Abstract
The types of neurotrophin receptors that are expressed in neuroblastomas have different prognostic implications; trkA is a marker of good prognosis, whereas trkB expression is associated with poor prognosis. This suggests that either the signaling that is mediated via these receptors modulates the biological features of neuroblastoma cells differently, or that distinct lineages of sympathoadrenal precursors have been transformed. In this report, we evaluate the biological effects after activation of trkA or trkB by their major ligands in SH-SY5Y human neuroblastoma cells. Both trkA and trkB induce differentiation, inhibit growth, and promote the survival of cells under conditions of nutrient deprivation. However, the up-regulation of insulin-like growth factor-II (IGF-II) expression is a predominant feature of trkA activation by nerve growth factor (NGF). The growth inhibition induced by blocking the insulin-like growth factor-I receptor suggests that IGF-II is a component of the effector mechanism of trkA activation by NGF in trkA-transfected cells. Although trkA and trkB expression is associated with different prognoses in neuroblastoma, our study indicates that the effects mediated by these receptors in vivo may be quite similar for certain subsets of neuroblastomas.
Neuroblastoma (NB), an embryonal tumor arising in the sympathetic nervous system, is characterized by the presence of subsets with clearly different clinical behavior. Prognostic markers include N-myc amplification, 1p deletion, DNA ploidy, and histopathology. The type of neurotrophin (NT) receptor present has also been found to have prognostic significance. 1,2 Previous analyses have shown that tumors expressing high levels of trkA are associated with favorable clinical outcomes, whereas those expressing full-length trkB mRNA and its primary ligand brain-derived neurotrophic factor (BDNF) are usually associated with a poor prognosis and N-myc amplification. 3-5 The different clinical implications of the NT receptor types in NB strongly suggest that the activation of trkA and trkB may exert different influences on the biological behavior of the transformed cells. Another possibility is that the transformed cells are derived from distinct lineages of neuroblasts.
NTs exert profound effects on the biological behavior of neuronal cells during the processes of development, differentiation, and survival in the central and peripheral nervous systems, by binding to at least two types of receptor. The nerve growth factor receptor (LNGFR) is a member of the tumor necrosis factor family of receptors, and trk is a member of the tyrosine kinase family of receptors. 6,7 The tyrosine kinase receptors selectively bind to their cognate ligands: trkA for nerve growth factor (NGF) and NT-3; trkB for BDNF, NT-4/5, and NT-3; and trkC for NT-3. 8-10 Because NTs and trks are expressed in the central and peripheral nervous system tumors of childhood, there have been many studies regarding the role of these receptor-ligand interactions in these tumors. 11,12
Many cell lines provide systems that have proved useful in the investigation of the major biological aspects of NB, because they retain the potential to respond to various biological response modifiers and growth factors. 13 In our study, we used a tetracycline-regulatable vector system, in which two components of the tetracycline-controlled transactivator (tTA) promotor system have been organized in a single vector, 14 to investigate the role of the activation of trkA and trkB in the SH-SY5Y (SY5Y) human neuroblastoma cell line. SY5Y is a neuronal subclone of the SK-N-SH cell line, which was established from a bone marrow aspirate of a thoracic NB. 15 SY5Y expresses low levels of trkA and p75 LNGFR and lacks endogenous expression of trkB or BDNF. 16
We examined how conditional activation of trkA and trkB affected the growth, survival, and differentiation of SY5Y cells. The effects of the activation of trkA and trkB signal transduction paths were quite similar for growth, survival, and differentiation; however, distinct differences were identified in the lineage-dependent markers. An increase in insulin-like growth factor-II (IGF-II) expression induced by trkA activation was reminiscent of the extra-adrenal NBs of infancy, which express high levels of trkA and show biochemical evidence of chromaffin differentiation. 17 Furthermore, the inhibition of growth by blocking of the IGF-I receptor suggested that up-regulation of IGF-II expression is a component of the effector mechanism of trkA activation by NGF. Despite the different clinical implications, the biological effects mediated by trkA and trkB overlap in the case of SY5Y cells.
Materials and Methods
Construction of Vectors and the Establishment of Stable Transfectants
Tetracycline-regulated vector, pBPSTR1, 14 was obtained as a gift from Dr. Steven Reeves. The vector contained the puromycin resistance gene. Full-length human trkA cDNA (2.7 kb) was subcloned into NotI, and a 3.1-kb fragment of rat trkB spanning a full coding region, which was generated by NotI-HpaI digestion of rat trkB cDNA (4.7 kb), was subcloned into the NotI-PmeI site of pBPSTR1. Stable transfectants were obtained from the human neuroblastoma cell line SH-SY5Y by transfection using lipofectAMINE (Life Technologies, Gaithersburg, MD). Subsequent selection was done with 0.5 μg/ml puromycin (Sigma, St. Louis, MO). Cells were grown in RPMI 1640 (Mediatech, Herndon, VA) containing 10% fetal bovine serum, 2 mmol/L glutamine, and antibiotics. The screening for tetracycline-dependent regulation of transfected gene expression was performed by Northern blot analysis. For the Northern blot analysis, total RNA was extracted using a RNeasy kit (Quiagen, Santa Clarita, CA) from cells grown in the presence (1 μg/ml) or absence of tetracycline (Sigma) for at least 2 days. Ten micrograms of RNA was electrophoresed in 1.2% formaldehyde agarose gel, transferred to a nitrocellulose membrane (Nytran, Schleicher and Schuell, Keene, NH), and hybridized at 42°C with [α-32P]dCTP-labeled trkA (2.7 kb EcoRI fragment) or trkB (3.1 kb) purified inserts in solutions containing 50% formamide, 1 mol/L NaCl, 10% dextran sulfate, 1% sodium dodecyl sulfate (SDS), and 250 μg/ml of salmon sperm DNA. Membranes were washed twice with 2× standard saline citrate (SSC), 0.1% SDS solution at room temperature for 15 minutes, and once with 0.5× SSC, 0.1% SDS for 15 minutes at 50°C. They were then exposed to Kodak XAR film at −70°C.
Functional Assessment of Signaling
To assess trkA and trkB autophosphorylation after NT stimulation, cells were plated in 100-mm tissue culture dishes (1 × 10 6 cells/dish) and cultured in the presence (1 μg/ml) and absence of tetracycline for 3 days. They were then harvested after stimulation with 100 ng/ml of 2.5S mouse NGF (Upstate Biotechnology, Lake Placid, NY) or recombinant human BDNF (Promega, Madison, WI) for 5 minutes. For the analysis of the early response gene profile after NGF and BDNF stimulation, 1 × 10 6 cells were plated in 100-mm tissue culture dishes in the presence or absence of tetracycline and were stimulated with 100 ng/ml of NTs on day 3 for the indicated periods of time. Cells were harvested, and RNA was extracted and analyzed for c-fos, c-jun, NGFI-A, NGFI-B/nur77, and NGFI-C by Northern blot analysis, according to the protocols described above. To assess the expression of endogenous NTs, selected clones were analyzed for the expression of NGF, BDNF, NT-4/5, and NT-3. NGF activity was assessed by application of the culture media to PC12 rat pheochromocytoma cells. Northern blot analysis was performed for BDNF, NT-4/5, and NT-3 expression. The level of LNGFR gene expression was also analyzed by Northern blot analysis.
Immunoblotting/Immunoprecipitation
For the protein analysis, cells were harvested in Tris-buffered saline (TBS) with cell scrapers and kept at −70°C until analysis. Cells were lysed in NP-40 lysis buffer containing 1% NP-40, 10% glycerol, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 500 μmol/L sodium orthovanadate in ice-cold TBS. Lysis was done on ice for 30 minutes, and the protein concentration was measured with the Bradford assay kit (Bio-Rad Laboratories, Hercules, CA). For the immunoprecipitation of phosphorylated trkA and trkB receptors, 500 μg of protein from each lysate was immunoprecipitated with polyclonal rabbit anti-pan-trk antibody (C-14) (Santa Cruz Biotechnology, Santa Cruz, CA) and protein A Sepharose (Pharmacia Biotech, Piscataway, NJ). Immunoprecipitates were electrophoresed in an 8% SDS-polyacrylamide gel, transferred to nitrocellulose, and subsequently probed with an anti-phosphotyrosine antibody (4G10) (Upstate Biotechnology). Signals were detected using enhanced chemiluminescence reagents (Amersham Life Science, Arlington Heights, IL).
Analysis of Differentiation Lineage-Dependent Markers
Changes associated with differentiation in neural cell adhesion molecule (NCAM) and GAP-43 were analyzed by Western blot analysis, using 40 μg of protein with mouse monoclonal antibodies to NCAM (Zymed Laboratories, South San Francisco, CA) and GAP-43 (Oncogene Research Products, Cambridge, MA), respectively. Expression of bcl-2, IGF-II, and chromogranin A was analyzed by Northern blot analysis. The levels of norepinephrine in the media in which the cells were cultured were measured by high-pressure liquid chromatography (Beckmann Instruments, Fullerton, CA) at the Department of Laboratory Medicine, Seoul National University Hospital.
Radioimmunoassay for Insulin-Like Growth Factor-II
Cells were split in a six-well plate (1 × 10 5 cells/well) in the absence of tetracycline. NGF was added to individual wells the next day at a concentration of 100 ng/ml. The cells were then cultured for 5 days. The radioimmunoassay of culture supernatants for IGF-II was performed using a DSL-9100 ACTIVE IGF-II coated-tube immunoradiometric assay kit (Diagnostic Systems Laboratories, Webster, TX), according to the manufacturer’s instructions. The free IGF-II level was measured in samples not treated by acid/ethanol extraction, to enable the detection of only free IGF-II, which is not complexed with insulin-like growth factor-binding proteins (IGFBPs). Total IGF-II level was measured after an acid/ethanol extraction step to separate IGFBPs. Assay sensitivity was 0.13 ng/ml, and intra-assay and interassay coefficients of variation were 4.3–7.2% and 6.3–10.4%, respectively. The assay has no detectable cross-reactivity for IGF-I or insulin up to respective concentrations of 480 μg/ml and 4.3 μg/ml.
[3H]dThymidine Uptake Analysis
Cells were cultured in the presence of tetracycline, and 5 × 10 3 cells were split in a 96-well plate in different concentrations of tetracycline (1 μg/ml, 1 × 10−3 μg/ml, 5 × 10−4 μg/ml, 0 μg/ml) in quadruplicate. The next day (day 0), NGF and BDNF were added to individual wells at concentrations of 100 ng/ml. Cells were then cultured for 3 days (day 3). Twenty hours before harvesting, 1 μCi of [3H]dthymidine (ICN, Costa Mesa, CA) was applied to each well. Cells were harvested 20 hours after [3H]dthymidine application with a cell harvester (Inotech, Lansing, MI), and radioactivity was measured with a liquid scintillation counter (Beckmann Instruments).
Viable Cell Counting and Cell Cycle Analysis
To assess the changes in the number of cells and in the cell cycle distribution after NT treatment, 1 × 10 4 cells were split in 24-well plates in the presence or absence of tetracycline. NTs were applied at a concentration of 100 ng/ml the next day (day 0). Cells were harvested on day 5. The number of viable cells was then counted and subsequently subjected to cell cycle analysis. Cells were stained with propidium iodide (50 μg/ml) for cell cycle analysis, which was performed with a Becton-Dickinson FACScan.
Analysis of Cell Survival
Cells were split in 24-well plates at a density of 1 × 10 5 cells per well in triplicate, in the presence or absence of tetracycline. To assess the effects of NTs on the survival of cells after nutrient deprivation, cells were washed twice with serum-free RPMI 1640 on the following day (day 0). They were then cultured in RPMI 1640 containing 0.5% fetal bovine serum with and without tetracycline. NTs were applied at a concentration of 100 ng/ml. Cells were kept under these conditions, and the proportion of viable and nonviable cells was evaluated by trypan blue exclusion on day 5.
Assessment of the Effects of IGF-I Receptor Blocking
To analyze the effects of IGF-I receptor blocking on growth, the cells were cultured in the absence of tetracycline, and mouse anti-IGF-I receptor monoclonal antibody (αIR-3; Oncogene Research Products) was applied to individual wells on days 0 and 2. Preincubation of SY5Y cells with 1 μg/ml of αIR-3 prevents autophosphorylation of the IGF-I receptor when stimulated with 100 ng/ml of IGF-II. To investigate whether the blocking of the IGF-I receptor counteracts NGF-induced differentiation of trkA transfected cells, 1 × 10 4 cells were split in a six-well plate in triplicate. The cells were then treated with NGF for 4 days in the presence and absence of αIR-3 at a concentration of 2 μg/ml. Neurite extension, which was defined as an elongation of the cell processes to more than two times the cell body diameter, was analyzed in at least 200 cells. To assess the effects of IGF-I receptor blocking on growth, [3H]dthymidine uptake analysis was performed as described above in the presence of different concentrations of αIR-3 (0, 0.5, 1, 2 μg/ml).
Results
Characteristics of TrkA and TrkB Transfected Clones
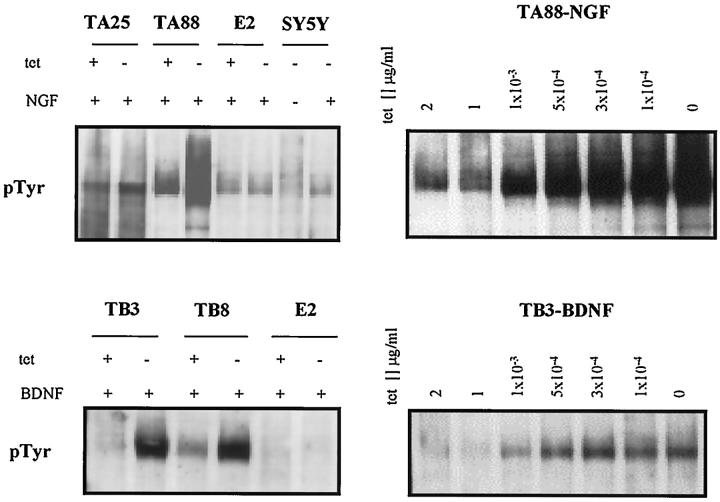
TrkA transfected clones SY5Y-TA25 (TA25) and SY5Y-TA88 (TA88), trkB transfected clones SY5Y-TB3 (TB3) and SY5Y-TB8 (TB8), and a mock transfected clone SY5Y-E2 (E2) were analyzed. The expression of trkA from transfected genes differed from the expression of endogenous trkA in terms of its regulatability and size. The expression of transfected gene trkA or trkB mRNA was conditionally regulated by the application of tetracycline in the range of 0–1 μg/ml (data not shown). Experimental clones showed readily detectable levels of receptor autophosphorylation when stimulated with cognate ligands, also in a dose-dependent manner (Figure 1) ▶ . E2 showed detectable levels of autophosphorylation after NGF treatment but not BDNF treatment. In the absence of tetracycline, trkA and trkB autophosphorylation increased 1.44-fold for TA25, 12.18-fold for TA88, 2.33-fold for TB8, and 7.95-fold for TB3 when compared to that measured in the presence of tetracycline, by quantitative densitometric analysis. The intensities of trkB autophosphorylation in TB3 and TB8, in the absence of tetracycline, were comparable to that of trkA autophosphorylation in TA25 in the absence of tetracycline. In the presence of tetracycline, TA25 and TA88 did not show detectable levels of receptor autophosphorylation without NGF stimulation. TA25 and TA88 did not express NT-3 or NT-4/5 mRNA. In addition, they did not produce a concentration of NGF in the conditioned media that would induce neurites in PC12 cells (data not shown). TB3, TB8, and E2 showed barely detectable levels of NT-4/5 on Northern blot analysis and did not express BDNF or NT-3 (data not shown).
Figure 1.
Expression of transfected trkA and trkB in SH-SY5Y neuroblastoma cells. Receptor autophosphorylation after NT treatment probed with 4G10 anti-phosphotyrosine antibody. Cells were cultured for 3 days in the presence (1 μg/ml) and absence of tetracycline in RPMI with 10% fetal bovine serum (FBS) and were stimulated for 5 minutes with NGF and BDNF (100 ng/ml). Five hundred micrograms of protein was immunoprecipitated with 1 μg of rabbit polyclonal anti-pan trk antibody (C-14) and was electrophoresed in 8% SDS-PAGE. E2 had approximately the same level of receptor autophosphorylation as the parental SH-SY5Y after NGF treatment. tet +: 1 μg tetracycline/ml; tet −: 0 μg tetracycline/ml.
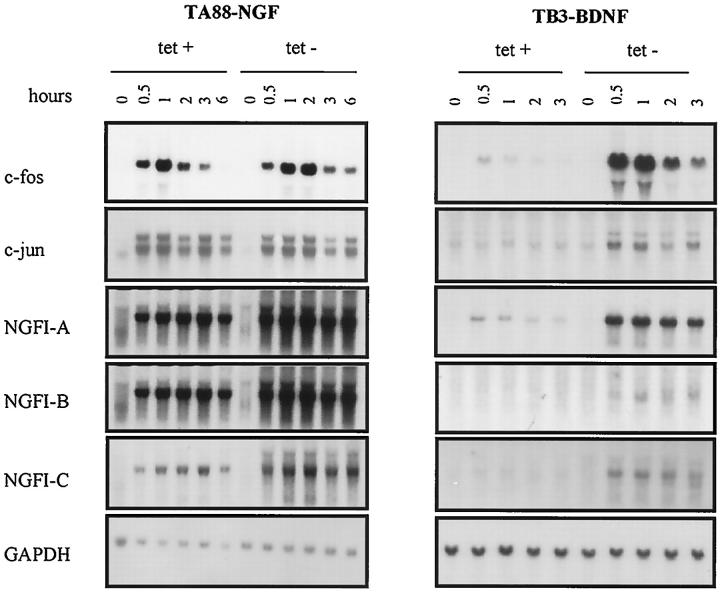
The activation of trkA and trkB induced c-fos, c-jun, NGFI-A, NGFI-B, and NGF-IC (Figure 2) ▶ . Induction of c-fos, c-jun, and NGFI-A by NGF in E2 was presumed to be via the activation of endogenous trkA. However, the response was transient when compared to those of other experimental clones, and gave no detectable message after 30 minutes. BDNF treatment of E2 did not induce c-fos. Although the application of tetracycline significantly repressed the expression of transfected genes, it was not sufficient to completely block trkA and trkB expression in transfected clones. Therefore, all of the experimental conditions were designated as either low (in the presence of 1 μg/ml of tetracycline) or high (in the absence of tetracycline) in terms of the expression of trkA and trkB.
Figure 2.
Induction of early response genes. Cells were cultured for 3 days in the presence (1 μg/ml) and absence of tetracycline in RPMI containing 10% FBS and were stimulated with cognate ligands at a concentration of 100 ng/ml. Profiles of the early response genes induced by NGF and BDNF are similar. The response was more sustained in the absence of tetracycline. Fifteen micrograms of total RNA was electrophoresed in 1.2% formaldehyde gel and subsequently transferred to nitrocellulose membrane. tet +: 1 μg tetracycline/ml; tet −: 0 μg tetracycline/ml.
Morphological Changes and Regulation of Lineage-Dependent Markers
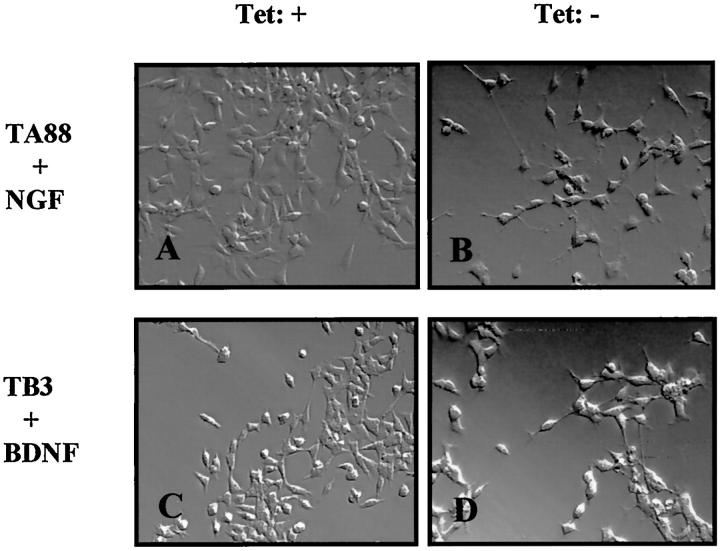
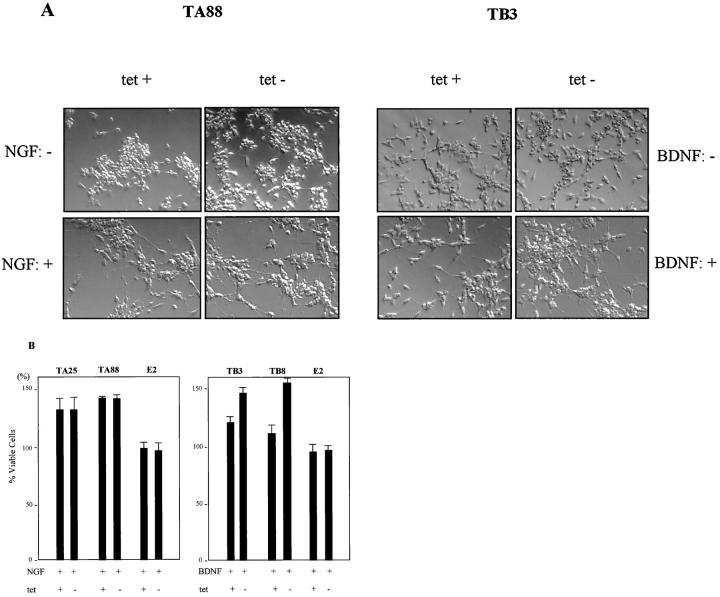
Treatment with NTs induced differentiation, characterized by neurite extension in both trkA and trkB transfected clones. The cells began to show scattered neurite outgrowth 2 days after NT application, and neuritogenesis was evident on day 3. With low trkA or trkB expression, all of the experimental cells showed a flattening of the soma with neurite formation. This was most evident in TA88 after NT treatment. High trkA or trkB expression was associated with the most differentiated features (Figure 3) ▶ . E2 cells did not show any significant alterations by NGF or BDNF.
Figure 3.
Morphological changes of trkA and trkB transfected cells after NT treatment. Cells were cultured in RPMI containing 10% FBS and were treated with NGF and BDNF (100 ng/ml) for 5 days in the presence (1 μg/ml) and absence of tetracycline. In the case of low trkA or trkB expression (A and C; in the presence of tetracycline), NT treatment induced a flattening of the soma and neurite formation. This was was more pronounced in the case of high trkA and trkB expression (B and D; in the absence of tetracycline). Tet +: 1 μg tetracycline/ml, Tet −: 0 μg tetracycline/ml.
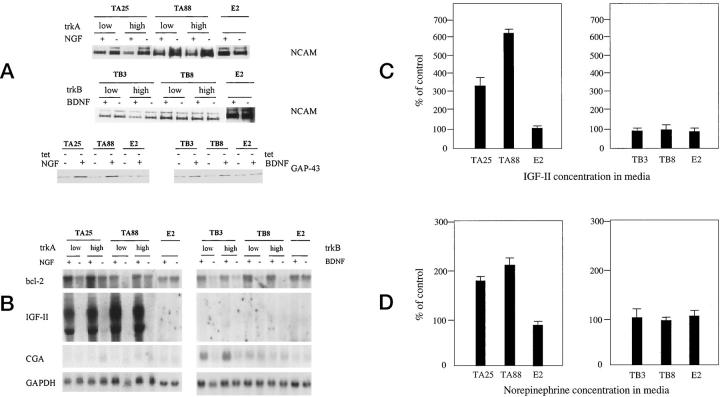
Because both trkA and trkB induced morphological differentiation, we tried to assess the biochemical changes associated with differentiation by monitoring the expression of lineage-dependent markers. NGF treatment of TA25 and TA88 was found to be characteristically associated with decreased expression of two isoforms of NCAM (145 kd and 185 kd), whereas the level of the expression of these proteins was not significantly altered by the activation of trkB in TB3 or TB8 (Figure 4A) ▶ .
Figure 4.
Changes in lineage-dependent differentiation markers. Cells were cultured in RPMI containing 10% FBS and treated with 100 ng/ml of NTs for 5 days in the presence (1 μg/ml) or absence of tetracycline. A: Immunoblot analysis of neural cell adhesion molecule (NCAM) was performed with mouse monoclonal antibody to NCAM. Forty micrograms of proteins was electrophoresed in 8% SDS-PAGE. TrkA-transfected clones showed decreases in both 185-kd and 145-kd isoforms of NCAM with NGF treatment. Reduced NCAM signal in TB3 with high trkB is due to an underloading of the protein. Immunoblot analysis of GAP-43 showed increased expression in TA25, TA88, TB3, and TB8 cells after NT treatment for 5 days in the absence of tetracycline. The GAP-43 expression level was not altered in E2 cells by NGF or BDNF. B: Northern blot analysis of bcl-2, IGF-II, and chromogranin A (CGA) expression. NGF and BDNF commonly increased bcl-2 expression of trkA and trkB transfected clones. Robust increases in IGF-II expression marked a characteristic feature of trkA transfected clones. Chromogranin A expression increased in one (TB3) of the trkB transfected clones. C: The results of radioimmunoassay using two-site immunoradiometric assay of IGF-II, which shows three- to sixfold increases in the concentration of IGF-II in the cell culture media in which TA25 and TA88 cells were grown in the absence of tetracycline, respectively. The concentration was adjusted according to the absolute cell numbers. The data were represented as a percentage of control (without NT treatment). D: Analysis of norepinephrine in media after 5 days of NT treatment in experimental and control cells by high-pressure liquid chromatography. An approximately twofold increase in norepinephrine concentration was found in TA25 and TA88 when the cells were treated with NGF (100 ng/ml) for 5 days in the absence of tetracycline. tet −: 0 μg tetracycline/ml.
The most striking alteration was that NGF caused a 7.33–9.26-fold increase in IGF-II mRNA expression in both TA25 and TA88 expressing either low or high levels of trkA (Figure 4B) ▶ . A detectable IGF-II mRNA message was found after 2 days of NGF treatment, and the radioimmunoassay results showed a chronological correlation. The biggest difference in IGF-II concentration was reached on day 5, after NGF treatment, increasing 3.3- and 6.2-fold in TA25 and TA88 cells, respectively (Figure 4C) ▶ . The highest concentrations of IGF-II in media on day 5 were 77 ng/ml and 89 ng/ml for TA25 and TA88, respectively. Up-regulation of IGF-II expression was not seen in BDNF-treated TB3 and TB8, or in NT-treated E2 cells. Treatment of TA25 and TA88 with NGF also resulted in a twofold increase in the secretion of norepinephrine into the media (179% of control in TA25 and 214% of control in TA88), whereas such changes were not seen in E2 cells and BDNF-treated TB3 and TB8 cells (Figure 4D) ▶ . The absolute concentration of norepinephrine in media after NGF treatment was more than 3000 pg/ml in both of the trkA-transfected cells on day 5. TB3 showed an increase in chromogranin A expression due to BDNF treatment. All of the trkA and trkB transfected clones showed increased expressions of GAP-43 and bcl-2 after NGF and BDNF treatments, respectively (Figure 4, A and B) ▶ . None of these significant biochemical changes were observed in E2, suggesting that the intensity and/or duration of signaling by endogenous trkA receptors may not be sufficient to elicit detectable biological changes.
Effects of TrkA and Trk B on Growth and Survival
When trkA was fully expressed and treated with NGF, TA25 and TA88 showed decreases in [3H]dthymidine uptake and cell numbers. With low trkA expression, NGF treatment of TA88 did show increases in [3H]dthymidine uptake and cell numbers (Figure 5A) ▶ . This suggests a differential modulation of growth by the same receptor. Activation of trkB in TB3 and TB8 resulted in decreased [3H]dthymidine uptake in a dose-dependent manner. The reduction of uptake ranged from 48% to 68% of controls (Figure 5B) ▶ . Cell numbers after 5 days of NT application were consistent with trends in cell growth as determined by [3H]dthymidine uptake analysis (Figure 5B) ▶ . Although activation of trkA and trkB significantly inhibited cell growth, flow cytometric analysis did not show significant changes in the distribution of the cell cycle in any of the clones. This suggests that growth inhibition by NTs is not associated with major changes in the cell cycle.
Figure 5.
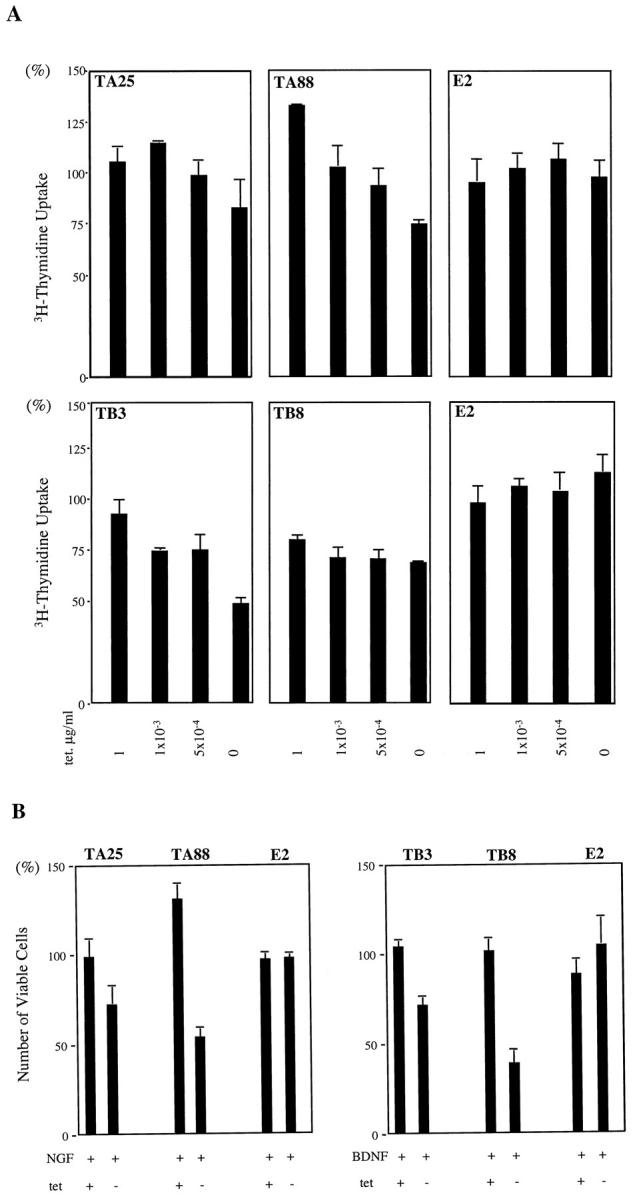
Effects of NGF and BDNF on cell proliferation. A: NGF treatment of trkA transfected clones decreased [3H]thymidine uptake with high trkA expression (in the absence of tetracycline). The values are quoted as a percentage of the control (without NT treatment). The cells were cultured in RPMI with 10% FBS for 3 days at the different tetracycline concentrations, which ranged from 0 to 1 μg/ml, and were labeled with 1 μCi of [3H]thymidine for 20 hours. Radioactivity was measured on day 4 after NT treatment (100 ng/ml). Interestingly, TA88 showed increased [3H]thymidine uptake with low trkA at a tetracycline concentration of 1 μg/ml. B: Viable cell numbers are quoted as a percentage of the control (without NT treatment). The cells were treated with NGF or BDNF (100 ng/ml) for 5 days in RPMI with 10% FBS in the presence (1 μg/ml) and absence of tetracycline. tet +: 1 μg tetracycline/ml; tet −: 0 μg tetracycline/ml.
NTs are known to protect neuronal cells from various kinds of stresses, and we tested the rescue effects of NGF and BDNF by measuring the viability of cells under conditions of nutrient deprivation. Serum concentration was lowered to 0.5%, and cells were cultured for 5 days in the absence and presence of NTs. Even low levels of trkA successfully protected the cells from serum starvation in TA25 and TA88 when NGF was available, whereas there was a clear dose-dependent effect in TB3 and TB8. The protective effects of NTs were associated with more pronounced effects on differentiation (Figure 6A) ▶ . The percentage of viable cells increased to 37% of controls in TA25 and to almost 45% in TA88 cells, regardless of the levels of trkA. In TB3 and TB8, the addition of BDNF rescued cells in a dose-dependent manner. BDNF stimulated a 15% increase in the cell survival of TB8 and a 22% increase in the cell survival of TB3 expressing low levels of trkB. This increased to 54% in TB8 and 45% in TB3 expressing high levels of trkB (Figure 6B) ▶ . The survival of E2 was not significantly affected by NGF or BDNF.
Figure 6.
Effects of NGF and BDNF on protection from nutrient deprivation. Cells were cultured for 5 days in RPMI containing 0.5% serum. Viable and nonviable cells were counted based on trypan blue exclusion. A: Nutrient deprivation was associated with more exaggerated differentiation after NGF and BDNF treatment. B: Fraction of viable cells represented as a percentage of the control (without NT treatment). Low trkA protected the cells to a level comparable to that of high trkA in each clone with NGF treatment, whereas trkB transfected clones showed a dose-dependent effect. tet +: 1 μg tetracycline/ml; tet −: 0 μg tetracycline/ml.
Effects of Insulin-Like Growth Factor-I Receptor Blocking
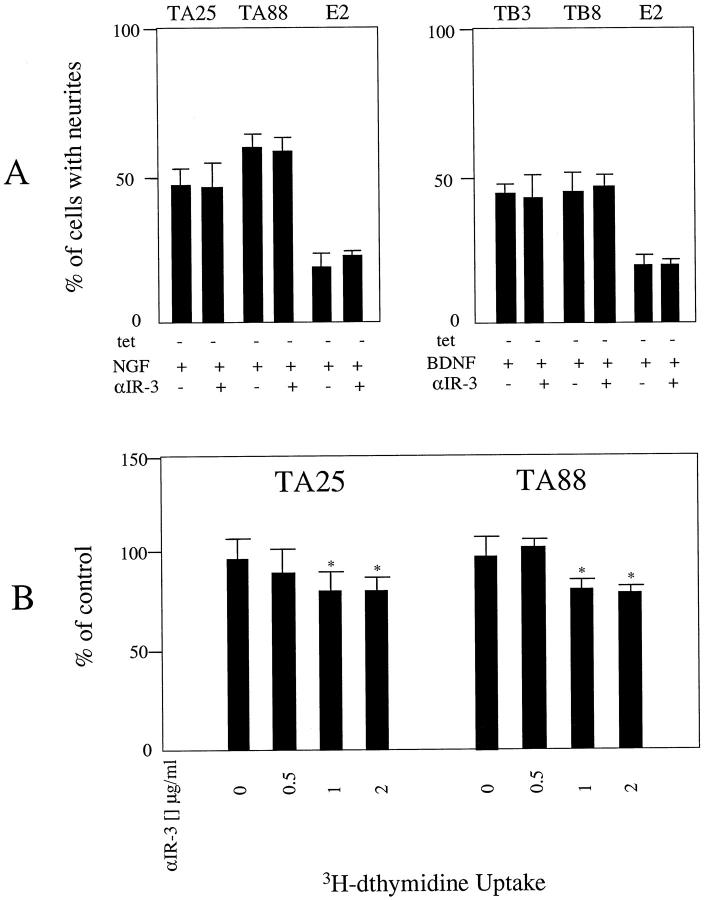
To assess whether up-regulation of IGF-II expression mediates biological effects after trkA activation, TA25 and TA88 cells were treated with NGF for 4 days in the presence and absence of αIR-3 at a concentration of 2 μg/ml. Treatment of the cells with αIR-3 did not counteract NGF-induced differentiation of the cells (Figure 7A) ▶ . However, there was a persistent decrease in [3H]dthymidine uptake up to 19% and 22% in TA25 and TA88 cells, respectively, when these cells were treated with αIR-3, which suggested that up-regulation of IGF-II expression is a part of the effector mechanism of trkA activation (Figure 7B) ▶ . These changes were not seen in BDNF-treated TB3, TB8, or NT-treated E2 cells.
Figure 7.
Blocking of IGF-I receptor with αIR-3 did not block NGF-induced differentiation of trkA-transfected SY5Y cells, but resulted in a decreased [3H]dthymidine uptake. A: Cells were cultured in RPMI with 10% FBS in the absence of tetracycline and the presence of NTs (100 ng/ml) for 4 days. The fractions of the cells showing neurite extension, which was defined as an elongation of the cell process to more than two times the cell body diameter, were determined for at least 200 cells. Addition of αIR-3 at a concentration of 2 μg/ml did not counteract NGF-induced differentiation of the trkA-transfected cells. B: Treatment of TA25 and TA88 with αIR-3 at different concentrations (0, 0.5, 1, 2 μg/ml) resulted in a decreased [3H]dthymidine uptake. The cells were grown in RPMI with 10% FBS and were treated with 100 ng/ml of NGF in the absence of tetracycline. Radioactivity was measured on day 4 after 20 hours of incubation with 1 μCi of [3H]dthymidine. *Differs from control at P < 0.05.
Discussion
During nervous system development, the expressions of NTs and their receptors are spatiotemporally regulated, and sympathetic neurons are largely trkA dependent. 18,19 However, reports of trkB expression during the development of the neural crest and sympathetic ganglia, 20,21 trkB and BDNF expression in NBs with poor prognoses, 5 and trkB induction during retinoic acid (RA)-induced neuronal differentiation in some NB cell lines 16 suggest the presence of trkB-dependent cells in the sympathetic nervous system.
Generally, the expression of trkA and that of trkB are mutually exclusive in primary NBs. 2 Although a majority of neuroblastoma cell lines express low levels of trkA, few of them are responsive to NGF in terms of induction of differentiation or changes in proliferation and survival. 22,23 Previous studies have reported the restoration of NGF responsiveness after trkA transfection in neuroblastoma cell lines and suggested that low levels of trkA, or defects in trkA signaling, may be responsible for the poor prognosis of these tumors. 23,24 On the contrary, in our previous analysis of the effects of trkB induced by RA, BDNF treatment enhanced the invasiveness and survival of neuroblastoma cell lines without growth inhibition. 25 Similar effects have been observed by others in the trkB-expressing SMS-KCN neuroblastoma cell line. 5 These results may explain why the expression of trkA and the expression of trkB are associated with different prognoses.
In this study, we developed an experimental system in which we can differentially modulate the expression of trkA and trkB. Clonal variability, differences in the level of expression of receptors in individual clones, and the relatively high level of trkA expression, even in the presence of tetracycline in trkA-transfected cells, can be potential biases. However, we identified novel aspects of the effects of trkA and trkB in SY5Y cells. SY5Y expresses low levels of LNGFR, which binds all NTs, and may influence and elicit biological responses both dependent and independent of trk activation. 26,27 Although it is possible that signaling via LNGFR may have influenced the effects by NGF and BDNF in our system, it is unlikely, as neither NGF nor BDNF significantly altered the expression level of LNGFR on Northern blot analysis (data not shown), and significant effects of NTs were not observed in E2.
Differentiation of adrenergic cells originating from the neural crest is characterized by its plasticity, because of the influence of environmental factors such as glucocorticoids and growth factors. 28 Sympathetic neuroblasts can differentiate into both neuronal (sympathetic ganglionic) and neuroendocrine (adrenal or extra-adrenal chromaffin) lineages. A recent analysis of a series of primary NBs and the sympathetic nervous system showed an increased expression of trkA, trkC, IGF-II, and chromogranins and a decreased expression of bcl-2 and NCAM with ongoing morphological differentiation in extra-adrenal NBs. 17 This pattern of gene expression corresponded to the antigenic profile of cells undergoing extra-adrenal chromaffin differentiation during human sympathetic nervous system development. A subsequent study demonstrated evidence of in vivo spontaneous neuronal to neuroendocrine lineage conversion in these NBs. 29 The changes in the biochemical phenotype marked by NCAM down-regulation, increased catecholamine secretion, and a robust increase in IGF-II expression after NGF treatment of trkA-transfected clones in the present study suggest that in certain extra-adrenal NBs, activation of trkA may contribute in part to the differentiation of neuroendocrine phenotypes, although the increases in GAP-43 and bcl-2 are consistent with the development of neuronal phenotypes.
Increased expression of bcl-2 by NGF and BDNF in trkA and trkB transfected clones and enhanced cell survival after nutrient deprivation are consistent with the observations in other studies. The rescue of PC12 cells and BDNF-dependent neurons from apoptosis by NGF and BDNF was dependent on the up-regulation of bcl-2. 30,31 It is interesting that trkA, a good prognostic marker in NB, is related to bcl-2 overexpression, although bcl-2 is not detected in tumors with a good prognosis. In contrast, bcl-2 expression is a major feature of NBs with unfavorable histologies and amplified N-myc. It also protects the NB cells from anticancer agents by producing a more drug-resistant phenotype. 32,33 Therefore, increased bcl-2 expression would be more consistent in NBs with poor prognoses, which tend to express both BDNF and trkB, and in which chemosensitivity is altered by the activation of trkB by BDNF. 34
The up-regulation of IGF-II expression, which can establish autocrine or paracrine loops of signaling in this IGF-I receptor-expressing cell line, 35,36 was found to be the major feature that distinguished trkA from trkB. This was the first demonstration of the novel link between trkA activation by NGF and IGF-II expression in NB. The insulin-like growth factor system is important during the course of human development, and cell types with abundant IGF-II expression are strikingly correlated to the organomegaly and tumor predisposition of the Beckwith-Wiedemann syndrome, a syndrome characterized by an increased incidence of embryonal tumors such as Wilms’ tumor and NB. 37 IGF-I and IGF-II have been reported to play an important role in the early development of chick sympathetic neurons. IGF-II has proven mitogenic effects, induces differentiation, and promotes the survival of cultured sympathetic neurons. 38,39 IGF-II expression was also found in a significant proportion of primary NBs and cell lines, suggesting an important role in the tumorigenesis and biology of NB. 40 However, NGF treatment of PC12 cells did not stimulate IGF-II expression (data not shown). Autocrine or paracrine loops between IGF-II and IGF-I receptors have been reported to induce proliferation and differentiation, prevent apoptosis, and enhance the tumorigenesis of SY5Y cells. 35,36,41 They also mediate the autonomous growth of another NB cell line, SK-N-AS, which expresses IGF-II abundantly. 42 Our findings suggest that up-regulation of bcl-2 and IGF-II expression are biologically relevant mechanisms that are coupled to trkA in NBs and possibly in the sympathetic nervous system. A recent report on the protease activity of 7S nerve growth factor for insulin-like growth factor-binding protein (IGFBP), which allows the level of free IGFs to increase, provides additional evidence that NGF can modulate the levels of IGF. 43 In the context that IGF-II expression has been repeatedly advocated as a marker for extra-adrenal chromaffin differentiation, 17,44 the findings from our study imply that up-regulation of IGF-II is not only a feature of the differentiated cells; it also has a role as a component of the effector mechanisms of trkA in these cells.
In contrast to previous studies, we found that the effects mediated by trkA and trkB receptors in SY5Y are overlapping. We showed that trkB activation is a growth-inhibiting signal for SY5Y, and that a particular level of trkA activation can be mitogenic. This strongly suggests that the effects of trkA and trkB activation can vary, depending on the biological phenotypes of individual NBs and the intensity of signaling in vivo. TrkA transfected SY5Y cells showed dissociation in the modulation of neuronal or neuroendocrine antigens and catecholamines. Moreover, the biochemical alterations seemed to be less dependent on the degree of morphological differentiation. These results are in line with those of previous observations of adrenal chromaffin cells, which demonstrated that distinct and independent modulations of peptidergic, catecholaminergic, and morphological properties of chromaffin cells were caused by different growth factors including NGF. 45 It seems reasonable to speculate that no single growth factor could allow the SY5Y cells to differentiate into a specific biological phenotype. However, the relationship between trkA and IGF-II, along with increased catecholamine synthesis, implies that trkA-transfected SY5Y cells may allow us to study the major biological aspects of extra-adrenal NBs of infancy, which are characterized by a favorable prognosis and extra-adrenal chromaffin differentiation.
Acknowledgments
The authors are grateful to Dr. Steven Reeves for providing the vector and helpful comments and to Ms. Lena Zee for her excellent technical assistance. We are also grateful to Dr. David Kaplan for helpful comments.
Footnotes
Address reprint requests to Dr. Chong Jai Kim, Department of Pathology, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul 110–799, Korea. E-mail: cjkim@plaza.snu.ac.kr.
References
- 1.Yamashiro DJ, Nakagawara A, Ikegaki N, Liu X, Brodeur GM: Expression of TrkC in favorable human neuroblastoma. Oncogene 1996, 12:37-41 [PubMed] [Google Scholar]
- 2.Brodeur GM, Nakagawara A, Yamashiro DJ, Ikegaki N, Liu X-G, Azar CG, Lee CP, Evans AE: Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol 1997, 31:49-55 [DOI] [PubMed] [Google Scholar]
- 3.Kogner P, Barbany G, Dominici C, Castello MA, Raschella G, Person H: Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Res 1993, 53:2044-2050 [PubMed] [Google Scholar]
- 4.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM: Association of high levels of expression of TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 1993, 52:1364-1368 [DOI] [PubMed] [Google Scholar]
- 5.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM: Expression and function of trkB and BDNF in human neuroblastomas. Mol Cell Biol 1994, 14:759-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snider WD: Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 1994, 77:627-638 [DOI] [PubMed] [Google Scholar]
- 7.Chao MV: The p75 neurotrophin receptor. J Neurobiol 1994, 25:1373-1385 [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Jing S, Nanduri V, O’Rourke E, Barbacid M: The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 1991, 65:189-197 [DOI] [PubMed] [Google Scholar]
- 9.Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T, Nikolics K, Parada LF: The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptors. Cell 1991, 65:895-903 [DOI] [PubMed] [Google Scholar]
- 10.Lamballe F, Klein R, Barbacid M: TrkC, a new member of the trk family of tyrosine kinases, is a receptor for neurotrophin-3. Cell 1991, 66:967-979 [DOI] [PubMed] [Google Scholar]
- 11.Hoehner JC, Olsen L, Sandstedt B, Kaplan DR, Påhlman S: Association of neurotrophin receptor expression and differentiation in human neuroblastoma. Am J Pathol 1995, 147:102-113 [PMC free article] [PubMed] [Google Scholar]
- 12.Washiyama K, Muragaki Y, Rorke LB, Lee VM-Y, Feinstein SC, Radeke MJ, Blumberg B, Kaplan DR, Trojanowski JQ: Neurotrophin and neurotrophin receptor proteins in medulloblastomas and other primitive neuroectodermal tumors of the pediatric central nervous system tumors. Am J Pathol 1996, 148:929-940 [PMC free article] [PubMed] [Google Scholar]
- 13.Abemayor E, Sidell N: Human neuroblastoma cell lines as models for the in vitro study of neoplastic and neuronal cell differentiation. Environ Health Perspect 1989, 80:3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulus W, Baur I, Boyce FM, Breakefield XO, Reeves SA: Self-contained, tetracycline-regulated retroviral vector system for gene delivery to mammalian cells. J Virol 1996, 70:62-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biedler JL, Helson L, Spengler BA: Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 1973, 33:2643-2652 [PubMed] [Google Scholar]
- 16.Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ: Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cell lines. Neuron 1993, 11:321-331 [DOI] [PubMed] [Google Scholar]
- 17.Hoehner JC, Gestblom C, Hedborg F, Sandstedt B, Olsen L, Påhlman S: A developmental model of neuroblastoma: differentiating stroma-poor tumors progress along an extraadrenal chromaffin lineage. Lab Invest 1996, 75:659-675 [PubMed] [Google Scholar]
- 18.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M: Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 1994, 368:246-249 [DOI] [PubMed] [Google Scholar]
- 19.Fagan AM, Zhang H, Landis S, Smeyne RJ, Silos-Santiago I, Barbacid M: TrkA, but not trkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci 1996, 16:6208-6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrhard PB, Otten U: Postnatal ontogeny of the neurotrophin receptors trk and trkB mRNA in rat sensory and sympathetic ganglia. Neurosci Lett 1994, 166:207-210 [DOI] [PubMed] [Google Scholar]
- 21.Kalcheim C, Gendreau M: Brain-derived neurotrophic factor stimulates survival and neuronal differentiation in cultured avian neural crest. Dev Brain Res 1988, 41:79-86 [DOI] [PubMed] [Google Scholar]
- 22.Azar CG, Scavarda NJ, Reynolds CP, Brodeur GM: Multiple defects of the nerve growth factor receptor in human neuroblastomas. Cell Growth Differ 1990, 1:421-418 [PubMed] [Google Scholar]
- 23.Lavenius E, Gestblom C, Johansson I, Nanberg E, Påhlman S: Transfection of TRK-A into human neuroblastoma cells restores their ability to differentiate in response to nerve growth factor. Cell Growth Differ 1995, 6:727-736 [PubMed] [Google Scholar]
- 24.Poluha W, Poluha DK, Ross AH: TrkA neurogenic receptor regulates differentiation of neuroblastoma cells. Oncogene 1995, 10:185-189 [PubMed] [Google Scholar]
- 25.Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ: Expression of brain-derived neurotrophic factor and p145 trkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res 1995, 55:1798-1806 [PubMed] [Google Scholar]
- 26.Lee K-F, Davies AM, Jaenisch R: p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development 1994, 120:1027-1033 [DOI] [PubMed] [Google Scholar]
- 27.Cortazzo MH, Kassis ES, Sproul KA, Shor NF: Nerve growth factor (NGF)-mediated protection of neural crest cells from antimitotic agent-induced apoptosis: the role of the low-affinity NGF receptor. J Neurosci 1996, 16:3895-3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doupe AJ, Landis SC, Patterson PH: Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci 1985, 8:2119-2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gestblom C, Hoehner JC, Hedborg F, Sandstedt B, Påhlman S: In vivo spontaneous neuronal to neuroendocrine lineage conversion in a subset of neuroblastomas. Am J Pathol 1997, 150:107-117 [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh S, Mitsui Y, Kitani K, Suzuki T: Nerve growth factor rescues PC12 cells from apoptosis by increasing amount of bcl-2. Biochem Biophys Res Commun 1996, 229:653-657 [DOI] [PubMed] [Google Scholar]
- 31.Allsopp TE, Kiselev S, Wyatt S, Davies AM: Role of bcl-2 in the brain-derived neurotrophic factor survival response. Eur J Neurosci 1995, 7:1266-1272 [DOI] [PubMed] [Google Scholar]
- 32.Castle VP, Heidelberger KP, Bromberg J, Ou X, Dole M, Nuñez G: Expression of the apoptosis-suppressing protein bcl-2, in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol 1993, 143:1543-1550 [PMC free article] [PubMed] [Google Scholar]
- 33.Dole M, Nuñez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, Castle VP: Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res 1994, 54:3253-3259 [PubMed] [Google Scholar]
- 34.Scala S, Wosikowski K, Giannakakou P, Valle P, Biedler JL, Spengler BA, Lucarelli E, Bates SE, Thiele CJ: Brain-derived neurotrophic factor protects neuroblastoma cells from vinblastine toxicity. Cancer Res 1996, 56:3737-3742 [PubMed] [Google Scholar]
- 35.Singleton JR, Randolph AE, Feldman EL: Insulin-like growth factor-I receptor prevents apoptosis and enhances neuroblastoma tumorigenesis. Cancer Res 1996, 56:4522-4529 [PubMed] [Google Scholar]
- 36.Martin DM, Feldman EL: Regulation of insulin-like growth factor-II expression and its role in autocrine growth of human neuroblastoma cells. J Cell Physiol 1993, 155:290-300 [DOI] [PubMed] [Google Scholar]
- 37.Hedborg F, Holmgren L, Sandstedt B, Ohlsson R: The cell type-specific IGF2 expression during early human development correlates to the pattern of overgrowth and neoplasia in the Beckwith-Wiedemann syndrome. Am J Pathol 1994, 145:802-817 [PMC free article] [PubMed] [Google Scholar]
- 38.Zackenfels K, Oppenheim RW, Rohrer H: Evidence for an important role of IGF-I and IGF-II for the early development of chick sympathetic neurons. Neuron 1995, 14:731-741 [DOI] [PubMed] [Google Scholar]
- 39.Recio-Pinto E, Rechler MM, Ishii DN: Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci 1986, 6:1211-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan KA, Castle VP, Hanash SM, Feldman EL: Insulin-like growth factor II in the pathogenesis of human neuroblastoma. Am J Pathol 1995, 147:1790-1798 [PMC free article] [PubMed] [Google Scholar]
- 41.Leventhal PS, Randolph AE, Vesbit TE, Schenone A, Windebank AJ, Feldman EL: Insulin-like growth factor-II as a paracrine growth factor in human neuroblastoma cells. Exp Cell Res 1995, 221:179-186 [DOI] [PubMed] [Google Scholar]
- 42.El-Badry OM, Romanus JA, Helman LJ, Cooper MJ, Rechler MM, Israel MA: Autonomous growth of a human neuroblastoma cell line is mediated by insulin-like growth factor II. J Clin Invest 1989, 84:829-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajah R, Bhala A, Nunn SE, Peehl DM, Cohen P: 7S nerve growth factor is an insulin-like growth factor-binding protein protease. Endocrinology 1996, 137:2676-2682 [DOI] [PubMed] [Google Scholar]
- 44.Hedborg F, Ohlsson R, Sandstedt B, Grimelius L, Hoehner JC, Påhlman S: IGF2 expression is a marker for paraganglionic/SIF cell differentiation in neuroblastoma. Am J Pathol 1995, 146:833-847 [PMC free article] [PubMed] [Google Scholar]
- 45.Ramírez-Ordoñez R, García-Arrarás JE: Peptidergic, catecholaminergic, and morphological properties of avian chromaffin cells are modulated distinctively by growth factors. Brain Res Dev Brain Res 1995, 87:160-171 [DOI] [PubMed] [Google Scholar]