Abstract
Two isoforms of the human cadherin-11/OB-cadherin gene, the intact and the variant forms, had been isolated from an osteosarcoma cDNA library. The intact form has a typical cadherin structure, whereas the variant form, generated by alternative splicing, encodes a cytoplasmic domain that is completely different from that of the intact form and lacks a homophilic cell-cell adhesion ability. At the protein level, the secreted form generated from the intact cadherin-11 is present. We examined the expression of the intact and the variant forms of cadherin-11 in 23 primary and metastatic osteosarcomas from 22 patients by reverse transcriptase-polymerase chain reaction (RT-PCR) analyses, revealing that all 23 tumors in the patients expressed the variant form and three of them expressed it prominently. On the other hand, Western blot analyses of six tumors showed that the secreted form was strongly expressed, and furthermore, expression of N-cadherin was extremely low. Overexpression of the intact cadherin-11 cDNA in osteosarcoma cell lines demonstrated that the secreted form is derived from the intact form of cadherin-11 in osteosarcoma. Immunohistochemically, cadherin-11, N-cadherin, and β-catenin were expressed at the cell surface of fetal osteoblasts, whereas in osteosarcoma cells, they were expressed only focally or weakly in the cytoplasm. Considering the function of cadherin in carcinomas, it is suggested that the anomalous expression of human cadherin-11 in osteosarcoma and the reduced expression of N-cadherin play a role in metastasis and the irregular morphology in the highly malignant mesenchymal tumor.
Osteosarcoma is a highly malignant bone-forming tumor frequently occurring in the metaphysis of long bones in individuals under 20 years of age. 1 In the past, the prognosis was so poor that the 5-year disease-free survival rate was less than 10%. The recent advances of adjuvant chemotherapy have resulted in improvement of the prognosis of osteosarcoma patients. 2 Lung metastasis through the bloodstream, however, is still one of the significant prognostic factors. The biological study of osteosarcoma, especially on the basic mechanism of metastasis, is important because of its bearing on patient survival. However, the underlying pathophysiology of osteosarcoma has been paid little attention in comparison with that of carcinoma.
Tumor metastasis consists of the following sequential steps: escape from the primary tumor, accommodation to interstitial tissues, invasion of vessels, and colonization of distant organs. The first two steps are known as local invasion. These processes are required for disruption of the normal tissue architecture that is maintained by the extracellular matrix (ECM). Cell adhesion molecules (CAMs) are thought to play a significant role not only in maintaining tissue architecture, but also in carcinoma progression, which includes change in morphology, invasion, and metastasis. Cadherin, a homophilic calcium-dependent cell adhesion molecule, has attracted particular attention with regard to altered expression in several different carcinomas. 3 Up to now more than 10 kinds of cadherins have been isolated and characterized. 4 In particular, E-cadherin has been subjected to vigorous investigation and has been identified as a tumor suppressor gene and a morphogenic factor in epithelial tumors. 5 Disruption of the cell-cell adhesion mediated by E-cadherin induces dissociation of tumor cells and results in regional invasion and metastasis. Catenins consisting of α-, β-, and γ-subunits interact with the cytoplasmic domain of cadherin, and the resulting complex forms a rigid adherens junction. Not only reduction of its expression or genomic mutation, but also dysfunction of catenins leads to the dissociation of tumor cells. Thus disturbance of the cell-cell adhesion mediated by E-cadherin induces regional invasion and metastasis of tumor cells. 6
Previously we isolated the first cadherin gene shown to be expressed in osteoblasts (termed osteoblast cadherin, OB-cadherin) from both the mouse osteoblastic cell line MC3T3-E1 and a human osteosarcoma cDNA library. 7 OB-cadherin is now known to be identical to cadherin-11 cloned from a brain cDNA library. We found that human cadherin-11 has three isoforms: the intact form, showing 50% amino acid identity to N-cadherin; the variant form, generated by alternative splicing; and the secreted form, caused by proteolysis. 8 The intact form of human cadherin-11 is 97% identical to the mouse counterpart and generates a 120-kd protein. It has a calcium-dependent homophilic cell-cell adhesive property in association with β-catenin, which is characteristic of the cadherin family. The cDNA structure of the variant form of human cadherin-11 demonstrated that the insertion of a 179-bp sequence located at the transmembrane domain causes a frameshift, resulting in a short cytoplasmic region that is different from the intact form. The variant form showed 85 kd in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Although the variant form shows no homophilic cell-cell adhesion property, it assists the cell-cell adhesion of the intact form when the intact and variant forms are coexpressed at similar levels. 8 The secreted form of cadherin-11 is an 80-kd protein and is found in supernatants of osteoblast-like cells and L-cell transfectants of intact cadherin-11. It is suggested that the secreted form is derived from the intact form by proteinase cleavage.
Although the human cadherin-11 gene was cloned from an osteosarcoma cDNA library, its expression in tissues of osteosarcomas has not yet been investigated. Moreover, it was reported that normal osteoblasts and human osteosarcoma cell lines express N-cadherin. 9 In this study, we examined the expression of cadherin-11 and N-cadherin in samples from patients with osteosarcoma. We demonstrate that both the intact form of cadherin-11 and N-cadherin are expressed at lower levels in osteosarcoma, whereas normal osteoblasts express them at high levels. The results suggest that the reduced expression of the intact forms of cadherin-11 and of N-cadherin causes the local invasion of osteosarcoma.
Materials and Methods
Cell Lines and Antibodies
Hos and Saos-2, osteosarcoma cell lines, and Ltk−, a mouse fibroblastic cell line, were obtained from RCB (Riken Cell Bank, Wako, Japan). HGH, the Giralidi heart cell line, and MRC-5, a lung fetal fibroblast cell line, were obtained from Dainihon Seiyaku (Osaka, Japan). Primary human fetal calvarial osteoblastic cells (CALs) and cadherin-11 cDNA-transfected Ltk− cells were described previously. 8 These cell lines were maintained in recommended growth media and incubated at 37°C with 5% CO2. Mouse monoclonal antibodies against human cadherin-11, 113H and 113E, 9 were kindly provided by Dr. St. John, ICOS Corporation (Bothell, WA), and antibodies against N-cadherin, P,E,N-cadherin (Takara Shuzo, Tokyo, Japan), β-catenin (Transduction Laboratories, Lexington, KY), G3PDH (Biogenesis, Sandon, NH), and a horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad, Hercules, CA) were purchased.
Tumor Samples from Patients
Tumors were obtained by surgical resection or during autopsy and then embedded in O.C.T. Compound (Miles, Elkhart, IN) and frozen at −80°C. All materials were histologically examined and used for further examinations. A total of 23 osteosarcoma samples from 22 patients were analyzed. Their relevant clinical and pathological details are summarized in Table 1 ▶ . All biopsy specimens were diagnosed to be conventional osteosarcoma, and preoperative chemotherapy was carried out in all cases. The secondary tumors often metastasized to lung.
Table 1.
Summary of Clinicopathological Data of Osteosarcoma Cases
| Samples | Age | Sex | Location | Metastasis | Outcome |
|---|---|---|---|---|---|
| OS-1 | 12 | M | L. femur* | + | DOD |
| OS-2 | 13 | M | L. femur* | + | DOD |
| OS-3 | 18 | M | L. femur* | + | DOD |
| OS-4 | 45 | M | L. calcaneus* | + | DOD |
| OS-5 | 10 | F | L. femur* | − | CDF |
| OS-6 | 26 | F | L. femur* | − | CDF |
| OS-7 | 17 | M | L. femur | + | DOD |
| OS-8 | 13 | F | L. femur* | + | DOD |
| OS-9 | 13 | F | L. femur* | − | CDF |
| OS-10 | 20 | M | Lung | + | DOD |
| OS-11 | 14 | F | Lung | + | AWD |
| OS-12 | 12 | F | Lung | + | NED |
| OS-13 | 17 | M | Lung | + | DOD |
| OS-14 | 75 | F | Lung | + | DOD |
| OS-15 | 15 | M | Lung | + | DOD |
| OS-16 | 14 | M | Chest wall | + | DOD |
| OS-17 | 29 | M | L. scapula | − | CDF |
| OS-18 | 9 | F | R. humerus* | + | AWD |
| OS-19 | 75 | F | L. scapula* | (same patient as OS-14) | |
| OS-20 | 44 | M | Lung | + | DOD |
| OS-21 | 18 | M | R. fibula* | + | DOD |
| OS-22 | 6 | F | R. femur | + | DOD |
| OS-23 | 12 | M | R. femur* | − | DOD |
*Primary tumor, state before chemotherapy.
L and R indicate left and right, respectively.
DOD, dead of disease; CDF, continuous disease free; AWD, alive with disease; NED, no evidence of disease.
RT-PCR and Southern Blot Analyses
First-strand cDNAs were prepared from 0.5 μg of total RNA using Super Script II RNaseH− reverse transcriptase (Life Technologies, Gaithersburg, MD) and 100 ng of oligo dT primer in a volume of 20 μl, except for biopsy samples, where less than 0.5 μg total RNA was extracted. All cDNAs were confirmed to be amplified with G3PDH primers by PCR. The nucleotide sequences of the primers were followed: cadherin-11 (5′-CGTGGAGGGTTCAGTCGGCAGA-3′ and 5′-TACTGATACTCAGGTTTGAT-3′). The PCR was carried out for 25 cycles to avoid saturation of the reaction. The PCR products were electrophoresed and subsequently blotted. The variant form of the cadherin-11 cDNA labeled with digoxygenin was used as a probe. A monoclonal antibody to digoxygenin and the chemiluminescent substrate were used for Southern blot analyses, using methods described elsewhere. 8
Western Blot Analyses
Tumors obtained by surgical resection were washed with phosphate-buffered saline and homogenized on ice. Samples (0.1–0.5 g) were mixed with 1 ml of cold TNE buffer containing 2% NP40, 20 mM Tris (pH 8.0), 150 mM NaCl, 5 mM EDTA, 2 mM NaN3, 0.1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, and 20 μg/ml aprotinin, and incubated for 1 hour at 4°C with a rotor mixer. After centrifugation at 15,000 rpm for 20 minutes, supernatants were recovered and the concentration of protein was determined with a protein assay kit (Bio-Rad). For each sample, an equal volume of 2× sample buffer was added and boiled at 95°C for 5 minutes. To obtain culture supernatant, 1 × 10 6 cells were cultured with Dulbecco’s minimum essential medium supplemented with 10% fetal calf serum overnight, and then the medium was replaced with fresh Dulbecco’s minimum essential medium and cells were cultured for additional 24 hours. Culture supernatants were concentrated from 10 ml to 200 μl by Centriflo (Amicon, Danvers, MA), and 50 μl of 5× sample buffer was added to each sample and boiled for 5 minutes. Samples were loaded and separated by electrophoresis on 7.5% or 10% polyacrylamide SDS gels, transferred onto nitrocellulose filters, and incubated with appropriate antibodies. The ECL system (Amersham Co., Buckinghamshire, England) was used to detect proteins recognized by antibodies.
Transfection of Human Cadherin-11 cDNA into Osteosarcoma Cell Lines
Transfection of the intact form of cadherin-11 cDNA into Hos and Saos-2 cell lines was performed as described previously. 8 In brief, the intact form of cadherin-11 cDNA in the expression vector driven by chicken β-actin and human cytomegalovirus enhancer was transfected using Lipofectamine reagent (Life Technology), and transfectants were selected in 800 μg/ml G418.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissues were cut into slices 4 μm thick. Immunohistochemical studies were performed by the peroxidase streptoavidin-biotin method with a LASB kit (DAKO, CA) or an automatic staining apparatus, the Nexus system (Bentana Medical System, Tucson, AZ). A monoclonal antibody against cadherin-11, 113E, which was generated using the human recombinant EC1–3 domain of cadherin-11 as an antigen, was used for the detection of all isoforms. Antibodies against β-catenin and P,E,N-cadherin were applied after antigen retrieval in the citrate acid buffer. Substitution of primary antibodies by normal serum was used as a negative control. The fetal calvaria, which was positive for the expression of cadherin-11 and N-cadherin, is the source of primary calvarial osteoblastic cells (CALs). For detecting N-cadherin, we used anti P,E,N-cadherin rabbit sera because there is no suitable antibody for N-cadherin with a specific immunohistochemistry.
Results
Preferential Expression of the Variant Form of Human Cadherin-11 mRNA in Osteosarcoma
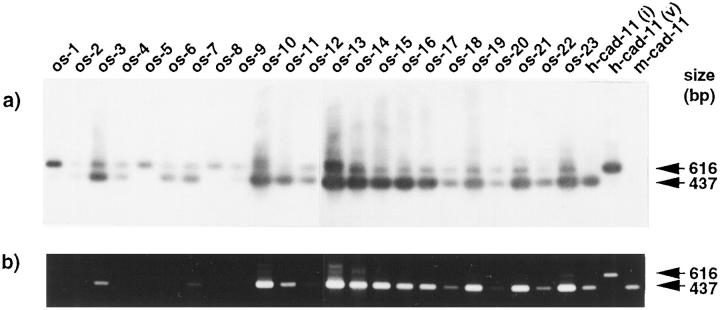
The expression of two isoforms of the human cadherin-11 mRNA was examined in osteosarcoma by RT-PCR (Figure 1) ▶ . Osteosarcoma tumors obtained by surgical resection or during autopsy were examined histologically. The relevant clinical and pathological details of 23 osteosarcoma samples from 22 patients are summarized in Table 1 ▶ . Previously, the intact and variant forms were found in bone tissues, and the variant form was generated by alternative splicing. 8 For detection of cadherin-11 transcription by RT-PCR analyses, we used PCR primers for the detection of both forms. Because the variant form has an additional 179 bp sequences, the variant form was detected as being 179 bp longer than the intact form. All tumors expressed the intact and variant forms of cadherin-11 mRNA (Figure 1) ▶ . We confirmed that the upper band shows the variant form by DNA sequencing (data not shown). Remarkably, some of them, OS-1, -5, and -8, prominently expressed the variant mRNA form. The RNA expression of the variant form of cadherin-11 in osteosarcoma samples was also confirmed by Northern blot analysis of several available samples (data not shown).
Figure 1.
RT-PCR analysis of the cadherin-11 mRNA in osteosarcomas. cDNAs were synthesized from total RNAs of 23 osteosarcoma samples (from OS-1 to OS-23) in Table 1 ▶ and amplified by PCR using cadherin-11-specific primers (b), and Southern blotting was performed for the human cadherin-11 gene (a) as previously described. 8 Size markers represent φX174 DNA fragments digested with HaeIII. h-cad-11(i), h-cad-11(v), and m-cad-11 indicate that cDNAs of the intact form and the variant form of human cadherin-11 and mouse cadherin-11, respectively, are used for positive and negative controls.
Protein Expression of Three Forms of Cadherin-11 and N-Cadherin in Osteosarcoma
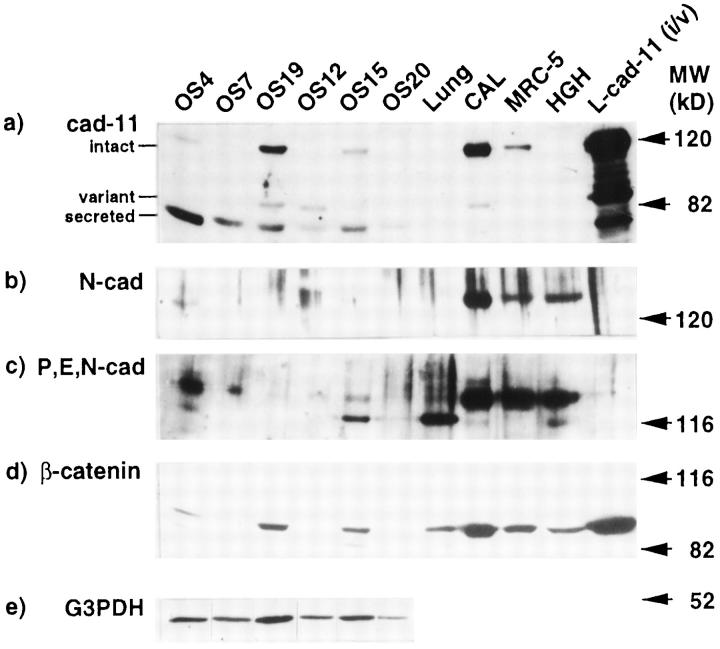
To verify the expression of the three forms of cadherin-11 and of β-catenin in osteosarcoma, we analyzed three primary tumors, OS-4, OS-7, and OS-19, and three metastatic tumors, OS-12, OS-15, and OS-20, by Western blot analyses with monoclonal antibodies against human cadherin-11 and β-catenin (Figure 2) ▶ . The intact and variant forms of cadherin-11 were 120 kd and 85 kd, respectively. The 80-kd form was assumed to be the secreted form of cadherin-11, based on both molecular weight on SDS-PAGE and indirect evidence by subsequent transfection experiments (shown in Figure 3 ▶ ). The 120-kd band was detected in all osteosarcoma samples, although signals from OS-7 and OS-20 were very weak. The 85-kd form was found in only a half of the osteosarcoma samples. A strong 80-kd band was detected in all osteosarcoma samples, whereas its expression in the fetal lung fibroblast cell line (MRC-5) and the human fetal calvarial cells (CALs) was very low. Stability of signals from G3PDH represents appropriate preparation of samples; thus the result is not an artifact. The expression level of β-catenin in each sample was proportional to that of the intact form of cadherin-11. Expression of N-cadherin was examined by commercially available antisera reacting to human N-cadherin or reacting to human P-, E-, and N-cadherin. Prominent expression of N-cadherin was found in OS-4, and low or no expression was found in the other five osteosarcoma samples by the anti-N cadherin antibody. The anti-P,E,N-cadherin antibody reacted to the 120-kd band of P-cadherin or E-cadherin and to the 140-kd band of N-cadherin. CAL, MRC-5, and the human heart cell line (HGH) used for control samples expressed a high level of N-cadherin and detectable levels of P- or E-cadherin. In conclusion, osteosarcoma expressed high levels of the secreted form of cadherin-11, and N-cadherin either was absent or was expressed at low levels.
Figure 2.
Protein expression of cadherins and β-catenin in osteosarcoma. Lysates from six osteosarcoma samples were tested by Western blotting with an anti-cadherin-11 antibody (a), anti-N-cadherin antisera (b), an anti-P,E,N-cadherin antibody (c), an anti-β-catenin antibody (d), and an anti-G3PDH antibody (e). Each lane contains 40 μg of protein. Cell lysates from both the intact and variant forms of L cell transfectants (L-cad11 (i/v)) were loaded, and three forms of cadherin-11, the intact (120 kd), the variant (85 kd), and the secreted (80 kd) forms, were detected. Lysates from human calvarial cells (CAL), the human lung fibroblast cell line (MRC-5), and the human Giralidi heart cell line (HGH) were loaded as positive controls.
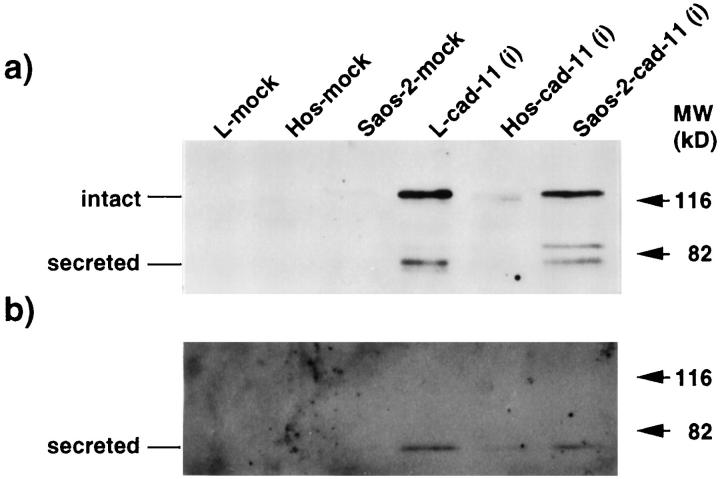
Figure 3.
Overexpression of human cadherin-11 cDNA in osteosarcoma cell lines. Transfection of the intact form of the cadherin-11 gene into the human osteosarcoma cell lines, HOS and Saos-2, was performed with methods described previously. 8 Panels show Western blot analyses of each cell lysate (a) and culture supernatants concentrated 20-fold (b). L cell, Hos, and Saos-2 transfectants with the vector alone (pCXN2) are termed L-mock, Hos-mock, and Saos-2-mock, respectively, and transfectants with the intact cadherin-11 expression vector are termed L-cad-11(i), Hos-cad-11(i), and Saos-2-cad-11(i), respectively. The intact form of cadherin-11 was found as a 120-kd band, and the secreted form was an 80-kd band.
Generation of the 80-kd Secreted Form from the Intact Form of Cadherin-11 in the Osteosarcoma Cell Lines
Because the high level expression of the 80-kd band was found in osteosarcomas, we attempted to determine whether the 80-kd band was the secreted form generated from the intact form of cadherin-11 by using osteosarcoma cell lines (Figure 3a) ▶ . An expression vector coding for the intact form of cadherin-11 was transfected into the osteosarcoma cell lines, Saos-2 and Hos, because they express endogenous cadherin-11 at very low levels. Previously we detected that L cell transfectants of the intact form of cadherin-11 expressed both the intact form (120 kd) and the secreted form (80 kd), 8 and therefore these transfectants were used as a positive control (L-cad11(i) in Figure 3 ▶ ) in this experiment. Overexpression of the intact form led to the production of two proteins, 88 kd and 80 kd, detected in cell lysates by Western blot analysis. The 80-kd band but not the 88-kd band was also found in the supernatants of cells (Figure 3b) ▶ . The 88-kd band may be another proteolytic protein from the intact form because we could not detect the mRNA of the variant form in transfectants (data not shown). Thus the result demonstrates that the secreted form is generated in osteosarcoma cells from the intact form of cadherin-11.
Strong Expression of Cadherin-11, N-Cadherin, and β-Catenin in Normal Osteoblasts but Faint Expression in Osteosarcoma Analyzed by Immunohistochemistry
To identify localization of cadherins in osteosarcoma and normal bone, including fetal calvaria (Figure 4, a–c) ▶ and adult reactive bone (Figure 4, d–f) ▶ , expression of cadherin-11 and N-cadherin was examined by immunohistochemistry. In normal bone, strong expression of cadherin-11 and β-catenin was detected at the cell membrane in both resting and forming osteoblasts along the surface of woven bone. Interestingly, immunoreactivity for cadherin-11 was also detected in immigrating osteocytes, which have abundant cytoplasm and large nuclei, but not in fibroblasts and mature osteocytes. Immunoreactivities of P,E,N-cadherin and β-catenin were also found in osteoblasts, but not in osteocytes. On the other hand, in osteosarcoma samples, OS-7 (Figure 4, g–i) ▶ and OS-12 (Figure 4, j–l) ▶ , we found only low levels of expression of cadherin-11 and β-catenin in cytoplasm and interstitium. Immunoreactivity of P,E,N-cadherin and β-catenin was found locally at the tumor cell surface (Figure 4, i, k, l) ▶ .
Figure 4.
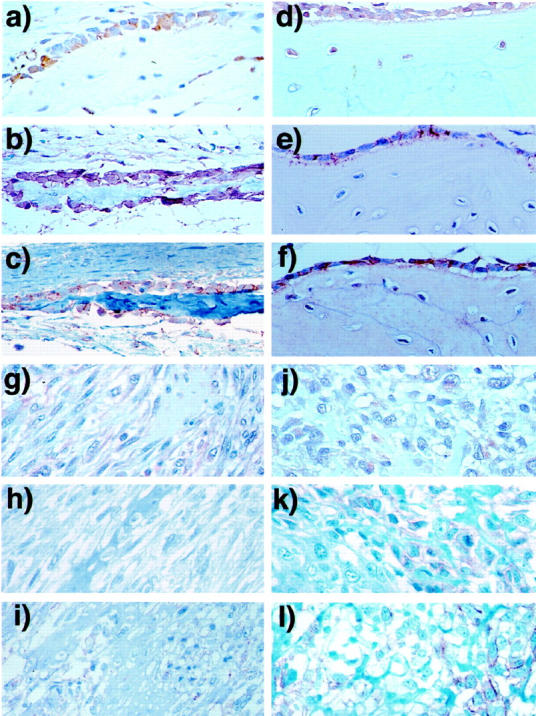
Immunohistochemistry of cadherin-11, N-cadherin, and β-catenin in normal bone tissues and osteosarcoma. Upper panels show the immunostaining of fetal calvaria in 19 weeks in gestation (a–c) and adult reactive bone (d–f), and lower panels show primary (OS7: g–i) and metastatic (OS12: j–l) osteosarcoma. Monoclonal antibody against cadherin-11(113E) (a, d, g, j), polyclonal rabbit antisera against P,E,N-cadherin (b, e, h, k), and monoclonal antibody against β-catenin (c, f, i, l) were used for immunohistochemistry with peroxidase streptavidin-biotin methods. Original magnification ×400.
Discussion
Cadherins are homophilic calcium-dependent cell adhesion molecules (CAMs) related to morphogenesis and organogenesis. 3 We had previously cloned the OB-cadherin gene, which belongs to type II cadherin superfamilies, from a mouse osteoblastic cell line and a human osteosarcoma cDNA library. 7 OB-cadherin is identical to cadherin-11, originally found in nervous tissues. 4 We also characterized the variant form of human cadherin-11, demonstrating that it is generated by alternative splicing and lacks the cytoplasmic domain. 8 During embryogenesis, cadherin-11 is expressed in various tissues of the mouse fetus, including the sclerotome and limbs, and thus is thought to be involved in morphogenesis. 10,11 MacCalman et al 12 and Shibata et al 13 also showed that cadherin-11 was widely expressed in various tissues and was strongly expressed in placenta enriched with cells derived from mesoderm. Thus cadherin-11 is primarily expressed in mesenchymal cells.
We demonstrated expression of the variant form of cadherin-11 in osteosarcoma, a mesoderm derivative tumor; the intact and variant forms of human cadherin-11 transcript were expressed in all 23 samples of osteosarcoma by RT-PCR analysis, and three samples among them dominantly expressed the variant form. Initially it was speculated that the high expression of the variant form in osteosarcoma was caused by mutation of DNA sequences. However, the result of that DNA sequencing of genomic fragments surrounding the insertion sequence of two tumors (OS-3 and OS-5) and normal tissues revealed them all to be identical denies this possibility (data not shown). Thus the expression of the variant form in osteosarcoma is attributed to an abnormality of alternative splicing that is same as the derivation of CD44 isoforms in gastric cancer. 14 Because the variant form is expressed in the tissues that are rich in immature mesenchymal cells, there is the possibility that the variant expression can be merely an oncofetal change of tumor. Our recent study investigating the function of the variant form revealed that it stabilizes the adhesion property of the intact form 8 ; however, in osteosarcoma, the variant form is dominantly or highly expressed and thus the adhesion property will be changed. Consequently, we must consider the fact that up-regulation of the variant form generated by alternative splicing is always concomitant with down-regulation of the intact form and therefore results in the disruption of cell adhesion.
A major form of cadherin-11 expressed in osteosarcoma was the secreted form generated from the intact form, which was confirmed by production of the secreted form after introduction of the intact form into osteosarcoma cell lines. The secreted form is also possibly generated from the variant form because the variant form was expressed on the surface together with the intact form. 8 Immunohistochemically, cadherin-11 was strongly expressed at the surface of osteoblasts, but faintly expressed in osteosarcoma, suggesting that the production of the secreted form of cadherin-11 leads to a decrease in the homophilic cell-cell adhesion property in osteosarcoma. The secreted form may be generated by proteinase action in the same way as formation of a soluble form of E-cadherin. 3,15 One possible candidate proteinase for production of the secreted form is MMP-3 (stromelysin-1), 16 because it is able to degrade E-cadherin to generate the soluble form, which can disrupt cell-cell adhesion of E-cadherin. 15 Recently, MMP-3 was detected in osteosarcoma by immunohistochemistry (data not shown). It is also known that the amount of the soluble E-cadherin was increased in the serum of cancer patients. 17 In a similar manner, the secreted form of cadherin-11 seemed to impede the function of the intact form in osteosarcoma, and it may be used as a tumor marker of osteosarcoma. Therefore, an efficient enzyme-linked immunosorbent assay system for detecting the secreted form is worth developing.
Normal osteoblasts along the surface of bone and unidirectional osteoid are linked to each other by adherens junctions. 18 Recent studies revealed that cadherins were located at the adherens junction and are even essential to its formation. 19 However, the intracellular adhesion machinery was not found in osteosarcoma, and thus neoplastic bone formation occurred. 20,21 In the case of various carcinomas, 3,5,6 several types of cadherins are related to metastasis and morphogenesis. For example, the dysfunction of E-cadherin in particular has been regarded as the cause of cell detachment from the primary tumor to the invasion area, which is an initial event of metastasis. Because a subtype of cadherin refers to the origin of cells or tissues, 3 both cadherin-11 and N-cadherin are expressed in osteosarcoma, which corresponds to the expression of E-cadherin in carcinoma, one of the epithelial tumors. Therefore, down-regulation of cadherin-11 and/or N-cadherin expression may be related to regional invasion of osteosarcoma. In addition, β-catenin was not expressed at the cell membrane of primary osteosarcoma in immunohistochemistry, which implies the instability of both cadherin-11 and N-cadherin in osteosarcoma.
Although we could not clearly show relationships between the expression level of cadherin-11 isoforms and clinical manifestation such as frequency of metastasis prognosis, OS-1 and OS-8, two of three patients who expressed the variant form prominently, died earlier than others in aggressive courses 17 and 20 months after operation, respectively (data not shown). The introduction of modern intensive chemotherapy has improved the prognosis. 2 Actually, in the surgical specimens of patients OS-5, -6, -9, and -17, who overcame the disease, the tumors showed broad necrosis, the remarkable result of preoperative chemotherapy (data not shown).
In conclusion, we showed the anomalous expression of cadherin-11 and the reduced expression of N-cadherin in osteosarcoma samples and proposed several mechanisms for its expression and dysfunction. Down-regulation of the intact form is induced by up-regulation of the variant form and by proteolytic cleavage of the intact form into the secreted form. Moreover, the secreted form may impede the function of the intact form. Thus the alteration of cadherin expression could be related to the metastasis and morphology of osteosarcoma. To clarify the importance of cadherins in the metastasis of osteosarcoma, an in vivo metastatic model using a transfectant of a cadherin-11 cDNA is required.
Acknowledgments
The authors thank Drs. Makoto Okazaki, Takuya Iijima, Kaori Tsuji, and Kaichi Ohira for technical and clinical assistance; Dr. Emma Furth for technical advice on immunohistochemistry; and Dr. André Lochter for critical reading of the manuscript.
Footnotes
Address reprint requests to Akira Kudo, Department of Life Science, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8501, Japan. E-mail: akudo@bio.titech.ac.jp.
Supported by grants from the Ministry of Education, Science and Culture of Japan and the Vehicle Racing Commemorative Foundation.
Masahiko Kuroda’s current address: Department of Pathology, Faculty of Medicine, Tokyo Medical University, Tokyo, Japan.
References
- 1.Schajowicz F: WHO: Histological Typing of Bone Tumors, ed 2. Berlin, Springer-Verlag, 1993
- 2.Uozaki H, Horiuchi H, Ishida T, Iijima T, Imamura T, Machinami R: Overexpression of resistance-related proteins (metallothioneins, glutathione-S-transferase pi, heat shock protein 27, and lung resistance-related protein) in osteosarcoma. Relationship with poor prognosis. Cancer 1997, 79:2336–2344 [DOI] [PubMed]
- 3.Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991, 251:1451-1455 [DOI] [PubMed] [Google Scholar]
- 4.Tanihara H, Sano K, Heimark RL, St. John T, Suzuki S: Cloning of five human cadherins clarifies characteristic features of cadherin extracellular domain and provides further evidence for two structurally different types of cadherin. Cell Adhes Commun 1994, 2:15-26 [DOI] [PubMed] [Google Scholar]
- 5.Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, Sugimura T, Hirohashi S: E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA 1994, 91:1858-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawanishi J, Kato J, Sasaki K, Fujii S, Watanabe N, Niitsu Y: Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the β-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol 1995, 15:1175-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki M, Takeshita S, Kawai S, Kikuno R, Tsujimura A, Kudo A, Amann E: Molecular cloning and characterization of cadherin-11, a new member of cadherin family expressed in osteoblasts. J Biol Chem 1994, 269:12092-12098 [PubMed] [Google Scholar]
- 8.Kawaguchi J, Takeshita S, Kashima T, Imai T, Machinami R, Kudo A: Expression and function of the splice variant of the human cadherin-11 gene in subordination to intact cadherin-11. J Bone Miner Res 1999, 14:764-775 [DOI] [PubMed] [Google Scholar]
- 9.Cheng S-L, Lecanda F, Davidson MK, Warlow PM, Zhang S-F, Zhang L, Suzuki S, John TS, Civitelli R: Human osteoblasts express a repertoire of cadherins, which are critical BMP-2-induced osteogenic differentiation. J Bone Miner Res 1998, 13:633-644 [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann I, Balling R: Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev Biol 1995, 169:337-346 [DOI] [PubMed] [Google Scholar]
- 11.Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M: Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol 1995, 169:347-358 [DOI] [PubMed] [Google Scholar]
- 12.MacCalman CD, Furth EE, Omigbodun A, Bronner M, Coutifaris C, Strauss JF: Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Dev Dyn 1996, 206:201-211 [DOI] [PubMed] [Google Scholar]
- 13.Shibata T, Ochiai A, Gotoh M, Machinami R, Hirohashi S: Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer Lett 1996, 99:147-153 [DOI] [PubMed] [Google Scholar]
- 14.Tahara E, Semba S, Tahara H: Molecular biological observations in gastric cancer. Semin Oncol 1996, 23:307-315 [PubMed] [Google Scholar]
- 15.Wheelock MJ, Buck CA, Bechtol KB, Damsky CH: Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem 1987, 34:187-202 [DOI] [PubMed] [Google Scholar]
- 16.Lochter A, Galosy S, Muschler J, Feedman N, Werb Z, Bissell MJ: Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 1997, 139:1861-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama M, Hirai S, Kamihagi K, Nakagawa K, Yasumoto M, Kato I: Soluble E-cadherin fragments increased in circulation of cancer patients. Br J Cancer 1994, 69:580-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palumbo C, Palazzini S, Marotti G: Morphological study of intercellular junctions during osteocyte differentiation. Bone 1990, 11:401-406 [DOI] [PubMed] [Google Scholar]
- 19.Tsukita S, Tsukita S, Nagafuchi A, Yonemura S: Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol 1992, 4:834-839 [DOI] [PubMed] [Google Scholar]
- 20.Williams AH, Schwinn CP, Parker JW: The ultrastructure of osteosarcoma. A review of twenty cases. Cancer 1976, 37:1293–1301 [DOI] [PubMed]
- 21.Grundmann E, Roessner A, Immenkamp M: Tumor cell types in osteosarcoma as revealed by electron microscopy. Implications for histogenesis and subclassification. Virchows Arch B Cell Pathol Incl Mol Pathol 1981, 36:257–273 [DOI] [PubMed]