Abstract
Our previous study demonstrated increases in epidermal growth factor receptor (EGF-R) phosphorylation and receptor tyrosine kinase and extracellular signal-regulated kinase (ERK1 and ERK2) activities in the ulcer margin of experimental gastric ulcer during healing. However, the intermediate steps linking activated receptor tyrosine kinase to ERKs during ulcer healing are as yet unknown. Raf-1 is upstream of mitogen-activated protein kinases (MAPK/ERK) and can be activated by Ras-dependent and/or Ras-independent mechanisms. Therefore, we studied Raf-1 activity, its potential activators protein kinase C (PKC) and Ras, and expression and associations of adapter proteins Shc, Grb2, and Sos during experimental gastric ulcer healing. To investigate if Raf-1–ERK activation is attributable to the epithelial component of ulcer margins, we studied the effect of EGF on PKC, Ras, and ERK activities in a rat gastric epithelial cell line (RGM1). Our results demonstrate that gastric ulceration significantly increases Raf-1 kinase activity, Grb2 and Ras protein, and Shc-Grb2 and Grb2-Sos complex levels. In contrast, PKC activity and protein level were significantly decreased in the ulcer margins. In RGM1 cells, EGF significantly increased Ras and ERK2 activities without affecting PKC activity. These findings indicate that Raf-1 activation during gastric ulcer healing is Ras mediated, involves Shc-Grb2-Sos, and is PKC-independent.
Ulcer healing requires interaction of various cellular and connective tissue components. 1,2 It involves reconstruction of glandular structures, reepithelialization of the mucosal surface, and restoration of the connective tissue components. 1-3 A number of growth factors, including epidermal growth factor (EGF) and transforming growth factor α (TGF-α), have been shown to participate in the repair of tissue injury by stimulating the cell proliferation and migration necessary for reepithelialization and ulcer healing. 4-7 Immunohistochemical studies have shown overexpression of EGF and its receptor (EGF-R) in epithelial cells lining ulcer margins and regenerating glands, 8-10 indicating that these cells are major targets for the proliferation-stimulating action of EGF.
Our recent study demonstrated that gastric ulceration triggers increased receptor tyrosine kinase activity, EGF-R phosphorylation, and extracellular signal regulated kinase 1 and 2 (ERK1 and ERK2) activity in epithelial cells of the ulcer margins. 11 Biochemical and genetic studies in various cell systems (other than gastric mucosa) have demonstrated that Raf-1 functions downstream of activated tyrosine kinases and Ras, but upstream of mitogen-activated protein kinase (MAPK) and MAPK kinase (MEK). 12,13 Raf-1 activity can be modulated by both Ras-dependent and Ras-independent pathways. 14 In addition to Ras, Raf-1 activators may include protein kinase C (PKC), activated tyrosine kinases, or as yet unidentified serine threonine kinases and phosphatases. 14 The intermediate steps linking activated receptor tyrosine kinase to MAP kinases (ERK1 and ERK2) during gastric ulcer healing in vivo remain unknown.
In some cellular sytems other than gastric cells (eg, Rat1, A431, and Her14 cells), EGF-R activation has been shown to cause binding of the SH2-containing adapter protein (Shc) to growth factor receptor-bound protein (Grb2), leading to recruitment of Son of sevenless (Sos) to the plasma membrane and Ras activation. 15-18 Phospholipase C-γ (PLC-γ) is another signaling protein that contains SH domains and is activated by tyrosine kinase. 19 PLC-γ1 can act either as an enzyme that generates diacylglycerol and IP3, leading to PKC activation, 20,21 or as an adapter protein by binding to activated growth factor receptors via its SH2 domains and a downstream molecule via its SH3 domains. 22 PKC can activate MAPK, which in turn activates the gene transcription involved in cell proliferation and differentiation. 23-27 Growth factor-mediated activation of the Raf-1–MAPK/ERK cascade may therefore involve either Ras, PKC, or both. 13,23-27 Previous studies have shown increased tyrosine kinase activity and PLC-γ1 phosphorylation during the early phase of acute injury repair in rat gastric mucosa. 28,29 However, involvement of Ras or PKC in signaling pathways during chronic gastric ulcer healing is not known.
The present study was aimed at determining whether during experimental gastric ulcer healing Raf-1 is activated and to identify potential activators of Raf-1 by examining the Ras and PKC protein levels, PKC activity, and Shc-Grb2, Grb2-Sos complex levels. To determine whether activation of the Raf-1–ERK cascade during gastric ulcer healing occurs predominantly in the epithelial component, we studied the effect of EGF on Ras activation, and PKC and ERK activities in an in vitro model using a rat gastric epithelial cell line (RGM1) derived from normal rat gastric mucosa.
Materials and Methods
Kinase-inactive MEK, mouse monoclonal anti-PKC (MC5; recognizes α, β, and γ isoforms), anti-Ras, rabbit polyclonal anti-ERK2, and anti-Sos antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-Shc antibody, mouse monoclonal anti-Grb2, and anti-c-Raf-1 antibodies were purchased from Transduction Laboratories (Lexington, KY). A Protein kinase C Assay System was purchased from Gibco BRL (Gaithersburg, MD). [γ-32P]ATP was purchased from Dupont NEN Research Products (Boston, MA), and all other molecular biology-grade chemicals were purchased from Sigma Chemical Company (St. Louis, MO).
Animals
This study was approved by the Subcommittee for Animal Studies of the VA Medical Center (Long Beach, CA). Male Sprague-Dawley rats (Crl:CD(SD)BR; Charles River Laboratories; Wilmington, MA) were fasted for 12 hours and underwent laparotomy under Nembutal anesthesia (60 mg/kg body weight). One hundred percent acetic acid (50 μl) was applied to the serosa of lower gastric corpus at the posterior wall through a polyethylene tube (4.0 mm i.d.) for 90 seconds. The serosal area was then washed with isotonic saline and the abdomen closed. Sham-operated rats underwent a similar procedure without acetic acid administration. Rats with gastric ulcers (n = 54) and sham-operated rats (n = 36) were euthanized 3 and 7 days after operation. Ulcer margins were carefully dissected from granulation tissue, snap-frozen in liquid nitrogen, and stored at −80°C.
Raf-1 Kinase Assay
Raf-1 kinase activity was determined using the method previously described. 30 In brief, Raf-1 was immunoprecipitated from protein normalized tissue lysates (0.03 mg), using protein A Sepharose-antibody complex, and washed four times in lysis buffer. After the final wash, the immunoprecipitated Raf-1 complexes were resuspended and incubated for 20 minutes at 25°C in 40 μl kinase buffer containing 30 mmol/L HEPES (pH 7.4), 7 mmol/L manganese chloride, 5 mmol/L magnesium chloride, 1 mmol/L dithiothreitol, 15 μmol/L ATP, 10 μCi of [γ-32P]ATP (3000 Ci/mmol; Dupont NEN), and 4 units of kinase-inactive MEK. To terminate the assay, 15 μl of 4× Laemmli sample buffer was added and heated for 5 minutes at 100°C and analyzed by sodium dodecyl sulfate-polyacrylamide gel electophoresis (SDS-PAGE) and autoradiography. Quantification was performed with a phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Effect of Exogenous EGF Treatment on Raf-1 Activity in Ulcer Margins
To determine whether exogenous EGF could modulate Raf-1 activity during the healing process, ulcer margins from 3-day ulcers were carefully dissected and incubated with 10 ng/ml of EGF in Dulbecco’s minimum essential medium (DMEM)/F12 serum-free medium for 15 and 30 minutes in a humidified chamber with 5% CO2. After incubations, tissue samples were snap-frozen in liquid nitrogen and homogenized in lysis buffer, and Raf-1 activity was determined, following the procedure outlined above.
Protein Kinase C Assay
PKC activity was determined using an assay system described in our previous study. 31 In brief, frozen tissue samples were lysed in 0.5 ml PKC homogenization buffer (20 mmol/L Tris-HCl, pH 7.4; 2 mmol/L EGTA; 2 mmol/L EDTA; 1% NP-40; 0.33 mol/L sucrose; 0.2 mmol/L sodium orthovanadate; 100 mmol/L sodium fluoride; 10 mmol/L sodium pyrophosphate; 10 μg/ml aprotinin; 10 μg/ml leupeptin; 1 mmol/L phenylmethylsulfonyl fluoride). The protein-normalized lysates were diluted in ice-cold dilution buffer (20 mmol/L Tris-HCl, pH 7.4; 0.2 mol/L NaCl; 0.5 mmol/L EGTA; 0.5 mmol/L EDTA; 10 mmol/L β-mercaptoethanol), and 25 μl (1 μg of protein) was assayed using the PKC assay system according to the manufacturer’s instructions (Gibco BRL, Gaithersburg, MD).
Immunoprecipitation
Frozen tissues were homogenized in ice-cold lysis buffer (20 mmol/L Tris-HCl (pH 7.5), 50 mmol/L NaCl, 50 mmol/L sodium fluoride, 30 mmol/L sodium pyrophosphate, 5 mol/L EGTA, 10% glycerol, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate, and 5 μg/ml aprotinin) and clarified by centrifugation at 14,000 rpm for 10 minutes. The protein concentration of the lysate was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Equal amounts of proteins were incubated with specific primary antibody immobilized onto protein A Sepharose for 2 hours at 4°C under gentle rotation. Beads were washed extensively with lysis buffer, and immune complexes were eluted by heating for 5 minutes at 95°C in Laemmli buffer and microcentrifuged. The supernatant was subjected to SDS-PAGE, followed by immunoblotting with specific antibodies (listed in Materials and Methods).
Western Blot Analysis
Western blot analysis was performed following the method previously described. 11 Tissue lysates containing equal amounts of proteins (0.15 mg) were subjected to SDS-PAGE and transferred onto a nitrocellulose membrane. Blots were stained with Ponceau Red to ensure equal loading and complete transfer of proteins. The membrane containing the transferred proteins was incubated with blocking buffer, subsequently washed, and incubated with specific primary antibodies (listed in Materials and Methods) for 1 hour at room temperature. Blots were washed and incubated with specific peroxidase-conjugated secondary antibodies for 1 hour at room temperature. After washing, bound antibody was visualized with the ECL detection system (Amersham Corp, IL) according to the manufacturer’s instructions. The density of the protein bands was analyzed using a laser densitometer (UltroScan XL Laser Densitometer; Pharmacia LKB Biotechnology, Uppsala, Sweden).
Ras Assay
Because there is no currently available method allowing determination of Ras activation in vivo, we examined Ras activation in rat gastric epithelial cells. RGM1 cells were plated in 100-mm tissue culture dishes and grown until ∼80% confluent in DMEM/F12 supplemented with 20% fetal bovine serum. The cells were serum-starved for 16 hours and metabolically labeled for an additional 8 hours in serum-free, phosphate-free DMEM containing 200 μCi/ml 32 PO4. To optimize the dose and time of EGF treatment on Ras activation, cells were treated with various concentrations of EGF (1–100 ng/ml) for 5–60 minutes. Ras activation was determined according to a previously described method. 32 Briefly, Ras proteins contained in the cell lysates were immunoprecipitated with rat monoclonal anti-Ras antibody (Y13–259; Santa Cruz Biotechnology, Santa Cruz, CA). The guanine nucleotides bound to the Ras proteins were eluted in 16 μl of 2 mmol/L EDTA, 5 mmol/L dithiothreitol, 1 mmol/L GTP, 1 mmol/L GDP, 0.2% SDS at 68°C for 20 minutes and fractionated by thin-layer chromatography. Quantification was performed with a phosphorimager (Molecular Dynamics). The percentage of GTP bound to Ras (as an indicator of Ras activation) was calculated as cpm in GTP/(cpm in GTP + cpm in GDP) normalized for moles of phosphate in each nucleotide.
ERK2 Assay
ERK activity in RGM1 cells was determined as in our previous study. 11 In brief, serum-starved cells were treated with various concentrations of EGF (1–100 ng/ml) for 5–60 minutes. After incubation, cells were washed in cold phosphate-buffered saline and lysed in lysis buffer (100 mmol/L NaCl, 50 mmol/L HEPES (pH 7.5), 1 mmol/L EDTA, 1% NP-40, 1 μmol/L pepstatin, 0.2 mmol/L phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 1 μg/ml leupeptin, 0.2 mmol/L sodium orthovanadate, 40 mmol/L paranitrophenyl phosphate). ERK2 was immunoprecipitated from protein-normalized cell lysates (0.03 mg), using protein A Sepharose-antibody complex. Beads were washed twice with lysis buffer and twice with wash buffer (10 mmol/L HEPES, 10 mmol/L magnesium acetate, pH 7.5). Kinase reactions were performed in 30 μl of tracer buffer (4.0 μCi/tube [γ-32P]ATP, 50 μmol/L ATP, 10 mmol/L magnesium acetate, 7.5 mmol/L HEPES, pH 7.5) and 10 μl of myelin basic protein (MBP) (3.0 mg/ml) for 30 minutes at 30°C. Then 20 μl of 2× Laemmli sample buffer was added to each tube, and the samples were boiled for 5 minutes and subjected to 10% SDS-PAGE. The gel was stained with Coomassie blue R250, dried, and autoradiographed. After this, individual bands were cut out and counted for isotope labeling by liquid scintillation spectrometry.
Statistical Analysis
All data are presented as mean ± SD. Student’s t-test was used to determine the statistical significance between ulcers and gastric tissues of sham-operated rats. One-way analysis of variance followed by Bonferroni correction was used for multiple comparisons. A P value of <0.05 was considered to be statistically significant.
Results
Gastric Ulceration Stimulates Raf-1 Kinase Activity
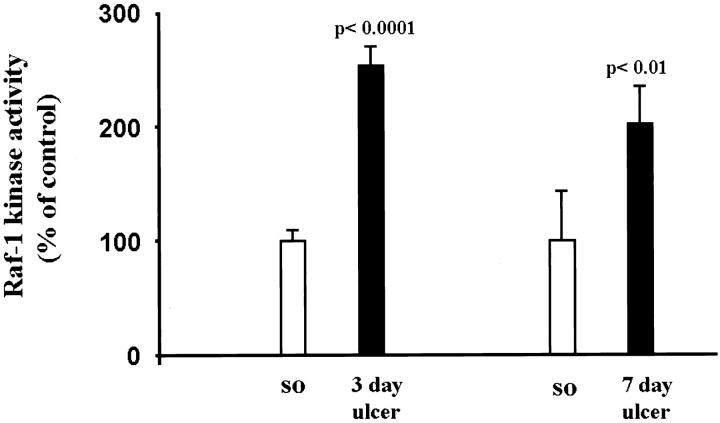
Because our previous study showed induction of the ERK cascade during gastric ulcer healing, 11 in the present study we attempted to determine the activators of this cascade. We determined the Raf-1 kinase activity by in vitro phosphorylation of inactive MEK, using immunoprecipitated Raf-1 kinase from normal (sham operated) and ulcerated gastric mucosa (Figure 1) ▶ . In ulcerated gastric mucosa there was about a twofold increase in Raf-1 activity versus controls (P < 0.01).
Figure 1.
Gastric ulceration stimulates Raf-1 kinase activity. Tissue lysates containing equal amounts of protein from normal (sham operated) and ulcerated gastric mucosa were immunoprecipitated with anti-Raf-1 antibody. Immunecomplexes were washed and subjected to in vitro kinase reaction, using [γ-32P]ATP and kinase inactive MEK as a substrate and analyzed by SDS-PAGE and autoradiography as described in Materials and Methods. Data are expressed as the mean ± SD (n = 6); each experiment was performed in triplicate.
Treatment of dissected ulcer margins with exogenous EGF for 15 minutes caused a 1.1-fold (statistically nonsignificant) increase in Raf-1 activity versus ulcer margins treated with medium alone. Treatment of dissected ulcer margins with exogenous EGF for 30 minutes did not cause any significant change compared with ulcer margins treated with medium alone.
Protein Kinase C Activity and Protein Level Are Down-Regulated during Gastric Ulcer Healing
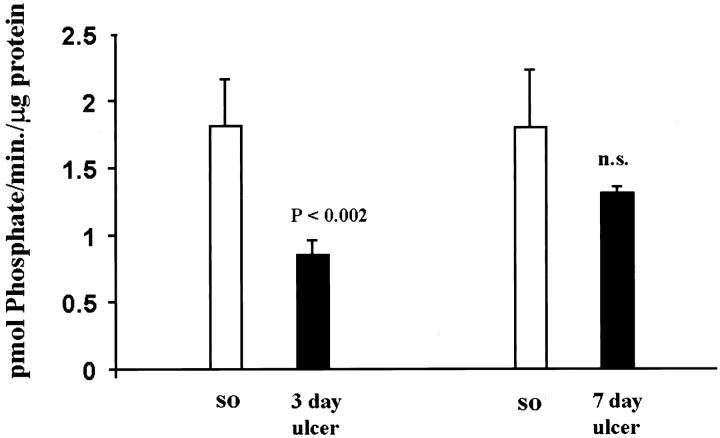
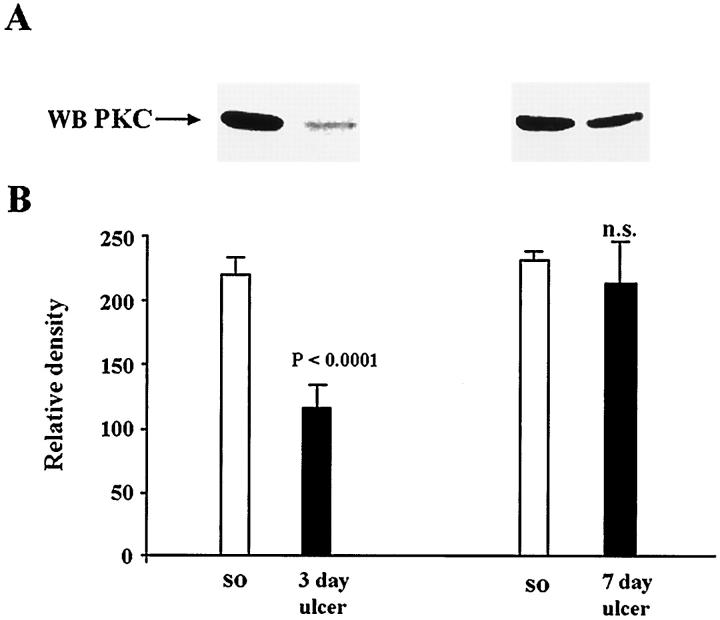
PKC has been shown to modulate ERK activity in several cell systems. 23-27 To investigate whether PKC is involved in ERK activation during gastric ulcer healing, we determined PKC activity and protein levels in normal and ulcerated gastric mucosa. In ulcerated gastric mucosa, PKC activity was significantly reduced at 3 days (2.1-fold decrease; P < 0.002) and 7 days (1.4-fold decrease) compared to controls (sham operated) (Figure 2) ▶ . PKC protein levels determined by Western blot analysis (Figure 3) ▶ showed a significant decrease at 3 days (1.9-fold decrease; P < 0.0001).
Figure 2.
Gastric ulceration down-regulates PKC activity. PKC activity was determined in normal (sham operated) and ulcerated gastric mucosa, using an assay kit as described in Material and Methods. Data are expressed as the mean ± SD (n = 6); each experiment was performed in triplicate.
Figure 3.
Gastric ulceration down-regulates PKC protein levels. Detergent-soluble lysates containing equal amounts of protein (0.15 mg) were subjected to Western blot analysis, using rabbit polyclonal anti-PKC antibody. A: Representative Western blot shows reduced PKC protein expression in ulcerated gastric mucosa when compared with normal (sham operated) controls. B: Quantitative analysis of PKC protein levels during ulcer healing. Data are expressed as the mean ± SD (n = 6).
Gastric Ulceration Triggers Shc Association with Grb2 Protein
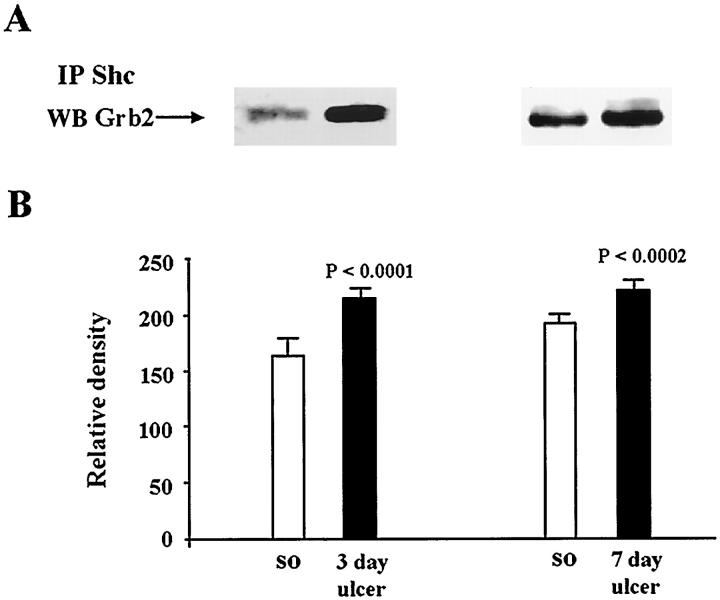
Because in some cell systems in vitro, growth factors such as EGF can induce activation of Ras through the recruitment of adapter proteins containing SH2 and SH3 domains such as Shc and Grb2, 15-17 in the present study we examined whether activation of Raf-1 during ulcer healing in vivo involves these adapter proteins. Immunoprecipitation of Shc protein followed by immunoblotting with anti-Grb2 antibody revealed that gastric ulceration significantly increases Shc binding to Grb2 at both 3 and 7 days (Figure 4) ▶ . At 3 days there was a 1.4-fold increase (P < 0.0001) and at 7 days there was a 1.2-fold increase (P < 0.0002) in Grb2 bound to Shc protein versus controls (sham operated).
Figure 4.
Analysis of Shc-Grb2 complexes in normal (sham-operated rats) and ulcerated gastric mucosa. Tissue lysates containing equal amounts of protein (0.4 mg/tube) were immunoprecipitated with anti-Shc antibody. Immunecomplexes were washed and subjected to SDS-PAGE and transferred onto nylon membranes. Blots were probed with mouse monoclonal anti-Grb2 antibody. A: Representative Western blot shows Grb2 bound to Shc in normal and ulcerated gastric mucosa. B: Quantitative analysis of Grb2 bound to Shc during ulcer healing. Data are expressed as the mean ± SD (n = 6).
Gastric Ulceration Increases Grb2 Association with Sos
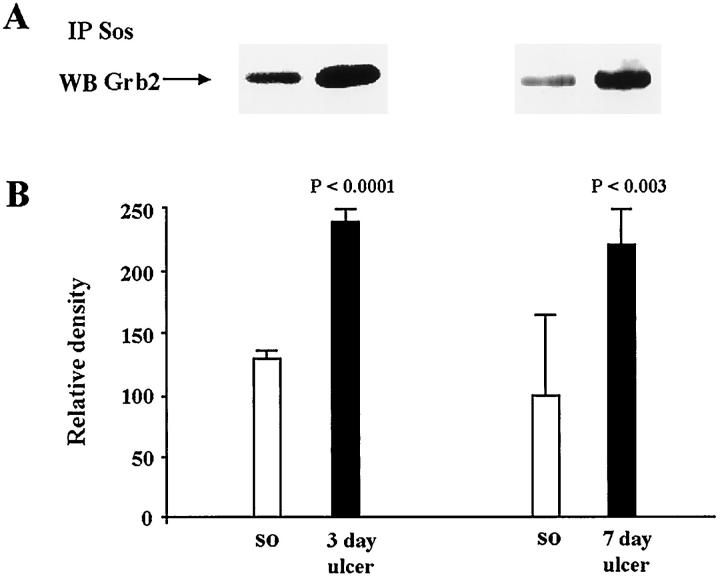
Because we found increased binding of Grb2 to Shc during ulcer healing, and because this association has been shown to result in recruitment of Sos and Ras activation, 15-18 we next examined whether the guanidine exchange factor Sos was bound to Grb2. This was analyzed by immunoprecipitation of Sos followed by immunoblotting with Grb2 antibody. In ulcerated gastric mucosa, the Grb2-Sos complex level was increased by 1.8-fold (P < 0.0001) at 3 days and by 2.2-fold (P < 0.002) at 7 days versus controls (sham operated) (Figure 5) ▶ .
Figure 5.
Analysis of Grb2-Sos complexes in normal (sham operated) and ulcerated gastric mucosa. Tissue lysates containing equal amounts of protein (0.4 mg/tube) were immunoprecipitated with anti-Sos antibody. Immunecomplexes were washed and subjected to SDS-PAGE and transferred onto nylon membranes. Blots were probed with mouse monoclonal anti-Grb2 antibody. A: Representative Western blot shows Grb2 levels that formed complex with Sos in normal and ulcerated gastric mucosa. B: Quantitative analysis of Grb2 bound to Sos during ulcer healing. Data are expressed as the mean ± SD (n = 6).
Shc, Grb2, Sos Protein Levels during Ulcer Healing
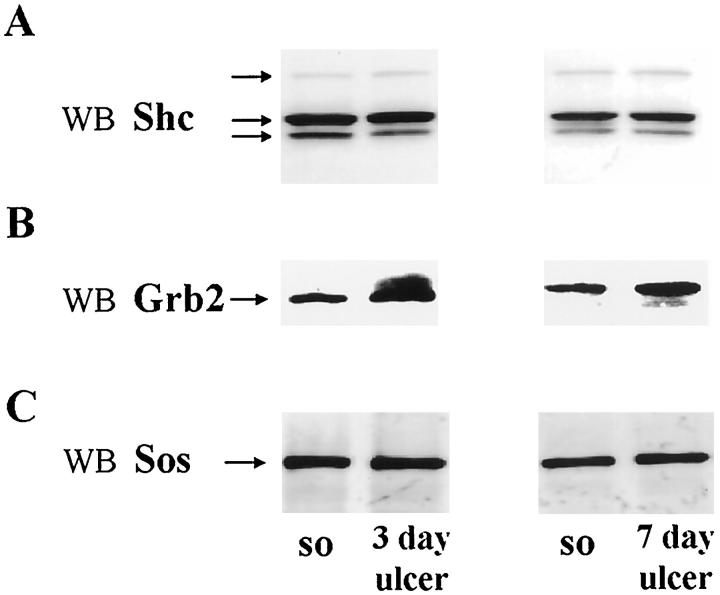
To determine whether the above increase in Shc-Grb2-Sos association reflects an increase in respective protein levels during ulcer healing, we performed Western blot analysis for Shc, Grb2, and Sos proteins. As shown in Figure 6A ▶ , all three isoforms of Shc protein (corresponding to 46, 52, and 66 kd) were expressed almost equally in both normal and ulcerated gastric mucosa. Grb2 showed substantial increases in protein levels in ulcerated mucosa versus controls at both 3 days (1.2-fold increase; P < 0.03) and 7 days (1.5-fold increase; P < 0.0001) (Figure 6B) ▶ . Gastric ulceration did not significantly affect Sos protein levels (Figure 6C) ▶ .
Figure 6.
Analysis of levels of adapter proteins Shc, Grb2, and Sos during gastric ulcer healing. Detergent-soluble lysates containing equal amounts of protein (0.15 mg) from normal (sham operated) and ulcerated gastric mucosa (n = 6) were subjected to Western blot analysis, using specific antibodies, as described in Materials and Methods. A: Representative Western blot shows Shc protein levels remaining unaltered during gastric ulcer healing. B: Representative Western blot shows substantial increase in Grb2 protein in ulcerated gastric mucosa at both 3 and 7 days after ulcer induction when compared with normal (sham operated) controls. C: Representative Western blot shows no significant change in Sos protein levels during ulcer healing.
Increased Ras Protein Expression during Gastric Ulcer Healing
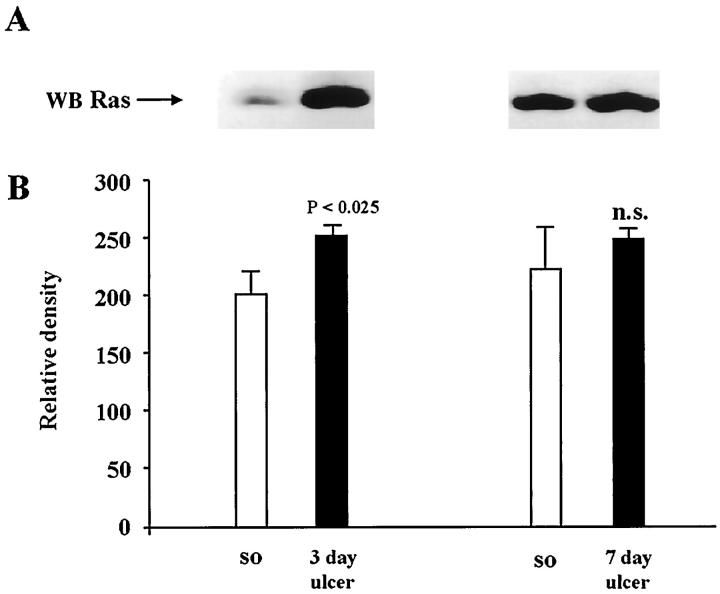
Because we observed significant increases in Shc-Grb2-Sos complex formation during ulcer healing, we attempted to determine whether Ras protein levels were affected. Immunoblot analysis showed that gastric ulceration significantly increased the Ras protein level at 3 days (P < 0.025) and sustained an increased level at 7 days (Figure 7) ▶ versus controls.
Figure 7.
Analysis of Ras protein expression during gastric ulcer healing. Detergent-soluble lysates containing equal amounts of protein (0.15 mg) were subjected to Western blot analysis using mouse monoclonal anti-Ras antibody. A: Representative Western blot shows reduced Ras protein expression in ulcerated gastric mucosa when compared with normal (sham operated) controls. B: Quantitative analysis of Ras protein levels during ulcer healing. Data are expressed as the mean ± SD (n = 6).
EGF Stimulates Ras Activation and ERK2 Activity but Not PKC Activity in Rat Gastric Epithelial Cells (RGM1) in Vitro
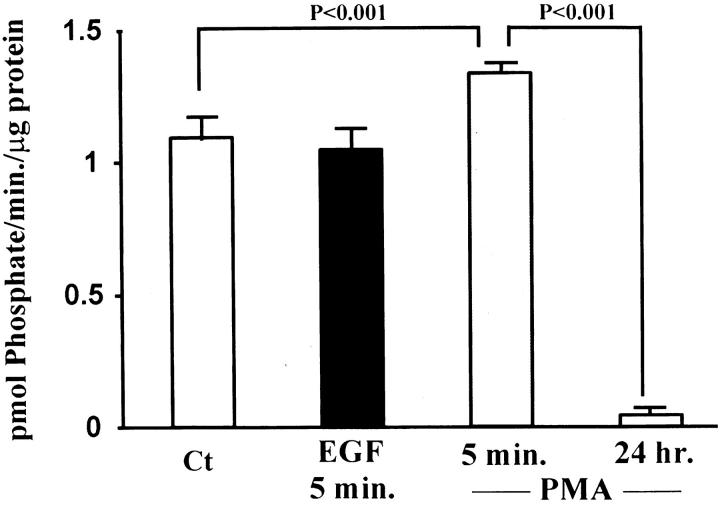
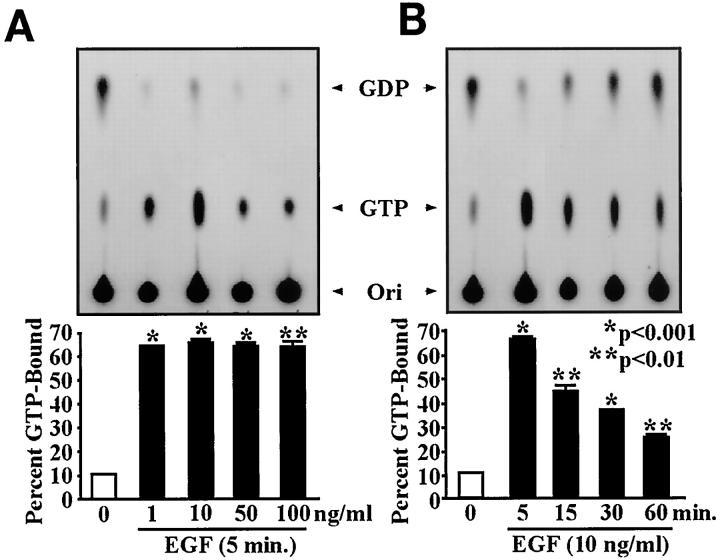
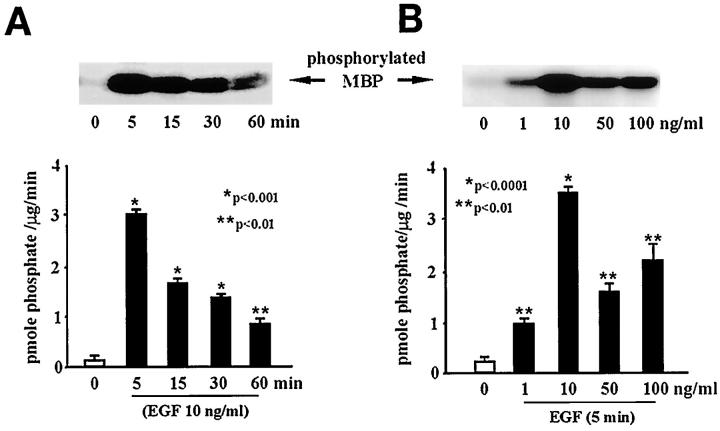
Previous studies showed that gastric ulceration triggers increased EGF-R expression 8 and ERK activity in epithelial cells of the ulcer margins. 11 In the present study we observed increased Ras protein and Raf-1 kinase activity levels in the ulcer margins. These findings prompted us to hypothesize that activation of the Ras–Raf-1–ERK cascade occurs predominantly in the epithelial component of the ulcer margins. To test this, we studied the effect of EGF on Ras activation and PKC and ERK activities in an epithelial cell line (RGM1) derived from normal rat gastric mucosa. EGF treatment (10 ng/ml; 5 minutes) did not alter PKC activity in rat gastric epithelial cells versus untreated controls (Figure 8) ▶ . However, treatment with 50 nM PMA for 5 minutes (positive control) significantly increased PKC activity. Prolonged treatment with 50 nM PMA (24 hours; negative control) significantly decreased PKC activity in RGM1 cells versus untreated controls, showing the efficacy of the signaling system in these cells. Dose-response studies for EGF-stimulated Ras activation and ERK2 activity (EGF 1–100 ng/ml; 5 minutes) demonstrated a maximum response at 10 ng/ml (Figures 9A and 10A) ▶ ▶ versus untreated controls. Time response studies for EGF-stimulated Ras activation and ERK2 activity (5–60 minutes; EGF 10 ng/ml) showed a maximum response at 5 minutes (Figures 9B and 10B) ▶ ▶ versus untreated controls.
Figure 8.
Effect of EGF treatment on PKC activity in a rat gastric epithelial cell line (RGM1). Serum-starved RGM1 cells were treated with EGF (10 ng/ml) for 5 minutes. Cell lysates were used to assess PKC activity, following the procedures described in Materials and Methods. EGF treatment of RGM1 cells does not significantly alter PKC activity when compared to untreated controls. Treatment of gastric epithelial cells with 50 nM PMA for 5 minutes (positive control) significantly increased PKC activity, whereas depletion of PKC by prolonged incubation (24 hours) with PMA (50 nmol/L) significantly decreased PKC activity. Data are expressed as the mean ± SD from three separate experiments performed in triplicate.
Figure 9.
Dose and time-dependent studies on EGF-stimulated Ras activation. Serum-starved RGM1 cells were treated with EGF 1–100 ng/ml for 5–30 minutes. Cell lysates were used to assess Ras activation levels, following the procedure described in Materials and Methods. EGF treatment significantly increased Ras activation, as assessed by the percentage of GTP binding versus controls. A: EGF treatment in doses ranging from 1 to 100 ng/ml caused a maximum response at 10 ng/ml. B: EGF treatment for various time intervals (5–60 minutes) caused a maximum response at 5 minutes. Data are expressed as the mean ± SD from three separate experiments.
Figure 10.
Dose- and time-dependent studies on EGF-stimulated ERK activity. Serum-starved RGM1 cells were treated with EGF 1–100 ng/ml for 5–30 minutes. Cell lysates were used to assess ERK activity levels, following the procedure described in Materials and Methods. EGF treatment significantly increased ERK2 activity, as assessed by levels of MBP phosphorylated by immunoprecipitated ERK2 versus untreated controls. A: EGF treatment in doses ranging from 1 to 100 ng/ml caused a maximum response at 10 ng/ml. B: EGF treatment for various time intervals (5–60 minutes) caused a maximum response at 5 minutes. Data are expressed as the mean ± SD from three separate experiments performed in triplicate.
Discussion
Several growth factors, including EGF, TGF-α, and basic fibroblast growth factor, have been shown to accelerate gastric injury repair and ulcer healing by stimulating cell migration and proliferation. 4-8 However, the cellular signaling pathways involved in these two processes in the in vivo condition are not fully elucidated. Our previous study demonstrated increased receptor tyrosine kinase activity and induction of the ERK cascade during ulcer healing. 11 Furthermore, it showed that treatment with Tyrphostin A46, a potent inhibitor of EGF-R kinase and EGF-R kinase-dependent cell proliferation, 33 dramatically delays ulcer healing, 11 clearly demonstrating the importance of the EGF-R–ERK signaling pathway in the healing process. Our present study shows that gastric ulceration increases Raf-1 kinase activity and Grb2 and Ras protein levels and triggers Shc-Grb2-Sos association. PKC activity and protein levels were down-regulated in ulcer margins. In a rat gastric epithelial cell line (RGM1), EGF significantly increased Ras activation and ERK activity, without affecting PKC activity, supporting our contention that activation of the Raf-1–ERK cascade occurs predominantly in the epithelial component of the ulcer margins. While EGF has been shown to play a crucial role in ulcer healing by activation of the ERK signaling pathway, 11 activation of this pathway by other growth factors may also be important. The gut epithelial motility itself (even in the absence of growth factors) might be expected to be associated with intracellular signals, 34 perhaps via integrin-mediated altered cell-matrix interaction. 35,36
The Ras and Raf-1 proto-oncogene products are key proteins in the transmission of a variety of intracellular proliferative and differentiation signals. Raf-1 and Ras serve as intermediates in these signaling pathways by connecting upstream tyrosine kinases with downstream serine/threonine kinases such as MAPK (or ERK) and MAPK kinase (MEK). 12,13 This phosphorylation cascade leads to activation of transcription factors involved in cell growth and differentiation. 12,13 Raf-1-dependent activation of ERK1 and ERK2 has been demonstrated in wounded intestinal epithelial monolayers, 37 in mechanically stretched rat cardiac myocytes, 38 and in shear stress exposed human endothelial cells, 39 but has not been explored during healing of injured gastric mucosa. In our present study, we demonstrated that gastric ulceration triggers a significant increase in Raf-1 activity. During ulcer healing the Raf-ERK signaling pathway is significantly activated, most likely by endogenous EGF produced by regenerating epithelial cells (ulcer-associated lineage). 9 Therefore, epithelial cells in the ulcer margin may have already attained submaximum stimulation due to overexpression of EGF and EGF-R 8,9 and may render them less susceptible to exogenous EGF. This could explain why treatment of ulcer margins with exogenous EGF causes only a very modest (nonsignificant) increase in Raf-1 activity when compared to untreated ulcer margins.
Potential activators of Raf-1 include protein kinase C and activated Ras. 14 While studies of a number of tissues have implicated PKC in the positive control of cell growth and transformation, some data also point to its involvement in cell growth inhibition and differentiation. 40 PKC represents a family of phospholipid-dependent serine/threonine kinases consisting of 11 members, including the conventional, novel, and atypical isozymes, which differ in their structural and biochemical properties. 41 Individual PKC isozymes are known to exhibit varying substrate specificities, tissue distributions, and subcellular localization. Although the understanding of biological functions of individual isozymes and the molecular regulatory pathways in which they participate remains limited, individual isozymes may play specific specialized roles in cell signaling.
Not only can PLC-γ1 activated by receptor tyrosine kinases generate DAG and subsequently activate PKC; it can also associate with SH2 domains of activated EGF-R and with both dynamin and Sos (through its SH3 domain) in EGF-stimulated Madin-Darby canine kidney cells. 22 Previous studies have shown increased tyrosine kinase activity and PLC-γ1 phosphorylation during the early phase of acute injury repair in rat gastric mucosa, which is a process that is entirely different from chronic gastric ulcer healing. 28,29 Our present finding that gastric ulceration down-regulates both PKC activity and protein levels indicates that ERK activation during gastric ulcer healing is PKC-independent. The observed reduction in both activity and protein levels of PKC could be due to either a lack or reduced availability of a co-factor necessary for PKC activation, 42 tightly controlled regulation preventing its inhibitory effect on cell cycle progression (as shown in intestinal epithelial cells (IEC-18)), 43 ] or loss of a particular cell type, eg, parietal cells, which have been shown to express high levels of PKC. 44 The latter possibility is supported by our previous study demonstrating a substantial reduction of the parietal cells in dilated glands lining the ulcer margin. 45 Furthermore, the substrate and the antibody used in the present study to measure PKC activity and the protein level, respectively, recognize only the conventional isoforms, α-, β-, and γ-isozymes (which share a high sequence homology), and not the novel isoforms, δ-, ε-, and ζ-isozymes (which have lower sequence homology). Therefore, a possibility that isozyme-specific PKC activation (eg, δ-, ε-, ζ-isozymes) may occur during chronic gastric ulcer healing cannot be completely ruled out by our studies.
Studies of various cell systems have shown that Shc, Grb2, and Sos link activated tyrosine kinases to Ras. 15,16 Previous studies have demonstrated increased receptor tyrosine kinase activity and increased EGF-R phosphorylation levels during gastric mucosal injury repair. 11,28,29 Our data demonstrating increased binding of adapter protein Grb2 to Shc and to Sos indicate their involvement as mediators of receptor tyrosine kinase signaling via Ras during ulcer healing. In the present in vivo study, we examined Ras protein expression during ulcer healing but were unable to determine Ras activation, because currently there is no available method allowing for this in an in vivo condition. Because the method of Ras activation measurement in vitro is well established, we used gastric epithelial RGM1 cells to study the effect of EGF on Ras activation. In this model, EGF dramatically increased Ras activation and ERK2 activity but not PKC activity, clearly indicating that in gastric epithelial cells, EGF-triggered induction of ERK activity is mediated by Ras and not by PKC.
In summary, this study demonstrates for the first time that during experimental gastric ulcer healing, Raf-1 activity is induced and that Raf-1 activation involves increased Shc-Grb2-Sos association. Furthermore, this study indicates that ERK activation during ulcer healing is not mediated by PKC. Similarly, treatment of rat gastric epithelial cells with EGF increased Ras and ERK activity but not PKC activity. Thus activation of the Raf-1–ERK cascade during gastric ulcer healing is Ras-mediated, independent of PKC, and is attributable to the epithelial component of ulcer margins.
Footnotes
Address reprint requests to Dr. Andrzej S. Tarnawski, Chief, Gastroenterology Section (111G), DVA Medical Center, 5901 East Seventh Street, Long Beach, CA 90822. E-mail: atarnawski@yahoo.com.
Supported by a Merit Review Award from the Department of Veterans Affairs to AST.
References
- 1.Tarnawski A: Cellular mechanisms of gastric ulcer healing. Domschke W Konturek SJ eds. The Stomach. 1993, :pp 177-192 Springer-Verlag, Berlin [Google Scholar]
- 2.Tarnawski A, Stachura J, Krause WJ, Douglass T, Gergely H: Quality of gastric ulcer healing: a new emerging concept. J Clin Gastroenterol 1991, 13:S42-S47 [DOI] [PubMed] [Google Scholar]
- 3.Wang JY, Johnson LR: Induction of gastric and duodenal mucosal ornithine decarboxylase during stress. Am J Physiol 1989, 257:G259-G265 [DOI] [PubMed] [Google Scholar]
- 4.Johnson LR: Regulation of gastrointestinal growth. Physiology of The Gastrointestinal Tract, vol 1, ed 3. Edited by LR Johnson. New York, Raven, 1994, pp 611–641
- 5.Konturek SJ, Dembinski A, Warzecha A, Brzozowski T, Gregory H: Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology 1988, 94:1300-1307 [DOI] [PubMed] [Google Scholar]
- 6.Podolsky DK: Peptide growth factors in the gastrointestinal tract. Physiology of the Gastrointestinal Tract, vol 1, ed 3. Edited by LR Johnson. New York, Raven, 1994, pp 129–167
- 7.Basson MD, Modlin IM, Madri JA: Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest 1992, 90:15-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H: Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology 1992, 102:695-698 [DOI] [PubMed] [Google Scholar]
- 9.Wright NA, Pike C, Elia G: Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature 1990, 43:82-85 [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Hansson H-A, Norstrom E, Helander HF: Immuno-reactivities for epidermal growth factor (EGF) and for EGF receptors in rats with gastric ulcer. Cell Tissue Res 1991, 265:211-218 [DOI] [PubMed] [Google Scholar]
- 11.Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski A: Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology 1998, 14:706-713 [DOI] [PubMed] [Google Scholar]
- 12.Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, Avruch J: Raf-1 activates MAP kinase-kinase. Nature 1992, 358:417-421 [DOI] [PubMed] [Google Scholar]
- 13.Dent P, Haser W, Haystead TAJ, Vincent LA, Roberts TM, Sturgill TW: Activation of mitogen-activated protein kinase by v-Raf in NIH 3T3 cells and in vitro. Science 1992, 257:1404-1407 [DOI] [PubMed] [Google Scholar]
- 14.Morrison DK: Mechanisms regulating Raf-1 activity in signal transduction pathways. Mol Reprod Dev 1995, 42:507-514 [DOI] [PubMed] [Google Scholar]
- 15.Buday L, Downward J: Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 1993, 73:611-620 [DOI] [PubMed] [Google Scholar]
- 16.Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J: The SH2 and SH3 domain-containing protein Grb2 links receptor tyrosine kinases to ras signaling. Cell 1992, 70:431-442 [DOI] [PubMed] [Google Scholar]
- 17.Pellici G, Lanfrancone L, Grignani G, McGlace J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pellici PG: A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 1992, 70:93-104 [DOI] [PubMed] [Google Scholar]
- 18.Pawson T, Schlessinger J: SH2 and SH3 domains. Curr Biol 1993, 3:434-442 [DOI] [PubMed] [Google Scholar]
- 19.Rhee SG: Inositol phospholipids-specific phospholipase C: interaction of the γ 1 isoform with tyrosine kinase. Trends Biochem Sci 1991, 16:297-301 [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Sotomayor SMT, Carpenter G: Non-catalytic activation of phospholipase C-γ in vitro by epidermal growth factor receptor. Biochem J 1993, 293:507-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meisenhelder J, Suh P, Rhee SG, Hunter T: Phospholipase C-γ is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell 1989, 57:1109-1122 [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Gluck S, Zhang L, Moran MF: Requirement for phospholipase C-γ1 enzyme activity in growth factor-induced mitogenesis. Mol Cell Biol 1998, 18:590-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sylvester PW, Birkenfeld HP, Hosick HL, Briski KP: Fatty acid modulation of epidermal growth factor-induced mouse mammary epithelial cell proliferation in vitro. Exp Cell Res 1994, 214:145-153 [DOI] [PubMed] [Google Scholar]
- 24.Kazlauskas A, Cooper JA: Protein kinase C mediates platelet-derived growth factor-induced tyrosine phosphorylation of p42. J Cell Biol 1988, 106:1395-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schonwasser DC, Marias RM, Marshall CJ, Parker PJ: Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 1988, 18:790-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blenis J: Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA 1993, 90:5889-5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis RJ: The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 1993, 268:14553-14556 [PubMed] [Google Scholar]
- 28.Relan NK, Fligiel SEG, Dutta S, Tureaud J, Chauhan DP, Majumdar APN: Induction of EGF-R tyrosine kinase during early reparative phase of gastric mucosa and effects of aging. Lab Invest 1995, 73:717-726 [PubMed] [Google Scholar]
- 29.Majumdar APN, Fligiel SEG, Jaszewski R, Tureaud J, Dutta S, Chelluderai B: Inhibition of gastric mucosal regeneration by tyrphostin: evaluation of the role of epidermal growth factor receptor tyrosine kinase. J Lab Clin Med 1996, 128:173-180 [DOI] [PubMed] [Google Scholar]
- 30.Morrison DK: Activation of Raf-1 by Ras in intact cells. Methods Enzymol 1995, 255:301-310 [DOI] [PubMed] [Google Scholar]
- 31.Jones MK, Sarfeh IJ, Tarnawski A: Induction of in vitro angiogenesis in the endothelial-derived cell line, EA hy926, by ethanol is mediated through PKC and MAPK. Biochem Biophys Res Commun 1998, 249:118-123 [DOI] [PubMed] [Google Scholar]
- 32.Downward J, Graves JD, Warne PH, Rayter S, Cantrell DA: Stimulation of p21ras upon T-cell activation. Nature 1990, 346:719-723 [DOI] [PubMed] [Google Scholar]
- 33.Harmon JW: Roles of growth factors in esophageal mucosal healing. J Gastroenterol Hepatol 1997, 12:A155 (Abstr) [DOI] [PubMed]
- 34.Perdikis DA, Basson MD: Basal nutrition promotes human intestinal epithelial (Caco-2) proliferation, brush border enzyme activity, and motility. Crit Care Med 1997, 25:159-165 [DOI] [PubMed] [Google Scholar]
- 35.Woodley DT: The Molecular and Cellular Biology of Wound Repair, ed 2. Edited by RAF Clark. New York, Plenum, 1996, pp 339–354
- 36.Martin P: Wound healing—aiming for perfect skin regeneration. Science 1997, 276:75-81 [DOI] [PubMed] [Google Scholar]
- 37.Goke M, Kanai M, Lynch-Devaney K, Podolsky DK: Rapid mitogen-activated protein kinase activation by transforming growth factor a in wounded rat intestinal epithelial cells. Gastroenterology 1998, 114:697-705 [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki T, Komuro I, Kudo S, Zuo Y, Shiojima I, Mizuno T, Takano H, Hiroi Y, Ueki K, Tobe K, Kadowaki T, Nagai R, Yazaki Y: Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J Clin Invest 1995, 96:438-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi M, Berk BC: Mitogen-activated protein kinase (ERK1/2) activation by shear stress and adhesion in endothelial cells. J Clin Invest 1996, 99:2623-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemens MJ, Trayner I, Menaya J: The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J Cell Sci 1992, 103:881-887 [DOI] [PubMed] [Google Scholar]
- 41.Dekker LV, Parker PJ: Protein kinase C—a question of specificity. Trends Biochem Sci 1994, 19:73-77 [DOI] [PubMed] [Google Scholar]
- 42.Nishizuka Y: The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988, 334:661-665 [DOI] [PubMed] [Google Scholar]
- 43.Frey MR, Saxon ML, Zhao X, Rollins A, Evans SS, Black JD: Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21waf1/cip1 and p27kip1 and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem 1997, 272:9424-9435 [DOI] [PubMed] [Google Scholar]
- 44.Tarnawski A, Hollander D, Krause WJ, Dabros W, Stachura J, Gergely H: Healed experimental gastric ulcers remain histologically and ultrastructurally abnormal. J Clin Gastroenterol 1990, 12:S139-S147 [DOI] [PubMed] [Google Scholar]
- 45.Anderson NG, Hanson PJ: Involvement of calcium-sensitive phospholipid-dependent protein kinase in control of acid secretion by isolated rat parietal cells. Biochem J 1985, 232:609-611 [DOI] [PMC free article] [PubMed] [Google Scholar]