Abstract
Neuroblastoma is the second most common solid tumor occurring in children. Amplification of the MYCN oncogene is associated with poor prognosis. To identify neuroblastoma tumors with MYCN amplification, we studied the number of copies of MYCN in interphase cells by fluorescence in situ hybridization in 20 neuroblastoma patients. MYCN amplification appeared in 7 tumor specimens. Interphase and metaphase studies showed a tumor cell population with both forms of amplification, double minutes and homogeneously staining regions, in two patients. These patients showed a smaller tumor cell subpopulation with the presence of more than one homogeneously staining region, suggesting that gene amplification was undergoing karyotype evolution.
Neuroblastoma (NB) is a pediatric solid tumor arising from the postganglionic sympathetic nervous system and is a leading cause of death in infants below 1 year of age. One of its most remarkable features is a striking clinical heterogeneity. Characterization of genetic alterations in NB have been helpful in predicting clinical outcome and stratifying therapy for the various genetic changes. MYCN amplification has proven to be an independent prognostic factor for identifying rapid tumor progression and predicting a very poor prognosis irrespective of age and clinical stage. 1-3 The MYCN gene is a cellular proto-oncogene of the MYC family of transcription factors. MYCN maps to the short arm of chromosome 2 at band 2p24. 4 Although its role in oncogenesis is thought to involve extrachromosomal elements called double minutes (DMs), it may also be integrated as reiterated amplicons within a chromosomal site as a homogeneously staining region (HSR). Amplicons in NBs range from 350 to 2000 kb with a consensus commonly amplified domain defined as 130 kb. 5 Although the precise details of the amplification mechanism are poorly understood, it is thought that a large region of genomic DNA, including the entire MYCN gene, becomes amplified initially as extrachromosomal DMs, 6-8 possibly persisting in this form. However, occasionally extrachromosomal DMs become linearly integrated into a chromosome, forming one HSR by a mechanism such as unequal sister chromatid exchange. 9 Brodeur 9 suggests that MYCN amplification is an intrinsic biological property of a subset of aggressive NBs and that tumors without amplification at diagnosis rarely, if ever, develop this abnormality subsequently. It is generally accepted that amplification of the MYCN oncogene is relevant to prognosis, 10,11 and most current treatment protocols require examination of MYCN amplification in NB tumors before treatment begins. 12 Previous studies using fluorescence in situ hybridization (FISH) analysis of MYCN have demonstrated its utility for NB tumors. 13-17 FISH directly reveals MYCN copy number on a per-cell basis and also shows whether amplification is present as HSR or DMs. The observation of tumors containing both cytological forms of gene amplification may indicate either a transition from DMs to HSRs or independent generation of both types of structures, with subsequent cell selection favoring the predominance of one form.
Materials and Methods
Twenty tumor samples from patients with clinical NB diagnosis were studied. Patient data are summarized in Table 1 ▶ . Neuroblastoma tumors were classified according to the International Staging System (INSS). 18 The histopathological evaluation was done by Shimada classification. 19 Among the 20 samples, 17 were obtained at diagnosis and 3 after chemotherapy. Samples were obtained by biopsy or surgery from the primary tumor in 17 cases, and in 3 cases from metastatic bone marrow aspirates. Malignant cell percentage in the bone marrow aspirates exceeded 50%. For FISH analysis the suspensions were performed from tumor tissue by mechanical and enzymatic disaggregation, and direct preparation for analysis of tumor or bone marrow cells was done using modifications of techniques described. 20,21 We used a MYCN DNA probe, digoxigenin-labeled; detection was obtained with fluorescein-conjugated sheep antibodies to digoxigenin (Oncor, Gaithersburg, MD), followed by counterstaining in propidium iodide solution containing antifade. We analyzed the cells in a Zeiss fluorescence photomicroscope equipped with fluorescein filter. A minimum of 100 interphase nuclei was scored per sample except for case 3, where study of only 22 nuclei was possible. Copy number was determined by counting and averaging the number of fluorescence signals per interphase nucleus. In cells with high levels of gene amplification, accurate scoring was impossible, then we included these cells, therefore, in a class of more than 50 copies. 14 We only studied slides with hybridization efficiencies higher than 90%. MYCN amplification was considered as number of copies per nuclei exceeding 10. 10 We analyzed 2000 cells from case 8 to find metaphase nuclei with both cytological structures of gene amplification.
Table 1.
Clinical and Biological Features of 20 Neuroblastoma Cases
| Patient/tumor | Tumor specimens/sex | Age at diagnosis (months) | Primary tumor | Stage system (INSS) | Shimada classification | MYCN amplification | Treatment | Status | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | CT | XRT | |||||||||
| 1 /NB | TF /M | 21 | adrenal | 3 | U | + | n.d. | + | + | DOD | 12 |
| 2 /NB | TF /F | 13 | adrenal | 1 | F | − | + | n.d. | n.d. | DF | 38 |
| 3 /NB | TF /F | 9 | paraspinal | 3 | F | − | n.d. | + | n.d. | DF | 36 |
| 4 /GNB | TF* /F | 43 | mediastinum | 3 | n.d. | − | + | + | n.d. | DF | 38 |
| 5 /NB | TF /F | 30 | adrenal | 3 | U | + | + | + | + | DOD | 17 |
| 6 /NB | TF /M | 35 | adrenal | 3 | U | + | + | + | + | DOD | 13 |
| 7 /NB | TF* /M | 11 | cervical | 4 | n.d. | − | + | + | n.d. | DF | 31 |
| 8 /NB | BM /M | 12 | adrenal | 4 | n.d. | + | n.d. | + | n.d. | DOD | 12 |
| 9 /NB | TF /M | 5 | paraspinal | 3 | F | − | + | + | n.d. | DF | 17 |
| 10 /NB | TF /M | 10 | adrenal | 2 | F | − | + | + | n.d. | DOI | 5 |
| 11 /NB | TF* /M | 15 | adrenal | 3 | F | − | + | + | n.d. | DF | 36 |
| 12 /GN | TF /F | 26 | paraspinal | 2 | n.d. | − | + | n.d. | n.d. | DF | 13 |
| 13 /NB | TF /M | 84 | adrenal | 3 | n.d. | − | n.d. | + | n.d. | T | 13 |
| 14 /NB | TF /M | 12 | adrenal | 3 | n.d. | + | n.d. | + | n.d. | DOD | 7 |
| 15 /NB | TF /F | 39 | adrenal | 3 | U | + | + | + | n.d. | LF | 7 |
| 16 /NB | BM /M | 30 | adrenal | 4 | n.d. | − | n.d. | + | n.d. | DF | 11 |
| 17 /NB | BM /M | 4 | adrenal | 4 | n.d. | + | n.d. | + | + | DOD | 5 |
| 18 /GNB | TF /M | 36 | mediastinum | 1 | n.d. | − | + | n.d. | n.d. | DF | 9 |
| 19 /GNB | TF /F | 82 | mediastinum | 1 | n.d. | − | + | n.d. | n.d. | DF | 5 |
| 20 /NB | TF /F | 24 | adrenal | 3 | F | − | + | + | n.d. | DF | 7 |
NB, neuroblastoma; GNB, ganglioneuroblastoma; GN, ganglioneuroma; TF, tumor fragment obtained at diagnosis; BM, bone marrow; M, male; F, female; n.d., not done; F, favorable; U, unfavorable; CT, chemotherapy; XRT, radiation therapy; DOD, died of disease; DF, disease-free; DOI, died of infection; LF, lost follow-up; T, in treatment.
*Sample was obtained after diagnosis.
Results and Discussion
We studied twenty tumors, 17 at diagnosis and 3 after therapy. MYCN amplification was seen in 7 of 17 patients at diagnosis (41%), a frequency surpassing that shown by the data of other authors, 22 and resulting either from the number of tumors studied or their stage of progression. Six patients with MYCN amplification had progressive and fatal disease, despite intensive therapy (Table 1) ▶ . All patients presenting MYCN amplification had stage 3 or 4 INSS classification. 18 Among the 13 tumor specimens without MYCN amplification, 5 were stage 1 or 2 (INSS) and 8 were stage 3 or 4 (INSS). Within follow-up time limits, just one patient (case 10, stage 2) died due to infection 5 months after diagnosis. Eleven are alive and disease-free, and one patient continues under therapy. Thus, MYCN amplification was correlated with rapid tumor progression and a poor outcome independent of tumor clinical stage or age of the patient at diagnosis.
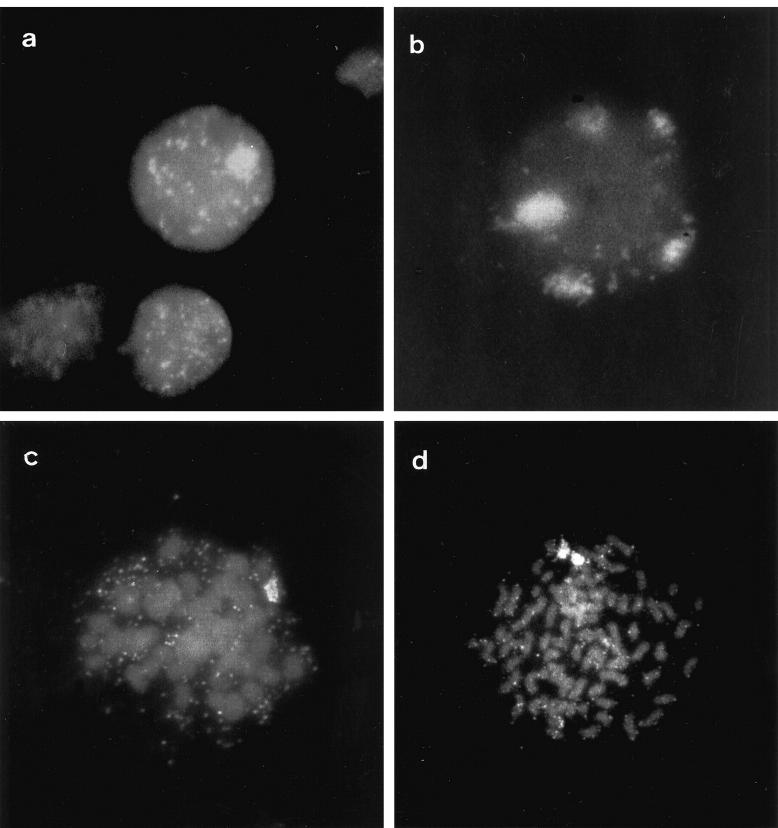
Table 2 ▶ shows cytogenetic data of the tumors with MYCN amplification. We found the MYCN gene most frequently amplified episomally in DMs, as seen in previous reports. 14,23 Intrachromosomal amplification as HSR is rare in comparison to DMs in NB, and is more often observed in vitro 24,25 in cell lines rather than in primary tumors. In three tumor samples we saw beside DMs, cells with only one HSR (cases 1, 6, and 8); the other two (cases 1 and 8) showed both structures in the same cells (Figure 1a) ▶ . NB cell lines have rarely been described as having two cell subpopulations, one with DMs and the other with HSR. 24,26 However, the same phenomenon was reported for other cell lines. 27-30 Although seldom observed in the same cell, 28,30 when present in the same cell population, they are mutually exclusive in individual cells. 26,29,31 We also observed cells with DMs and more than one HSR (Figure 1b) ▶ . Presence of 2 or 3 HSRs per nucleus has been seen in NB cell lines, 4,32,33 but not in direct preparations from tumors. We believe that our observations correspond to what happens in vivo because we did not culture our tumor samples in any way, thus avoiding in vitro selection bias. In an in vitro transformed mouse salivary gland epithelial cell line, DMs were observed in 100% of cells at an early passage level. 29,34 After approximately 17 in vitro passages a subpopulation of cells devoid of DMs appeared, whereas in DM-negative cells one HSR was present. 29
Table 2.
Copy Number of DMs per Cell, Interphase Nuclei with HSRs, and Nuclei with Both Structures
| Patient no. | DMs | HSR | DMs + 1 HSR | DMs + 2 HSRs | DMs + 5 HSRs | Total no. of cells analyzed |
|---|---|---|---|---|---|---|
| 1 | 93 | 5 | 4 | 1 | 1 | 104 |
| 5 | 100 | 0 | 0 | 0 | 0 | 100 |
| 6 | 96 | 4 | 0 | 0 | 0 | 100 |
| 8 | 94 | 2 | 4 | 0 | 0 | 100 |
| 14 | 100 | 0 | 0 | 0 | 0 | 100 |
| 15 | 100 | 0 | 0 | 0 | 0 | 100 |
| 17 | 100 | 0 | 0 | 0 | 0 | 100 |
DMs, double minutes; HSR, homogeneously staining region.
Figure 1.
Fluorescent in situ hybridization with probe MYCN. a: Interphase nucleus with DMs and one HSR from case 1. b: Interphase nucleus with DMs and five HSRs from the same patient. c: Metaphase cell with DMs and one HSR from case 8. d: Metaphase cell from the same case with DMs and two HSRs.
In case 8, we demonstrated in metaphase nuclei the presence of both structures in the same cell. The results of our analysis of 2000 nuclei from this case are shown in Table 3 ▶ and Figures 1c and 1d ▶ . MYCN amplification in these tumors (cases 1, 6, and 8) presented cells where both forms of amplification (DMs and HSR) are detectable by interphase FISH analyses. We have seen cells where HSR appears as a distinct domain of signals together with DMs as double-specked signals throughout the same nuclei. These observations confirm the putative mechanism of gene amplification which proposes extrachromosomal DMs reintegrating into another chromosomal site and amplifying by a mechanism such as unequal sister chromatid exchange, generating a HSR. 33,35 The occurrence of both cytological structures in the same cell, as observed, indicates that a transition may occur from DM to HSR bearers; the alternative hypothesis that both cytological forms exist, with cell selection eventually favoring HSR, is less likely. However, the gene amplification mechanism is not yet well understood. High levels of gene amplification in tumors of patients who died after intensive therapy may account for the presence of more than one integration site, and we are tempted to conclude that this contributed to the rapid cytogenetic evolution of gene amplification.
Table 3.
Cytogenetic Forms of Gene Amplification in Metaphase and Interphase Nuclei of Case 8
| Nucleus | DMs | HSR | DMs + 1 HSR | DMs + 2 HSRs | Total of 2000 cells analyzed |
|---|---|---|---|---|---|
| Interphase | 1974 | 9 | 11 | 0 | 1994 |
| Metaphase | 4 | 0 | 1 | 1 | 6 |
| Total | 1978 | 9 | 12 | 1 | 2000 |
DMs, double minutes; HSR, homogeneously staining region.
Acknowledgments
We thank Dr. Jeremy Squire for his valuable suggestions.
Footnotes
Address reprint requests to Silvia Regina Caminada de Toledo, Disciplina de Genética, Departamento de Morfologia, Universidade Federal de São Paulo, Rua Botucatu, 740, Pavilhão Leitão da Cunha, Vila Clementino, São Paulo, Brazil. E-mail: srcp.morf@epm.br.
Supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, Brazil (CNPq), Coordenação de Aperfeio̧amento de Pessoal de Nível Superior, Brazil (CAPES), Financiadora de Estudos e Projetos, Brazil (FINEP), and Grupo de Apoio 20 Adolescente e a Criança com Câncer, Brazil (GRAACC).
References
- 1.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM: Amplification of N-myc in untreated human neuroblastoma correlates with advanced disease stage. Science 1984, 224:1121-1124 [DOI] [PubMed] [Google Scholar]
- 2.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D: Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med 1985, 313:11111-11116 [DOI] [PubMed] [Google Scholar]
- 3.Look AT, Hayes FA, Shuster JJ, Douglas EC, Catleberry RD, Bowman LC, Smith EI, Brodeur GM: Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol 1991, 9:581-591 [DOI] [PubMed] [Google Scholar]
- 4.Schwab M, Varmus HE, Bishop JM, Grzeschik KH, Naylor SL, Sakaguchi AY, Brodeur G, Trent J: Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature 1984, 308:288-291 [DOI] [PubMed] [Google Scholar]
- 5.Hiemstra J, Schneider S, Brodeur GM: High-resolution mapping of the N-myc amplicon core domain in neuroblastomas. Prog Clin Biol Res 1994, 385:51-57 [PubMed] [Google Scholar]
- 6.Brodeur GM, Seeger RC: Gene amplification in human neuroblastomas: basic mechanisms and clinical implications. Cancer Genet Cytogenet 1986, 19:101-111 [DOI] [PubMed] [Google Scholar]
- 7.Amler LC, Schwab M: Amplified N-myc in human neuroblastoma cells is often arranged as clustered tandem repeats of differentlys recombined DNA. Mol Cell Biol 1989, 9:4903-4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider S, Hiemstra J, Zehnbauer B, Taillon-Miller P, Paslier D, Vogelstein B, Brodeur GM: Isolation and structural analysis of a 1.2-megabase N-myc amplicon from neuroblastoma. Mol Cell Biol 1992, 12:5563-5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodeur GM: Molecular basis for heterogeneity in human neuroblastomas. Eur J Cancer 1995, 31A:505-510 [DOI] [PubMed] [Google Scholar]
- 10.Seeger RC, Wada R, Brodeur GM, Moss TJ, Bjork RL, Sousa L, Slamon DJ: Expression of N-myc by neuroblastomas with one or multiple copies of the oncogene. Prog Clin Biol Res 1988, 271:41-49 [PubMed] [Google Scholar]
- 11.Brodeur GM: Neuroblastoma: clinical significance of genetic abnormalities. Cancer Surv 1990, 9:673-688 [PubMed] [Google Scholar]
- 12.Cohn SL, Look AT, Joshi VV, Holbrook T, Salwen H, Chagnovich D, Chester L, Rowe ST, Valentine MB, Komuro H, Castleberry RP, Bowman LC, Rao PV, Seeger RC, Brodeur GM: Lack of correlation of n-myc gene amplification with prognosis in localized neuroblastoma: A Pediatric Oncology Group study. Cancer Res 1995, 55:721-726 [PubMed] [Google Scholar]
- 13.Cohen PS, Seeger RC, Triche TJ, Israel MA: Detection of n-myc gene expression in neuroblastoma tumors by in situ hybridization. Am J Pathol 1988, 131:391-397 [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro DN, Valentine MB, Rowe ST, Sinclair AE, Sublett JE, Roberts WM, Look AT: Detection of n-myc gene amplification by fluorescence in situ hybridization. Am J Pathol 1993, 142:1339-1346 [PMC free article] [PubMed] [Google Scholar]
- 15.Leong PK, Thorner P, Yeger H, Ng K, Zhang Z, Squire J: Detection of MYCN gene amplification and deletions of chromosome 1p in neuroblastoma by in situ hybridization using routine histologic sections. Lab Invest 1993, 69:43-50 [PubMed] [Google Scholar]
- 16.Misra DN, Dickman PS, Yunis EJ: Fluorescence in situ hybridization (FISH) detection of MYCN oncogene amplification in neuroblastoma using paraffin-embedded tissues. Diagn Mol Pathol 1995, 4:128-135 [DOI] [PubMed] [Google Scholar]
- 17.Squire JA, Thorner P, Marrano P, Parkinson D, Ng YK, Gerrie B, Chilton-MacNeill S, Zielenska M: Identification of MYCN copy number heterogeneity by direct FISH analysis of neuroblastoma preparations. Mol Diagnosis 1996, 1:281-289 [DOI] [PubMed] [Google Scholar]
- 18.Brodeur GM, Seeger RC, Barrett A, Berthold F, Castleberry RP, D’Angio G, De Bernardi B, Evans AE, Favrot M, Freeman AI, Haase G, Hartmann O, Hayes FA, Helson L, Kemshead J, Lampert F, Ninane J, Ohkawa H, Philip T, Pinkerton CR, Pritchard J, Sawada T, Siegel S, Smith EI, Tsuchida Y, Voute PA: International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol 1988, 6:1874-1881 [DOI] [PubMed] [Google Scholar]
- 19.Shimada H, Chatten J, Newton WA, Jr, Sachs N, Hamoudi AB, Chiba T, Marsden HB, Misugi K: Histopathologic prognostic factors in neuroblastic tumors: Definition of subtypes of ganglioneuroblastoma and age-linked classification of neuroblastomas. J Natl Cancer Inst 1984, 73:405-416 [DOI] [PubMed] [Google Scholar]
- 20.Mandhal N: Methods in solid tumour cytogenetics. Human Cytogenetics. A Practical Approach. Volume II, Malignancy and Acquired Abnormalities, 2nd ed. Edited by Rooney DE, Czepulkowski BH. New York, IRL Press, 1992, pp 155–187
- 21.Czepulkowski BH, Bhatt B, Rooney DE. Basic techniques for the preparation and analysis of chromosomes from bone marrow and leukaemic blood. Human Cytogenetics. A Practical Approach. Volume, II Malignancy and Acquired Abnormalities, 2nd ed. Edited by Rooney DE, Czepulkowski BH. New York, IRL Press, 1992, pp 1–25
- 22.Brodeur GM: Clinical and biological aspects of neuroblastoma. Vogelstein B Kinzler KW eds. The Genetic Basis of Human Cancer. 1998, :pp 691-711 McGraw-Hill, New York [Google Scholar]
- 23.Brodeur GM, Fong C: Molecular biology and genetics of human neuroblastoma. Cancer Genet Cytogenet 1989, 41:153-174 [DOI] [PubMed] [Google Scholar]
- 24.Brodeur GM, Green AA, Hayes FA, Williams KJ, Williams DL, Tsiatis AA: Cytogenetic features of human neuroblastomas and cell lines. Cancer Res 1981, 41:4678-4686 [PubMed] [Google Scholar]
- 25.Windle BE, Wahl GM: Molecular dissection of mammalian gene amplification: new mechanistic insights revealed by analyses of very early events. Mutation Res 1992, 276:199-224 [DOI] [PubMed] [Google Scholar]
- 26.Balaban-Malenbaum G, Gilbert F: Double minute chromosomes and the homogeneously staining regions in chromosomes of a human neuroblastoma cell line. Science 1977, 198:739-741 [DOI] [PubMed] [Google Scholar]
- 27.Levan A, Levan G, Mandahl N: A new chromosomes type replacing the double minutes in a mouse tumor. Cytogenet Cell Genet 1978, 20:12-23 [DOI] [PubMed] [Google Scholar]
- 28.Quinn LA, Moore GE, Morgan RT, Woods LK: Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res 1979, 39:4914-4924 [PubMed] [Google Scholar]
- 29.Cowell JK: A new chromosome region possibly derived from double minutes in an in vitro transformed epithelial cell line. Cytogenet Cell Genet 1980, 27:2-7 [DOI] [PubMed] [Google Scholar]
- 30.George DL, Powers VE: Amplified DNA sequences in Y1 mouse adrenal tumor cells: association with double minutes and localization to a homogeneously staining chromosomal region. Proc Natl Acad Sci USA 1982, 79:1597-1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George DL, Francke U: Homogeneously staining chromosome regions and double minutes in a mouse adrenocortical tumor cell line. Cytogenet Cell Genet 1980, 28:217-226 [DOI] [PubMed] [Google Scholar]
- 32.Balaban G, Gilbert F: Homogeneously staining regions in direct preparations from human neuroblastomas. Cancer Res 1982, 42:1838-1842 [PubMed] [Google Scholar]
- 33.Amler LC, Shibasaki Y, Savelyeva L, Schwab M: Amplification of the n-myc gene in human neuroblastomas: tandemly repeated amplicons within homogeneously staining regions on different chromosomes with the retention of single copy gene at the resident site. Mutation Res 1992, 276:291-297 [DOI] [PubMed] [Google Scholar]
- 34.Cowell JK: Chromosome abnormalities associated with salivary gland epithelial cell lines transformed in vitro and in vivo with evidence of a role for genetic imbalance in transformation. Cancer Res 1981, 41:1508-1517 [PubMed] [Google Scholar]
- 35.Brodeur GM, Hogarty MD: Gene amplification in human cancers: biological and clinical significance. Vogelstein B Kinzler KW eds. The Genetic Basis of Human Cancer. 1998, :pp 161-172 McGraw-Hill, New York [Google Scholar]