Abstract
Mixed medullary-follicular carcinomas (MMFCs) are tumors of the thyroid that display morphological and immunohistochemical features of both medullary and follicular neoplasms. The histogenetic origin and possible molecular mechanisms leading to MMFCs are still unclear. To address these questions, we have isolated the two histological components of 12 MMFCs by (laser-based) microdissection, analyzed them for mutations in the RET proto-oncogene and allelic losses of nine loci on six chromosomes, and studied the clonal composition of MMFCs in female patients. Our results provide strong evidence that the follicular and medullary components in MMFCs are not derived from a single progenitor cell, because the seven tumors amenable for analysis consistently exhibited a different pattern of mutations, allelic losses, and clonal composition. We also demonstrate that follicular structures in MMFCs are often oligo/polyclonal and more frequently exhibit hyperplastic than neoplastic histological features, indicating that at least a subset of MMFCs are composed of a medullary thyroid carcinoma containing hyperplastic follicles.
Medullary thyroid carcinomas (MTCs) comprise 5–10% of all thyroid carcinomas. Although a majority of these tumors occur sporadically, about 20% have a familial background. 1 MTCs are assumed to evolve from the neural crest or ultimobranchial body-derived C-cells 2,3 and are regarded as being closely related to tumors of the disseminated neuroendocrine system. In the late 1970s it was noted that the histological appearance of MTC may be “atypical” and that follicular structures can be encountered in these tumors in addition to typical medullary features. 4 Subsequently it was shown that in addition to the characteristic calcitonin immunoreactivity in such atypical MTCs, thyroglobulin (Tg) was detectable in the foci having a follicular appearance, and that the same histological and immunohistochemical pattern was also present in their metastatic lesions. Hence it was proposed that these tumors might represent a new entity, which was termed “mixed medullary-follicular carcinoma” (MMFC). 5,6 In the second edition of Histological Typing of Thyroid Tumors, 7 Hedinger and associates defined MMFC as “tumors which show the morphological features of both a medullary carcinoma with immunoreactivity for calcitonin and a follicular carcinoma with immunoreactivity for thyroglobulin,” both in the primary tumor and metastatic lesions. Since these early reports, several authors have published results for mixed tumors fulfilling these WHO criteria. More recently there have also been reports on mixed tumors with papillary patterns 8-11 and MMFCs with a familial setting. 12-14 However, MMFCs exhibiting a dual endocrine and neuroendocrine differentiation appear to be rather rare, with little more than 30 well-documented tumors on record to date 5,6,8-13,15-22 . The existence of the entity of MMFC was further consolidated by demonstrating 1) Tg mRNA expression in follicular components 11,13 and 2) mixed patterns of follicles and MTCs in organ metastases. 16 Therefore, these data excluded absorption of Tg or cross-reaction of Tg antibodies to high-molecular-weight 27S Tg (so called C-Tg) in C-cells as causes for Tg positivity in MMFCs and the presence of nonneoplastic follicles, which rarely occur in regional lymph nodes, 23 as a cause for mixed patterns in lymph node metastases of MMFCs.
Because of the variety of patterns and admixed components in MMFCs, several terms have been proposed to designate these neoplasms, including “mixed follicular–parafollicular carcinoma,” 20 “compound medullary-papillary carcinoma,” 8 “composite carcinomas of the thyroid,” 24 “differentiated thyroid carcinoma, intermediate type,” 25 and “stem cell carcinoma.” 9 The last term was proposed because the majority of authors believe that MMFCs are derived from neoplastically transformed uncommitted stem cells with the capacity to differentiate into tumor components with morphological and histochemical characteristics of both follicular and medullary neoplasms of the thyroid. 9,10,12,13,17,18,20-22 This hypothesis was further supported by reports demonstrating Tg and CT coexpression in tumor cells of MMFCs 13,26 and the development of occasional mixed tumors with Tg-positive cells in a transgenic murine model for MTC. 27 However, the stem cell theory has never been proved by direct evidence. Thus the embryological background and possible molecular mechanisms leading to MMFC are still unclear.
The aim of the present study was to determine whether the two tumor components in MMFC are derived from the same cell clone or whether they arise independently. For this purpose, the two tumor components of each MMFC were separated by (laser-based) microdissection and individually analyzed for allelic losses at nine different chromosomal loci, somatic mutations of the RET and gsα gene, as well as clonal composition in female patients. 28,29
Materials and Methods
Materials
A total of 12 MMFCs of the thyroid were collected from the files of the Mayo Clinic (Rochester, MN) (five cases), the Department of Pathology, University of Turin (Turin, Italy) (six cases), and the Department of Pathology, University of Zürich (Zürich, Switzerland) (one case). Tumor specimens had been fixed in 10% buffered formaldehyde solution and embedded in paraffin according to standard procedures.
All tumors had previously been studied by conventional histology, immunohistochemistry, or in situ hybridization. 5,11 Ten tumors met all histopathological criteria for the diagnosis of MMFC as defined by the WHO classification. 7 One tumor (file number T5; Table 1 ▶ , Figure 1 ▶ ), which presented as a primary tumor with components of a MTC and follicular variant of a papillary carcinoma, exhibited papillary features in the lymph node metastases together with a medullary component. Another tumor (file number T2) was included in the study because of the mixed medullary-papillary features encountered in the lymph node metastases despite the fact that two separate primary papillary and medullary carcinomas were identified simultaneously in the same thyroid lobe (Figure 1) ▶ .
Table 1.
Clinicopathological Data
| No. | Sex/age | Location/size (cm) | Pathology | Metastasis/recurrences | Follow-up, years |
|---|---|---|---|---|---|
| T1 | M/68 | L/4 | MTC with Tg-positive follicles (5%)*+ solid follicular carcinoma (40%) | Lymph nodes (mixed) (10%) | NED 1 |
| T2 | F/53 | L/5 | Papillary carcinoma and MTC | Lymph node (mixed) (5%) | DOD 8 |
| T3 | M/55 | n.n. | MTC with Tg-positive follicles (15%)+ focus of follicular carcinoma | None | NED 5 |
| T4 | M/74 | R/2 | MTC with Tg-positive follicles (25%) | None | DOD 1 |
| T5 | M/68 | Isthmus/3 | MTC with Tg-positive follicles (15%) | Lymph nodes (mixed) (10%) | DOD 8 |
| Local recurr. (2y)/bone (3y) | |||||
| T6 | M/68 | n.n. | Oxyphilic carcinoma (80%)+ MTC | Lung | DOD 1 |
| M7 | F/26 | L/2.5 | MTC with Tg-positive follicles (10%) | None | NED 0.4 |
| M8 | F/49 | R/1+ L/0.2 MEN2A | MTC with Tg-positive follicles (15%) | None | NED 3.2 |
| M9 | F/42 | L/5.5 | MTC with Tg-positive follicles (15%) | Mediastinum | n.n. |
| M10 | M/58 | L/n.n. | MTC with Tg-positive follicles (25%) | None | NED 1 |
| M11 | M/69 | R/1.7 + L/1.1 MEN2A | MTC with Tg-positive follicles (10%) | Lymph nodes (mixed) (5%) | AWD 3 |
| Z12 | M/35 | R/5 | MTC with Tg-positive follicles (15%) | Lymph nodes (mixed) (5%) | n.n. |
| Local recurr. (1y)/liver (2y) |
L, left lobe; R, right lobe; n.n., not known; MTC, medullary thyroid carcinoma; Tg, thyroglobulin; NED, no evidence of disease; AWD, alive with disease; DOD, died of disease; y, years.
*Approximate percentage of the follicular component.
Figure 1.
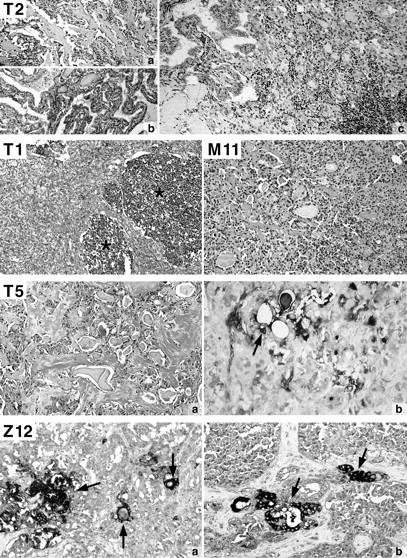
Histological appearance of some studied mixed medullary-follicular thyroid carcinomas. T2: Two separate primary tumors (a: medullary thyroid carcinoma; b: papillary carcinoma) and collision tumor in the lymph node metastasis (c). T1: Mixed tumor with a solid follicular tumor component (*). M11: Mixed tumor with approximately 10% thyroglobulin-positive follicles. T5: Mixed tumor with thyroglobulin-positive (arrow; b) follicles and amyloid stroma in the medullary part (a). Z12: Thyroglobulin-positive follicles (arrows) in a mixed tumor (a: primary; b: lymph node metastases). T2, T1, M11, T5a: Hematoxylin and eosin (H&E) ×200. T5b, Z12: Double immunostaining with black reaction product for thyroglobulin and red for calcitonin, ×200.
Clinical data were collected and follow-up information was obtained for all but two patients (Table 1) ▶ .
Immunohistochemistry
Freshly cut sections from archival paraffin blocks were stained with hematoxylin and eosin (H&E) and used for light microscopic examination. Serial sections (4 μm) of all 12 tumors were cut and mounted on Superfrost Plus glass slides (Menzel Gläser, Germany). Immunoreactive sites for calcitonin (CT) (polyclonal, 1:200; Dako, Glostrup, Denmark) and thyroglobulin (Tg) (monoclonal, 1:50; Dako) were detected using the avidin-biotin-complex (ABC) systems. 30
Double immunostaining of CT and Tg was performed using the APAAP system 31 and naphthol phosphate/fast red TR (Dako) as a chromogen for CT, and subsequently the ABC system and diaminobenzidine (Walter, Kiel, Germany) for Tg immunohistochemistry.
Molecular Analyses
DNA Extraction
Tumor DNA was isolated from five 10-μm paraffin sections of each tumor as described. 29 In 11 of the 12 cases, nontumorous tissue of the same patient was also available, and DNA was extracted as well.
Selected cases were submitted to laser-based microdissection (PALM Laser-Microbeam Systems GmbH, Germany) 32,33 to obtain isolated single neoplastic follicles and single nests of medullary carcinoma. The dewaxed and hematoxylin-stained tissue was placed in 0.5-ml Eppendorf tubes (Eppendorf GmbH, Hamburg, Germany) and boiled for 10 minutes at 94°C in 18 μl 1× polymerase chain reaction (PCR) buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl; GeneAmp, Perkin Elmer, Roche, NJ). Then 0.5 μg of proteinase K diluted in 1 μl of double-distilled water (ddH2O) was added to each sample and incubated at 55°C for 24 hours. Then samples were incubated for 10 minutes at 94°C to inactivate the proteinase K. Next 7.5 μl of the solution was added to the appropriate PCR mixture for further PCR amplification, under the conditions described below.
For clonality analysis, 29 microdissected tissue samples were placed in 0.5-ml Eppendorf tubes and boiled for 10 minutes at 94°C in 18 μl 1× restriction enzyme Buffer L (Boehringer Mannheim GmbH, Mannheim, Germany). After proteinase K treatment, as described above, 10 U of HpaII (in 1 μl of ddH2O) was added, and the samples were incubated at 37°C overnight. Then inactivation of the enzyme was performed at 94°C for 10 minutes, and 7.5 μl of the final solution was used as a template for further PCR amplification.
Nonisotopic PCR-SSCP and HDE RET Mutation Analysis
All 12 cases were screened for somatic or germline mutations of the RET proto-oncogene in exons 10, 11, 15, and 16 by the PCR-based single-strand conformation polymorphism (SSCP) and heteroduplex gel electrophoresis (HDE) analysis as recently described, 34 using primers and PCR conditions as detailed in Table 2 ▶ . Two tumors (file numbers M8 and M11) were from known MEN 2A patients.
Table 2.
PCR Primers
| Primer | Orientation | Sequence | PCR product (bp) | Ann. T† |
|---|---|---|---|---|
| RET primers (exons 10, 11, 15, 16) | ||||
| 10 | Sense | 5′-GCGCCCCAGGAGGCTGAGTG-3′ | ||
| Antisense | 5′-CGTGGTGGTCCCGGCCGCC-3′ | 182 | 63°C | |
| 10N* | Sense | 5′-CTCAGGGGGCAGCATTGTT-3′ | ||
| Antisense | 5′-CACTCACCCTGGATGTCTT-3′ | 132 | 56°C | |
| 11 | Sense | 5′-CCTCTGCGGTGCCAAGCCTC-3′ | ||
| Antisense | 5′-TGTGGGCAAACTTGTGGTAGCA-3′ | 155 | 58°C | |
| 15 | Sense | 5′-GACTGCTGCTATTTTTCCTC-3′ | ||
| Antisense | 5′-ATGGTGCACCTGGGATCCCT-3′ | 199 | 53°C | |
| 15N* | Sense | 5′-TGCTATTTTTCCTCACA-3′ | ||
| Antisense | 5′-CCGGGACTGGGCAC-3′ | 155 | 48°C | |
| 16 | Sense | 5′-AGGGATAGGGCCTGGGCTT-3′ | ||
| Antisense | 5′-TAACCTCCACCCCAAGAG-3′ | 192 | 56°C | |
| 16N* | Sense | 5′-AGAGTTAGAGTAACTTCAATGTC-3′ | ||
| Antisense | 5′-TAACCTCCACCCCAAGAGA-3′ | 151 | 45°C | |
| Gsα gene primers (exons 8 and 9) | ||||
| GsE8 | Sense | 5′-TTACTGTTTCGGTTGGCT-3′ | ||
| Antisense | 5′-AGAGGGACTGGGGTGAA-3′ | 192 | 52°C | |
| GsE8N* | Sense | 5′-CGGTTGGCTTTGGTGAGA-3′ | ||
| Antisense | 5′-CAGAAACCATGATCTCTGT-3′ | 163 | 52°C | |
| GsE9 | Sense | 5′-TTTCTTGACATTCACCCCAG-3′ | ||
| Antisense | 5′-AGCGACCCTGATCCCTAA-3′ | 186 | 56°C | |
| GsE9N* | Sense | 5′-CACCCCAGTCCCTCTGGAA-3′ | ||
| Antisense | 5′-CGTTCTTTACGAACAGCCA-3′ | 140 | 62°C | |
| Androgen receptor primers (exon 1) | ||||
| AR | Sense | 5′-GAGGAGCTTTCCAGAATCTG-3′ | ||
| Antisense | 5′-GATGGGCTTGGGGAGA-3′ | 233 | 59°C | |
| ARN* | Sense | 5′-TCCAGAATCTGTTCCAGAGC-3′ | ||
| Antisense | 5′-TGGGGAGAACCATCCTCACC-3′ | 216 | 59°C |
*Nested primers.
†Specific annealing temperature.
Blood-derived DNA of healthy persons and from patients carrying RET point mutations were used as negative and positive controls, respectively.
Restriction Analysis
Twenty microliters of PCR products from RET exons 15 and 16 was digested overnight at 37°C in 50 μl of medium and low-salt buffer containing 10 U of the restriction enzyme AluI (exon 15) and FokI (exon 16), respectively (Boehringer Mannheim). Restriction fragments were analyzed by 6% polyacrylamide gel electrophoresis and silver staining. 35
Nonisotopic PCR-SSCP Gsα Gene Mutation Analysis
Four MMFCs (file numbers T1–3 and T6) with a clearly distinct neoplastic follicular or papillary component were analyzed for mutations in exons 8 and 9 of the Gsα gene. Oligonucleotide primers flanking exons 8 and 9 (Table 2) ▶ were added in a concentration of 1 mmol/L in a 50-μl mixture containing 100–300 ng of template DNA, 0.2 mmol/L dNTPs, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, and 1 U of Taq DNA polymerase (Boehringer Mannheim). Thirty-five cycles of amplification were performed with a programmable thermal cycler (GeneAmp PCR System 9600), and the SSCP method was performed as described for the RET analysis. Nested primers were used for an additional amplification in cases with low DNA yield.
Loss of Heterozygosity Analysis
Eleven MMFC (T2–Z12), from which additional nontumorous tissue from the same patient was available, were examined for loss of heterozygosity (LOH) in microsatellite regions of chromosomes 1, 3, 7, 10, 11, and 22, using a recently described PCR-based approach. 36,37 The primer pairs D1S188, D3S1110, D3S1100, D7S480, D7S490, D22S257, and D22S1043 were purchased from Research Genetics (Huntsville, AL). The chromosomal location, the annealing temperatures, and the sequence of the other two primers, D10S564 and D11S4936, are detailed in Table 3 ▶ . For LOH analysis, only patients heterozygous for a given DNA sequence were considered informative, whereas the presence of either homozygosity or an unclear distinction between paternal and maternal alleles was considered uninformative. A twofold difference in relative allele intensity ratios between tumor DNA and normal DNA was considered as allelic loss.
Table 3.
Microsatellite Primers
| Primer | Chromosomal location | PCR product (bp) | Ann. T† |
|---|---|---|---|
| D1S188 | 1p32 | 149–173 | 55°C |
| D3S1110 | 3p25.3-p25.1 | ∼66 | 50°C |
| D3S1100 | 3p22-p21.3 | 154–170 | 52°C |
| D7S480 | 7q31-q35 | 189–206 | 55°C |
| D7S490 | 7q31-q35 | 92–106 | 55°C |
| D10S564 | 10q23 | 252–262 | 55°C |
| (Sense: 5′-TGGGAATGTGTCTTTATCCA-3′ | |||
| Antisense: 5′-AGCTCTAACATAGAGGCCAGAT-3′)* | |||
| D11S4936 | 11q13 | ∼142 | 55°C |
| (Sense: 5′-GCTTGCAGTGAGCCGAGATTG-3′ | |||
| Antisense: 5′-CAAAACAACGACACAAAAAAGCC-3′)* | |||
| D22S257 | 22q11.2 | 108–130 | 55°C |
| D22S1043 | 22q13 | 108–130 | 55°C |
*Sequences of the related primer.
†Specific annealing temperature.
Clonality Analysis
The clonal composition of the tumors from the four female patients (T2, M7, M8, M9) was studied by analyzing the inactivation patterns of a polymorphic X-linked region encoding the androgen receptor (AR) gene as recently described. 29 Patients were considered heterozygous if PCR amplification of digested and undigested DNA from nonneoplastic tissue showed two major PCR products. Genomic DNA from paraffin-embedded tissue of a monoclonal non-Hodgkin lymphoma (female) patient and of a healthy male individual were used as controls for HpaII digestion.
Results
The clinicopathological findings and the morphological patterns of the tumors, which have been discussed in detail in previous reports, 5,11 are shown in Figure 1 ▶ and are summarized in Table 1 ▶ .
The ratio of the two tumor components varied from 10% to 80% for the medullary portion either separate from (two cases) or intermingled with the follicular component (eight cases) or both (two cases). When clearly distinct from the medullary portion, the follicular component presented various morphological features, ranging from well-differentiated papillary and follicular carcinoma to oxyphilic and poorly differentiated (solid) carcinoma. In five cases, mixed features were found in the lymph node metastases (T1, T2, T5, M11, Z12). In patient T2, a mixed papillary/medullary lymph node metastasis was present, although the primary tumors were topographically separated (Figure 1) ▶ . In patient T5, a lymph node metastasis with features of a cystic papillary carcinoma associated with a MTC in the cyst wall developed from a MTC with prominent follicular features (follicular variant of papillary carcinoma) in the primary tumor (not shown). The other three tumors (T1, M11, and Z12) with lymph node metastases exhibited similar histological features in the primary tumors and their metastases (eg, Z12, Figure 1 ▶ ).
All tumors stained positively for calcitonin in the medullary but not in the follicular component, whereas thyroglobulin immunostaining was observed both in follicular (all tumors) and in solid tumor areas (7/12 cases).
Mutation Analysis
The results are summarized in Table 4 ▶ , and representative examples are shown in Figures 2 and 3 ▶ ▶ . The sequence of RET exons 10, 11, 15, and 16 was studied in tumor DNA of all tumors and in the related germline DNA from normal tissue of 11 patients. Two of our patients (M8 and M11) were known MEN2A disease gene carriers.
Table 4.
Results
| No. | Sample | RET | Gsα gene | LOH | Clonality (HUMARA) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon | Type | D1S188 | D3S1110 | D3S1100 | D7S480 | D7S490 | D10S564 | D11S4936 | D22S257 | D22S1043 | ||||
| T1 | MTC | — | — | nm | nm | nm | nm | nm | nm | nm | nm | nm | ||
| FOL | — | — | ||||||||||||
| T2 | MTC | 16 (M918T) | S | □ | □ | □ | □ | □ | ni | ni | □ | □ | ni | |
| FOL | — | — | □ | ▪ | □ | □ | □ | ni | ni | □ | □ | ni | ||
| T3 | MTC | — | — | □ | □ | □ | □ | □ | □ | □ | □ | □ | ||
| FOL | — | — | □ | □ | □ | □ | □ | □ | □ | □ | □ | |||
| T4 | MTC | — | np | np | □ | □ | ▪ | np | □ | np | np | |||
| FOL(μdissect.) | □ | □ | ||||||||||||
| T5 | MTC | 15 (A883F)* | S | np | □ | np | ▪ | ▪ | np | □ | □ | □ | ||
| FOL(μdissect.) | — | □ | □ | |||||||||||
| M6 | MTC | — | — | □ | □ | □ | □ | □ | □ | □ | □ | □ | ||
| FOL | — | — | □ | □ | □ | □ | □ | □ | □ | □ | □ | |||
| M7 | MTC | 11 (C634W) | G | □ | □ | □ | □ | □ | ni | □ | □ | □ | M | |
| FOL(μdissect.) | P | |||||||||||||
| MIXED | P | |||||||||||||
| M8 | MTC | 10 (C618A) | G | np | □ | □ | np | np | □ | □ | □ | □ | M | |
| FOL(μdissect.) | M | |||||||||||||
| MIXED | P | |||||||||||||
| M9 | MTC | — | ▪ | □ | ni | □ | □ | □ | □ | □ | □ | M | ||
| FOL(μdissect.) | □ | P | ||||||||||||
| MIXED | P | |||||||||||||
| M10 | MTC | — | □ | □ | □ | □ | □ | □ | □ | □ | □ | |||
| M11 | MTC | 10 (C618S) | G | □ | □ | □ | □ | □ | □ | ni | □ | □ | ||
| Z12 | MTC | 16 (M918T) | S | □ | □ | □ | □ | □ | □ | np | np | np | ||
| FOL(μdissect.) | — |
MTC, medullary component; FOL, follicular component; MIXED, mixed component; S, somatic; G, germline; *mutation present in subclones of tumor cells; np, no successful PCR; ni, not informative; nm, no normal tissue available; □, retention of heterozygosity; ▪, loss of heterozygosity; M, monoclonal; P, polyclonal.
Figure 2.
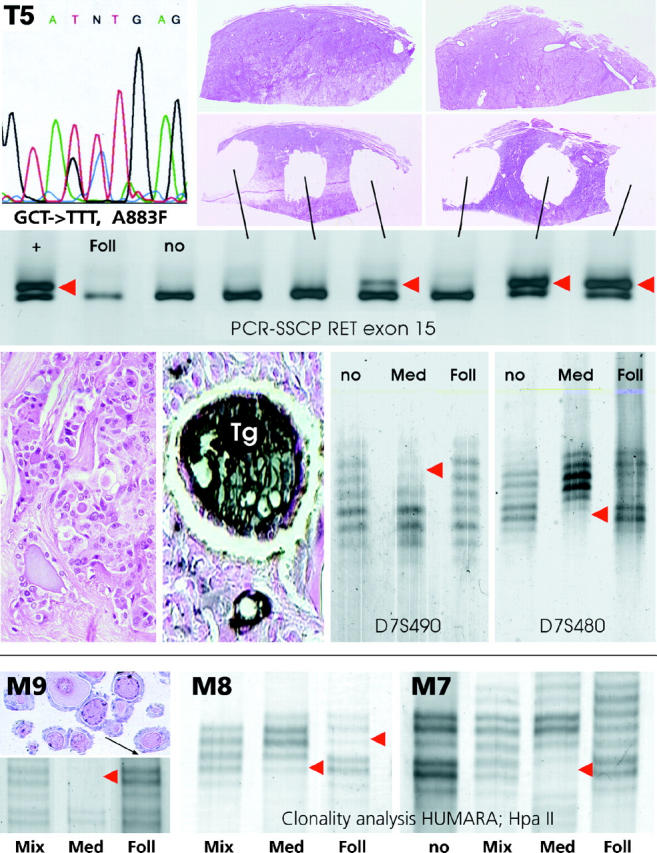
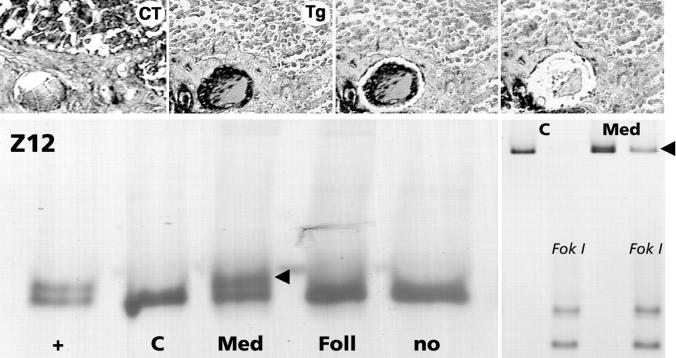
Mutation analysis (top) and loss of heterozygosity analysis (middle) of microdissected medullary and follicular components from a mixed medullary-follicular carcinoma (T5). Note that in the medullary carcinoma portion the single-strand conformation analysis of RET exon 15 exhibits aberrant band patterns (red arrowheads) in a subset of the tumor (which is indicative of the presence of a somatic A883F missense mutation; upper left) but not in the follicular component (Foll) or nontumorous tissue (no). Note also that a loss of heterozygosity at the microsatellite loci D7S490 and D7S480 is only detectable in the medullary portion (Med) of the tumor (red arrowheads) and not in the microdissected thyroglobulin-positive (Tg) follicular part (Foll). Bottom, M9, M8, M7: Comparison of the X-chromosomal inactivation (clonality) pattern in the microdissected medullary carcinoma and follicular components of three mixed medullary-follicular thyroid carcinomas from informative female patients (for details see Materials and Methods). Note that all three tumors exhibit a monoclonal pattern in the medullary portion (Med), which is defined by the loss of one allele after HpaII digestion of DNA (red arrowheads), whereas a polyclonal pattern similar to that in normal tissue (no) or whole tumor extract (Mix) is encountered in the follicular component (Foll) of two tumors (M9 and M7), and a monoclonal pattern with inactivation of the opposite allele is present in the third tumor (M8).
Figure 3.
Mutation analysis of RET exon 16 in the microdissected calcitonin-positive (CT) medullary and thyroglobulin-positive (Tg) follicular portions of a mixed medullary-follicular thyroid carcinoma (lymph node metastasis; Z12). Note that a heteroduplex formation (lower left, red arrowhead) is only detectable in the medullary (Med) but not in the follicular (Foll) tumor component. The presence of a somatic M918T point mutation was confirmed by FokI restriction analysis of PCR products (lower right, red arrowhead).
PCR-SSCP analysis of RET exon 10 revealed single-strand conformation variants (SSCVs) in both tumor and germline DNA of two different patients, and the sequence analysis of PCR products revealed two different germline missense mutations at codon 618, C618A (M8) and C618S (M11). The analysis of RET exon 11 revealed a SSCV in one patient (M7), and the sequencing analysis revealed a mutation in normal and tumor DNA at codon 634 (C634W), defining this patient as a thus far unknown MEN2A disease gene carrier. The analysis of RET exon 15 revealed abnormal SSCP patterns in tumor DNA of one patient (T5). Because no SSCP variation could be detected in the DNA from a metastasis of the same patient, we dissected six different regions of the primary tumor and were able to demonstrate the presence of the abnormal band only in three subpopulations of tumor cells (Figure 2 ▶ , T5). Sequence analysis revealed the presence of a mutation at codon 883 (A883F), which was confirmed by AluI restriction enzyme digestion (not shown). Microdissected follicles from the positive MMFC regions, however, showed no SSCP alterations.
The heteroduplex assay for exon 16 showed abnormal patterns in tumor DNA of two patients (T2 and T12). In one tumor (T2) aberrant band patterns (heteroduplices) were present in PCR products from DNA of the MTC but not of the papillary component. The same result was obtained for the other tumor (Z12), in which follicles were microdissected from the lymph node metastasis (Figure 3) ▶ . The presence of a M918T mutation at codon 918 in the positive samples was confirmed by FokI restriction enzyme analysis.
No mutations were found in the Gsα gene in any MMFC (data not shown).
LOH Analysis
We analyzed allelic losses of nine loci on six chromosomes in the MTC and follicular components of all MMFCs. Three tumors exhibited LOH in the MTC portion (one each at D1S188 and D7S490; and one tumor at both D7S480 and D7S490), which was not present in the respective microdissected follicular component of the MMFC (Table 4 ▶ , Figure 2 ▶ ). One additional MMFC had a LOH at D3S1110 in the follicular tumor, part which was not detectable in the MTC component (data not shown).
Clonality Analysis
Four MMFCs were from female patients, and three of these were informative for PCR-based clonality analysis by identification of X-chromosomal inactivation. In all three MMFCs, a monoclonal X-chromosomal pattern was identified in the medullary portion, but the respective follicular components exhibited a polyclonal pattern in two patients (Figure 2 ▶ ; M9, M7) and a monoclonal pattern with inactivation of the allele opposite that of the medullary component in the remaining MMFC (Figure 2 ▶ ; M8).
Discussion
The most interesting and equally controversial aspect of MMFC is its histogenetic and pathogenetic origin. The various hypotheses proposed to explain the occurrence of MMFCs and the molecular changes to be expected are listed in Table 5 ▶ . We report here the first molecular analysis of the two components in MMFC of the thyroid. Our data strongly suggest that the follicular and medullary components in MMFCs are not derived from a single stem cell, because they consistently exhibited different patterns of RET proto-oncogene mutation, LOH, and X-chromosomal inactivation (clonality) in seven tumors that were suitable for clonal analysis or had detectable molecular aberrations at the genes and loci investigated (Table 4) ▶ . Furthermore, we demonstrate that the follicular structures in MMFCs are often polyclonal and more frequently exhibit hyperplastic than neoplastic histological characteristics.
Table 5.
Hypotheses about the Pathogenesis of Mixed Thyroid Tumors
| Hypothesis | Expected results from the two components | |
|---|---|---|
| Clonality assay | LOH/mutation assay | |
| “Stem cell” theory | Same clonality | Same (similar) pattern |
| “Divergent differentiation” | Same clonality | Different (similar) pattern |
| “Field effect” | Different clonality | Different pattern |
| “Collision effect” | Different clonality | Different pattern |
| “Hostage” theory | Different clonality | Different pattern |
The currently favored “stem cell” theory of MMFC is based on studies of chicken and dog thyroid in which ultimobranchial remnants can give rise to both thyroid follicles and C-cells, 38,39 a finding that could be confirmed for humans as well. 3 Thus it was proposed that the neoplastic transformation of an uncommitted stem cell from the ultimobranchial body might give rise to MTC with dual (endocrine and neuroendocrine) differentiation. According to the stem cell hypothesis, the two tumor components of MMFCs should exhibit the same X-inactivation pattern (as a sign for clonal growth), and molecular alterations such as LOH and mutational status should be very similar (Table 5) ▶ . However, our findings clearly demonstrate differences for both the clonal and the LOH/mutation pattern of the two tumor components. Specifically, in three MMFCs of female patients the MTC component exhibited a monoclonal pattern, whereas the follicle part displayed a polyclonal pattern in two tumors and a monoclonal pattern with inactivation of the opposite allele in one tumor. Furthermore, whereas three MMFCs demonstrated a LOH at different loci in the medullary part, a normal composition was detectable in the follicular part of the same patients. In an additional tumor we found a LOH at the locus D3S1110 in the follicular component but not in the MTC part. Our mutation analysis revealed additional differences: three sporadic MMFCs contained somatic RET mutations that could not be detected in their follicular components or in one examined lymph node metastasis. In summary, different molecular and clonal patterns were found in the two components of all seven MMFCs amenable for molecular analysis by three independent molecular approaches.
Therefore, the present results do not support the “stem cell” theory of development of MMFC. Likewise, our findings seem to exclude the “divergent differentiation” theory, assuming that some MTC cells differentiate toward a follicular phenotype by the acquisition of additional molecular defects (Table 5) ▶ . Such tumors should exhibit a common clonality pattern in the two tumor components, which was not detectable in the MMFCs studied.
Another pathogenetic mechanism that has been put forward for MMFC is the “collision theory” (Table 5) ▶ . It assumes that two independently arising tumors, either a MTC and a follicular carcinoma, or a MTC and a papillary carcinoma, collide in the thyroid. In fact, several authors have reported such neoplasms, 40-43 and others have pointed out that such tumors may imitate a primary MMFC. 8,24 Our series included one patient who had two topographically separated primaries consisting of a MTC and a papillary carcinoma and mixed metastases in regional lymph nodes. This indicates that a MTC can entrap and disseminate foci of another thyroid neoplasms. The same holds true for residual thyroid follicles, which can become entrapped by MTCs. Thus MTC exhibits a tumor growth different from that of most other human cancers, which usually destroy the tissue in which they arise.
Our molecular analysis does not entirely rule out the “field effect” hypothesis (Table 5) ▶ , although we did not find a common LOH or mutation in the components investigated. The field effect hypothesis assumes a common oncogenic stimulus triggering neoplastic transformation of both follicular and C-cells, 24 which leads to the simultaneous development of two different tumor components. 44
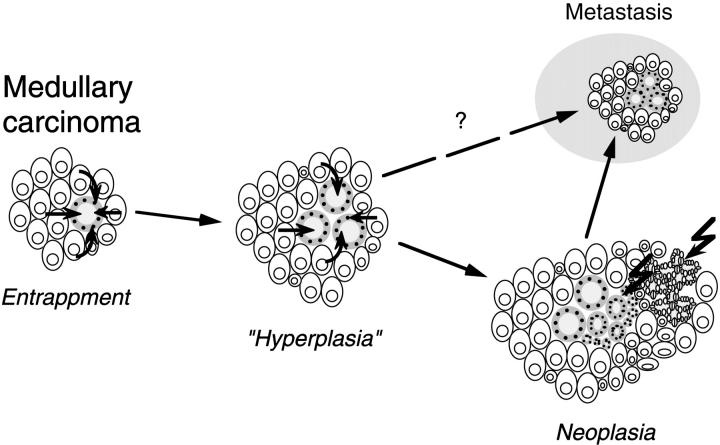
Yet another pathogenetic mechanism may exist in at least one subset of MMFC. As already pointed out, MTCs may exhibit entrapped, well-preserved residual follicles. Although it is common to see such residual follicles at the periphery of classical MTCs, it is typical for MMFCs that the follicular structures are found deep within the tumor and that they exhibit cytological features different from that of the surrounding nonneoplastic follicles. It is conceivable that some unknown trophic factors keep the entrapped follicles alive and might even stimulate them to proliferate, and conversely that some follicles might secrete substances preventing them from being destroyed by the MTC. 45 Such a “symbiosis” of neoplastic C-cells and follicular cells might lead to a combined growth of follicular and C-cell structures and result in the mixed tumor pattern of the majority of MMFCs. The microenvironment provided by the MTC might result in hyperplastic and adenomatous follicular foci, which eventually may become neoplastic through the acquisition of molecular defects (Figure 4) ▶ . Such a scenario could explain the spectrum of follicular patterns and the occasional neoplastic foci in MMFCs as well as the clonal and molecular changes found in this study. The question that arises from this “hostage theory” is whether such follicular structures can metastasize together with the MTC component (Figure 4) ▶ . This aspect, however, cannot be answered at present and will require additional detailed analysis.
Figure 4.
Diagram illustrating the “hostage theory” of the evolution of mixed medullary-follicular thyroid carcinomas. Entrapped nonneoplastic follicles are stimulated by trophic factors leading to hyperplastic follicular foci (“hyperplasia”). Acquired genetic defects in follicular cells lead to neoplastic transformation and development of follicular or papillary carcinoma components that can give rise to mixed metastases.
An important question remains: namely whether follicles with Tg immunoreactivity in MMFC are really neoplastic or merely hyperplastic or adenomatous, as suggested by Sobrinho-Simões. 46 Thus, in the group of reported “true” MMFCs, the majority of tumors exhibited follicular components in both primary stage and metastases that have to be classified as hyperplastic or adenomatous by conventional histology 4,6,13-18,20,22,26 (similar to our three cases amenable for clonal analysis), and only the minority of tumors contained morphologically undoubtably neoplastic non-MTC components. 19,21 Follicular structures fulfilling the conventional criteria of neoplastic follicular lesions in our series were identified in four MMFCs. Unfortunately, none of the four tumors were amenable for clonality analysis (three MMFCs from male patients and one not informative). Thus no definite answer about the clonal composition of the latter tumors can be given. One of those tumors, however, exhibited a RET mutation and a LOH at D7S480 and D7S490 in the medullary component but not in the follicular carcinoma portion.
One might argue that our molecular results are more easily explained by the fact that we have only accidentally analyzed entrapped residual follicles. However, we have only microdissected follicular structures deep within the MMFC, which exhibited a histology different from that of the peritumoral nonneoplastic follicles (eg, enlarged or clear nuclei), or which occurred in clusters. Thus we believe that our results are obtained from follicular components typical for MMFCs. Other technical reasons, eg, resulting in false clonality patterns, can be excluded because appropriate controls have been included in each assay and methods have been evaluated and tested in previous studies. 29,37
From all of the data of the literature and the results of our study, it appears that the group of so-called MMFCs is in fact a rather heterogeneous group with various morphological features and different pathogenetic mechanisms. It includes 1) neoplasms that have to be classified as collision tumors, ie, that are composed of two separately arising tumors with different morphologies and an intermingled growth; 2) “true” mixed carcinomas with features as defined by the WHO classification 7 ; and 3) MTCs with Tg-expressing cells that might be derived from a common stem cell. 26 However, the latter tumor type should be separated from MMFCs because they do not present histological evidence of follicles or papillae.
In summary, our present investigation provides molecular evidence that Tg-positive follicles or clusters of follicles and the medullary portion in MMFCs are not derived from a common stem cell. We therefore postulate that the pathogenetic mechanisms of MMFC might be different from those ones previously proposed. These findings provide new insights into the development of tumors with mixed morphology and raise the possibility that trophic factors secreted by neoplastic and host cells might have a significant role in the tumorigenesis of MMFC.
Acknowledgments
We thank Seraina Muletta-Feurer and Katrin Rütimann for technical support, André Barghorn for helpful discussions and technical advice, Madeleine Pfaltz for providing tissue samples, the group of Dieter Zimmermann for performing the sequence analyses, and Norbert Wey and Ida Schmieder for photographic and computer-assisted reproductions.
Footnotes
Address reprint requests to Dr. Paul Komminoth, Division of Cell and Molecular Pathology, Department of Pathology, Schmelzbergstrasse 12, University of Zürich, CH-8091 Zürich, Switzerland. E-mail: paul.komminoth@pty.usz.ch.
Dedicated to the memory of the late Prof. Ch. Hedinger (December 5, 1917-January 12, 1999), former head of the Institute of Pathology at the University of Zürich, Zürich, Switzerland.
Supported in part by grants from the Italian Ministry of University and Research (M.V., M.P.).
References
- 1.Murray D: The thyroid gland. Kovacs K Asa S eds. Functional Endocrine Pathology. 1991, :pp 293-374 Blackwell Scientific, Boston [Google Scholar]
- 2.Le Douarin NM: The Neural Crest. 1982. Cambridge University Press, Cambridge
- 3.Williams ED, Toyn CE, Harach HR: The ultimobranchial gland and congenital thyroid abnormalities in man. J Pathol 1989, 159:135-141 [DOI] [PubMed] [Google Scholar]
- 4.Bussolati G, Monga G: Medullary carcinoma of the thyroid with atypical patterns. Cancer 1979, 44:1769-1777 [DOI] [PubMed] [Google Scholar]
- 5.Pfaltz M, Hedinger CE, Muhlethaler JP: Mixed medullary and follicular carcinoma of the thyroid. Virchows Arch A Pathol Anat Histopathol 1983, 400:53-59 [DOI] [PubMed] [Google Scholar]
- 6.Hales M, Rosenau W, Okerlund MD, Galante M: Carcinoma of the thyroid with a mixed medullary and follicular pattern: morphologic, immunohistochemical, and clinical laboratory studies. Cancer 1982, 50:1352-1359 [DOI] [PubMed] [Google Scholar]
- 7.Hedinger C, Williams E, Sobin L: Histological typing of thyroid tumours. ed 2 World Health Organization International Histological Classification of Tumors, 1988, Springer-Verlag, Berlin
- 8.Matias-Guiu X, Caixas A, Costa I, Cabezas R, Prat J: Compound medullary-papillary carcinoma of the thyroid: true mixed versus collision tumour. Histopathology 1994, 25:183-185 [DOI] [PubMed] [Google Scholar]
- 9.Lax SF, Beham A, Kronberger SD, Langsteger W, Denk H: Coexistence of papillary and medullary carcinoma of the thyroid gland-mixed or collision tumour? Clinicopathological analysis of three cases. Virchows Arch 1994, 424:441-447 [DOI] [PubMed] [Google Scholar]
- 10.Albores-Saavedra J, Gorraez de la Mora T, de la Torre-Rendon F, Gould E: Mixed medullary-papillary carcinoma of the thyroid: a previously unrecognized variant of thyroid carcinoma. Hum Pathol 1990, 21:1151-1155 [DOI] [PubMed] [Google Scholar]
- 11.Papotti M, Negro F, Carney JA, Bussolati G, Lloyd RV: Mixed medullary-follicular carcinoma of the thyroid. A morphological, immunohistochemical and in situ hybridization analysis of 11 cases. Virchows Arch 1997, 430:397-405 [DOI] [PubMed] [Google Scholar]
- 12.Mizukami Y, Michigishi T, Nonomura A, Nakamura S, Noguchi M, Hashimoto T, Itoh N: Mixed medullary-follicular carcinoma of the thyroid occurring in familial form. Histopathology 1993, 22:284-287 [DOI] [PubMed] [Google Scholar]
- 13.Noel M, Delehaye MC, Segond N, Lasmoles F, Caillou B, Gardet P, Fragu P, Moukhtar MS: Study of calcitonin and thyroglobulin gene expression in human mixed follicular and medullary thyroid carcinoma. Thyroid 1991, 1:249-256 [DOI] [PubMed] [Google Scholar]
- 14.Kovacs CS, Mase RM, Kovacs K, Nguyen GK, Chik CL: Thyroid medullary carcinoma with thyroglobulin immunoreactivity in sporadic multiple endocrine neoplasia type 2-B. Cancer 1994, 74:928-932 [DOI] [PubMed] [Google Scholar]
- 15.de Micco C, Chapel F, Dor AM, Garcia S, Ruf J, Carayon P, Henry JF, Lebreuil G: Thyroglobulin in medullary thyroid carcinoma: immunohistochemical study with polyclonal and monoclonal antibodies. Hum Pathol 1993, 24:256-262 [DOI] [PubMed] [Google Scholar]
- 16.Ruhlmann J, Vogel J, Bockisch A, Biersack HJ: Metastases of a medullary carcinoma of the thyroid (follicular variant). Diagnosis and therapy using radioiodine. Dtsch Med Wochenschr 1987, 112:1170-1172 [DOI] [PubMed] [Google Scholar]
- 17.Harach HR: Thyroglobulin in human thyroid follicles with acid mucin. J Pathol 1991, 164:261-263 [DOI] [PubMed] [Google Scholar]
- 18.Massart C, Gibassier J, Lucas C, Le Gall F, Giscard-Dartevelle S, Bourdiniere J, Moukhtar MS, Nicol M: Hormonal study of a human mixed follicular and medullary thyroid carcinoma. J Mol Endocrinol 1993, 11:59-67 [DOI] [PubMed] [Google Scholar]
- 19.Parker LN, Kollin J, Wu SY, Rypins EB, Juler GL: Carcinoma of the thyroid with a mixed medullary, papillary, follicular, and undifferentiated pattern. Arch Intern Med 1985, 145:1507-1509 [PubMed] [Google Scholar]
- 20.Ljungberg O, Ericsson UB, Bondeson L, Thorell J: A compound follicular-parafollicular cell carcinoma of the thyroid: a new tumor entity? Cancer 1983, 52:1053-1061 [DOI] [PubMed] [Google Scholar]
- 21.Tanda F, Massarelli G, Mingioni V, Bosincu L, Moroni RV, Cossu A: Mixed follicular-parafollicular carcinoma of the thyroid: a light, electron microscopic and histoimmunologic study. Surg Pathol 1990, 3:65-74 [Google Scholar]
- 22.Mizukami Y, Nonomura A, Michigishi T, Noguchi M, Ishizaki T: Mixed medullary-follicular carcinoma of the thyroid gland: a clinicopathologic variant of medullary thyroid carcinoma. Mod Pathol 1996, 9:631-635 [PubMed] [Google Scholar]
- 23.Rosai J, Carcangiu ML, DeLellis RA: Tumors of the thyroid gland. Atlas of Tumor Pathology, series 3, vol 5. Washington, DC, Armed Forces Institute of Pathology, 1990
- 24.Apel RL, Alpert LC, Rizzo A, LiVolsi VA, Asa SL: A metastasizing composite carcinoma of the thyroid with distinct medullary and papillary components. Arch Pathol Lab Med 1994, 118:1143-1147 [PubMed] [Google Scholar]
- 25.Ljungberg O, Bondeson L, Bondeson AG: Differentiated thyroid carcinoma, intermediate type: a new tumor entity with features of follicular and parafollicular cell carcinoma. Hum Pathol 1984, 15:218-228 [DOI] [PubMed] [Google Scholar]
- 26.Holm R, Sobrinho-Simões M, Nesland JM, Sambade C, Johannessen JV: Medullary thyroid carcinoma with thyroglobulin immunoreactivity. A special entity? Lab Invest 1987, 57:258-268 [PubMed] [Google Scholar]
- 27.Johnston D, Hatzis D, Sunday ME: Expression of v-Ha-ras driven by the calcitonin/calcitonin gene-related peptide promoter: a novel transgenic murine model for medullary thyroid carcinoma. Oncogene 1998, 16:167-177 [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Piao Z, Park C, Chung WY, Park CS: Clinical significance of clonality in thyroid nodules. Br J Surg 1998, 85:1125-1128 [DOI] [PubMed] [Google Scholar]
- 29.Perren A, Roth J, Muletta-Feurer S, Saremaslani P, Speel EJM, Heitz PU, Komminoth P: Clonal analysis of sporadic pancreatic endocrine tumours. J Pathol 1998, 186:363-371 [DOI] [PubMed] [Google Scholar]
- 30.Hsu S, Raine L, Fanger H: Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 31.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KA, Stein H, Mason DY: Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984, 32:219-229 [DOI] [PubMed] [Google Scholar]
- 32.Schütze K, Clement-Sengewald A: Catch and move-cut or fuse. Nature 1994, 368:667-669 [DOI] [PubMed] [Google Scholar]
- 33.Zitzelsberger H, Kulka U, Lehmann L, Walch A, Smida J, Aubele M, Lörch T, Höfler H, Bauchinger M, Werner M: Genetic heterogeneity in a prostatic carcinoma and associated prostatic intraepithelial neoplasia as demonstrated by combined use of laser- microdissection, degenerate oligonucleotide primed PCR and comparative genomic hybridization. Virchows Arch 1998, 433:297-304 [DOI] [PubMed] [Google Scholar]
- 34.Komminoth P, Roth J, Muletta FS, Saremaslani P, Seelentag WK, Heitz PU: RET proto-oncogene point mutations in sporadic neuroendocrine tumors. J Clin Endocrinol Metab 1996, 81:2041-2046 [DOI] [PubMed] [Google Scholar]
- 35.Komminoth P, Kunz EK, Matias-Guiu X, Hiort O, Christiansen G, Colomer A, Roth J, Heitz PU: Analysis of RET protooncogene point mutations distinguishes heritable from nonheritable medullary thyroid carcinomas. Cancer 1995, 76:479-489 [DOI] [PubMed] [Google Scholar]
- 36.Görtz B, Roth J, Krähenmann A, de Krijger RR, Muletta-Feurer S, Rütimann K, Saremaslani P, Speel EJM, Heitz PU, Komminoth P: Mutations and allelic deletions of the MEN1 gene are associated with a subset of sporadic endocrine pancreatic and neuroendocrine tumors and not restricted to foregut neoplasms. Am J Pathol 1999, 154:429-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Görtz B, Muletta-Feurer S, De Krijger RR, Rütimann K, Speel EJM, Saremaslani P, Roth J, Heitz PU, Komminoth P: Analysis of MEN-1 gene mutations in sporadic neuroendocrine and adrenocortical tumors. Int J Cancer 1998, 80:373-379 [DOI] [PubMed] [Google Scholar]
- 38.Leblanc B, Paulus G, Andreu M, Bonnet MC: Immunocytochemistry of thyroid C-cell complexes in dogs. Vet Pathol 1990, 27:445-452 [DOI] [PubMed] [Google Scholar]
- 39.Kameda Y: Immunohistochemical study of cyst structures in chick ultimobranchial glands. Arch Histol Jpn 1984, 47:411-419 [DOI] [PubMed] [Google Scholar]
- 40.Lamberg B-A, Reissel P, Stenman S, Koivuniemi A, Ekblom M, Mäkinen J, Franssila K: Concurrent medullary and papillary thyroid carcinoma in the same thyroid lobe and in siblings. Acta Med Scand 1981, 209:421-424 [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, Yoshimi N, Kanai N, Mori H, Nagai K, Fujii A, Sakata S, Tokimitsu N: Simultaneous occurrence of medullary and follicular carcinoma in the same thyroid lobe. Hum Pathol 1989, 20:83-86 [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Campora R, Lopez-Garrido J, Martin-Lacave I, Miralles-Sanchez EJ, Villar JL: Concurrence of a symptomatic encapsulated follicular carcinoma, an occult papillary carcinoma and a medullary carcinoma in the same patient. Histopathology 1992, 21:380-382 [DOI] [PubMed] [Google Scholar]
- 43.Pastolero GC, Coire CI, Asa SL: Concurrent medullary and papillary carcinomas of thyroid with lymph node metastases. A collision phenomenon. Am J Surg Pathol 1996, 20:245-250 [DOI] [PubMed] [Google Scholar]
- 44.Triggs SM, Williams ED: Experimental carcinogenesis in the thyroid follicular and C cells. A comparison of the effect of variation in dietary calcium and of radiation. Acta Endocrinol (Copenh) 1977, 85:84-92 [PubMed] [Google Scholar]
- 45.Galera-Davison M, Fernandez A, Salguera M, Martin-Lacave I, Gonzalez-Camora R: Simultaneous hyperplasia of follicular and parafolliular cells in experimental hypothyroidism. Lab Invest 1988, 58:33A [Google Scholar]
- 46.Sobrinho-Simões M: Mixed medullary and follicular carcinoma of the thyroid. Histopathology 1993, 23:287-289 [DOI] [PubMed] [Google Scholar]