B cell malignancy in Sjogren’s syndrome. J Rheumatol 1995, 22:1674-1680 [PubMed] [Google Scholar]
- 18.Ott G, Katzenberger T, Greiner A, Kalla J, Rosenwald A, Heinrich U, Ott MM, Müller Hermelink HK: The t(11,18)(q21,q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res 1997, 57:3944-3948 [PubMed] [Google Scholar]
- 19.Prabhu RM, Medeiros LJ, Kumar D, Drachenberg CI, Papadimitriou JC, Appelman HD, Johnson LB, Laurin J, Heyman M, Abruzzo LV: Primary hepatic low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) associated with primary biliary cirrhosis. Mod Pathol 1998, 11:404-410 [PubMed] [Google Scholar]
- 20.Greiner A, Seeberger H, Knörr C, Starostik P, Müller Hermelink HK: MALT-type B cell lymphomas escape the censoring FAS-mediated apoptosis. Blood 1998, 92:484 [Google Scholar]
- 21.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf Peeters C, Falini B, Gatter KC, Müller Hermelink HK, et al: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 22.Greiner A, Marx A, Müller Hermelink HK, Schmausser B, Petzold K, Kruger H: Vascular autoantibodies in amyotrophic lateral sclerosis. Lancet 1992, 340:378-379 [DOI] [PubMed] [Google Scholar]
- 23.Greiner A, Schuster V, Bohm E, Kreth HW, Hermelink HK, Kreipe H: Regulary organized lymphoid tissue and CD40 ligand expression in a male fetus carrying the defective gene for X-linked lymphoproliferative disease (XLP). Histopathology 1997, 30:481-482 [DOI] [PubMed] [Google Scholar]
- 24.Friedland JS, Shattock RJ, Johnson JD, Remick DG, Holliman RE, Griffin GE: Differential cytokine gene expression and secretion after phagocytosis by a human monocytic cell line of Toxoplasma gondii compared with Mycobacterium tuberculosis. Clin Exp Immunol 1993, 91:282-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma V, Knobloch TJ, Benjamin D: Differential expression of cytokine genes in HIV-1 tat transfected T and B cell lines. Biochem Biophys Res Commun 1995, 208:704-713 [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Rousset F: Growing human B lymphocytes in the CD40 system. Nature 1991, 353:678-679 [DOI] [PubMed] [Google Scholar]
- 27.Arpin C, Dechanet J, van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ: Generation of memory B cells and plasma cells in vitro. Science 1995, 268:720-722 [DOI] [PubMed] [Google Scholar]
- 28.Arpin C, Banchereau J, Liu YJ: Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J Exp Med 1997, 186:931-940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greiner A, Marx A, Heesemann J, Leebmann J, Schmausser B, Müller Hermelink HK: Idiotype identity in a MALT-type lymphoma and B cells in Helicobacter pylori associated chronic gastritis. Lab Invest 1994, 70:572-578 [PubMed] [Google Scholar]
- 30.Dallma MJ, Porter ACG: Semi-quantitative PCR of gene expression. Mcpherson MJ Quirke P Taylor GR eds. PCR—A Practical Approach. 1991, :pp 215-224 Oxford University Press, London [Google Scholar]
- 31.Grammer AC, Bergman MC, Miura Y, Fujita K, Davis LS, Lipsky PE: The CD40 ligand expressed by human B cells costimulates B cell responses. J Immunol 1995, 154:4996-5010 [PubMed] [Google Scholar]
- 32.Fischbach W, Tacke W, Greiner A, Müller Hermelink HK: Regression of immunoproliferative small intestinal disease after eradication of Helicobacter pylori. Lancet 1997, 349:31-32 [DOI] [PubMed] [Google Scholar]
- 33.Hussell T, Isaacson PG, Crabtree JE, Spencer J: Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol 1996, 178:122-127 [DOI] [PubMed] [Google Scholar]
- 34.Ashany D, Song X, Lacy E, Nikolic Zugic J, Friedman SM, Elkon KB: Th1 CD4+ lymphocytes delete activated macrophages through the Fas/APO-1 antigen pathway. Proc Natl Acad Sci USA 1995, 92:11225-11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demanet C, Brissinck J, Leo O, Moser M, Thielemans K: Role of T-cell subsets in the bispecific antibody (anti-idiotype × anti-CD3) treatment of the BCL1 lymphoma. Cancer Res 1994, 54:2973-2978 [PubMed] [Google Scholar]
- 36.Racila E, Scheuermann RH, Picker LJ, Yefenof E, Tucker T, Chang W, Marches R, Street NE, Vitetta ES, Uhr JW: Tumor dormancy and cell signaling. II. Antibody as an agonist in inducing dormancy of a B cell lymphoma in SCID mice. J Exp Med 1995, 181:1539-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schattner EJ, Mascarenhas J, Bishop J, Yoo DH, Chadburn A, Crow MK, Friedman SM: CD4+ T-cell induction of Fas-mediated apoptosis in Burkitt’s lymphoma B cells. Blood 1996, 88:1375-1382 [PubMed] [Google Scholar]
- 38.Friesen C, Herr I, Krammer PH, Debatin KM: Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Med 1996, 2:574-577 [DOI] [PubMed] [Google Scholar]
- 39.Voorzanger-Rousselot N, Favrot M, Blay JY: Resistance to cytotoxic chemotherapy induced by CD40 ligand in lymphoma cells. Blood 1998, 92:3381-3387 [PubMed] [Google Scholar]
- 40.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S: The CD40 antigen and its ligand. Annu Rev Immunol 1994, 12:881-922 [DOI] [PubMed] [Google Scholar]
- 41.Brugnoni D, Rossi G, Tucci A, Cattaneo R, Airo P: Study of CD40 ligand expression in B-cell chronic lymphocytic leukemia. Haematologica 1995, 80:440-442 [PubMed] [Google Scholar]
- 42.Trentin L, Zambello R, Sancetta R, Facco M, Cerutti A, Perin A, Siviero M, Basso U, Bortolin M, Adami F, Agostini C, Semenzato G: B lymphocytes from patients with chronic lymphoproliferative disorders are equipped with different costimulatory molecules. Cancer Res 1997, 57:4940-4947 [PubMed] [Google Scholar]
- 43.Greiner A, Marx A, Schmausser B, Müller Hermelink HK: The pivotal role of the immunoglobulin receptor of tumor cells from B cell lymphomas of mucosa associated lymphoid tissue (MALT). Adv Exp Med Biol 1994, 355:189-193 [DOI] [PubMed] [Google Scholar]
- 44.Qin Y, Greiner A, Trunk MJ, Schmausser B, Ott MM, Müller Hermelink HK: Somatic hypermutation in low-grade mucosa-associated lymphoid tissue-type B-cell lymphoma. Blood 1995, 86:3528-3534 [PubMed] [Google Scholar]
- 45.Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR: Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasion? Nature Med 1996, 2:1361-1366 [DOI] [PubMed] [Google Scholar]
- 46.Gronbaek K, Straten PT, Ralfkiaer E, Ahrenkiel V, Andersen MK, Hansen NE, Zeuthen J, Hou Jensen K, Guldberg P: Somatic Fas mutations in non-Hodgkin’s lymphoma: association with extranodal disease and autoimmunity. Blood 1998, 92:3018-3024 [PubMed] [Google Scholar]
- 47.Plumas J, Jacob MC, Chaperot L, Molens JP, Sotto JJ, Bensa JC: Tumor B cells from non-Hodgkin’s lymphoma are resistant to CD95 (Fas/Apo-1)-mediated apoptosis. Blood 1998, 91:2875-2885 [PubMed] [Google Scholar]
- 48.Hauer AC, Finn TM, MacDonald TT, Spencer J, Isaacson PG: Analysis of TH1 and TH2 cytokine production in low grade B cell gastric MALT-type lymphomas stimulated in vitro with Helicobacter pylori. J Clin Pathol 1997, 50:957-959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King C, Davies J, Mueller R, Lee MS, Krahl T, Yeung B, O’Connor E, Sarvetnick N: TGF-β1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity 1998, 8:601-613 [DOI] [PubMed] [Google Scholar]
- 50.Garcia B, Rodriguez R, Angulo I, Heath AW, Howard MC, Subiza JL: Differential effects of transforming growth factor-beta 1 on IgA vs. IgG2b production by lipopolysaccharide-stimulated lymph node B cells: a comparative study with spleen B cells. Eur J Immunol 1996, 26:2364-2370 [DOI] [PubMed] [Google Scholar]
- 51.Defrance T, Carayon P, Billian G, Guillemot JC, Minty A, Caput D, Ferrara P: Interleukin 13 is a B cell stimulating factor. J Exp Med 1994, 179:135-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cayeux S, Richter G, Noffz G, Dorken B, Blankenstein T: Influence of gene-modified (IL-7, IL-4, and B7) tumor cell vaccines on tumor antigen presentation. J Immunol 1997, 158:2834-2841 [PubMed] [Google Scholar]
- 53.D’Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G: T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol 1997, 158:962-967 [PubMed] [Google Scholar]
- 54.Grafton G, Goodall M, Gregory CD, Gordon J: Mechanisms of antigen receptor-dependent apoptosis of human B lymphoma cells probed with a panel of 27 monoclonal antibodies. Cell Immunol 1997, 182:45-56 [DOI] [PubMed] [Google Scholar]