Abstract
Pulmonary fibrosis is the pathological result of a diverse group of insults. Common features of this group of diseases include chronic inflammation and immune cell activation. The pathogenesis of pulmonary fibrosis is not well defined and the prognosis is poor, highlighting the need for good animal models to elucidate the cellular and molecular events that lead to pulmonary fibrosis. This paper provides insight on a newly described model of pulmonary fibrosis using a single intratracheal challenge with fluorescein isothiocyanate (FITC). Balb-c and C57BL6 mice given intratracheal FITC develop acute lung injury followed by chronic inflammation. Significant increases in lung collagen content compared to saline-treated mice are noted at day 21 after inoculation. T-cell-deficient animals develop similar increases in lung collagen content compared to immunocompetent controls despite the abrogation of specific anti-FITC serum antibodies. Thus, the induction of fibrosis in FITC-challenged mice is not dependent on T cell immunity. Persistent chronic inflammation and acute lung injury may be the inciting events for the development of lung fibrosis in this model.
Pulmonary fibrosis is the consequence of a various insults to the lung. It is likely that a complex set of cell-cell interactions is involved in determining whether the tissue response to a given insult is healing, leading to restoration of the normal alveolar architecture, or progression to pulmonary fibrosis. Chronic inflammation and immune cell activation are both thought to play an important role in the pathological processes that lead to pulmonary fibrosis. Unfortunately, the inciting events and signals that perpetuate the immune cell activation are largely unknown. Understanding these events could lead to novel therapeutic approaches for a human disease process that has a poor prognosis and a poor response to current therapies.
Human pulmonary fibrosis often begins insidiously without a defined acute injury. The disease is chronic, progressive, and difficult to treat. Models of the human disease are necessary to examine the cellular events that lead to progressive disease. The existing animal models are poor mimics of the human disease. Many models represent acute injury and healing with fibrosis that is self-limited and not progressive, as the human disease is. It is also difficult to determine whether the fibrosis is a direct result of the injury or a response to the ensuing inflammation at a site distant from the injury in these animal models. Attempts to implicate specific immune activation in animal models of fibrosis have met with limited success. The best demonstration of involvement of specific immunity in fibrosis models comes from the hapten-mediated model using DNTP. 1-3
A recent study reported preliminary work on a novel model of fibrosis using the skin-sensitizing hapten fluorescein isothiocyanate (FITC). 4 This study demonstrated fibrosis in the lungs of both mice and rats given a single intratracheal instillation of FITC. The authors speculated that hapten-induced lymphocyte responses could explain the development of the fibrotic lesion. If this were true, this model could be a useful tool for examining the role of specific immune activation in the development of lung fibrosis.
To evaluate this model further we performed experiments to examine whether the hapten FITC given as an single intratracheal inoculation would lead to a reproducible quantifiable fibrosis in both Balb-c and C57BL-6 mice and, furthermore, to determine the role of T cell immune responses to the fluorescein hapten in the development of the fibrotic lesion.
Materials and Methods
Animals
Specific-pathogen-free male Balb-c and C57BL6 mice weighing 18 to 20 g were purchased from Jackson (Bar Harbor, ME) or Charles Rivers Laboratories (Wilmington, MA). Balb-c SCID mice and C57BL6 mice carrying a selective deletion of the recombination activating gene (RAG-KO) were purchased from Taconic (Germantown, NY). Mice were kept in isolator cages and received water and chow ad libitum. All protocols were approved by the animal care committees at the University of Michigan School of Medicine.
FITC Preparation and Experimental Protocol
FITC (14 mg, Sigma #F-7250, St. Louis, MO) was added to 10 ml of PBS in a 50-ml conical tube and vortexed. The mixture was sonicated (Sonics and Material Vibra Cell, Danbury, CT) at 50% power for 30 seconds. The mixture was transferred to multiuse vials.
Animals were anesthetized with pentobarbital. A small skin incision was made in the neck and the trachea exposed by blunt dissection. Anesthetized animals were restrained on a surgical board at approximately a 60° angle, with the trachea held in line by a surgical thread around the upper incisors. FITC or PBS (50 μl) was delivered via a 26-gauge needle inserted directly into the trachea. To ensure proper mixing and uniform FITC delivery, vials containing FITC were vortexed vigorously just before withdrawing a dose. Animals were monitored closely after surgery. Wet chow was given to all animals during the first week and animals losing 20% of body weight were given intraperitoneal injections of normal saline (1.5 ml). At various times after surgery animals were killed with intraperitoneal pentobarbital overdose. Serum, lungs, and spleen were harvested for analysis.
Determination of Hydroxyproline Content of Lung
The hydroxyproline content of mouse lung was determined by standard methods. 5 The pulmonary vasculature was perfused with PBS until free of blood. Lung tissue was excised, trimmed free of surrounding conducting airways, and homogenized in 1.8 ml 0.5 mol/L glacial acetic acid. This mixture was dried in a speed vacuum and weighed. The dried sample was redissolved sample in 2 ml of 6N HCl and hydrolyzed at 110°C overnight. Seven-μl samples were transferred to Eppendorf tubes, dried, and resuspended in citrate-acetate buffer (5% citric acid, 1.2% glacial acetic acid, 7.24% sodium acetate, and 3.4% NaOH dissolved in distilled water and adjusted to pH 6.0). Freshly prepared chloramine-T solution (0.282 g Chloramine-T (Sigma), 2 ml n-propanol, 2 ml distilled H2O QS to 20 ml with citrate-acetate buffer), 150 μl, was added and the sample allowed to stand at room temperature for 20 minutes. Freshly prepared Ehrlich’s solution (4.5 g 4-dimethylaminobenzaldehyde (Aldrich, Milwaukee, WI) dissolved in 18.6 ml n-propanol, 7.8 ml of 70% perchloric acid) 150 μl was added and the sample heated to 65°C for 15 minutes. Samples were transferred to a 96-well plate and absorbance at 550 nm was recorded. A standard curve of samples with known quantity of hydroxyproline (Sigma) was generated for each assay. Absorbance of unknown samples were compared to the standard curve and hydroxyproline content per lung was calculated.
Measurement of Anti-FITC IgG
As an antigenic target, FITC-labeled albumin (Sigma) was diluted in 0.05 Mol/L carbonate/bicarbonate buffer (pH 9.6) to 5 μg/ml 50 μl of this solution was added to each well of a 96-well NUNC ELISA plate and allowed to stand overnight at 4°C. The plate was blocked with 50 μl of blocking buffer (PBS plus 2% nonfat dry milk) for 1 hour at room temperature. The plate was washed once with wash buffer (PBS, 0.5% Tween 20, pH 7.4) before duplicate 100-μl samples of individual mouse sera (1:200 in dilution buffer/wash buffer plus 2% FBS) were added and incubated for 2 hours at room temperature. The plate was washed with wash buffer ×3 before adding 100 μl of goat anti-murine IgG-HRP (1:2000 in dilution buffer). The plate was washed with wash buffer ×3 before applying 190 μl of OPD (Sigma Fast) for 5 to 30 minutes. The reaction was quenched with 45 μl of 3 Mol/L H2SO4 before reading the absorbance at 490 nm.
T Cell Subset Depletion
In some experiments T cell subsets were depleted with monoclonal antibodies as previously described. 6 Animals were treated with intraperitoneal injections of 300 ug of GK1.5 (anti CD4) IgG on days −2, 0, 7, 14 or 500 μg of 2.43 (anti-CD8) IgG day −2, 0, 14. On day 0 the animals were given intratracheal FITC or PBS. Spleen and hilar LN cells were harvested on day 21 and subject to flow cytometry to confirm the efficacy of T cell subset depletion.
Lung Histology
Lungs were perfused free of blood and inflated with 10% formalin. The trachea was tied and the inflated lungs suspended in formalin overnight. The lung was transferred to 70% alcohol for 24 hours before embedding in paraffin. Sections 5 to 7 μm thick were placed on slides. Slides were deparaffinized and stained with hematoxylin and eosin or Masson’s trichrome.
Statistics
Data are expressed as mean ± SE. Groups were compared using a two-tailed t-test for unpaired samples using Statview 4.5. Analysis of variance (ANOVA) was used to compare multiple groups. Comparisons were deemed statistically significant when P < 0.05.
Results
Time Course of Injury after Intratracheal FITC
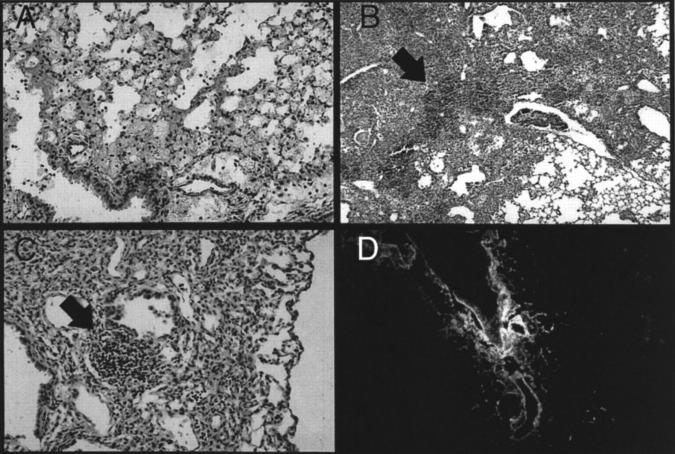
Early after instillation, Balb-c mice developed a pattern of injury consistent with acute lung injury. Alveolar wall edema, eosinophilic alveolar exudate, hemorrhage, and acute inflammation characterized the first several days after injury (Figure 1A) ▶ . Mice receiving intratracheal FITC demonstrated evidence of significant illness including ruffled fur, shivering, rapid respiratory rate, and weight loss. This pattern was present for the first week after inoculation and then resolved. After 1 week, surviving mice lost all external signs of illness and rapidly regained the weight lost during the acute phase. By day 5 to 7 the histological pattern changed to show dense consolidation with a mixture of acute inflammatory cells (PMNs) and mononuclear cells (Figure 1B) ▶ . Dense collections of mononuclear cells appeared in areas of inflammation (Figure 1B ▶ , arrow). These collections persisted in the lung throughout the 2l days examined in these experiments. At day 21 patchy areas of increased extracellular matrix deposition were noted. These areas were less cellular than seen at earlier time points; however, mononuclear cell aggregates persisted (Figure 1C ▶ , arrow).
Figure 1.
Intratracheal FITC causes acute lung injury, chronic persistent inflammation, and pulmonary fibrosis. A single dose of intratracheal FITC was administered to Balb-c mice. Mice were killed at various times and the lungs were examined for histological changes. A: Acute lung injury demonstrated by alveolar wall edema, alveolar exudate, and acute inflammation 1 day after inoculation. B: Dense consolidation with a mixture of both acute and chronic inflammatory cells on day 7. Dense collections of mononuclear cells are present (arrow). C: Residual patchy bronchocentric inflammation at day 21 and increased extracellular matrix. Collections of mononuclear cells persist (arrow). D: Deparaffinized section of lung examined directly with the fluorescent microscope demonstrates persistent FITC staining in the peribronchial areas.
The use of FITC allowed us to recognize areas of deposition based on characteristic green fluorescence. At day 21, persistent fluorescein staining was found especially in the peribronchial areas (Figure 1D) ▶ . At all time points the areas of injury and persistent inflammation demonstrated staining with fluorescein on adjacent sections. Mice that received intratracheal PBS showed no evidence of inflammation at any of the time points (data not shown).
Quantification of Lung Collagen Deposition
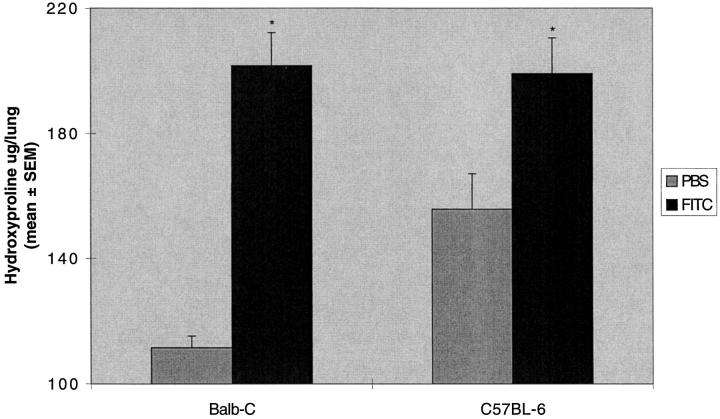
To quantify collagen production in the lung we performed a hydroxyproline assay as a surrogate marker of collagen deposition. Hydroxyproline content in the lungs of Balb-c mice treated with FITC was nearly double that of PBS treated mice (201 ± 10 versus 111 ± 4 μg/lung; Figure 2 ▶ ). Because of the relative sensitivity of various mouse strains to agents used in animal models of fibrosis, 7 we also studied the effects of intratracheal FITC on C57BL6 mice. The lung hydroxyproline content of these mice also was increased albeit at a lower level than that seen in the Balb-c mice (199 ± 11 versus 155 ± 11 μg/lung; Figure 2 ▶ ). Histological examination of the lungs of C57BL6 mice demonstrated the same pattern described above for Balb-c mice; however, a qualitative assessment of the extent of the injury and early inflammation seen on histological sections was not as severe in the C57BL6 mice. Thus, both Balb-c and C57BL6 mice developed fibrosis to an intratracheal instillation of FITC but the Balb-c mice demonstrated a larger response in terms of hydroxyproline accumulation in the lung compared to an equivalent dose delivered to a C57BL6 mouse.
Figure 2.
Increased lung hydroxyproline content after intratracheal FITC. A single dose of intratracheal FITC or PBS was administered to Balb-c or C57BL-6 mice. Mice were killed at day 21 and lungs were assayed for hydroxyproline content. Significant increases in lung hydroxyproline content were demonstrated in Balb-c and C57BL-6 mice. *P < 0.05 compared to same strain PBS control, unpaired t-test; n = 5–8 animals; representative data from seven experiments.
Quantification of Anti-FITC Immune Response
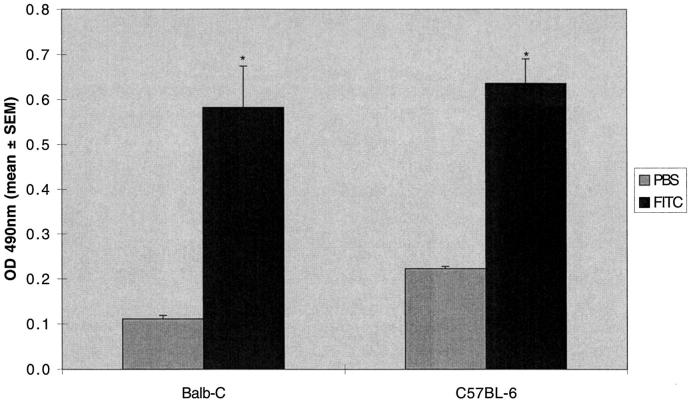
Serum from both Balb-c and C57BL6 mice was evaluated for the presence of anti-fluorescein antibodies using a qualitative ELISA. Using a secondary antibody that recognizes all murine IgG subclasses, we demonstrated significant production of fluorescein specific antibodies in both Balb-c and C57BL6 mice (Figure 3) ▶ .
Figure 3.
Increased serum anti-fluorescein IgG after intratracheal FITC. A single dose of intratracheal FITC or PBS was administered to Balb-c or C57BL-6 mice. Mice were killed at day 21 and serum was assayed for fluorescein specific antibodies. Increased production of serum anti-fluorescein IgG was noted in both Balb-c and C57BL-6 mice. *P < 0.05 compared to same strain PBS control, unpaired t-test; n = 5–8 animals; representative data from seven experiments.
T Cell Dependence
Having demonstrated that intratracheal FITC results in acute injury, followed by chronic inflammation and reproducible fibrosis and in both Balb-c and C57BL6 mice, we next sought to test the hypothesis that specific immune responses to the fluorescein hapten were the driving force for the development of fibrosis. We sought to test this hypothesis in three ways by performing the experiment in the presence of antibodies to deplete T cell subsets, in SCID mice, and in RAG-KO mice.
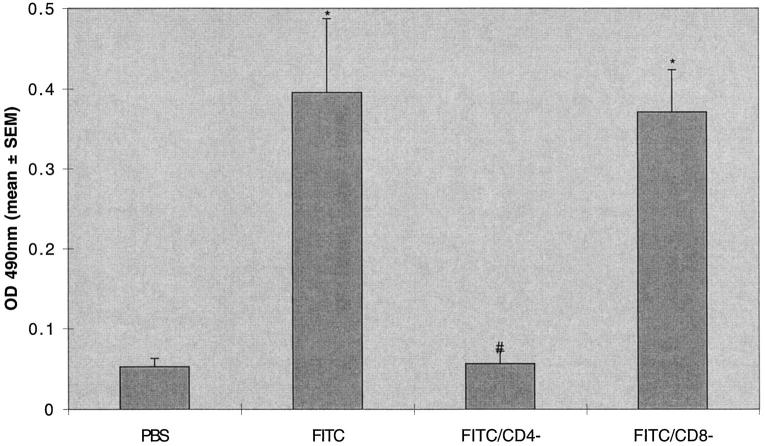
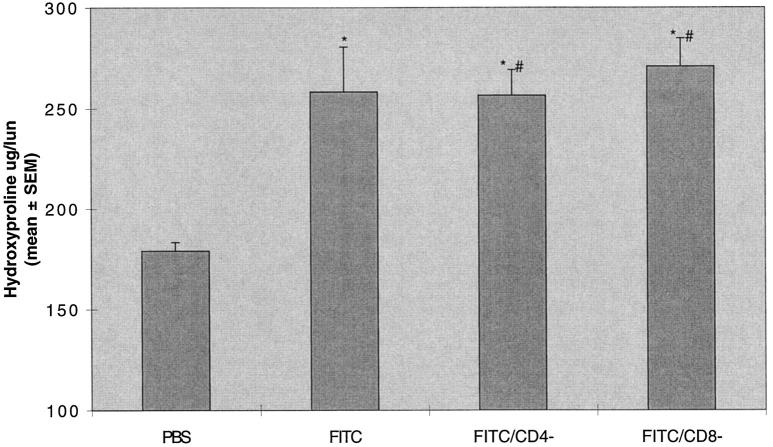
T cell subset depletion has been used to evaluate the contribution of T cell subset to immune responses to pathogens in mice. 6 In these experiments we treated animals 2 days before and at intervals after the instillation of intratracheal FITC. The treatment protocol resulted in near complete ablation (<2% of cells in lymphocyte gate staining positively; data not shown) of CD4 cells (GK1.5-treated animals) and CD8 cells (2.43-treated animals) in isolated spleen cells. The ablation of CD4 cells led to a absence of production of anti-fluorescein IgG, whereas ablation of CD8 cells had no effect on anti-fluorescein IgG production (Figure 4) ▶ . Despite depletion of CD4 cells and absence of specific anti-FITC immunoglobulin production, mice treated with intratracheal FITC developed lung fibrosis identical to mice treated with FITC and a control immunoglobulin (258 ± 22 versus 256 ± 13 μg/lung). Ablation of CD8 cells similarly had no effect on lung fibrosis (270 ± 14 μg/lung; Figure 5 ▶ ).
Figure 4.
Depletion of CD4 but not CD8 T cell subsets ablate the production of serum anti-fluorescein IgG after intratracheal FITC. Mice were treated with GK1.5 (anti CD4) or 2.43 (anti-CD8) monoclonal antibodies 2 days before and at intervals after a single dose of intratracheal FITC or PBS in Balb-c mice. Mice were killed at day 21 and serum was assayed for fluorescein-specific antibodies. Increased production of serum anti-fluorescein IgG was noted in FITC-treated mice. This production was ablated in CD4-depleted but not CD8-depleted mice. *P < 0.05 compared to PBS treated mice, ANOVA; #P < 0.05 compared to FITC-treated mice with normal T cell subsets, ANOVA; n = 5–8 animals; representative data from three experiments.
Figure 5.
Depletion of T cell subsets does not alter lung hydroxyproline content after intratracheal FITC. Mice were treated with GK1.5 (anti CD4) or 2.43 (anti-CD8) monoclonal antibodies 2 days before and at intervals after a single dose of intratracheal FITC in Balb-c mice. Mice were killed at day 21 and lungs were assayed for hydroxyproline content. Depletion of either CD4 or CD8 T cell subsets did not alter the increase in lung hydroxyproline content after intratracheal FITC. *P < 0.05 compared to PBS-treated mice, ANOVA; #P not significant compared to FITC-treated mice with normal T cell subsets, ANOVA; n = 18 to 30 animals, pooled data from three identical experiments.
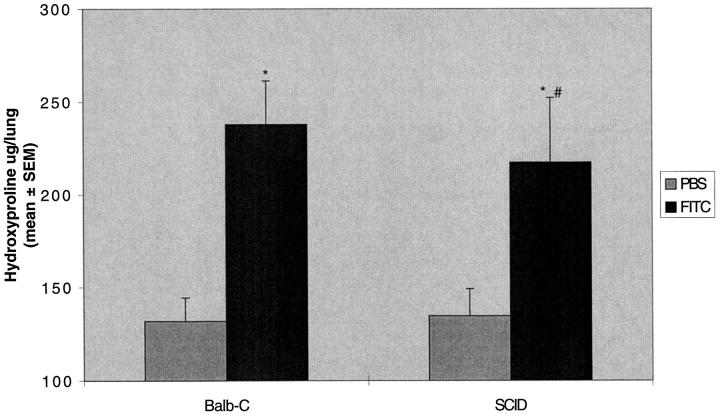
Antibody depletion of T cell subsets in these experiments may have been incomplete. Therefore to further test the hypothesis, we tested mice with congenitally deficient immune systems. SCID mice have a small epithelial thymus containing sparse lymphoid elements and a virtual absence of T and B cells in the blood and in all peripheral lymphoid tissues due to defective rearrangement of antigen receptor genes. 8 After intratracheal instillation of FITC, SCID mice did not develop antibody to fluorescein (Figure 6) ▶ . Lung hydroxyproline was not different when compared to normal Balb-c mice treated with FITC (237 ± 23 versus 217 ± 34 μg/lung; Figure 7 ▶ ). Thus, immune-deficient SCID mice develop fibrosis when given a single dose of intratracheal FITC despite the absence of specific immune response generation.
Figure 6.
SCID mice do not develop anti-fluorescein IgG after intratracheal FITC. Balb-c SCID or normal Balb-c mice were treated with a single dose of intratracheal FITC or PBS. Mice were killed at day 21 and serum was assayed for fluorescein-specific antibodies. Increased production of serum anti-fluorescein IgG was noted in normal Balb-c mice but not SCID mice. *P < 0.05 compared to PBS-treated Balb-c mice, ANOVA; #P < 0.05; compared to FITC-treated Balb-c mice, ANOVA; n = 5–8 animals.
Figure 7.
SCID mice manifest increased lung hydroxyproline content after intratracheal FITC. Balb-c SCID or normal Balb-c mice were treated with a single dose of intratracheal FITC or PBS. Mice were killed at day 21 and lungs were assayed for hydroxyproline content. Increased hydroxyproline content was noted in both Balb-c and SCID mice treated with FITC compared to PBS-treated controls. There was no difference in lung hydroxyproline content between normal Balb-c and SCID mice treated with FITC. *P < 0.05 compared to PBS-treated same strain control, ANOVA; #P not significant compared to FITC-treated Balb-c mice, ANOVA; n = 5–8 animals.
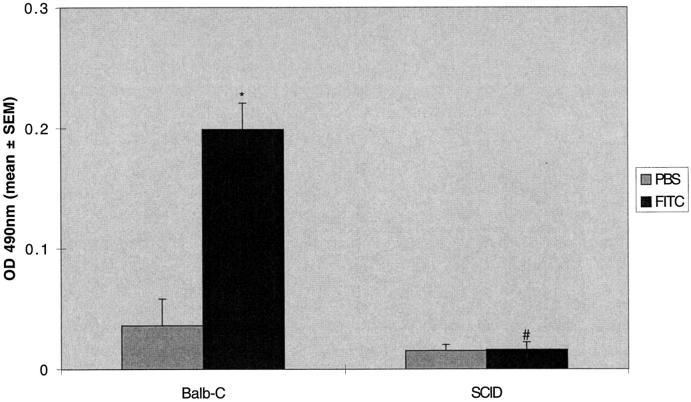
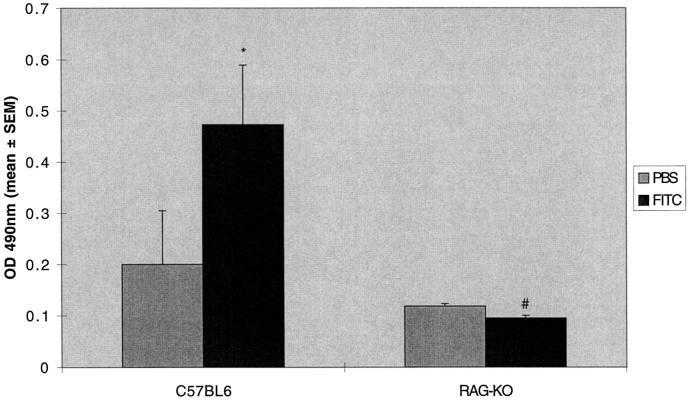
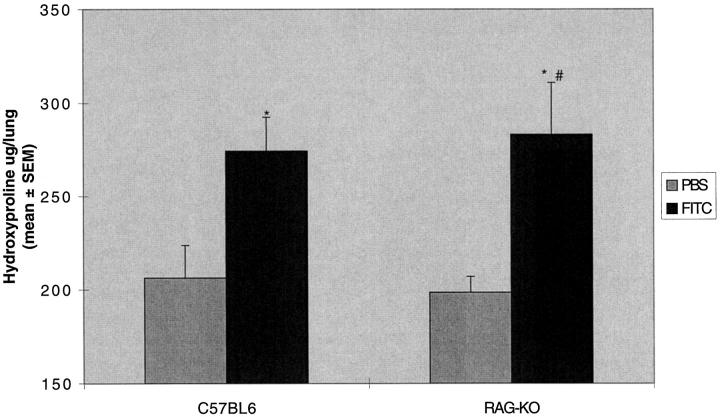
SCID mice are known to have an incomplete block in antigen receptor rearrangement, resulting in the presence of B cells, immunoglobulins, and some functional T cells, and are thus “leaky” to some immune functions. 9 We therefore tested the response to FITC in mice with a targeted deletion of recombination-activating genes necessary for T cell antigen receptor rearrangement. These mice (RAG-KO) are completely lacking in functional T cells. 10 The results were similar to the T cell subset depletion and SCID mice experiments. The RAG-KO mice were unable to mount a specific IgG response (Figure 8) ▶ , but developed lung fibrosis identical to control C57BL6 mice (283 ± 27 versus 274 ± 18 μg/lung; Figure 9 ▶ ).
Figure 8.
RAG-KO mice do not develop anti-fluorescein IgG after intratracheal FITC. RAG-KO or normal C57BL-6 mice were treated with a single dose of intratracheal FITC or PBS. Mice were killed at day 21 and serum was assayed for fluorescein-specific antibodies. Increased production of serum anti-fluorescein IgG was noted in normal C57BL-6 mice but not in RAG-KO mice. *P < 0.05 compared to PBS-treated C57BL-6 mice, ANOVA; #P < 0.05 compared to FITC-treated C57BL-6 mice, ANOVA; n = 5–8 animals.
Figure 9.
RAG-KO mice manifest increased lung hydroxyproline content after intratracheal FITC. RAG-KO or normal C57BL-6 mice were treated with a single dose of intratracheal FITC or PBS. Mice were killed at day 21 and lungs were assayed for hydroxyproline content. Increased hydroxyproline content was noted in both RAG-KO or normal C57BL-6 mice treated with FITC compared to PBS-treated controls. There was no difference in lung hydroxyproline content between normal RAG-KO or normal C57BL-6 mice treated with FITC. *P < 0.05 compared to same strain PBS control, ANOVA; #P not significant compared to FITC-treated normal C57BL-6 mice, ANOVA; n = 5–8 animals.
Discussion
These studies extend the development of a model of pulmonary fibrosis in the mouse induced by the fluorescein hapten FITC. The three major findings from our studies are: 1) a single intratracheal instillation of FITC leads to a reproducible lung injury, quantifiable increases in lung collagen content, and evidence of specific immunity to the fluorescein hapten; 2) both Balb-c and C57BL6 mice strains are susceptible to this insult; and 3) specific immunity is not required for the development of the fibrotic lesion, because mice depleted of CD4 cells or genetically immunodeficent (SCID and RAG-KO) that are challenged with FITC do not develop specific immunity to fluorescein, but have similar increases in lung collagen content compared to immunocompetent control mice. This model has interesting features and is a useful alternative to the existing rodent models of pulmonary fibrosis.
Intratracheal instillation of FITC leads to pulmonary fibrosis. Animal models of lung fibrosis are poor mimics of human disease. Human pulmonary fibrosis is often insidious in onset and progressive in nature and in many instances appears to be immunologically mediated. A new animal model of lung fibrosis that was immunologically mediated, antigen-specific, progressive, and allowed for localization of injury would be an important advance in determining the mechanism of injury in human disease. A recent report by Roberts et al 4 described preliminary studies about a promising model that would fit these requirements. Their studies showed histological evidence of fibrosis in the lung after a single instillation of FITC. 4 Our experiments extend these observations. As in the previous report, we have shown that intratracheal instillation of FITC leads to an acute lung injury followed by chronic patchy bronchocentric inflammation. Ultimately, this results in patchy pulmonary fibrosis. The injury, inflammation, and fibrosis are localized to areas of FITC deposition, confirming that these areas were involved in the initial injury. However, to be useful, a model of lung fibrosis must yield data from which the amount of fibrosis in the lung can be quantified. The widely accepted method of quantifying lung fibrosis is the measurement of hydroxyproline content of hydrolyzed lung as a surrogate marker of collagen production. Our experiments demonstrate that intratracheal FITC leads to reproducible and quantitative increases in hydroxyproline accumulation in the lung. This increase is similar in magnitude to that reported with bleomycin-induced fibrosis in mice. 11 FITC is a known skin-sensitizing agent capable of inducing specific immune responses. 12 Our studies confirm that intratracheal instillation of FITC leads to a specific immune response. The induction of anti-fluorescein-specific immunity is demonstrated by serum IgG present 21 days after a single intratracheal instillation. Consequently, a single instillation of the fluorescein hapten leads to the induction of a specific immune response and a reproducible increase in lung collagen content. The fibrotic lesion in response to FITC is similar to that seen in existing models of lung fibrosis.
Intratracheal instillation of FITC leads to pulmonary fibrosis in both Balb-c and C57BL6 mice. Strain differences in susceptibility to bleomycin have been noted previously. In response to bleomycin, C57BL6 mice are more susceptible to the development of fibrosis and Balb-c mice are relatively resistant. 7 In our experiments, both Balb-c and C57BL6 mice develop pulmonary fibrosis in response to a single dose of intratracheal FITC. In contrast to bleomycin, the degree of fibrosis in response to a given dose of FITC is worse in Balb-c mice compared to C57BL6 mice. That both strains of mice develop fibrosis in response to FITC is important, given the increasing availability of mice with targeted genetic mutations. As an example, we used mice that had a targeted deletion of the RAG gene for these studies. These mice were bred on a C57BL6 background. The ability to work with both strains of mice offers an advantage for these types of studies. In addition to quantifiable increases in collagen content, the IgG response was similar in both Balb-c and C57BL6 mice. Thus, intratracheal FITC induces a specific immune response and a reproducible quantitative lung fibrosis in two mouse strains.
FITC-induced pulmonary fibrosis does not depend on T-cell-specific immunity. The initial report on this model speculated that FITC-induced fibrosis would be driven by specific immunity to the fluorescein hapten. 4 This was a logical conclusion, especially because mice and hamsters sensitized to the skin hapten DNTP develop lung fibrosis after an intratracheal rechallenge with the hapten. 1-3 Our studies demonstrate that mice depleted of CD4 cells do not develop a specific immune response, as demonstrated by an absence of anti-fluorescein IgG. Similar results are found with genetically deficient SCID and RAG-KO mice. However, despite the abrogation of specific immunity, in each of these experiments identical increases in hydroxyproline are found when compared to immunocompetent controls. Thus, development of fibrosis in this model is independent of T-cell-specific immunity. This is a surprising result given the previous studies with other hapten models of lung fibrosis. 1-3 A recent report on bleomycin-treated mice demonstrated that T cells are not required for the induction of fibrosis, 11 suggesting the development of fibrosis in that model is not dependent on specific immunity. Similar mechanisms may be implicated in FITC-induced fibrosis.
Despite the lack of T cell dependence, FITC-induced fibrosis is still a useful addition to existing models of lung fibrosis. Bleomycin-induced fibrosis is the most commonly used lung fibrosis model in the rodent. 12-16 FITC-induced fibrosis has several potential advantages compared to bleomycin. Like most animal models of fibrosis, the bleomycin insult results in an acute injury leading to fibrosis that is transient in nature. Intratracheal FITC produces a chronic persistent inflammation, which may be due to the fluorescein deposition in the lung interstitium. Persistent inflammation could lead to an altered fibroblast phenotype that leads to increased collagen production. If the fibrotic lesion in FITC-treated mice is progressive, as suggested previously, 4 this would fit with the notion that chronic inflammation plays a role in fibrosis. Although our studies do not address the long term progression of fibrosis in the lung in response to FITC, this model is amenable to studying the role of persistent chronic inflammation and its effect on fibroblast biology. Another advantage of FITC over bleomycin is that the former confers the ability to identify areas of injury by fluorescence. This allows us to track an area of initial injury at a time remote from the injury. It may be possible to exploit this finding to evaluate the injury/repair process in the lung. The final advantage of FITC over bleomycin is that FITC is less expensive. Thus, even though the fibrotic lesion does not depend on the development of specific immunity, FITC-induced fibrosis remains a useful model with several potential advantages over existing models of lung fibrosis.
The protocol for giving FITC is complex, because this molecule is relatively insoluble. Indeed, the particulate nature of intratracheal FITC may be an important part of the injury as dose reductions below the solubility threshold result in no fibrosis in either Balb-c or C57BL6 mice (data not shown.) Changing the particulate number or surface characteristics by sonication in our experiments clearly increased the degree of acute injury. We elected to sonicate this material to allow for a better dispersion of insoluble material in the lung to give a more uniform and reproducible injury. Sonication resulted in a significant increase in early mortality for intratracheal inoculation, requiring a 50% reduction in dose compared to the previous report. 4 It may be possible to manipulate this model to maximize the specific immunity aspects (by prior sensitization) and minimize the acute lung injury (decrease the intratracheal dose or change the protocol for FITC preparation) and bring forth an immune-specific lung fibrosis. Further experiments are required to test these notions.
In summary, these experiments provide important insights into a newly described model of pulmonary fibrosis in the mouse. The single intratracheal instillation of FITC led to a reproducible, quantitative pulmonary fibrosis in both Balb-c and C57BL6 mice. Contrary to our original hypothesis, this fibrosis is not dependent on the development of specific immunity to the fluorescein hapten. However, this model has unique features that can be exploited to evaluate the role of chronic inflammation and acute lung injury in the development of lung fibrosis. These studies could provide useful insights into the mechanisms leading to human pulmonary fibrosis.
Footnotes
Address reprint requests to Paul J. Christensen, M.D., Pulmonary Section (111G), VAMC, 2215 Fuller Road, Ann Arbor, Michigan 48105.
Supported by grants from the Department of Veterans Affairs Career Development Award Merit Review (to P. J. C.) and the National Heart, Lung and Blood Institute Specialized Center of Research, Pathobiology of Fibrotic Lung Disease 1P50-HL56402–1 (to P. J. C. and G. B. T.).
Dr. Goodman’s current address is Monsanto, Mail one BB56, 700 Chesterfield Parkway, St. Louis, MO 63198.
References
- 1.Kimura R, Hu H, Stein-Streilein J: Tolerance to hapten prevents specific delayed type hypersensitivity and pulmonary interstitial fibrosis in the mouse model. Chest 1993 (suppl), 103:122–124S [DOI] [PubMed]
- 2.Kimura R, Hu H, Stein-Streilein J: Delayed-type hypersensitivity responses regulate collagen deposition in the lung. Immunology 1992, 77:550-555 [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H, Stein-Streilein J: Hapten-immune pulmonary interstitial fibrosis (HIPIF) in mice requires both CD4+ and CD8+ T lymphocytes. J Leukoc Biol 1993, 54:414-422 [DOI] [PubMed] [Google Scholar]
- 4.Roberts SN, Howie SE, Wallace WA, Brown DM, Lamb D, Ramage EA, Donaldson K: A novel model for human interstitial lung disease: hapten-driven lung fibrosis in rodents. J Pathol 1995, 176:309-318 [DOI] [PubMed] [Google Scholar]
- 5.Thrall R, McCormack J, Jack R, McReynolds R, Ward P: Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol 1979, 95:117-128 [PMC free article] [PubMed] [Google Scholar]
- 6.Beck JM, Newbury RL, Palmer BE, Warnock ML, Byrd PK, Kaltreider HB: Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J Lab Clin Med 1996, 128:477-487 [DOI] [PubMed] [Google Scholar]
- 7.Baecher-Allan CM, Barth RK: PCR analysis of cytokine induction profiles associated with mouse strain variation in susceptibility to pulmonary fibrosis. Regional Immunol 1993, 5:207-217 [PubMed] [Google Scholar]
- 8.Schuler W, Weiler IJ, A. S, Phillips RA, Rosenberg N, Mak TW, Kearney JF, Perry RP, Bosma MJ: Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell 1986, 46:963–972 [DOI] [PubMed]
- 9.Bosma MJ: B and T cell leakiness in the scid mouse mutant. Immunodefic Rev 1992, 3:261-276 [PubMed] [Google Scholar]
- 10.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE: RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992, 68:869-877 [DOI] [PubMed] [Google Scholar]
- 11.Helene M, Lake-Bullock V, Zhu J, Hao H, Cohen DA, Kaplan AM: T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol 1999, 65:187-195 [DOI] [PubMed] [Google Scholar]
- 12.Sikic BI, Young DM, Mimnaugh EG, Gram TE: Quantification of bleomycin pulmonary toxicity in mice by changes in lung hydroxyproline content and morphometric histopathology. Cancer Res 1978, 38:787-792 [PubMed] [Google Scholar]
- 13.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P: Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate: functional and morphological studies. J Exp Med 1987, 166:1654-1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiser KM, Last JA: Pulmonary fibrosis in experimental acute respiratory disease. Am Rev Respir Dis 1981, 123:58-63 [DOI] [PubMed] [Google Scholar]
- 15.Brown RF, Drawbaugh RB, Marrs TC: An investigation of possible models for the production of progressive pulmonary fibrosis in the rat: the effects of repeated intratracheal instillation of bleomycin. Toxicology 1988, 51:101-110 [DOI] [PubMed] [Google Scholar]
- 16.Adamson IY, Bowden DH: The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 1974, 77:185-197 [PMC free article] [PubMed] [Google Scholar]