Abstract
We recently observed that growth inhibition of esophageal cancer cells by retinoic acid (RA) was associated with both constitutive expression and RA-induced up-regulation of RA receptor β (RAR-β). Cell lines that did not express RAR-β were also resistant to RA. To explore the expression of RAR-β mRNA in vivo, we analyzed esophageal tissue specimens from 16 normal mucosae, 30 dysplastic lesions, and 157 esophageal tumors by in situ hybridization. RAR-β was detected in 88% (14/16) of normal esophageal tissues and in 96% (96/100) of distant normal esophageal mucosa from cancer specimens. In contrast, RAR-β was expressed in only 57% (17/30) of dysplastic lesions and in 54% (84/157) of carcinomas. Among esophageal carcinomas RAR-β mRNA was expressed in 62% (26/42) of well-differentiated, 54% (27/50) of moderately differentiated, and only 29% (4/14) of poorly differentiated SCCs. Our data suggest that the loss of RAR-β expression is an early event associated with esophageal carcinogenesis and the status of squamous differentiation.
Retinoids, a group of natural and synthetic analogues of vitamin A, can modulate cell growth and differentiation in vitro and in vivo. 1 They have been shown to suppress or reverse epithelial carcinogenesis and to prevent the development of invasive cancer in many animal models for skin, lung, oral cavity, and esophagus neoplasms. 2 In the esophageal mucosa of experimental animals, vitamin A deficiency induces hyperkeratotic change. 3 In a Chinese clinical trial using the synthetic retinoid N-4-(ethoxycarbophenyl)retinamide, the incidence of cancer in the treatment group with severe esophageal dysplasia was 43.2% lower than that of the group treated with placebo. 4 However, in two other trials in Linxian, China, in which a combination of vitamin A and other nutrients was given, results were inconclusive. 5,6 Therefore, further studies of the retinoid signal transduction pathway are necessary to understand the potential role of retinoids in esophageal carcinogenesis and chemoprevention.
Retinoids exert their biological effects by binding to specific nuclear retinoid receptors that belong to the steroid hormone receptor superfamily. These receptors are ligand-activated, DNA-binding, trans-acting, transcription-modulating proteins. 7 The retinoid receptors include retinoic acid (RA) receptors (RARs) and retinoid X receptors (RXRs), each of which includes three subtypes (α, β, and γ). 7 We recently demonstrated that growth inhibition of esophageal cancer cell lines by RA was associated with expression and RA-induced up-regulation of RAR-β. Cell lines that failed to express RAR-β were resistant to RA, and only these cell lines were able to form colonies in soft agar. 8 These findings implied that the in vivo status of RAR-β expression may be important for the use of retinoids in chemoprevention and chemotherapeutic clinical trials of esophageal cancer. In this study, we used in situ hybridization to detect RAR-β expression in normal, dysplastic, and malignant esophageal tissue specimens to determine its involvement in esophageal tumorigenesis.
Materials and Methods
Tissue Specimens
Tissue specimens taken from 16 normal mucosae, 30 moderate or severe dysplastic lesions, and 157 esophageal tumors were obtained from the Cancer Institute and Hospital of China and Guangzhou Nanfang Hospital, China, respectively. These specimens included 71 distant normal squamous mucosae and 29 distant columnar mucosae. All samples were routinely fixed in 10% buffered formalin, embedded in paraffin, and cut into 4-μm sections. One of each of these sections was stained with hematoxylin and eosin for classification.
In Situ Hybridization
Levels of RAR-α, -β, and -γ and RXR-α mRNAs were measured by using a previously described method of nonradioactive in situ hybridization. 9,10 The quality and specificity of the digoxigenin-labeled anti-sense and sense riboprobes were determined using Northern blotting, and the specificity of the binding of antisense riboprobes was verified using negative control sections. Briefly, the tissue sections first underwent the treatment with 0.2 N HCl and proteinase K, respectively, after deparaffinization and rehydration. The slides were then postfixed with 4% paraformaldehyde and acetylated in freshly prepared 0.25% acetic anhydride in a 0.1 mol/L triethanolamine buffer. The slides were then prehybridized at 42°C with a hybridization solution containing 50% deionized formamide, 2× standard saline citrate, 2× Denhardt’s solution, 10% dextran sulfate, 400 μg/ml yeast tRNA, 250 μg/ml salmon sperm DNA, and 20 mmol/L dithiothreitol in diethylpyrocarbonate-treated water. Next the slides were incubated in 50 μl per slide hybridization solution containing 20 ng of a freshly denatured dig-cRNA probe at 42°C for 4 hours. After that, the slides were washed for 2 hours in 2× SSC containing 2% normal sheep serum (NSS) and 0.05% Triton X-100 and then for 20 minutes at 42°C in 0.1× SSC. For color reaction, the slides were incubated for 30 minutes at 23°C in 0.1 mol/L maleic acid and 0.15 mol/L NaCl (pH 7.5, buffer 1) containing 2% NSS and 0.3% Triton X-100 and then incubated overnight at 4°C with a sheep anti-digoxigenin antibody. After washing in buffer 1 twice, the color was developed in a chromogen solution (45 μl of nitroblue tetrazolium and 35 μl of an X-phosphate solution in 10 ml of buffer 2, which consisted of 0.1 mol/L Tris, 0.1 mol/L NaCl, and 0.05 mol/L MgCl2 (pH 9.5) for 4 hours with occasional observation for color development. The slides were then mounted with a coverglass in Aqua mounting medium (Fisher, Houston, TX).
Review and Scoring of Sections
The stained sections were reviewed and scored independently by two pathologists (H.Q., X-C. X.) with an Olympus microscope. The sections were signed as positively or negatively staining. The positive staining means 10% or more epithelial cells stained positive. Statistical analysis was performed using the chi-squared (χ2) test to determine the association between normal or distant normal tissues and tumors. P values were generated using Statistica version 3.0a for Macintosh computer (StatSoft, Tulsa, OK).
Results
Specimens from 16 normal mucosae, 30 moderate to severe dysplastic lesions, and 157 esophageal tumors were used in this study (Table 1) ▶ . The normal mucosae from 16 subjects were histologically verified and were a part of a clinical chemopreventive trial in a region of northern China with a high prevalence of esophageal cancer. RAR-α, RAR-γ, and RXR-α were expressed in all 16 normal esophageal mucosae; RAR-β was expressed in 14 of the 16 samples (Table 2 ▶ and Figure 1A ▶ ).
Table 1.
Patient Clinicopathological Characteristics
| Characteristic | Normal (N = 16) | Dysplastic (N = 30) | SCC* (N = 123) | AC (N = 29) | AC-SCC (N = 5) |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median (range) | 52 (45–68) | 56 (43–68) | 57 (28–81) | 63 (39–75) | 64 (52–66) |
| Sex | |||||
| Male | 7 (44%) | 13 (43%) | 95 (77%) | 20 (69%) | 4 (80%) |
| Female | 9 (56%) | 17 (57%) | 28 (23%) | 9 (31%) | 1 (20%) |
| Tumor grade | |||||
| WD† | 42 (34%) | 3 (10%) | |||
| MD | 50 (41%) | 14 (48%) | |||
| PD | 14 (11%) | 10 (35%) | |||
| Unknown | 17 (14%) | 2 (7%) | 5 (100%) |
*SCC, Squamous cell carcinoma; AC, adenocarcinoma; AC-SCC, adenosquamous cell carcinoma.
†WD, MD, and PD: well, moderate, and poorly differentiated tumor, respectively.
Table 2.
Expression of Retinoid Receptors in Normal, Dysplastic, and Malignant Esophageal Tissues
| Receptor | % (positive/total) | |||
|---|---|---|---|---|
| Normal | Distant normal | Dysplastic | Tumor | |
| RAR-β | 87.5 (14/16) | 96.0 (96/100) | 56.7 (17/30)* | 53.5 (84/157)†‡ |
| RXR-α | 100 (16/16) | 100 (100/100) | 96.6 (28/29) | 97.5 (153/157) |
*P < 0.034 by χ2 test between normal tissues and dysplastic tissues.
†P < 0.009 by χ2 test between normal tissues and tumor tissues.
‡P < 0.00001 by χ2 test between distant normal tissues and tumor or dysplastic tissues.
Figure 1.
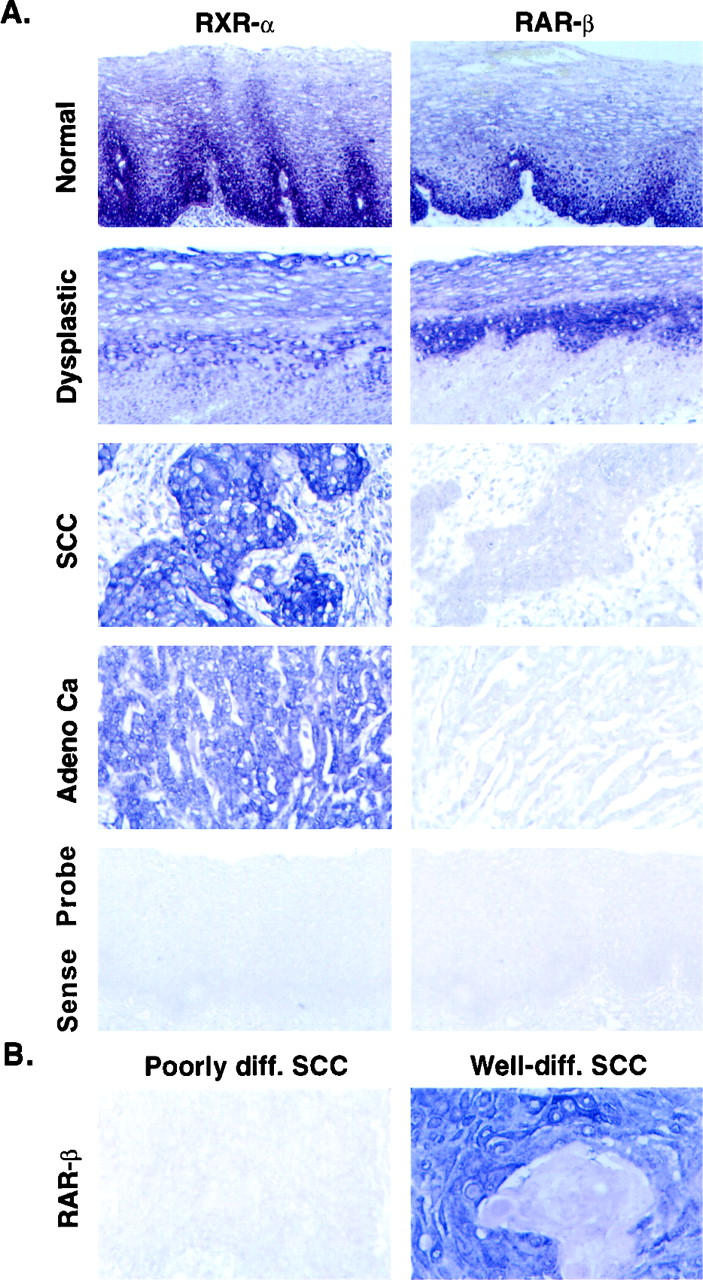
A: Localization of RAR-β and RXR-α mRNAs in consecutive sections of formalin-fixed and paraffin-embedded surgical specimens from normal and distant normal tissues as well as esophageal cancers by in situ hybridization with antisense or sense digoxigenin-labeled riboprobes. B: Differential expression of RAR-β in well-differentiated and poorly differentiated squamous cell carcinomas of human esophagus by in situ hybridization with RAR-β antisense probe.
Specimens of dysplastic lesions were obtained from 30 subjects as part of the same chemopreventive trial; these specimens showed histologically moderate to severe dysplastic changes. RAR-α, RAR-γ, and RXR-α were expressed in most (96–100%) of the specimens, whereas RAR-β was expressed in only 17 (56.7%) of 30 (Table 2 ▶ and Figure 1A ▶ ).
For the study of receptor expression in esophageal cancer specimens, we first analyzed 40 cases from northern and southern China. The preliminary results showed that RAR-α, RAR-γ, and RXR-α were expressed in almost all of these 40 specimens (data not shown). We therefore decided to exclude RAR-α and RAR-γ from subsequent study and only investigate the expression of RAR-β and RXR-α (as control) in the 157 tumors. In distant normal squamous mucosae (N = 71) and distant columnar mucosa specimens (N = 29) that were also collected from the 157 patients with tumors, 96% of the specimens expressed RAR-β. In the 157 tumors themselves, RAR-β was expressed in only 84 specimens (53.5%) (Table 2 ▶ and Figure 1 ▶ ). The differences in RAR-β expression levels were significant by χ 2 test between normal and abnormal tissues (Table 2) ▶ .
We further analyzed RAR-β expression in the three histological subtypes of esophageal cancer (ie, SCC, adenocarcinoma, and adenosquamous carcinoma). No difference in the positivity of RAR-β expression was detected among these subtypes of esophageal cancers. RAR-β expression, however, was associated with the degree of squamous cell differentiation: RAR-β mRNA was expressed in 62% (26/42) of well-differentiated SCCs and in 54% (27/50) of moderately differentiated SCCs, but in only 29% (4/14) of poorly differentiated SCCs. The degree of squamous differentiation was defined by a pathologist’s diagnosis in the individual cases and confirmed by the authors (H.Q., X-C. X.). The differences in RAR-β expression were significant between well-differentiated and poorly differentiated SCCs (P < 0.03) (Table 3 ▶ and Figure 1B ▶ ).
Table 3.
Positivity of RAR-β in Esophageal Squamous Cell Carcinomas
| Tumor differentiation | % (positive/total) |
|---|---|
| Well-differentiated SCC* | 61.9 (26/42) |
| Moderately differentiated SCC | 54.0 (27/50) |
| Poorly differentiated SCC | 28.6 (4/14) |
*SCC, Squamous cell carcinoma. P < 0.03 by χ2 test between well-differentiated and poorly differentiated SCCs.
Discussion
This is the first detailed and systematic study of RAR-β expression in normal, distant normal, dysplastic, and malignant esophageal tissues. We found that RAR-β expression was lost in nearly 50% of the invasive esophageal cancers and in 43% of the moderate to severe dysplastic lesions. These findings are similar to those of our previous studies of head and neck cancer, 9,10 non-small cell lung cancer, 11 and breast cancer. 12 The current findings in esophageal carcinogenesis expand the earlier results in other cancers and provide a new insight into the biology of carcinogenesis and RAR-β expression. The etiology of esophageal cancer in China is very different from that of all of the other studied cancers. In the United States, there is a similar etiological association between tobacco and alcohol use and esophageal and head and neck cancers. In China, the etiology of esophageal cancer seems to be related to profound nutritional deficiencies and other factors unrelated to the use of tobacco or alcohol. Therefore, our current RAR-β findings suggest that loss of this receptor is a common event across cancers of different sites and etiologies. Certain malignant/premalignant lesions (eg, colon cancer) retain RAR-β expression; however, this is the most frequently lost retinoid receptor in different epithelial cancers evaluated to date. Future study should establish the precise importance of loss of RAR-β in different cancer settings. In addition, we report for the first time that RAR-β expression is associated with esophageal squamous cell differentiation. Our previous studies with other cancers 9-12 did not show such an association with degree of squamous differentiation. This study suggests that loss of expression of RAR-β is an early event associated with esophageal carcinogenesis and squamous differentiation.
Various studies have clearly demonstrated that altered expression of retinoid receptors is associated with malignant transformation in human cells. 8-15 Altered expression of RAR-β is a common event in different types of tumors, including head and neck, lung, and breast tumors, 9-14 although lost expression of RAR-α or RAR-γ has also been reported. 15 A recent paper of ours 8 showed that the sensitivity of esophageal cancer cells to RA was correlated not only with the constitutive expression but also with RA-induced up-regulation of RAR-β. Cell lines that failed to express RAR-β were resistant to RA and could form colonies in soft agar. Two recent clinical trials of 13-cis RA in patients with advanced esophageal cancer were unsuccessful, 16,17 indicating that loss of RAR-β expression could contribute to resistance to treatment with 13-cis RA in this cancer. These results suggest that RA chemotherapy of esophageal cancer may be less effective. Nevertheless, our current data are suggestive of a potential benefit of RA in premalignant esophageal carcinogenesis, ie, RA chemoprevention. We showed that RAR-β is expressed in 96% of high-risk normal tissue specimens and 57% of dysplastic lesions. It could be inferred that these and the in vitro data suggest that RA chemopreventive approaches should target high-risk populations that express RAR-β.
Retinoids modulate epithelial cell growth and differentiation and suppress carcinogenesis in vitro and in vivo. 1-4 Physiologically, retinoids can prevent abnormal squamous differentiation of epithelial cells in nonkeratinizing tissues and are able to reverse squamous metaplasia that develops during vitamin A deficiency. 1 In animal experiments and clinical trials, retinoids can restore premalignant and malignant lesions to the normal nonkeratinizing phenotype and responsiveness to normal growth-control mechanisms, thereby suppressing carcinogenesis and preventing squamous cell carcinoma development. 1,2 These effects may be mediated by RAR-β, because lung cancer cells expressing a transfected RAR-β exhibited decreased tumorigenicity in nude mice 18 and transgenic mice expressing antisense RAR-β2 developed lung cancer. 19
Although loss of RAR-β expression appears to be a common event in esophageal squamous carcinomas, adenocarcinomas, and other cancers, 8-15 the underlying mechanism is largely unknown. 15 Loss of heterozygosity on chromosome 3p was detected in only approximately 30% of esophageal cancers, 20 and there was no correlation with RAR-β expression (our unpublished data). The RAR-β gene promoter includes a retinoic acid response element (RARE) that can be activated by retinoids through RAR-RXR heterodimers. In a recent study, Liu et al 21 found that the loss of all-trans RA-induced βRARE transcriptional activation may be responsible for the loss of RAR-β gene expression. Inactivation of nuclear retinoid receptor coactivators or activation of their corepressors may also account for altered expression of RAR-β. Finally, RAR-β expression is dependent on cellular levels of RA. Loss of RAR-β expression in premalignant oral lesions correlated with a low cellular level of RA. 22 All together this indicates that multiple mechanisms may be involved in the loss of RAR-β gene expression, which is an early event in carcinogenesis in head and neck, lung, and esophageal tissues and may be a useful intermediate biomarker in chemoprevention studies.
Acknowledgments
We thank Mr. Hong Wu for his help in cutting tissue sections.
Footnotes
Address reprint requests to Dr. Xiaochun Xu, Department of Clinical Cancer Prevention, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Box 236, Houston, TX 77030. E-mail: xxu@notes.mdacc.tmc.edu.
Supported in part by National Cancer Institute grant CA74835 and by grants from the Physicians Referral Service and the Office of the Vice President for Cancer Prevention, University of Texas M. D. Anderson Cancer Center and from the Chinese National Scientific Research Foundation (96-906-01-02) and China NSFC (39870838).
References
- 1.Lotan R: Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta 1980, 605:33-91 [DOI] [PubMed] [Google Scholar]
- 2.Moon RC, Mehta RG, Rao KVN: Retinoids and cancer in experimental animals. ed 2 Sporn MB Roberts AB Goodman DS eds. The Retinoids—Biology, Chemistry, and Medicine, 1994, :pp 537-596 Raven Press, New York [Google Scholar]
- 3.Inayama Y, Kitamura H, Shibagaki T, Usuda Y, Ito T, Nakatani Y, Kanisawa M: In vivo growth and differentiation potential of tracheal basal cells of rabbits in vitamin A deficiency. Int J Exp Pathol 1996, 77:89-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J: Highlights of the cancer chemoprevention studies in China. Prev Med 1993, 22:712-722 [DOI] [PubMed] [Google Scholar]
- 5.Blot WJ, Li JY, Taylor PR: Nutritional intervention trials in Linxian, China: supplementation with specific vitamin/mineral combination, cancer incidence and disease-specific mortality in the general population. J Natl Cancer Inst 1993, 85:1483-1492 [DOI] [PubMed] [Google Scholar]
- 6.Li JY, Taylor PR, Li B: Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst 1993, 85:1492-1498 [DOI] [PubMed] [Google Scholar]
- 7.Chambon P: A decade of molecular biology of retinoic acid receptors. FASEB J 1996, 10:940-954 [PubMed] [Google Scholar]
- 8.Xu X-C, Liu X, Tahara E, Lippman SM, Lotan R: Expression and up-regulation of retinoic acid receptor-β is associated with retinoid sensitivity and colony formation in esophageal cancer cell lines. Cancer Res 1999, 59:2477-2483 [PubMed] [Google Scholar]
- 9.Xu X-C, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R: Differential expression of nuclear retinoic acid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res 1994, 54:3580-3587 [PubMed] [Google Scholar]
- 10.Lotan R, Xu X-C, Lippman SM, Ro JY, Lee JS, Lee JJ, Hong WK: Suppression of retinoic acid receptor β in premalignant oral lesions and its upregulation by isotretinoin. N Engl J Med 1995, 332:1405-1410 [DOI] [PubMed] [Google Scholar]
- 11.Xu X-C, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, Kurie JM, Hong WK, Lotan R: Suppression of nuclear retinoic acid receptor β in non-small cell lung cancer in vivo: implications in lung cancer development. J Natl Cancer Inst 1997, 89:624-629 [DOI] [PubMed] [Google Scholar]
- 12.Xu X-C, Sneige N, Liu X, Nandagiri R, Lee JJ, Lukmanji F, Hortobagyi G, Lippman SM, Dhingra K, Lotan R: Progressive decrease in nuclear retinoic acid receptor β messenger RNA level during breast carcinogenesis. Cancer Res 1997, 57:4992-4996 [PubMed] [Google Scholar]
- 13.Hu L, Crowe DL, Rheinwald JG, Chambon P, Gudas LJ: Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res 1991, 51:3972-3981 [PubMed] [Google Scholar]
- 14.Gebert JF, Moghal N, Frangioni JV, Sugarbaker DJ, Neel BG: High frequency of retinoic acid receptor β abnormalities in human lung cancer. Oncogene 1991, 6:1859-1868 [PubMed] [Google Scholar]
- 15.de The H: Altered retinoic acid receptor. FASEB J 1996, 10:955-960 [DOI] [PubMed] [Google Scholar]
- 16.Enzinger PC, Ilson DH, Saltz LB, Martin LK, Kelsen DP: Phase II clinical trial of 13-cis-retinoic acid, and interferon-α-2a in patients with advanced esophageal carcinoma. Cancer 1999, 85:1213-1217 [DOI] [PubMed] [Google Scholar]
- 17.Slabber CF, Falkson G, Burger W, Schoeman L: 13-cis-Retinoic acid and interferon α-2a in patients with advanced esophageal cancer: a phase II trial. Invest. New Drugs 1996, 14:391–394 [DOI] [PubMed]
- 18.Houle B, Rochette-Egly C, Bradley WE: Tumor-suppressive effect of the retinoic acid receptor β in human epidermoid lung cancer cells. Proc Natl Acad Sci USA 1993, 90:985-989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berard J, Laboune F, Mukuna M, Masse S, Kothary R, Bradley WEC: Lung tumors in mice expressing an antisense RAR-β2 transgene. FASEB J 1996, 10:1091-1097 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Tokino T, Isomura M, Inazawa J, Aoki T, Mori T, Shimada M, Miura K, Suzuki K: Multistep carcinogenesis of esophageal carcinoma. Tahara E eds. Molecular Pathology of Gastroenterological Cancer. 1997, :pp 15-21 Springer-Verlag, Tokyo [Google Scholar]
- 21.Liu Y, Lee MO, Wang HG, Li Y, Hashimoto Y, Klaus M, Reed JC, Zhang XK: Retinoic acid receptor β mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol 1996, 16:1138-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X-C, Zile MH, Lippman SM, Lee JS, Lee JJ, Hong WK, Lotan R: Anti-retinoic acid (RA) antibody binding to human premalignant oral lesions, which occurs less frequently than binding to normal tissue, increases after 13-cis-RA treatment in vivo and is related to RA receptor β expression. Cancer Res 1995, 55:5507-5511 [PubMed] [Google Scholar]


