Abstract
Proliferation in the setting of longstanding chronic inflammation appears to predispose to carcinoma in the liver, large bowel, urinary bladder, and gastric mucosa. Focal prostatic atrophy, which is associated with chronic inflammation, is highly proliferative (Ruska et al, Am J Surg Pathol 1998, 22:1073–1077); thus the focus of this study was to more fully characterize the phenotype of the atrophic cells to assess the feasibility of the proposal that they may be targets of neoplastic transformation. The π-class glutathione S-transferase (GSTP1), a carcinogen-detoxifying enzyme, is not expressed in >90% of prostate carcinomas (CaPs). GSTP1 promoter hypermethylation, which appears to permanently silence transcription, is the most frequently detected genomic alteration in CaP (Lee et al, Proc Natl Acad Sci USA 1994, 91:11733–11737; >90% of cases). In high-grade prostatic intraepithelial neoplasia (PIN), this alteration is present in at least 70% of cases (Brooks et al, Cancer Epidemiol Biomarkers Prev, 1998, 7:531–536). Although normal-appearing prostate secretory cells rarely express GSTP1, they remain capable of expression, inasmuch as GSTP1 promoter hypermethylation is not detected in normal prostate. Fifty-five lesions from paraffin-embedded prostatectomy specimens (n = 42) were stained for GSTP1, using immunohistochemistry. Adjacent sections were stained for p27Kip1, Ki-67, androgen receptor (AR), prostate-specific antigen (PSA), prostate-specific acid phosphatase (PSAP), Bcl-2, and basal cell-specific cytokeratins (34βE12). With normal prostate epithelium as the internal standard, staining was scored for each marker in the atrophic epithelium. The lesions showed two cell types, basal cells staining positive for 34βE12, and atrophic secretory-type cells staining weakly negative for 34βE12. All lesions showed elevated levels of Bcl-2 in many of the secretory-type cells. All lesions had an elevated staining index for the proliferation marker Ki-67 in the secretory layer and decreased expression of p27Kip1, a finding reminiscent of high-grade PIN (De Marzo et al, Am J Pathol 1998, 153:911–919). Consistent with partial secretory cell differentiation, the luminal cells showed weak to moderate staining for androgen receptor and the secretory proteins PSA and PSAP. All atrophic lesions showed elevated GSTP1 expression in many of the luminal secretory-type cells. Because all lesions are hyperproliferative, are associated with inflammation, and have the distinct morphological appearance recognized as prostatic atrophy, we suggest the term “proliferative inflammatory atrophy” (PIA). Elevated levels of GSTP1 may reflect its inducible nature in secretory cells, possibly in response to increased electrophile or oxidant stress. Elevated Bcl-2 expression may be responsible for the very low apoptotic rate in PIA and is consistent with the conclusion that PIA is a regenerative lesion. We discuss our proposal to integrate the atrophy and high-grade PIN hypotheses of prostate carcinogenesis by suggesting that atrophy may give rise to carcinoma either directly, as previously postulated, or indirectly by first developing into high-grade PIN.
Chronic inflammation of longstanding duration has been linked to the development of carcinoma in several organ systems. 1-3 The proposed mechanism of carcinogenesis involves repeated tissue damage and regeneration in the presence of highly reactive oxygen and nitrogen species. These reactive molecules, such as H202 and nitric oxide (NO), are released from the inflammatory cells and can interact with DNA in the proliferating epithelium to produce permanent genomic alterations such as point mutations, deletions, and rearrangements. 2,3 This inflammation-carcinoma sequence as been invoked as a potential mechanism with regard to prostatic carcinogenesis. 4-9 Interestingly, focal prostatic glandular atrophy, which has been put forth previously as a potential precursor of prostatic adenocarcinoma, 10,11 occurs in close association with chronic inflammation. 5,12,13
Atrophy of the prostate is identified as a reduction in the volume of preexisting glands and stroma and can be divided into two major patterns, diffuse and focal. 12,14 Diffuse atrophy results from a decrease in circulating androgens and involves the entire prostate in a relatively uniform manner. 15 In contrast, focal atrophy is not related to decreased circulating androgens, and it occurs as patches of atrophic epithelium within a background of surrounding normal-appearing nonatrophic epithelium. 12 Franks 10 indicated that focal prostatic atrophy lesions occur chiefly in the “outer” portion of the prostate (referred to by McNeal as the “peripheral zone”) 12 and that they increase in frequency with advancing age. Others confirmed these findings. 11,13,16,17
How might atrophic cells be linked to carcinoma, which also occurs principally in the peripheral zone? While most focal prostatic atrophy lesions have been considered to be quiescent, 13 cells in some atrophy lesions appear proliferative. 10,11,18,19 In a comparison between benign nonatrophic epithelium and focal prostatic atrophy, Ruska et al recently demonstrated that, while there was no increase in the apoptotic index, atrophy exhibited a markedly increased immunohistochemical staining index for the proliferation marker, Ki-67. 20 This finding supports the contention that focal atrophy represents either a de novo proliferative lesion or a regenerative lesion resulting from replacement of cellular loss, as suggested previously. 11
Most cell division in the normal human prostate epithelium occurs in the basal cell compartment. 21,22 Yet high-grade PIN, the presumed precursor of many prostatic adenocarcinomas, 23 and adenocarcinoma cells possess phenotypic and morphological features of secretory cells. Thus cell proliferation has been shifted up from the basal into the secretory compartment in high-grade PIN and in carcinoma. 21,22 Based on this “topographic infidelity of proliferation” (TIP), 24 as well as patterns of cytokeratin expression, 25 it has been postulated that the prostatic cell type that is the target of neoplastic transformation is an intermediate cell, with some features of basal cells and some of secretory cells.
No prior studies have specifically examined the immunophenotype of the cells within focal atrophy of the prostate. To better elucidate the cell types present and to further explore the possibility that cells in focal atrophy of the prostate may be related to carcinoma and high-grade PIN, we performed a detailed morphological and immunohistochemical analysis. We examined expression of both basal cell-specific and secretory cell-specific markers. In addition, we examined the expression patterns of other molecular markers implicated in prostatic carcinogenesis: p27Kip1, Bcl-2, and the π-class glutathione S-transferase (GSTP1).
p27Kip1 is a cyclin-dependent kinase inhibitor whose expression is reduced in the majority of prostatic adenocarcinomas 26-32 and in high-grade PIN. 26 GSTP1, which functions as an inducible phase II detoxifying enzyme for reactive oxygen species and organic electrophiles, 33,34 is inactivated by promoter hypermethylation in human prostatic carcinoma. 35-37 Expression of GSTP1 is also absent in high-grade PIN, 37 with promoter hypermethylation occurring in at least 70% of cases. 37 In this study, we report a down-regulation of p27Kip1 in prostatic atrophy, consistent with its proposed role as a suppressor of prostatic epithelial cell proliferation. We also report increased expression of Bcl-2, which is consistent with the observed very low levels of apoptosis. 20 Finally, there was a striking increase in the expression of GSTP1 in many of the atrophic cells, indicative of a stress-induced response. Potential mechanisms of formation of prostatic atrophy are discussed, as well as the implications for prostatic carcinogenesis.
Materials and Methods
Antibodies
The anti-androgen receptor antibody was obtained and used as undiluted mouse monoclonal antibody hybridoma supernatant from clone AR-441 (a gift from Dean P. Edwards, Ph.D., University of Colorado HSC, Denver, CO). Antibodies against prostate-specific antigen (PSA) (mouse monoclonal, clone ER-PR8, dilution 1:50), prostate-specific acid phosphatase (PSAP) (rabbit polyclonal, dilution 1:10,000), Bcl-2 (mouse monoclonal, dilution 1:25), CD20 (mouse monoclonal, clone L26, dilution 1:200), CD3 (mouse monoclonal, clone UCHT1, dilution 1:150), and CD68 (mouse monoclonal, clone KP-1, dilution 1:4000) were from Dako (Carpinteria, CA). Anti-p27Kip1 (mouse monoclonal, dilution 1:800) and anti-PCNA (mouse monoclonal, dilution 1:250) were from Transduction Laboratories (Lexington, KY). Anti-Ki-67 (mouse monoclonal, clone Mib-1, dilution 1:100) was from Immunotech (Miami, FL). The basal cell-specific cytokeratin antibody (mouse monoclonal, clone 34βE12, dilution 1:50) was from Enzo Biochem (Farmingdale, NY). Anti-cytokeratins 8 and 18 (mouse monoclonal, clone Cam 5.2, prediluted) was from Becton Dickinson (Franklin Lakes, NJ). Anti-topoisomerase II α (mouse monoclonal, clone AB-1, dilution 1:100) was from Calbiochem (San Diego, CA). Anti-GSTP1 (rabbit polyclonal, dilution 1:40,000) was from Medical and Biological Laboratories (Watertown, MA).
Immunohistochemistry
Immunohistochemistry was performed using the Biotek Techmate 1000 (Ventana Medical Systems, Tucson, AZ) robotic immunostainer as described. 38 Briefly, all primary antibody incubations were carried out for 45 minutes at room temperature, except for GSTP1, which was at 4°C overnight. Biotinylated secondary antibody incubation was carried out for 30 minutes at room temperature. Histochemical localization using avidin-biotin horseradish peroxidase complex (ABC) was carried out using 3,3′-diaminobenzidine tetrahydrochloride (DAB) as the chromagen. Slides were couterstained with hematoxylin. For 34βE12 and Cam 5.2 cytokeratin staining, the sections were pretreated with protease type 27 (Sigma, St. Louis, MO) at 2 mg/ml for 20 minutes at 37°C before incubation with the primary antibodies.
Surgical Specimens
Formalin-fixed, paraffin-embedded tissues were obtained from The Johns Hopkins Hospital. All specimens were from radical prostatectomies (n = 42 patients) and consisted of portions of tissue dissected immediately after surgical removal and immersed in 10% neutral buffered formalin.
Scoring of Morphological and Immunohistochemical Features
Intensity of inflammation was recorded using a numerical 0–6 scale, with 0 representing no inflammation, 1–2 representing mild, 3–4 representing moderate, and 5–6 representing severe inflammation. All lesions were heterogeneous in terms of inflammation, in that different areas within an individual focus of atrophy had different amounts of inflammatory cells. Thus the scores recorded represented an overall average for each lesion. For each lesion we recorded the size and the following variables, using a 0–6 scale for each variable, where 0 is negative and 6 is the highest value: the extent of epithelial disruption, the relative number of intraluminal macrophages, and the extent of periglandular fibrosis. For each lesion we also recorded whether any part of the lesion was adjacent to or was away from high-grade PIN or carcinoma.
Apoptosis was accessed by light microscopic examination for apoptotic bodies, using hematoxylin and eosin (H&E)-stained sections. For both PIA and adjacent normal epithelium, we recorded the number of apoptotic bodies within the epithelium per 20 high-power fields (hpf), using an Olympus BX-40 microscope with a 40× objective.
The intensity of immunohistochemical staining for GSTP1 and Bcl-2 was scored on a numerical 0–6 scale system, with 0 representing background staining in benign normal-appearing nonatrophic secretory cells, 1–2 representing mild elevations, 3–4 representing moderate elevations, and 5–6 representing marked elevations. GSTP1 and Bcl-2 staining was also heterogeneous in that individual atrophy lesions contained areas with intense staining and other areas with somewhat less staining. Because lesions were heterogeneous, the overall score assigned represented the average for the entire lesion. For p27Kip1, AR, PSA, and PSAP, an identical scoring approach was used, except that negative numbers were used to indicate reduced expression. In 22 lesions, a Ki-67 labeling index was determined by counting the number of positively staining cells per total cells counted, with a minimum of 500 cells counted (range 500-2000). For this, the entire lesion was scanned at ×400 magnification with a BLISS imaging microscope (Bacus Laboratories, Lombard, IL). A minimum of five screen shots representing individual microscopic fields at ×400 were selected for counting at random. Each screen shot, consisting of a microscopic field, was copied and transferred to Adobe Photoshop 5.0 for Microsoft Windows 95/98. As the individual cells were counted, each nucleus was overlayed with a small green dot. In addition, cells staining positively for Ki-67 were overlayed with a red dot. By saving the file to disk, we obtained a permanent record of exactly which cells in the lesion were counted. In these 22 cases, normal epithelium away from the lesion was used as an internal reference for each lesion, and cells were similarly counted. Statistical analysis was carried out using the STATA 5.0 software package for Microsoft Windows 95.
Results
Many of the morphological features of PIA—focal prostatic atrophy in association with chronic inflammation—have been described. 5,10,13,16 The key identifying feature of focal prostatic atrophy is recognized at low power and consists of an overall hyperchromatic appearance of the involved glands, occurring often as a discrete focus among benign, normal-appearing glands (Figure 1) ▶ . The majority of the PIA lesions (n = 41/55) were considered simple atrophy as described, 20 which consisted of glands with variable acinar caliber (Figures 1–5) ▶ ▶ ▶ . The majority of acini in simple atrophy acini were stellate in architecture and lacked papillary infoldings. Often the lesions contained corpora amylacea of varying sizes. Three of the lesions (n = 3/55) were classified as postatrophic hyperplasia (PAH), 39 consisting of foci of crowded glands with small-caliber, round atrophic acini. Some of the lesions (n = 11/55) contained both PAH and simple atrophy.
Figure 1.
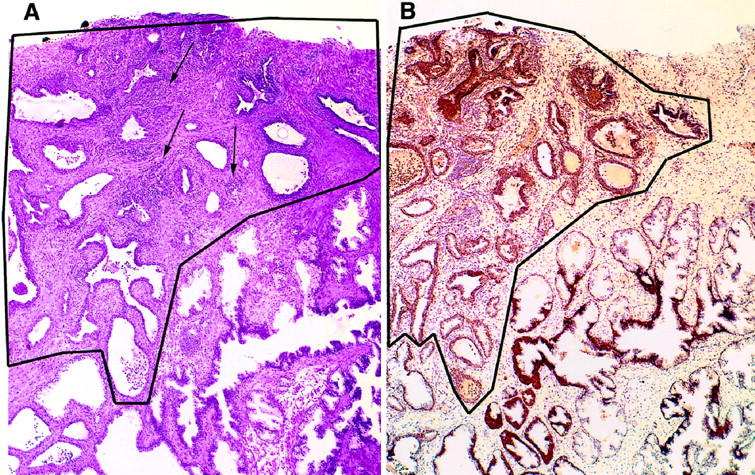
Proliferative inflammatory atrophy (PIA) of the prostate. A: Low-magnification view of a focus of PIA (outlined area) occurring adjacent to benign normal appearing glands (lower right). Arrows indicate collections of chronic inflammatory cells (predominantly lymphocytes). This lesion was classified as having marked chronic inflammation. H&E, ×40. B: Section adjacent to that shown in A, stained with anti-GSTP1 polyclonal antibody. Immunoperoxidase, ×40.
Figure 2.

Two cell layers in PIA. Medium-power view of section of PIA stained with the 34BE12 monoclonal antibody against basal cells. Arrows indicate atrophic secretory-type cells in the gland lumen. Arrowheads indicate basal cells. Inset: Higher power view of boxed area. Immunoperoxidase, ×200. Inset, ×400.
Figure 3.
Expression of markers of proliferation and differentiation in PIA. A: Increased expression of proliferation associated marker, Ki-67, in secretory-type cells. B: Decreased expression of cyclin-dependent kinase inhibitor p27kip1 in secretory-type cells. C: Expression of PSA in secretory-type cells. A–C: Immunoperoxidase, ×200. Arrows indicate luminal cells.
The vast majority of acini (>90% in all lesions) contained at least two layers of epithelial cells (Figure 2) ▶ . The luminal cells appeared as attenuated cuboidal secretory-type cells that were generally hyperchromatic but which contained somewhat more cytoplasm than the basal layer of cells. As compared with surrounding normal epithelium, the cytoplasm in the luminal cells ranged from markedly reduced and basophilic to slightly reduced and pale to clear (partial atrophy 40 ; see Figure 5 ▶ ). Most lesions contained at least some atrophic acini that were so attenuated as to appear to have only one layer of markedly flattened epithelial cells. The latter structures were often cystically dilated and have been referred to as cystic atrophy. 10 No cases consisted purely of cystic atrophy.
Figure 5.

Heterogeneity of expression of GSTP1 in PIA. Note areas of positive staining intermixed with areas of negative staining for GSTP1. The inset is a higher power view from the boxed area, showing the border zone between these areas. This lesion was classified as having mild chronic inflammation. Immunoperoxidase, ×100. Inset: Arrow indicates an atrophic secretory cell staining negatively, and the arrowhead indicates a nearby atrophic secretory cell staining positively. Magnification, ×400.
All cases contained chronic inflammation (Figure 1) ▶ , consisting of an infiltrate of lymphocytes with varying numbers of macrophages involving both the epithelium and surrounding stroma to varying degrees (Table 1) ▶ . The majority (∼80–85%) of the lymphocytes represented CD3-positive T cells with scattered and variable numbers of CD20-positive B cells and CD68-positive macrophages (data not shown). While none of the cases contained granulomata, some of the lesions contained a few eosinophils and plasma cells. In 33/55 (60%) lesions, there was acute (active) inflammation involving the stroma, acinar lumens, and epithelium (Table 2) ▶ . There was a strong correlation between the extent of acute and chronic inflammation (r = 0.683, P < 0.0001, n = 55). Furthermore, there was a correlation between the amount of intraluminal macrophages and the overall extent of acute (r = 0.4978, P = 0.0001, n = 55) and chronic (r = 0.4666, P = 0.003, n = 55) inflammation.
Table 1.
Extent of Chronic Inflammation in PIA Lesions
| Severity | n | Percentage of total |
|---|---|---|
| Mild | 27 | 49 |
| Moderate | 21 | 38 |
| Marked | 7 | 12.7 |
Table 2.
Extent of Acute Inflammation in PIA Lesions
| Severity | n | Percentage of total |
|---|---|---|
| None | 22 | 40 |
| Mild/moderate | 24 | 43.6 |
| Marked | 9 | 16.3 |
Direct evidence of tissue injury was present in 26 lesions (47%), where acinar outlines, in association with the inflammatory infiltrate, were at times disrupted or ulcerated. These features have been described previously for chronic prostatitis, 5 and in the present study, the term “epithelial disruption” is used. Most areas of epithelial disruption were in contact with intraluminal corpora amylacea and often showed intraluminal degenerating cells admixed with macrophages and/or polymorphonuclear leukocytes. Indirect evidence for tissue injury in focal prostatic atrophy is that often the stroma showed loss of normal smooth muscle differentiation (confirmed with anti-desmin antibody staining) with concomitant fibrosis (60% of lesions) in these regions, as seen in this study and by others. 10,39
Immunohistochemical staining for basal cell-specific cytokeratins 41 (34βE12) revealed a predominantly intact basal cell layer in most acini from most lesions (Figure 2) ▶ . In the vast majority of acini, the luminal layer of cuboidal cells contained reduced immunoreactivity for 34βE12 or were completely negative for this marker (Figure 2) ▶ . Rarely both the luminal and basal-most layers of cells stained strongly for 34βE12. The cuboidal atrophic luminal cells consistently stained intensely with the monoclonal antibody Cam 5.2. 42 This antibody recognizes cytokeratins 8 and 18, which are highly expressed in secretory cells, with somewhat weaker expression in basal cells (De Marzo et al, unpublished observations). 43
Many of the luminal cells also stained positively for nuclear AR, with the majority of the cells staining weakly and some staining strongly (Figure 4A) ▶ . The level of AR appeared to be related to the extent of clear cytoplasm present; in general those cells with the clearest cytoplasm contained the strongest AR staining. As further evidence for partial secretory cell differentiation in PIA, positive staining for PSA and PSAP was found in the luminal layer of cells, although staining was generally weak in intensity as compared to surrounding normal glands (Figure 3C) ▶ . There was a positive correlation between the extent of AR expression and the extent of expression of the secretory markers PSA (r = 0.5894, P = 0.0001, n = 38) and PSAP (r = 0.5748, P = 0.0002, n = 38). There were weak negative correlations between extent of expression of AR and the extent of acute (r = −0.2847, P = 0.0369) and chronic (r = −0.3579, P = 0.0035, n = 54) inflammation.
Figure 4.

Expression of androgen receptors and Bcl-2 in PIA. A: Medium-power view of AR expression in PIA. B: Adjacent section showing expression of Bcl-2. The solid line demarcates a single acinus with heterogeneous staining. Arrowheads indicate weak staining for AR but strong staining for Bcl-2. Immunoperoxidase, ×100.
As noted previously by staining against the proliferation marker Ki-67, 20 the atrophic lesions showed a variable, yet marked, increase in the staining index as compared with normal-appearing epithelium (median fold increase = 10.71, range 2.291–79.09, n = 22). In PIA, both basal and secretory-type cells showed an elevated Ki-67 staining index (Figure 3A) ▶ . Immunohistochemistry against two other markers of proliferating cells, PCNA 44 and topoisomerase II α, 45 was performed in 10 of the same cases on adjacent sections, and a pattern of increased staining in PIA similar to that obtained with Ki-67 was noted. Consistent with an overall increase in the proportion of cells that are proliferating in PIA, all lesions showed reduced levels of the cyclin-dependent kinase inhibitor p27Kip1 in many of the attenuated secretory-type cells, as compared with surrounding normal-appearing nonatrophic secretory cells (Figure 3B) ▶ .
In agreement with prior studies showing very low levels of apoptosis by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) method, 20 very few apoptotic bodies were identified within the epithelial layers in the PIA lesions examined (n = 55, mean = 0.072 apoptotic bodies/20 hpf) or in the surrounding normal appearing prostate epithelium (n = 41, mean = 0.146 apoptotic bodies/20 hpf). There was no significant difference between the number of apoptotic bodies in PIA versus matched normal epithelial (paired Student’s t-test, t = 0.703, df = 40, P = 0.4863). There were occasional apoptotic bodies found within the lumens of some of the PIA lesions; however, it was not possible to determine whether these represented epithelial or inflammatory cells.
As shown previously, staining against Bcl-2 in normal prostate epithelium was strong in the basal cells and negative to weak in the majority of secretory cells. 46-49 In all PIA lesions evaluated (n = 55), although staining was heterogeneous, there was an overall increase in staining as compared to secretory cells of the adjacent normal epithelium (Figure 4B) ▶ . Similar to that reported in normal prostate epithelium, 50 in all specimens examined for both markers (n = 51), there was an inverse relation between the expression of Bcl-2 and AR in the epithelial cells of PIA (Figure 4) ▶ in many of the glands. There was a significant relation between the extent of Bcl-2 expression and the type of lesion, with those lesions containing PAH showing higher overall levels of Bcl-2 (mean Bcl-2 score = 4.8, n = 14) than lesions not containing PAH (mean Bcl-2 score = 3.8, n = 38) (two-tailed Student’s t-test, t = −3.1046, df = 50, P = 0.0031).
As compared with the vast majority of normal-appearing nonatrophic secretory epithelial cells of the prostate that do not express GSTP1 (Figure 1B) ▶ , 35,36,51-53 all PIA lesions showed elevated levels of GSTP1 in many of the secretory-type cells (Figures 1B and 5) ▶ ▶ . There was a range of expression of GSTP1, and often the individual glands showed a pattern of cells staining positively for GSTP1 in the vicinity of cells staining negatively (Figure 5) ▶ . GSTP1 expression levels in PIA were not associated with acute or chronic inflammation, Ki-67 index, or fibrosis. As with Bcl-2, there was an association between the levels of GSTP1 and the type of lesion, with those lesions containing PAH showing higher levels of GSTP1 (mean GSTP1 score = 4.36, n = 11) than those not containing PAH (mean GSTP1 score = 3.49, n = 41) (two-tailed Student’s t-test, t = −2968, df = 53, P = 0.0045). Levels of GSTP1 expression in PIA were unrelated to whether the lesions were adjacent to or remote from high-grade PIN or carcinoma.
Discussion
In this paper we propose the term “proliferative inflammatory atrophy” (PIA) to designate discrete foci of proliferative glandular epithelium with the morphological appearance of simple atrophy, 20 or postatrophic hyperplasia, 39 occurring in association with inflammation. The key features of this lesion are the presence of two distinct cell layers, mononuclear and/or polymorphonuclear inflammatory cells in both the epithelial and stromal compartments, and stromal atrophy with variable amounts of fibrosis. The morphology of PIA is consistent with McNeal’s description of postinflammatory atrophy, 13 with that of chronic prostatitis described by Bennett et al, 5 and with the lesion referred to previously as “lymphocytic prostatitis” by Blumenfeld et al. 54 As well as showing increased staining for cell proliferation markers, 20 the key immunophenotypic features of PIA that we now describe are increased staining for GSTP1 and Bcl-2 and decreased staining for the cyclin-dependent kinase inhibitor p27Kip1.
In normal, nonatrophic prostatic epithelium, GSTP1, which is an inducible enzyme that appears to protect cells from DNA damage, is expressed predominantly in basal cells. 35-37 The fact that many of the secretory-type cells in PIA express elevated levels of GSTP1 is highly suggestive of a stress-induced response in these cells.
Although we expect that many of the cells in PIA are largely protected from incurring oxidative or electrophilic DNA damage as a result of increased expression of GSTP1, some of the proliferative secretory-type cells that lack expression of GSTP1 (Figure 5) ▶ may be targets for genetic alterations and hence neoplastic transformation. Currently we are performing microdissection and molecular analysis to determine the status of methylation of the GSTP1 promoter in PIA.
How might the prior hypothesis indicating that focal atrophy gives rise to carcinoma 10 be reconciled with the modern contention that most carcinomas, at least in the peripheral zone of the prostate, arise from high-grade PIN? 23 We suggest that PIA may indeed give rise to carcinoma directly as hypothesized previously 10 or that PIA may lead to carcinoma indirectly via development into PIN. Four separate findings provide supportive evidence for this novel hypothesis that PIA may represent a PIN precursor: 1) We demonstrate that a shift in the topographic fidelity of proliferation occurs in PIA. 2) We show that the phenotype of many of the cells in PIA is most consistent with that of an immature secretory-type cell, 24-26,55 similar to that for the cells of high-grade PIN and carcinoma. 3) PIA, high-grade PIN, and carcinoma all occur with high prevalence in the peripheral zone and low prevalence in the central zone of the human prostate. 13 4) In preliminary studies, we find in randomly sampled PIA lesions from radical prostatectomy patients that 34.5.% (n = 19/55, data not shown) show areas of atrophy merging directly with areas of high-grade PIN within the same glands. This preliminary finding appears at odds with a recent study, 17 and we are currently employing fully embedded prostates with and without PIN and carcinoma to determine the frequency and extent of PIA and the frequency with which PIN lesions arise within foci of PIA.
If cells in PIA are proliferating rapidly, why are the lesions not apparently growing in volume? Because there appears to be no increase in the rate of apoptosis in focal atrophy of the prostate, then either Ki-67 staining does not reflect cell proliferation, or cellular loss is balancing proliferation but the loss is not occurring via an apoptotic mechanism. Because other “proliferation markers” were also elevated in the present study, we submit that it is highly unlikely there is not an increased proliferative rate in PIA. Furthermore, others have used more direct measures of mitosis to indicate that focal prostatic atrophy has increased cell replication over that of normal-appearing epithelium. 18 Because our preliminary studies show frequent Cam 5.2-positive epithelial cells and CD68-positive macrophages in the lumens of atrophic glands, as opposed to normal-appearing glands (data not shown), we favor the concept of regeneration in PIA, 11 with the loss of epithelial cells occurring through direct cell injury, in which the injured cells are shed intraluminally and expressed in the ejaculate or engulfed by intraluminal macrophages. It has been previously pointed out that regenerating epithelium is expected to suppress programmed cell death, at least temporarily, to replace lost cells. 56 The increase in Bcl-2 expression in PIA may explain the very low levels of apoptosis and supports the concept that PIA is a regenerative lesion. The marked increase in the proliferation index in PIA may reflect a response to local growth factor release due to lost epithelial cells. Alternatively, more direct growth-promoting factors, such as platelet-derived growth factor, that are released from the inflammatory cells may stimulate epithelial proliferation. 57,58
Still open to debate is whether the inflammation produces tissue damage and regenerative atrophy or whether some other insult induces the tissue damage and/or atrophy directly, with inflammation occurring secondarily. Preliminary support indicating that inflammation occurs before PIA is provided by Bennett et al, 6 who found in autopsies of 125 young males that more foci of inflammation were present at a younger age than foci of atrophy, which tended to occur somewhat later. Additional studies using animal models of inflammation and atrophy may help resolve this question.
In summary, we confirm that essentially all forms of focal atrophy (PIA) are proliferative, the vast majority are associated with inflammation, and many of the proliferating cells appear to have an immature secretory cell phenotype—a phenotype with similarities to PIN and prostate carcinoma. We interpret this very common lesion in a new light and postulate that it arises in the setting of increased oxidative stress, most likely derived from the proximate inflammatory cells. Furthermore, we postulate that PIA may represent a precursor lesion to prostatic intraepithelial neoplasia and, therefore, prostatic carcinoma.
Acknowledgments
We thank Donald S. Coffey, Ph.D., for insightful discussions in all phases of this work. We thank Matthew Putzi, M.D., for careful reading of the manuscript. We thank Jack Clark, M.D., for helpful discussions and for diligent editing of the manuscript. We thank Josephine Geh for performing the immunohistochemistry for this paper.
Note added in proof: While this manuscript was in press, it came to our attention that a previously published manuscript appears to have been the first to show an elevated Ki-67 staining index in focal atrophy of the prostate (Feneley MR, Young MP, Chinyama C, Kirby RS, Parkinson MC: Ki-67 expression in early prostate cancer pathological lesions. J Clin Pathol 1996, 49:741–748).
Footnotes
Address reprint requests to Dr. Angelo M. De Marzo, Department of Pathology, Ross 512B, 720 Rutland Ave., Baltimore, MD 21205. E-mail: ademarz@welchlink.welch.jhu.edu.
Supported in part by United States Public Health Service Specialized Program in Research Excellence (SPORE) in Prostate Cancer Grants P50CA58236 and 1K08CA78588-01 (to A. M. D.).
References
- 1.Ames BN: Mutagenesis and carcinogenesis: endogenous and exogenous factors. Environ Mol Mutagen 1989, 14:66-77 [DOI] [PubMed] [Google Scholar]
- 2.Weitzman SA, Gordon LI: Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood 1990, 76:655-663 [PubMed] [Google Scholar]
- 3.Bartsch H, Frank N: Blocking the endogenous formation of N-nitroso compounds and related carcinogens. IARC Sci Publ 1996, 139:189-201 [PubMed] [Google Scholar]
- 4.Smith CJ, Gardner WA, Jr: Inflammation-proliferation: possible relationships in the prostate. Prog Clin Biol Res 1987, 239:317-325 [PubMed] [Google Scholar]
- 5.Bennett BD, Richardson PH, Gardner WA: Histopathology and cytology of prostatitis. Lepor H Lawson RK eds. Prostate Diseases. 1993, :pp 399-414 W. B. Saunders Company, Philadelphia, [Google Scholar]
- 6.Bennett BD, Culberson DE, Petty CS, Gardner WA: Histopathology of prostatitis. J Urol 1990, 143:265A [Google Scholar]
- 7.Gardner WA, Bennett BD: The prostate-overview: recent insights and speculations. Weinstein RS Gardner WA eds. Pathology and Pathobiology of the Urinary Bladder and Prostate. 1992, :pp 129-148 Williams and Wilkins, Baltimore, [PubMed] [Google Scholar]
- 8.Platz EA: Prostatitis and prostate cancer. New Dev Prostate Cancer Treatment 1998, 3:71-73 [Google Scholar]
- 9.De Marzo AM, Coffey DS, Nelson WG: New concepts in tissue specificity for prostate cancer and benign prostatic hyperplasia. Urology 1999, 53:29-39 [DOI] [PubMed] [Google Scholar]
- 10.Franks LM: Atrophy and hyperplasia in the prostate proper. J Pathol Bacteriol 1954, 68:617-621 [DOI] [PubMed] [Google Scholar]
- 11.Liavag I: Atrophy and regeneration in the pathogenesis of prostatic carcinoma. Acta Pathol Microbiol Scand 1968, 73:338-350 [DOI] [PubMed] [Google Scholar]
- 12.McNeal JE: Normal histology of the prostate. Am J Surg Pathol 1988, 12:619-633 [DOI] [PubMed] [Google Scholar]
- 13.McNeal JE: Prostate. ed 2 Sternberg SS eds. Histology for Pathologists, 1997, :pp 997-1017 Lippincott-Raven, Philadelphia [Google Scholar]
- 14.McNeal JE: Aging and the prostate. Brocklehurst JC eds. Urology in the Elderly. 1984, :pp 193-202 Churchill Livingstone, Edinburgh [Google Scholar]
- 15.McNeal JE: Regional morphology and pathology of the prostate. Am J Clin Pathol 1968, 49:347-357 [DOI] [PubMed] [Google Scholar]
- 16.Gardner WA, Jr, Culberson DE: Atrophy and proliferation in the young adult prostate. J Urol 1987, 137:53-56 [DOI] [PubMed] [Google Scholar]
- 17.Billis A: Prostatic atrophy: an autopsy study of a histologic mimic of adenocarcinoma. Mod Pathol 1998, 11:47-54 [PubMed] [Google Scholar]
- 18.Liavag I: Mitotic activity of prostatic epithelium. A study by means of Colcemid. Acta Pathol Microbiol Scand 1968, 73:19-28 [DOI] [PubMed] [Google Scholar]
- 19.Stiens R, Helpap B, Bruhl P: The proliferation of prostatic epithelium in chronic prostatitis. Urol Res 1975, 3:21-24 [DOI] [PubMed] [Google Scholar]
- 20.Ruska KM, Sauvageot J, Epstein JI: Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol 1998, 22:1073-1077 [DOI] [PubMed] [Google Scholar]
- 21.Bonkhoff H, Stein U, Remberger K: The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 1994, 24:114-118 [DOI] [PubMed] [Google Scholar]
- 22.McNeal JE, Haillot O, Yemoto C: Cell proliferation in dysplasia of the prostate: analysis by PCNA immunostaining. Prostate 1995, 27:258-268 [DOI] [PubMed] [Google Scholar]
- 23.Bostwick DG: Prospective origins of prostate carcinoma. Prostatic intraepithelial neoplasia and atypical adenomatous hyperplasia. Cancer 1996, 78:330-336 [DOI] [PubMed] [Google Scholar]
- 24.De Marzo AM, Nelson WG, Meeker AM, Coffey DS: Stem cell features of benign and malignant prostate epithelial cells. J Urol 1998, 160:2381-2392 [DOI] [PubMed] [Google Scholar]
- 25.Verhagen AP, Ramaekers FC, Aalders TW, Schaafsma HE, Debruyne FM, Schalken JA: Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res 1992, 52:6182-6187 [PubMed] [Google Scholar]
- 26.De Marzo A, Meeker A, Epstein J, Coffey D: Prostate stem cell compartments: expression of p27Kip1 in normal, hyperplastic and cancer cells. Am J Pathol 1998, 153:911-919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, deKernion JB, Loda M, Reiter RE: Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol 1998, 159:941-945 [PubMed] [Google Scholar]
- 28.Cheville JC, Lloyd RV, Sebo TJ, Cheng L, Erickson L, Bostwick DG, Lohse CM, Wollan P: Expression of p27kip1 in prostatic adenocarcinoma. Mod Pathol 1998, 11:324-328 [PubMed] [Google Scholar]
- 29.Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM: Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res 1998, 58:542-548 [PubMed] [Google Scholar]
- 30.Guo YP, Sklar GN, Borkowski A, Kyprianou N: Loss of the cyclin-dependent kinase inhibitor P27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res 1997, 3:2269-2274 [PubMed] [Google Scholar]
- 31.Cote RJ, Shi Y, Groshen S, Feng AC, Cordon-Cardo C, Skinner D, Lieskovosky G: Association of p27Kip1 levels with recurrence and survival in patients with stage C prostate carcinoma. J Natl Cancer Inst 1998, 90:916-920 [DOI] [PubMed] [Google Scholar]
- 32.Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, Scher HI: Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst 1998, 90:1284-1291 [DOI] [PubMed] [Google Scholar]
- 33.Coles B, Ketterer B: The role of glutathione and glutathione transferases in chemical carcinogenesis. Crit Rev Biochem Mol Biol 1990, 25:47-70 [DOI] [PubMed] [Google Scholar]
- 34.Rushmore TH, Pickett CB: Glutathione S-transferases, structure, regulation, and therapeutic implications. J Biol Chem 1993, 268:11475-11478 [PubMed] [Google Scholar]
- 35.Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Isaacs WB, Nelson WG: Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA 1994, 91:11733-11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WH, Isaacs WB, Bova GS, Nelson WG: CG island methylation changes near the GSTP1 gene in prostatic carcinoma cells detected using the polymerase chain reaction: a new prostate cancer biomarker. Cancer Epidemiol Biomarkers Prev 1997, 6:443-450 [PubMed] [Google Scholar]
- 37.Brooks JD, Weinstein M, Lin X, Sun Y, Pin SS, Bova GS, Epstein JI, Isaacs WB, Nelson WG: CG island methylation changes near the GSTP1 gene in prostatic intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev 1998, 7:531-536 [PubMed] [Google Scholar]
- 38.Silberman MA, Partin AW, Veltri RW, Epstein JI: Tumor angiogenesis correlates with progression after radical prostatectomy but not with pathologic stage in Gleason sum 5 to 7 adenocarcinoma of the prostate. Cancer 1996, 79:772-779 [DOI] [PubMed] [Google Scholar]
- 39.Cheville JC, Bostwick DG: Postatrophic hyperplasia of the prostate. A histologic mimic of prostatic adenocarcinoma. Am J Surg Pathol 1995, 19:1068-1076 [DOI] [PubMed] [Google Scholar]
- 40.Oppenheimer JR, Wills ML, Epstein JI: Partial atrophy in prostate needle cores: another diagnostic pitfall for the surgical pathologist. Am J Surg Pathol 1998, 22:440-445 [DOI] [PubMed] [Google Scholar]
- 41.Brawer MK, Peehl DM, Stamey TA, Bostwick DG: Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res 1985, 45:3663-3667 [PubMed] [Google Scholar]
- 42.Makin CA: Monoclonal antibodies raised to colorectal carcinoma antigens. Ann R Coll Surg Engl 1986, 68:298-301 [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Hao J, Liu X, Dalkin B, Nagle RB: Differential expression of cytokeratin mRNA and protein in normal prostate, prostatic intraepithelial neoplasia, and invasive carcinoma. Am J Pathol 1997, 150:693-704 [PMC free article] [PubMed] [Google Scholar]
- 44.Kurki P, Ogata K, Tan EM: Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J Immunol Methods 1988, 109:49-59 [DOI] [PubMed] [Google Scholar]
- 45.Lynch BJ, Guinee DG, Jr, Holden JA: Human DNA topoisomerase II-α: a new marker of cell proliferation in invasive breast cancer. Hum Pathol 1997, 28:1180-1188 [DOI] [PubMed] [Google Scholar]
- 46.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML: Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992, 52:6940-6944 [PubMed] [Google Scholar]
- 47.Kyprianou N, Tu H, Jacobs SC: Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol 1996, 27:668-675 [DOI] [PubMed] [Google Scholar]
- 48.Colombel M, Vacherot F, Diez SG, Fontaine E, Buttyan R, Chopin D: Zonal variation of apoptosis and proliferation in the normal prostate and in benign prostatic hyperplasia. Br J Urol 1998, 82:380-385 [DOI] [PubMed] [Google Scholar]
- 49.Bubendorf L, Sauter G, Moch H, Jordan P, Blochlinger A, Gasser TC, Mihatsch MJ: Prognostic significance of Bcl-2 in clinically localized prostate cancer. Am J Pathol 1996, 148:1557-1565 [PMC free article] [PubMed] [Google Scholar]
- 50.Bonkhoff H, Fixemer T, Remberger K: Relation between Bcl-2, cell proliferation, and the androgen receptor status in prostate tissue and precursors of prostate cancer. Prostate 1998, 34:251-258 [DOI] [PubMed] [Google Scholar]
- 51.Canada AT, Roberson KM, Vessella RL, Trump DL, Robertson CN, Fine RL: Glutathione and glutathione S-transferase in benign and malignant prostate cell lines and prostate tissues. Biochem Pharmacol 1996, 51:87-90 [DOI] [PubMed] [Google Scholar]
- 52.Moskaluk CA, Duray PH, Cowan KH, Linehan M, Merino MJ: Immunohistochemical expression of pi-class glutathione S-transferase is down-regulated in adenocarcinoma of the prostate. Cancer 1997, 79:1595-1599 [DOI] [PubMed] [Google Scholar]
- 53.Cookson MS, Reuter VE, Linkov I, Fair WR: Glutathione S-transferase PI (GST-pi) class expression by immunohistochemistry in benign and malignant prostate tissue. J Urol 1997, 157:673-676 [PubMed] [Google Scholar]
- 54.Blumenfeld W, Tucci S, Narayan P: Incidental lymphocytic prostatitis. Selective involvement with nonmalignant glands. Am J Surg Pathol 1992, 16:975-981 [PubMed] [Google Scholar]
- 55.Verhagen AP, Aalders TW, Ramaekers FC, Debruyne FM, Schalken JA: Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate 1988, 13:25-38 [DOI] [PubMed] [Google Scholar]
- 56.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 57.Vlahos CJ, Kriauciunas TD, Gleason PE, Jones JA, Eble JN, Salvas D, Falcone JF, Hirsch KS: Platelet-derived growth factor induces proliferation of hyperplastic human prostatic stromal cells. J Cell Biochem 1993, 52:404-413 [DOI] [PubMed] [Google Scholar]
- 58.Gleason PE, Jones JA, Regan JS, Salvas DB, Eble JN, Lamph WW, Vlahos CJ, Huang WL, Falcone JF, Hirsch KS: Platelet derived growth factor (PDGF), androgens and inflammation: possible etiologic factors in the development of prostatic hyperplasia. J Urol 1993, 149:1586-1592 [DOI] [PubMed] [Google Scholar]



