Abstract
As barriers to xenotransplantation are surmounted, such as suppression of hyperacute rejection allowing improved graft survival, it becomes important to define longer-term host-xenograft interactions. To this end we have prepared in baboons high titer anti-α-Galactosyl (αGal) and anti-porcine aortic endothelial cell antibodies, similar to human natural xenoantibodies and reactive with epitopes of thyroglobulin, laminin, and heparan sulfate proteoglycans. When injected into pigs with a protocol similar to that used in the rat to show the nephritogenic potential of heterologous anti-laminin and anti-heparan sulfate proteoglycan antibodies, baboon immunoglobulins bound first to renal vascular endothelium, and later to interstitial cells, especially fibroblasts and macrophages, and to antigens in basement membranes and extracellular matrix, where they colocalized with laminin- and heparan sulfate proteoglycan-antibodies, and with bound Griffonia simplicifolia B4. A similar binding was observed in other organs. The pigs did not develop an acute complement-dependent inflammation, but rather chronic lesions of the basement membranes and the extracellular matrix. Incubation of renal fibroblasts with baboon anti-α-Galactosyl antibodies resulted in increased synthesis of transforming growth factor-β and collagen, suggesting a possible basis for the fibrotic response. The results demonstrate that in this experimental model a consequence of αGal antibody interaction with porcine tissues, is immunoreactivity with αGal on matrix molecules and interstitial cells, priming mechanisms leading to fibrosis resembling that in chronic allograft rejection. The possibility that similar lesions may develop in long-surviving pig xenografts is discussed.
For several reasons the pig is the donor animal of choice for human xenotransplantation. Therefore, close study of the mechanisms of porcine xenograft rejection is crucial. The most relevant information has been derived from the pig-to-baboon model. After transplantation, pig kidneys and hearts, and, less rapidly, lungs and livers are hyperacutely rejected. This phenomenon is initiated by binding of baboon natural IgM to the vascular endothelium of the graft, by local activation of complement, and additional cytotoxic T lymphocytes injury induced by host’s natural killer cells, (CTL), and polymorphonucleus leukocytes (PMNs). 1 An IgG response occurs after a first xenograft, or when hyperacute rejection is prevented by depletion of xenoantibodies and complement. 2-4 In the latter conditions IgG may contribute to activate endothelial cells and cooperate with IgM in the development of the acute vascular rejection (or delayed xenograft rejection) that follows. 2,3 The principal target of human and baboon IgM and IgG is the Galα1–3Galβ1–4 GlcNAc-R (αGal) epitope 5-7 present on glycoproteins and glycolipids on pig endothelial cells. 7
The rapid and destructive nature of unmodified xenograft rejection does not allow determination of whether the antibodies that interact with endothelium can bind and induce injury to other cells and extracellular compartments. To obtain such additional information we have prepared in baboons antibodies to αGal/bovine serum albumin (BSA) and to porcine aortic endothelial cells (PAEC), termed baboon anti-αGal and baboon anti-PAEC, respectively, similar to human xenoantibodies, and reactive with epitopes of laminin and heparan sulfate proteoglycans (HSPG). We injected these antibodies into pigs and studied the consequences.
The results show that the baboon antibodies with αGal reactivity bind first to endothelium, erythrocytes, and platelets, and later to αGal epitopes on fibroblasts and macrophages and to the extracellular matrix, especially to laminin and heparan sulfate proteoglycans, and that the pigs develop basement membrane and fibrosclerotic lesions. Incubation of pig fibroblasts with anti-αGal antibodies increased their synthesis of transforming growth factor β (TGF-β) and collagen, suggesting a basis for the fibrotic response. Therefore, in this experimental model baboon antibody with αGal reactivity binds to basement membranes and extracellular matrix, setting off mechanisms that give rise to local fibrosclerotic lesions.
Materials and Methods
Animals
Baboons (Papio anubis), 25 to 31 kg body weight, were obtained from the Biomedical Research Foundation (Houston, TX) and from LEMSIP, New York University (Tuxedo, NY). Miniature pigs six week old were purchased from Harlan Sprague Dowley, Sinclair Research, Inc. (Columbia, MO) and used when their body weight was 3 to 13 kg. The protocol for animal experiments was formally approved by the Columbia University Review Board.
Antigen Preparation and Immunization
Four healthy baboons (two naive and two that had previously received heterotopic pig heart transplants, subsequently removed) were immunized with 1 mg αGal conjugated to BSA (14 atom spacer, termed αGal/BSA; Dextra Laboratories, Reading, UK) in an equal volume of incomplete Freund’s adjuvants (IFA) (Sigma) at multiple s.c. and intradermal sites, boosted after 3 weeks by subcutaneous 500 μg of αGal/BSA in IFA. A fifth (naïve) baboon was immunized with 3–4 million PAEC in IFA, and subsequently boosted as given above for αGal/BSA. After the first bleeding, the baboons received booster injections every 4 weeks and were bled every 6 weeks (<10% of blood volume). The same protocol was used to immunize a sheep with BSA and pigs with baboon γ-globulins. Anti-rabbit angiotensin-converting enzyme (ACE) antibody was prepared in goats as previously described. 8 Pre-immune sera from naïve baboons, normal baboon sera, and αGal antibody-negative sera from normal rabbits and pigs were used as controls. The γ-globulin fractions were obtained by precipitation in 50% ammonium sulfate; IgG was isolated using ImmunoPure immobilized protein A column (Pierce, Rockford, IL). The flow-through, devoid of IgG, was used as baboon anti-αGal IgM. Total protein concentration was measured by a standard Bradford protein assay (BioRad, Hercules, CA). The concentration of γ-globulins, IgG, and IgM was determined by radial immunodiffusion, 9 and that of pig anti-baboon antibody in the sera of pigs injected with baboon γ-globulin by Ouchterlony’s immunodiffusion. αGal antibody was affinity purified from baboon anti-αGal serum on a 0.2 ml αGal-conjugated silica beads column (Synsorb 115, Chembiomed, Edmonton, AB, Canada) as previously described. 10 Titers of adsorbed and subsequently eluted antibody and of the flow-through were determined by enzyme-linked immunosorbent assay (ELISA) on mouse laminin and PAEC. Silica beads conjugated with Galβ1–4-GlcNac-R epitopes were used as specificity control. Other baboon anti-αGal aliquots were depleted of αGal reactivity by extensive absorption with α-Gal/BSA and with αGal-rich rabbit erythrocytes 7,10 and were also tested by ELISA.
Other Antibodies, Lectins, Components of the Extracellular Matrix, and Chemicals
All conjugated and some unconjugated affinity-purified antibodies were purchased from Sigma Chemical Co. (St. Louis, MO), except biotin-conjugated rat anti-mouse IgG (H+L). AP-conjugated goat anti-human IgG and AP-conjugated streptavidin which were from Zymed Laboratories, Inc. (South San Francisco, CA). Sheep anti-human C3c, and sheep anti-pig C3 were obtained from The Binding Site (San Diego, CA), and FITC-conjugated rabbit anti-human C3c from Dako (Carpinteria, CA). Anti-porcine IgA monoclonal antibody was obtained from Serotec (Oxford, UK). Rabbit anti-bovine type II collagen, rabbit anti-bovine type II collagen, and monoclonal antibodies to HSPG core protein (Perlecan), to rat fibronectin, and to human β3 integrin, were purchased from Chemicon International, Inc. (Temecula, CA), and anti-mouse Englebert-Holm-Swerm (EHS) HSPG from Seikagaku (Tokyo). Biotin- and FITC-conjugated Bandeireea griffonia simplicifolia isolectin B-4, and FITC-avidin D were from Vector Laboratories (Burlingame, CA). The rabbit anti-rat basement membrane HSPG was a gift of Dr. Marilyn G. Farquhar. 11 All polyclonal antisera to human proteins were crossreactive with baboon proteins in double immunodiffusion techniques. When appropriate, antibody and antisera were absorbed with pig serum before use.
Biocoat matrigel thin layer plates were from Becton Dickinson (Franklin Lakes, NJ). Human plasma fibronectin, bovine plasma fibronectin, mouse laminin from mouse EHS tumor, laminin from human placenta, HSPG from mouse EHS tumor, bovine chondroitin sulfate A, bovine hyaluronate, bovine type I, II, and III collagen, pig type I and II collagen, chicken type II collagen, and porcine thyroglobulin were all obtained from Sigma, and bovine type III collagen from Chemicon International (Temecula, CA). The 54-kd rabbit tubular basement membrane nephritogenic antigen 12 was a gift of Drs. Butkowski and Charonis.
Agarose anti-human IgM (μ chain-specific), biotin (long arm) NHS (Blanks) N-hydroxysuccininidyl-6-(biotinamide)hexanoate, o-phenyledadiamine dihydrochloride, 3-3′-dimethoxybenzidine, ovalbumin, pig albumin, BSA, pig thyroglobulin, bovine thyroglobulin, protease inhibitors cocktail, and p-nitrophenylphosphate, α-galactosidase, and β-galactosidase, and collagenase type VII were obtained from Sigma. Other reagents were Accray Assay Human IgG RID Kit, Accray Assay Human IgM RID kit, Micro-Ouchterlony Kit (ICN, Costa Mesa, CA) and nitrocellulose membranes, gels, and Tris-glycine (BioRad).
Blood Counts, Chemistry, and Urinalysis
Peripheral erythrocytes, lymphocytes, and platelets were enumerated, the glucose, BUN, creatinine, and CH50 measured, and the clotting profile was studied by the Bioveterinary Services Department of Roche Biomedical Laboratories (Raritan, NJ). Urinary protein excretion and sediments were examined using conventional methods.
Cultures of PAEC and Fibroblasts from the Renal Medulla
Porcine aortas and kidneys were obtained from a local abattoir. PAEC 8 and fibroblasts 13 were prepared and characterized by methods previously described. For fibroblasts, the inner stripe of the renal medulla was dissected in sterile conditions, minced, passed through a 106-μm mesh (Fisher Scientific, Pittsburgh, PA), and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS).
Endothelial Cell Cytotoxicity
PAEC (third passage) were incubated at 37°C for 1 hour in 10 μmol/L Calcein AM (Molecular Probes, Eugene, OR), washed, and incubated for 1 hour at 37°C with baboon anti-αGal or baboon anti-PAEC γ-globulin or IgG fractions plus fresh or heat-decomplemented rabbit serum. The supernatants were collected and the absorbance was measured at 485 nm in a CytoFluor multi-well fluorescent-plate reader (PerSeptive Biosystems, Framingham, MA). Specific lysis was calculated subtracting the dye released by cells incubated with the antibody and complement from that released from cells incubated with phosphate buffered saline (PBS), divided by the value of maximal dye release from cells incubated with 1% saponin.
ELISA
αGal reactivity on immobilized αGal/BSA, mouse laminin, porcine thyroglobulin, and single components of the extracellular matrix was measured by ELISA according to Engvall and Perlmann. 14 Binding of antibody to PAEC was studied as described by Platt et al. 15 The reactivity of the antibody with murine extracellular matrix was determined using Matrigel plates (Becton Dickinson). The expression of αGal epitopes on porcine cells or tissues was measured by inhibition ELISA with anti-αGal monoclonal antibody (M86) prepared in α1–3,galactosyltransferase knockout mice immunized with rabbit erythrocyte membranes. 16 Controls were normal rabbit and pig serum, sheep anti-BSA serum, the baboon anti-αGal flow through of αGal immunoadsorption column, and baboon anti-αGal absorbed with αGal/BSA, or rabbit erythrocytes.
Western Blot Analysis
Sixty μg PAEC lysates, and 6 μg of single components of the extracellular matrix were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions on 4–15% gradient gels. 17 The proteins were transferred to nitrocellulose membranes incubated with primary antibody at concentrations indicated in the legends of Figures 1C and 2C ▶ ▶ , followed by the appropriate secondary biotin-conjugated antibody and AP-conjugated streptavidin. Control reagents were as for ELISA.
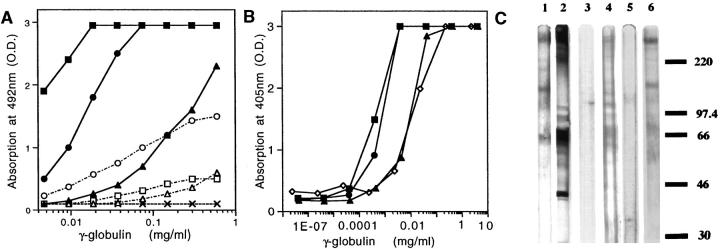
Figure 1.
Serological characterization of baboon anti-αGal and baboon anti-PAEC. A: αGal reactivity of IgG and IgM by ELISA with αGal/BSA. The titers of baboon anti-αGal IgG (▪) and baboon anti-PAEC IgG (•) are increased 32- to 200-fold as compared to normal baboon IgG (▴). The titers of baboon anti-αGal IgM (□) and baboon anti-PAEC IgM (○) are increased 2- to 32-fold. (▵), normal baboon IgM; (X) sheep anti-BSA IgG. B: Binding of baboon anti-αGal and baboon anti-PAEC γ-globulins to PAEC measured by ELISA. Baboon anti-αGal (▪) and baboon anti-PAEC (•) have similar reactivity, which is 27 and 19 times higher than that of normal baboon serum (▴), respectively. The binding of goat anti-ACE (⋄) is like that of normal baboon serum. C: SDS-PAGE analysis of PAEC. The Western blots were probed with 10 μg/ml of the IgG fractions of baboon anti-αGal (lane 1), and baboon anti-PAEC (lane 2); 1:10 dilution of normal rabbit serum (lane 3) , pre-immune baboon serum (lane 4), and pre-immune baboon serum absorbed with rabbit erythrocytes (lane 5); and 10 μg/ml affinity-purified baboon anti-αGal (lane 6). Baboon anti-αGal and affinity-purified baboon anti-αGal recognize bands of apparent 70, 125–135, and 225 kDa. Baboon anti-PAEC recognizes more bands than baboon anti-αGal, including a 44-kd band. αGal reactivity was absorbed out by rabbit erythrocytes (not shown).
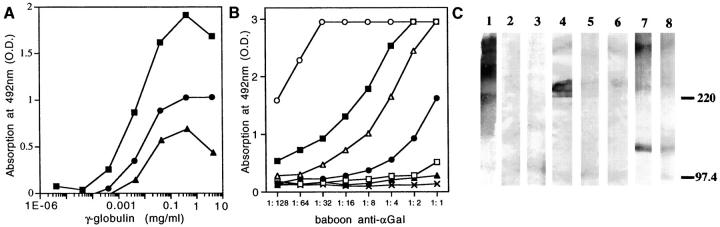
Figure 2.
Reactivity of baboon anti-αGal and baboon anti-PAEC with the extracellular matrix. A: ELISA reactivity of baboon anti-αGal, baboon anti-PAEC, and pre-immune baboon γ-globulins with Matrigel. Binding of baboon anti-αGal (▪) was 328-fold, and that of baboon anti-PAEC (•) 19-fold higher than that of pre-immune baboon γ-globulin (▴), (P < 0.0001) B: Binding of baboon anti-αGal to αGal/BSA, pig thyroglobulin, and to components of the extracellular matrix, measured by ELISA. The highest binding was for mouse laminin (▪), followed by porcine thyroglobulin (▵), mouse HSPG (•), and bovine fibronectin (□). The antibody did not react with the nephritogenic tubular basement membrane antigen (▴) and with human laminin (X), or with bovine chondroitin sulfate, hyaluronate, bovine collagen I, II, and III, pig collagen I and II, and BSA (not shown). (○) αGal/BSA. C: Western blot analysis of extracellular matrix components probed with 10 μg/ml baboon anti-αGal IgG. This antibody recognized epitopes expressed on mouse laminin (lane 1), bovine fibronectin (lane 4), and mouse HSPG (lane 7), not epitopes on human laminin (lane 3), and human fibronectin (lane 6). Absorption with rabbit erythrocytes abolished reactivity with laminin (lane 2), bovine fibronectin (lane 5) and, partially, mouse HSPG (lane 8).
Detection of Anti-Baboon IgG in Pig Sera
The presence of antibodies against baboon IgG in sera of pigs injected with baboon γ-globulin preparations was determined using double immunodiffusion.
PAl-1/Luciferase (PAIL) Assay for TGF-β on Supernatants of Cultured Renal Fibroblasts
Fibroblasts (8th–10th passage) were placed into 6-well plates, 5 × 10 5 cells/well, and cultured in DMEM with 5% FCS. After seeding, the fibroblasts were incubated for 24 hours in DMEM with 0.5% FCS, followed by incubation for 60 hours in DMEM with 0.5% FCS containing 1 mg/ml of the γ-globulin preparation. The supernatants were collected and assayed for TGF-β. 18 Briefly, mink lung epithelial cells (MLECs-clone 32) transfected with a fragment of the human plasminogen activator inhibitor-1 gene fused to the firefly luciferase reporter gene from Dr. Daniel Rifkind (Department of Cell Biology, New York University Medical Center) were plated and incubated with test samples for 14 hours. Luciferase activity was measured using the Luciferase Assay System E1500 (Promega, Madison, WI) and Lumat LB 9501 (Wallac, Gaithersburg, MD). Standard curve was with TGF-β1 (R&D Systems, Minneapolis, MN).
Quantitation of Collagen Produced by Cultured Renal Fibroblasts
Collagen synthesis was quantified by proline incorporation. 19 Fibroblasts were prepared and made quiescent as described in the previous paragraph, and incubated for 24 hours in DMEM/0.5% FCS, 50 μg/ml ascorbic acid, and 1 mg/ml of the γ-globulin preparation. This medium was replaced for 12 hours with DMEM/0.5% FCS, 50 μg/ml ascorbic acid, 50 μg/ml β-aminoproprionitrile, 1 mg/ml of the γ-globulin preparations, and 100 μCi/ml of L-[2,3,4,5-3H] Proline (Amersham, Little Chalfont, UK). The supernatant proteins were precipitated with trichloroacetic acid (TCA), dissolved in 0.2 mol/L NaOH, neutralized, and incubated with or without 27 IU of collagenase VII (Sigma), followed by the TCA precipitation. The radioactivity of supernatant and pellet were counted using a liquid scintillation counter 1219 Rackbeta (Wallac). Collagen synthesis was calculated on the assumption that the proline content of collagen is 5.4 times that of other proteins.
Tissue Preparations for Morphology and Immunohistochemistry
Autopsy or biopsy samples of lung, kidney, heart, liver, and intestine were immediately fixed in 10% buffered formalin to be processed for light microscopy. Others were fixed in 2% glutaraldehyde and processed for electron microscopy. 20 For immunohistochemistry, fresh frozen sections or formalin-fixed paraffin sections were used. Frozen sections were stained or double-stained with FITC- or TRITC-conjugated antibody. For direct staining, purified IgG were conjugated with FITC, TRITC, or biotin, then extensively absorbed with normal pig serum. For indirect immunohistochemistry the sections were first incubated with primary antibody, followed by appropriate FITC-, TRITC-, or biotin-conjugated secondary antibody. The sections were examined in a Nikon epifluorescence and phase contrast microscope, or a Zeiss LSM 410 laser scanning confocal microscope. Controls were done with baboon anti-αGal absorbed with αGal/BSA and rabbit erythrocytes, sheep anti-BSA, substitution of PBS for the primary antibody and digestion of tissue sections with α- or β-galactosidase (1 U α-galactosidase and 2 U β-galactosidase in 100 mmol/L NaCl, 50 mmol/L sodium acetate, pH 5.0 at 37°C for 2 hours; control was buffer without enzyme). The ability of baboon anti-αGal, baboon anti-PAEC to fix complement was evaluated in vitro by indirect immunofluorescence. 21
Experiments in Living Pigs (Table 1) ▶
Table 1.
Injected γ-Globulin Preparation, Dose, and Time of Sacrifice
| Group | Pig no. | Preparation injected | Total dose of γ-globulin (mg/kg) | Time of continuous infusion (hours) | Time of sacrifice after end of infusion |
|---|---|---|---|---|---|
| I | 1 | baboon anti-αGal | 200 | 6 | 40 minutes |
| 2 | baboon anti-PAEC | 200 | 6 | 40 minutes | |
| 3 | goat anti-ACE | 200 | 6 | 40 minutes | |
| II | 4 | baboon anti-αGal | 890 | 72 | 10 hours |
| 5 | baboon anti-PAEC | 850 | 72 | 1 hour | |
| 6 | baboon anti-PAEC | 950 | 70 | 20 minutes* | |
| 7 | goat anti-ACE | 970 | 72 | 6 hours | |
| 8 | sheep anti-BSA | 970 | 72 | 14 hours | |
| III | 9 | baboon anti-αGal/goat anti-ACE | 540/40 | 72 | 30 days |
| 10 | goat anti-ACE | 40 | 72 | 30 days | |
| 11 | baboon anti-αGal | 1250 | 72 | 90 days | |
| 12 | baboon anti-PAEC | 970 | 72 | 120 days | |
| 13 | goat anti-ACE | 970 | 72 | 92 days | |
| 14 | sheep anti-BSA | 970 | 72 | 93 days | |
| IV | 15 | baboon anti-αGal/baboon anti-PAEC | 540/555 | 72 | |
| pig anti-baboon γ-globulin | 300 | 30† | 18 hours | ||
| 16 | baboon anti-αGal/baboon anti-PAEC | 555/415 | 72 | ||
| pig anti-baboon γ-globulin | 30 | 25‡ | 1 hour |
*Died 70 hours after beginning of injection.
†Pig anti-baboon γ-globulin given 60 hours after last injection of baboon anti-αGal and baboon anti-PAEC.
‡Pig anti-baboon γ-globulin given 66 hours after last injection of baboon anti-αGal and baboon anti-PAEC; died 1 hour after pig anti-baboon γ-globulin injection.
Five γ-globulin preparations were used: baboon anti-αGal, baboon anti-PAEC, two controls (sheep anti-BSA and goat anti-ACE, which cross-reacts with PAEC), and pig anti-baboon. The baboon anti-PAEC was used to determine whether antibodies with multiple endothelial specificities induced lesions more severe than or different from baboon anti-αGal. Four groups of pigs received immunoglobulins as follows: Group I, (pigs 1 and 2), injected over 6 hours to visualize early binding site; pig 3 was the ACE control. Group II, (pigs 4, 5, and 6), infused with antibody for 72 hours to determine the effect of the longest possible αGal antigen-antibody interaction before additional heterologous protein would have induced serum sickness; pigs 7 and 8 were the ACE and BSA controls, respectively. Group III, (pigs 9, 11, and 12) that were mononephrectomized, infused with antibody for 72 hours, and studied 30 to 120 days later for long-term consequences. To enhance cross-linking of tissue-bound antibodies in the autologous phase, 22 pigs were immunized s.c. with baboon γ-globulin in IFA 2 days before the infusion, on a schedule like that used with rats to test the pathogenetic potential of anti-laminin 23 and anti-HSPG 24 antibodies. In pig 9, injected with baboon anti-αGal, vascular permeability was enhanced by concomitant injection of an amount of goat anti-ACE comparable to that inducing in rabbits a minimal increase in glomerular permeability without anatomic lesions20; as control, pig 10 received only the same small dose of goat anti-ACE; pigs 13 and 14 were ACE and BSA controls for pigs 9, 11, and 12. Group IV, included pigs 15 and 16 used as positive control for serum sickness, and to study complement fixation after passive transfer of pig anti-baboon γ-globulins, as described elsewhere23; baboon anti-αGal plus baboon anti-PAEC was infused for 72 hours followed, 3 days later, by pig anti-baboon γ-globulin.
Statistical Analysis
Statistical analyses used ANOVA and unpaired t-test. P < 0.05 was considered a statistically significant difference between values. All measurements were in triplicate and all assays were repeated at least three times with similar results.
Results
Serological Characterization of the Antisera
The concentration of baboon anti-αGal, baboon anti-PAEC, and goat anti-ACE γ-globulins was 30 to 40 mg/ml. The highest dilution producing unequivocal immunofluorescence in pig tissue sections was 0.63 μg/ml. Sheep anti-BSA (300 μg/ml) was the control. The concentration of pig anti-baboon γ-globulin was 20 mg/ml and its staining titer, on sections of pig kidney containing deposits of baboon γ-globulin, was 8 μg/ml.
Immunoreactivity on αGal/BSA was studied by ELISA; IgG titers were increased 32- to 200-fold, and IgM titers were increased 2- to 32-fold as compared to naive baboon or preimmune sera. Similar results were obtained with αGal-bearing proteins, such as pig thyroglobulin and mouse laminin. Sheep anti-BSA was not reactive (Figure 1A) ▶ . Complementary assays were developed to evaluate immunoreactivity and lysis of PAEC. The two γ-globulin fractions had similar titers, which were 20 to 27 times higher than in normal baboon sera (Figure 1B) ▶ . Baboon anti-PAEC and baboon anti-αGal induced 54- and 20-fold more cell lysis than normal baboon sera, respectively, but none with heat-inactivated rabbit serum (not shown). Baboon anti-αGal and baboon anti-PAEC also fixed complement on kidney sections; sections incubated with the tested γ-globulin preparations and rabbit or porcine serum, then stained for rabbit or pig C3, revealed immune deposits in the brush border of proximal tubules, in glomerular and tubular basement membranes, and in the extracellular matrix, but not when heat-inactivated rabbit or porcine serum was used (not shown). Immunoblotting of PAEC with baboon anti-αGal IgG, or affinity-purified baboon anti-αGal IgG, displayed several glycoproteins with apparent molecular weights of 70, 125–135, and 225 (Figure 1C ▶ , Lanes 1 and 6, respectively). Baboon anti-PAEC displayed more intense staining of immobilized PAEC extract, and a prominent 44-kd band, not observed with baboon anti-αGal (Figure 1C ▶ , Lane 2). As expected, pre-immune baboon serum showed a similar pattern of immunoreactive bands, which was prevented by absorption with rabbit erythrocytes (Figure 1C ▶ , Lanes 4 and 5, respectively). Normal rabbit serum revealed no bands (Figure 1C ▶ , Lane 3).
Reactivity with the extracellular matrix was studied by ELISA on murine basement membrane-like matrix of EHS tumor (Matrigel); baboon anti-αGal, baboon anti-PAEC, and baboon pre-immune γ-globulins showed reactivity, though baboon anti-αGal had the highest titer (Figure 2A) ▶ . The presence of αGal epitopes in components of the extracellular matrix was also studied by ELISA; mouse laminin, and mouse HSPG were most reactive and, minimally, bovine fibronectin (Figure 2B) ▶ . In immunoblotting αGal antibody was bound to immobilized mouse laminin (Figure 2C ▶ , Lane 1), bovine fibronectin (Figure 2C ▶ , Lane 4), and mouse HSPG (Figure 2C ▶ , Lane 7), but not to human laminin and fibronectin (Figure 2C ▶ , Lanes 3 and 6, respectively). Absorption of baboon anti-αGal with αGal/BSA or rabbit erythrocytes almost completely abolished this binding (Figure 2C ▶ , Lanes 2, 5, and 8).
Binding of Baboon Anti-αGal and Baboon Anti-PAEC to Normal Pig Tissues
On kidney sections as substratum, the staining titer of baboon anti-αGal and baboon anti-PAEC IgG was 200-fold higher than that of preimmune baboon IgG, which bound mainly to tubular brush border. The staining pattern with baboon anti-PAEC was comparable to that with baboon anti-αGal, although weaker in epithelial cells. The IgM fractions of naïve pigs or pre-immune sera did not stain the tissues, whereas those of baboon anti-αGal and baboon anti-PAEC stained similarly to IgG, but weaker.
In kidney, lung, heart, liver, and intestine, dual fluorescence confocal microscopy, colocalized αGal with laminin and HSPG in the basement membranes. Fibronectin partially colocalized with αGal in the basement membranes, but was mainly expressed in the septa and in the media of the arteries, where αGal was not (or only weakly) detected. Moreover, baboon anti-αGal bound to several epithelial cells, fibroblasts, macrophages, and chondrocytes, whereas baboon anti-PAEC bound to the same cells and structures, but provided a stronger staining of endothelial cells (not shown).
The specificity of baboon anti-αGal and baboon anti-PAEC for αGal was shown by their colocalization with Griffonia simplicifolia B4, and by examination of sections predigested with α- or β-galactosidase; digestion with α-galactosidase, but not with β-galactosidase, abolished staining. In contrast, α-galactosidase digestion only partially reduced the staining capacity of baboon anti-PAEC. Moreover, absorption with αGal/BSA and αGal-rich rabbit erythrocytes abolished the ability of baboon anti-αGal to stain and reduced that of baboon anti-PAEC. Sheep anti-BSA did not stain (not shown).
Clinical Observations after Antibody Infusion
Shortly after the beginning of the injection of baboon anti-αGal, baboon anti-PAEC, and goat-anti-ACE, the pigs developed episodes of tachypnea, agitation, and occasionally, pulmonary edema. With the exception of pigs 6 and 16 (which died at the end of day 4 and after beginning of passive transfer of pig anti-baboon γ-globulin, respectively) these respiratory crises were overcome by decreasing or stopping the delivery of antibody. Infusion of sheep anti-BSA (pigs 8 and 14) did not induce respiratory distress. In pigs injected with baboon anti-αGal and baboon anti-PAEC the signs of pulmonary distress were associated with decreased number of circulating erythrocytes (23% pig 1, 84% pig 4), leukocytes (72% pig 1 and 60% in pig 6), and platelets (83% pig 4). In contrast, blood cell counts remained normal in pig 8, injected with sheep anti-BSA. CH50 decreased 35% in pig 6, injected with baboon anti-PAEC, 65% in pig 4, injected with baboon anti-αGal, and 100% in pig 7, injected with goat anti-ACE. After day 4, slight proteinuria (+ to ++) developed in all pigs injected with baboon anti-αGal, baboon anti-PAEC, and goat anti-ACE, but not with sheep anti-BSA. Serum creatinine, BUN, and liver enzymes remained normal in all animals.
Salient features of results for each group are described below.
Group I
Pigs Injected for 6 Hours with Baboon Anti-αGal (Pig 1) or Baboon Anti-PAEC (Pig 2)
Lungs showed congestion and patchy edema. Some alveolar capillaries were occluded by aggregated erythrocytes and platelets. Baboon IgG, but not IgM, was bound to endothelium and to platelets (Figure 3A) ▶ . Pig C3 was present only in aggregated platelets. Kidneys showed only aggregated platelets and erythrocytes in capillaries and venules. Baboon IgG, but not IgM, stained the endothelium of peritubular capillaries, the glomerular capillary walls, and, less intensely, tubular basement membranes (Figure 3, B and C) ▶ . Pig C3 was deposited only in glomeruli of the pig injected with baboon anti-PAEC. Pig IgG was absent. In other organs aggregated erythrocytes and platelets were in the capillaries, and baboon IgG was deposited on endothelium.
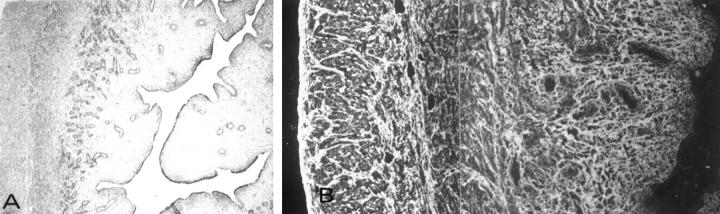
Figure 3.
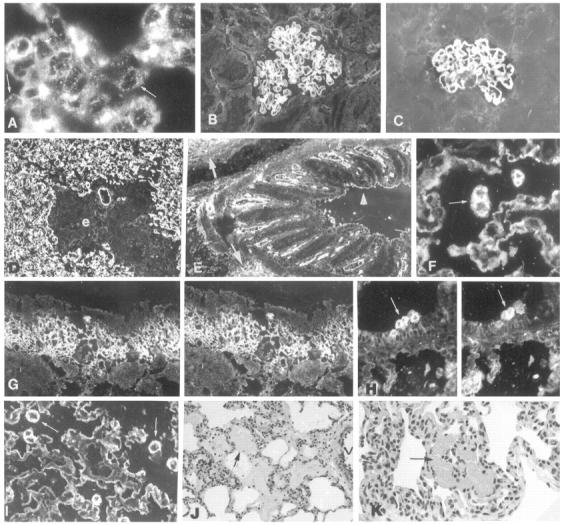
Morphology and immunopathology in pigs Groups I (A–C) and II (D–K). A: Six hours after the beginning of infusion, fine granular deposits of baboon anti-αGal IgG are found in the alveolar endothelium (arrows) of pig 1; the coarse deposits are in aggregated erythrocytes and platelets. Linear deposits of baboon IgG are in glomerular capillary walls of pigs 1 (B) and 2 (C). D: Pig 5 has diffuse deposits of baboon anti-PAEC IgG in the alveolar capillaries and in a small vessel; baboon IgG, and αGal (not shown), are not detectable in areas of perivascular edema (e). E: In pig 4, baboon anti-αGal IgG binds to the vessels of a bronchus, to surrounding extracellular matrix (arrows), and to the plasma membranes of epithelial cells (arrowhead). F: In pig 4, baboon anti-αGal IgG binds to alveolar epithelium and to macrophages (arrows); G and H are lung sections double-stained for αGal (left panels) and baboon anti-αGal IgG (right panels), showing that baboon IgG co-localizes with αGal in the lamina propria of a bronchus, and on mononuclear cells in the lumen of a bronchus. In I: In pig 4, the strong expression of αGal in the alveolar epithelium and at the surface of macrophages (arrows) indicates that αGal was not modulated. In the lung of pig 4, there is perivascular alveolar edema (J, arrow) and focal hemorrhage (K, arrow). V, vessel. Original magnifications, A, ×800; B and C, ×400; D, ×200; E–K, ×400.
Pig Injected for 6 Hours with Goat Anti-ACE (Pig 3)
In lungs there was severe edema, hemorrhage, and accumulation of inflammatory cells. Fine granular deposits of goat IgG and pig C3 were in the alveolar endothelium, and fibrin thrombi occluded the capillaries. The kidneys had granular deposits of ACE and goat IgG in glomerular and some tubular basement membranes.
Group II
Pigs Injected for 72 Hours with Baboon Anti-αGal (Pig 4) or Baboon Anti-PAEC (Pigs 5 and 6)
Circulating antibodies to pig IgG were not detectable. In lungs there was alveolar, subpleural, and perivascular edema and focal hemorrhage, greater after baboon anti-PAEC (Figure 3, J and K) ▶ . Mononuclear cells and occasional erythrocytes were present in the bronchial walls and lumens. Both baboon anti-αGal and baboon anti-PAEC bound at the same sites, but baboon anti-PAEC stained endothelia more strongly. By confocal microscopy baboon IgG co-localized with laminin in pulmonary basement membranes and peribronchial extracellular matrix; it was also bound to alveolar epithelium, capillaries and venules in the visceral pleura, alveolar septa, peribronchial vessels and plasma membrane of bronchial epithelial cells, chondrocytes, and matrix of the cartilage. Baboon IgG and αGal colocalized in alveolar macrophages and in peribronchial and endobronchial mononuclear cells, presumably monocyte/macrophages (Figure 3, F–H) ▶ . The kidneys were morphologically normal. There were deposits of baboon IgG in glomerular basement membrane and at the surface of fibroblast-like cells in the interstitium of the medulla, and lesser ones in tubular basement membranes and basolateral compartments of proximal tubules, but, surprisingly, not in the brush border of proximal tubules, which contain large amount of αGal. Baboon IgG colocalized with αGal in the walls of sinusoids and hepatic septa; in the intestine in the lamina propria of villi, the basement membranes and the lamina propria of choroid plexus, ciliary body, aortic endothelium, and media were also stained.
Pig Injected for 72 Hours with Goat Anti-ACE (Pig 7)
Changes in lungs were similar to but milder than in the pig injected with goat anti-ACE for 6 hours (Group I, pig 3). In kidney, glomeruli had minimal morphological changes but, by immunofluorescence, diffuse granular deposits of ACE and goat IgG were seen in the capillary walls, corresponding to discrete dense deposits in the filtration slits, by electron microscopy.
Pig Injected for 72 Hours with Sheep Anti-BSA (Pig 8)
All organs appeared normal.
Group III
Pigs Injected for 72 Hours with Baboon Anti-αGal (Pigs 9 and 11) and Baboon Anti-PAEC (Pig 12), Sacrificed 4 to 17 Weeks Later
All pigs had low levels of circulating antibodies against baboon IgG. The lesions were more severe in pig 9, to which a small dose of goat anti-ACE was given in order to make extravascular αGal more accessible to antibody. In the lungs of pig 9 there was severe fibrosclerosis around bronchioles, bronchi, medium-sized vessels, and in interlobular septa. The alveolar basement membrane was focally thickened, the alveolar septa enlarged, with collagen fibrils (Figure 4, A–D) ▶ . Alveolar basement membranes were similarly affected in pigs 11 and 12. In sclerotic areas the perineurium of nerves was thickened and collagenous (Figure 4H) ▶ . Baboon IgG was bound to the alveolar capillary walls, alveolar septa, peribronchial matrix, bronchial capillaries, perineurium, and endoneurium (Figure 4, F and G) ▶ . Some deposits of pig IgA were present in the bronchi, but there was no baboon IgM or pig C3. Deposits of pig IgG were absent or minimal and focal. All fibrotic tissues were stained by anti-type I collagen (Figure 4E) ▶ , and less intensely, by anti-type III collagen antibody. In kidneys of all three pigs, glomeruli had increased mesangial matrix with distorted or collapsed capillary walls, glomerulo-capsular adhesions, and focal sclerosis. The glomerular basement membranes were focally thickened, with formation of small spikes and deposits of foreign material between the endothelium and the basement membrane, in the mesangium, and, more rarely, between epithelium and basement membrane. There was periglomerular and medullary sclerosis, especially in the inner stripe. Some arteries were thickened and sclerotic (Figure 5, A, C, D ▶ –F, H and I). Baboon IgG was bound to glomerular capillary walls (Figure 5B) ▶ , mesangium, and Bowman’s capsule. Baboon IgG was also bound to fibroblasts in the interstitium of the medulla (Figure 5G) ▶ , whereas baboon IgM and pig C3 were absent. Deposition of pig IgG was absent or minimal and focal. Increased collagen I and less collagen III expanded the interstitium of the medulla and the adventitia of vessels (Figure 5J) ▶ . In the small intestine of pig 9 severe fibrosis had developed, mainly in the lamina propria, and less in the submucosa, with great distortion of the mucosal structure. The fibrotic lamina propria was strongly stained by type I and type III collagen antibody (Figure 6) ▶ . In all pigs, baboon but not pig IgG was bound to intestinal basement membranes and extracellular matrix, especially in the small intestine.
Figure 4.
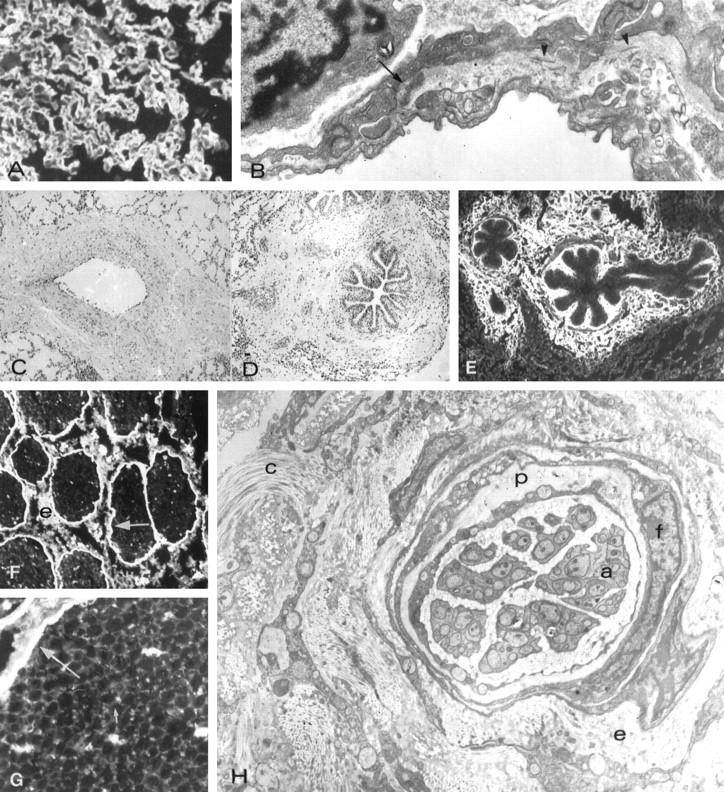
Morphology and immunopathology in lungs of pigs Group III. (Pig N09) A: Thickened alveolar capillary walls stained by anti-laminin antibody. B: Electron micrograph of a thickened alveolar basement membrane containing a dense deposit (arrow) and collagen fibrils (arrowheads). C: Mural and adventitial sclerosis of a pulmonary vein. D: Peribronchial sclerosis. E: Type I collage in the bronchial lamina propria and in the peribronchial matrix. F: Binding of baboon anti-αGal to perineurium (arrow) and epinerium (e) uf unmyelinated nerve. G: Binding of baboon anti-αGal to the perineurium (large arrow) and endoneurium (small arrow). H: Electron micrograph showing the thickened and distorted perineurium (p) and sclerosis of the endoneurium (e), which contains increased amount of collagen (C). f, indicated fibroblast-liek perineurial cells; a, islands of unmyelinated axons. Original magnifications, A, C, D, E, ×300; B, ×20,000; F, ×600; G, ×1000; H, ×25,000.
Figure 5.
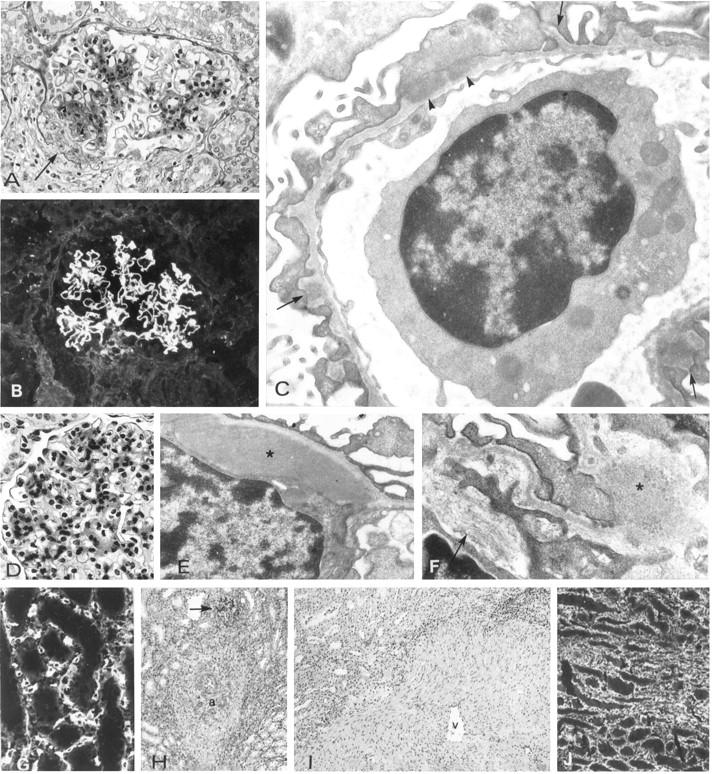
Morphology and immunopathology in kidneys of Group III pigs.
Lesions in the kidneys of pigs 11 (A–F) and 12 (G–I). A: Mesangial sclerosis and glomerulo-capsular adhesion (arrow). B: Deposits of baboon IgG in the glomerular capillary walls. C:: Electron micrograph showing glomerular basement membrane spikes (arrows), and subepithelial deposits of foreign material (arrowheads). D: Mesangial cell proliferation and sclerosis. E and F: Electron micrographs showing mesangial deposits (asterisks) and bundles of fibrils (arrows). G: Binding of baboon anti-αGal to fibroblasts in the inner stripe of the medulla, which strongly express αGal epitopes. H: Sclerosis around a medullary artery. The arrow indicates a glomerulus. I: Sclerosis around a medullary vein (v). J: Deposits in type I collagen in the medulla. A, B, D, G, ×600; C, E, F, 25.000; H, I, J ×200.
Figure 6.
Morphological and immunopathological findings in the small intesting of pig 9. A: Severe sclerosis in the lamina propria with distortion of mucosal structure. B: Diffuse deposits of type I collagen. Original magnifications: A, ×200; B, ×600.
Pigs Injected with Goat Anti-ACE (Pigs 10 and 13) for 72 Hours and Sacrificed 4 to 13 Weeks Later
The organs of pig 10 were normal. Pig 13 had developed some small subepithelial granular deposits of goat IgG and pig IgG; all other organs were normal.
Pig Injected with Sheep Anti-BSA (Pig 14) for 72 Hours and Sacrificed 13 Weeks Later
All organs were normal.
Group IV
Pigs Injected with Baboon Anti-αGal/Baboon Anti-PAEC and Pig Anti-Baboon γ-Globulin (Pigs 15 and 16, Passive Transfer)
The lesions were more severe in pig 16, which died, probably of serum sickness. The lungs were diffusely edematous and hemorrhagic. The alveolar capillaries were occluded by erythrocytes, polymorphonuclear leukocytes, and mononuclear cells. The kidneys exhibited proliferative and exudative glomerulonephritis. Diffuse, coarse/granular deposits of baboon IgG, pig IgG, and C3 were present in alveolar and glomerular capillary walls and in the walls of small vessels (not shown).
Effect of Baboon Anti-αGal on Renal Fibroblast Production of TGF-β and Collagen
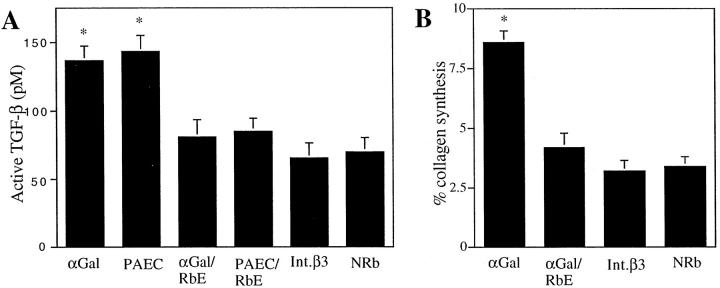
Because baboon anti-αGal bound to fibroblasts and to the extracellular matrix, and widespread fibrotic lesions were observed in pigs infused with baboon anti-αGal and baboon anti-PAEC, we tested the hypothesis that the interaction of αGal antibodies with fibroblasts elicits expression of profibrotic cytokine/growth factors, such as TGF-β, and/or causes increased production of collagen. Cells cultured from the inner stripe of the renal medulla had morphological aspects and a growth cycle characteristic of fibroblasts, were stained by baboon anti-αGal, by anti-β3 integrin and anti-vimentin antibodies, but not cytokeratin antibody, which is reactive with renal tubular epithelial cells. 25 In culture supernatants of fibroblasts incubated with baboon anti-αGal and anti-PAEC, total TGF-β was twofold (60 hours) higher than in supernatants of fibroblasts incubated with normal rabbit γ-globulins. This effect was reduced by absorption of baboon antibodies with rabbit erythrocytes. Anti-β3 integrin antibody did not increase the production of TGF-β (Figure 7A) ▶ . Baboon anti-αGal, but not anti-β3 integrin antibody, also induced increased production of collagen. This effect of baboon anti-αGal was blocked by preabsorption with rabbit erythrocytes (Figure 7B) ▶ .
Figure 7.
Effect of baboon anti-αGal on renal fibroblast production of TGF-β (A) and collagen (B) A: After 60 hours of incubation with anti-αGal (αGal) and anti-PAEC (PAEC), active TGF-β in culture supernatants was twofold higher as compared to incubation with αGal antibody-free normal rabbit γ-globulin (NRb) or anti-β3 integrin (Int. β3) (*P < 0.01), and significantly higher than incubation with baboon anti-αGal absorbed with rabbit erythrocytes (αGal/RbE) or anti-PAEC absorbed with rabbit erythrocytes (PAEC/RbE) (*P < 0.05). B: Incubatin with baboon anti-αGal (αGal) induced a 2.5-fold increase in collagen synthesis, as compared to normal rabbit γ-globulin (NRb) (*P < 0.01). Baboon anti-αGal absorbed with αGal/RbE and Int. β3 did not induce a comparable stimulation. Data represent the mean ± SE.
Discussion
We have studied the interaction of baboon anti-αGal antibody with pig tissues in a nondestructive model that allows assessment of long-term consequences of the antigen-antibody reaction. We observed fibrosclerotic lesions associated with, and probably consequent upon, deposition of the antibodies in basement membranes and extracellular matrix. We demonstrate αGal epitopes in the extracellular matrix, especially in laminin and HSPG. We will consider how the results of this model may relate to prolonged survival of pig organs in primates.
Characteristics of Baboon Antisera and Their Suitability as a Model for Elicited Human Xenoantibodies
Immunization of baboons with αGal/BSA or PAEC resulted in increased levels of αGal IgG and IgM. This is consistent with the immune response observed in cynomolgus monkeys transplanted with porcine or bovine cartilage, 26 patients transplanted with fetal porcine islet cells, 27 or those treated with porcine liver perfusion. 28 The observation that baboon anti-PAEC had high αGal titer confirms that αGal is a major xenoantigen of PAEC.4–7,29–31 ELISA reactivity was also increased for Matrigel, which contains laminin, with 50 to 60 αGal epitopes per molecule16; type IV collagen; and HSPG, with a ratio of 1:0.6:0.03, respectively. 32 By immunohistochemical titration, using a section of pig kidney as substratum, the reactivity of baboon anti-αGal and baboon anti-PAEC IgG was about 200-fold greater than that of human natural xenoantibodies.
Western blot analysis of PAEC extracts using baboon anti-αGal and baboon anti-PAEC identified bands with masses similar to those identified with human natural xenoantibodies. These are 125 to 135-kd, presumably integrins; 230-kd likely either von Willebrand factor or fibronectin; and bands of 34- to 76-kd glycoproteins. 33,34 Moreover, baboon anti-PAEC immunoprecipitates another abundantly glycosylated 44-kd protein. 35 Baboon anti-αGal, baboon anti-PAEC, and human natural xenoantibodies may have similar specificities, because the specificity of natural xenoantibodies and that of sera of patients immunized by cross-perfusion with pig liver is the same. 28 The reactive epitope of the glycoproteins identified by human natural xenoantibodies is αGal,4–7,30,34 which is broadly represented in phylogeny. 7,10 Fixation of xenoantibodies to PAEC, however, is not solely dependent on αGal, since removal of αGal decreases its fixation only by 75 to 80%. 34 That the baboons had developed a sustained antibody response was shown by the increased binding of baboon anti-αGal and baboon anti-PAEC to PAEC, and by greater complement-mediated cytotoxicity, than normal baboon sera. Both antisera could fix complement in vitro as in sections of normal pig tissue.
Binding Sites of Baboon Anti-αGal and Baboon Anti-PAEC to Sections of Normal Pig Tissues
Immunoreactive epitopes were identified in plasma membranes of endothelial and some epithelial cells, blood cells, macrophages, chondrocytes, and fibroblasts. In solid tissues they stained vascular endothelia, as previously shown with Bandeiraea (Griffonia) simplicifolia 1 isolectin B4 and αGal antibody isolated from normal human sera. 36-38 However, baboon anti-αGal and baboon anti-PAEC also stained the basement membranes and the extracellular matrix, where they colocalized with antibodies to laminin, HSPG, perlecan, and partially to fibronectin. The principal immunological target was αGal, because the staining was identical to that obtained with Bandeiraea (Griffonia) simplicifolia B4, which is specific for αGal epitopes39; was eradicated by digestion of tissues with α-galactosidase, but not by β-galactosidase; was abolished by absorption of baboon anti-αGal with αGal/BSA, but not with BSA; and was also abolished by absorption with αGal-rich rabbit erythrocytes. In contrast, the α-galactosidase digestion markedly inhibited, but did not abolish, the staining with baboon anti-PAEC, consistent with recognition of epitopes other than αGal. Differences between our immunohistochemical findings and previous reports may be ascribed mostly to the high titer and avidity of the baboon antisera, which were used at optimal staining concentrations 350 to 400 times lower than those of αGal antibodies isolated from normal human sera. 37 It could be possible that, in vivo, not all the epitopes visualized in tissue sections are readily accessible to antibody.
Usefulness and Limitations of the Protocol Used for the Experiments Performed in Vivo
One of aims of this study was to find out whether baboon anti-αGal, which react with αGal epitopes on matrix glycoproteins of several animal species, induced in pig matrix lesions similar to those described in animals injected with heterologous anti-matrix antibodies. 23,24,40-45 Baboon anti-PAEC was used to determine whether an antiserum with multiple specificities, including αGal, induced lesions similar to or different from baboon anti-αGal. We infused baboon γ-globulins i.v. for a relatively short period of time in order to prevent acute serum sickness, and serum sickness never occurred in pigs of Groups I, II, and III, as shown by the sharp linear immunofluorescence binding of baboon antibody to pig basement membranes, 46 without pig IgG, and by absence of acute inflammatory lesions. 47 And we could also compare the effects of baboon anti-αGal and baboon anti-PAEC with those natural xenoantibodies. 30,48
The major limitation was that, aside from antibody, pig rather than baboon immune reactants were involved. Moreover, the effect of baboon antibodies was diluted in the entire blood and body of the pig instead of being focused on a single organ. Lastly, the binding of antibodies to blood cells and their agglutination at the beginning of the injections could cause acute pulmonary symptoms and release of mediators and cytokines in the circulation.
Clinical Observations
In pigs injected with baboon anti-αGal and baboon anti-PAEC the early signs of pulmonary distress were mainly due to erythrocyte and platelet agglutination and pulmonary small vessel embolism, as shown in the pulmonary morphology of pig 6, which died suddenly at the beginning of the infusion, or pigs that were sacrificed after 6 hours (Group I). This is consistent with the observed decrease in blood cell counts. Similar pulmonary symptoms developed in control pig 3, injected with goat anti-ACE, in which the binding of antibody to pulmonary endothelium with local fixation of complement induced local accumulation and degranulation of inflammatory cells, especially platelets and monocyte/macrophages, as in rabbits injected with goat anti-ACE antibody. 49,50 Control pigs 7 and 13, also injected with goat anti-ACE, developed pulmonary signs analogous to pig 3. In contrast, pigs injected with sheep anti-BSA remained normal in all these respects.
In Vivo Localization of Baboon Anti-αGal and Baboon Anti-PAEC
In pigs injected for 6 hours (Group I) baboon IgG, most strongly baboon anti-PAEC IgG, was bound mainly to the endothelium, as seen in pig organs transplanted into unmodified baboons, confirming that the endothelium is the principal and immediate target of circulating xenoantibodies. 1,48 In the kidney, baboon IgG was bound to the endothelium, but also to glomerular and tubular basement membrane.
After 72 hours (Group II), baboon IgG, but not pig C3, was localized in alveolar basement membranes, in the alveolar septa, and on the plasma membrane of alveolar epithelial cells. This delayed fixation of the IgG is probably due to restrictive blood to airspace barrier posed by the alveolar capillary walls; when the permeability is not increased, about 24 hours are required for the transfer of IgG from the circulation to the alveolar space. 51 Baboon IgG was also bound to renal basement membranes, to the basolateral compartment of the cells of proximal tubules, and to αGal-positive cells in the medulla. These findings are like those in other model systems. Fixation of IgG and IgM to basement membranes and extracellular matrices of heart, lung, liver, pancreas, intestine, and aortic adventitia was described in pig organs which had been extracorporeally perfused with human blood, 52 fixation of rat IgG and IgM to the extracellular matrix of hamster or guinea pigs aortas was shown to occur after transplantation into rats, 53 and rabbit IgG was bound to fibroblasts and extracellular matrices of pig patellar tendon and cartilage transplanted into rabbits. 54 It is notable that in rats with allograft rejection glomerulosclerosis, serum antibodies reactive with cryptic basement membrane antigens and proteoglycans (biglycan and decorin) are bound to the basement membranes of the kidney. 55,56
Four weeks after infusion of baboon anti-αGal and baboon anti-PAEC (Group III), baboon IgG was bound to pig lung and renal basement membranes in a pattern like that of animals given antibodies reactive with basement membranes or some of their purified components, laminin, 23,40-43 type IV collagen, 40,41 HSPG, 24,44 or fibronectin. 45 It is well established that damage sufficient to alter morphology and function of basement membranes and extracellular matrices can be induced by antibody deposition alone, independent of complement activation and inflammation. 57
Consequences of a Prolonged Antigen-Antibody Interaction in Vivo and Mechanisms of Tissue Damage
That baboon anti-αGal and baboon anti-PAEC induce a similar effect is in agreement with previous studies showing that the lesions in pig cells or tissues caused by human natural xenoantibodies, or sera of baboons or man sensitized by porcine grafts, are mainly due to αGal antibodies. 5-7,29-31
With the exception of antibody to α3 chain type IV collagen, the Goodpasture’s antigen, 58 all other antibodies to defined components of the extracellular matrix provoke mild tissue lesions, detectable only by electron microscopy. 24,42,44 This is probably due to the abundance and very widespread distribution of matrix antigens, the amount of tissue-fixing antibody being too low to reach the threshold necessary to fix complement. 59 For example, sheep anti-laminin antibody can fix C in vitro but, when injected i.v. into rats, does not induce morphological and functional changes, even when the autologous phase is actively or passively boosted. Severe complement-dependent lesions, however, develop with a more focused immunological attack, as when kidneys with planted sheep anti-laminin antibodies are transplanted into naïve recipients passively immunized with rat anti-sheep IgG. 23 Likewise, the effects of baboon anti-αGal and baboon anti-PAEC antibodies are dissipated throughout the pig body, and early fixation to blood cells may remove some high-affinity antibodies, so that antibodies bound to any tissue were insufficient in quality, amount, and concentration to fix C1q. That insufficient complement fixation was due to a limitation of the model, and not to intrinsic deficiency of baboon antibodies, was shown by studies in vitro, and by the observation that when a control passive acute serum sickness was induced (Group IV), complement was locally activated and acute pulmonary and glomerular lesions ensued.
Lesions of basement membranes and the extracellular matrix were similar, though more severe, than those of animals given anti-laminin 42 or anti-HSPG 44 antibodies which do not, or only poorly, fix complement in vivo. These antibodies also bind to fibroblast surface and probably affect their function. A similar effect seems to have been exerted by baboon anti-αGal and baboon anti-PAEC when they are bound in vivo to αGal epitopes on laminin and HSPG, interfering with the critical function of these two glycoproteins in the supramolecular assembly of an integrated basement membrane and matrix network. 60 The autologous phase, with in situ formation of immune complexes, might have contributed to the development of the lesions. In pigs, however, deposits of pig IgG in tissues were either absent or minimal and focal. Therefore, even if a cross-linking effect of pig IgG cannot be excluded, there is no evidence of it.
The possibility that cytokines/mediators released from platelets, lymphocytes, and monocytes during their early and transitory agglutination in pulmonary capillaries might have contributed to the pathogenesis of the lesions deserves consideration. The control experiments most appropriate to rule out the possible effect of these mediators would have required injection of heterologous antibodies to pig blood cell surface antigens, but devoid of αGal reactivity, but such antibodies are not available. However, pigs injected with anti-ACE antibodies also developed acute pulmonary symptoms, due to interaction with pulmonary endothelium, activation of complement, and local accumulation and degranulation of blood cells, especially platelets and monocyte/macrophages. Absence of basement membrane and fibrosclerotic lesions in these control pigs support the interpretation that fixation of αGal antibodies to the extracellular matrix is the primary pathogenetic mechanism. This interpretation is strengthened by the similarity with lesions induced in the rat by heterologous anti-laminin 42 and anti-HSPG 44 antibodies, and by apparent absence of matrix lesions induced by cytokines/mediators released in the circulation when antibody or immune complexes are not bound to tissues.
Binding of αGal antibodies to αGal on fibroblast and macrophage surfaces, might stimulate a fibrogenic response, consistent with our observation that binding of baboon anti-αGal to renal fibroblasts increased their production of active TGF-β. Enhanced production of collagen could result from stimulation by TGF-β, 61-63 and/or other effects of antibody engagement of cell surface αGal. Similar mechanisms were invoked to explain the fibrosclerotic response of rats injected with anti-thymocyte 63 or anti-glomerular basement membrane 64 antibodies.
How the Results May Relate to Transplanted Pig Organs Surviving in Primates
The probability that in acute vascular and chronic rejection αGal antibodies may bind to αGal epitopes on laminin, HSPG, and fibronectin should be contemplated, as should their perturbing effect on αGal-positive cells involved in the synthesis and remodeling of the extracellular matrix. This hypothesis gains additional credibility from the consideration that pigs were injected with αGal antibodies only for 72 hours, whereas functioning xenografts may be exposed to a longer and more focused attack. Thus, overcoming the hurdles of hyperacute and acute vascular rejection would be insufficient to prevent the subsequent development of fibrosclerotic lesions similar to those restricting the survival of allografts, 65,66 unless the expression of αGal epitopes in the xenograft 67 or the ability of the host to generate αGal antibodies 68 are substantially inhibited or eradicated.
Acknowledgments
We thank Dr. Peter R. B. Caldwell for the generous gift of ACE antibody, Dr. Marilyn G. Farquhar for the HSPG antibody, Drs. Ralph J. Butkowski and Aristidis S. Charonis for the TBM antigen, Dr. Daniel B. Rifkin for MLECs-clone 32 cells, Drs. Bernard F. Erlanger, Elvin A. Kabat, Paul D. Killen, and Hynda K. Kleinman for advice and criticism, and Ms. Theresa Swayne for expert technical help.
Footnotes
Address reprint requests to Dr. Giuseppe Andres, via Gerolamo Belloni 38, Roma, 00191 Italy.
Supported by National Institutes of Health grants DK-36807-25/27 (to G. A.) and HL-42507-PERC (to D. S.).
The first two named authors contributed equally to this work.
References
- 1.Platt JL: Antibodies in graft rejection. Bach FH Auchincloss H, Jr eds. Transplantation Immunology. 1995, :pp 113-129 Wiley-Liss & Sons, New York [Google Scholar]
- 2.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC: Delayed xenograft rejection. Immunol Today 1996, 17:379-383 [DOI] [PubMed] [Google Scholar]
- 3.Platt JL, McGregor CGA: Acute vascular rejection. Xenotransplantation 1998, 5:169-175 [DOI] [PubMed] [Google Scholar]
- 4.McCurry KR, Parker W, Cotterell AH, Weidner BC, Linn SS, Daniels LJ, Holzhnecht ZE, Byrne GW, Diamond LE, Logan JS, Platt JL: Humoral responses in pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol 1997, 58:91-105 [DOI] [PubMed] [Google Scholar]
- 5.Good AH, Cooper DCK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontage LR: Identification of carbohydrate structures which bind human anti-porcine antibodies: implication for discordant xenografting in man. Transplant Proc 1992, 24:559-562 [PubMed] [Google Scholar]
- 6.Sandrin M, Vaugham HA, Dabkowski PL, McKenzie IFC: Anti-pig IgM antibodies in human serum react predominantly with Galα1-3Gal epitopes. Proc Natl Acad Sci USA 1993, 90:11391-11395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galili U: The α Galactosyl epitope (Galα1-3Galβ1-4GlcNAc-R) and the natural anti-Gal antibody. Blamcher A Klein J Socha WW eds. Molecular Biology and Evolution of Blood Groups and MHC antigens in Primates. 1997, :pp 236-253 Springer, Berlin [Google Scholar]
- 8.Yuzawa Y, Brentjens JR, Brett J, Caldwell PRB, Esposito C, Fukatsu A, Godman G, Stern D, Andres G: Antibody-mediated redistribution and shedding of endothelial antigens in the rabbit. J Immunol 1993, 150:5633-5646 [PubMed] [Google Scholar]
- 9.Mancini G, Carbonara AO, Heremans JF: Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry 1965, 2:235-254 [DOI] [PubMed] [Google Scholar]
- 10.Galili U, Shohat SB, Kobrin A, Stults CLM, Macher BA: Man, apes and old world monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J Biol Chem 1988, 263:17755-17762 [PubMed] [Google Scholar]
- 11.Stow JL, Sawada H, Farquhar MG: Basement membrane heparan sulfate proteoglycans are concentrated in the laminae rarae and in podocytes of the rat renal glomerulus. Proc Natl Acad Sci USA 1985, 82:3296-3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butkowski RJ, Langveld JP, Wieslander J, Brentjens JR, Andres G: Characterization of a tubular basement membrane component reactive with autoantibodies associated with tubulointerstitial nephritis. J Biol Chem 1990, 265:21091-21098 [PubMed] [Google Scholar]
- 13.Kelley J, Fabisiak JP, Hawes K, Absher M: Cytokine signaling in lung: transforming growth factor-β secretion by lung fibroblasts. Am J Physiol 1991, 260:L123-L128 [DOI] [PubMed] [Google Scholar]
- 14.Engvall E, Perlmann P: Enzyme-linked immunosorbent assay (ELISA): quantitative assay of immunoglobulin G. Immunochemistry 1971, 8:871-874 [DOI] [PubMed] [Google Scholar]
- 15.Platt JL, Turman MA, Noreen HG, Fischel RJ, Bolman RM, Bach FH: An ELISA assay for xenoreactive natural antibodies. Transplantation 1990, 49:1000-1001 [DOI] [PubMed] [Google Scholar]
- 16.Galili U, LaTemple DC, Radic MZ: A sensitive assay for measuring α-gal epitope expression on cells by monoclonal anti-Gal antibody. Transplantation 1998, 65:1129-1132 [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Harpel JG, Metz CN, Nannes I, Loskutoff DJ, Rifkind DB: An assay for TGF-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 1994, 216:276-284 [DOI] [PubMed] [Google Scholar]
- 19.Peterkofsky B, Chojkier M, Beteman J: Determination of collagen synthesis in tissue and cell culture systems. Furthmayr H eds. Immunochemistry of the Extracellular Matrix. 1982, :pp 20-31 FL, CRC Press, Boca Raton [Google Scholar]
- 20.Matsuo S, Fukatsu A, Taub LM, Caldwell PRB, Brentjens JR, Andres G: Glomerulonephritis induced in the rabbit by anti-endothelial antibodies. J Clin Invest 1987, 79:1798-1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkholder PM: Complement fixation in diseased tissues. I. Fixation of guinea pig complement in sections of kidney from humans with membranous glomerulonephritis and rats injected with anti-rat kidney serum. J Exp Med 1961, 114:605-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unanue ER, Dixon FJ: Experimental glomerulonephritis. VI. The autologous phase of nephrotoxic serum nephritis. J Exp Med 1965, 121:715-725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feintzeig ID, Abrahmson DR, Cybulsky AV, Dittmer JE, Salant DJ: Nephritogenic potential of sheep antibodies against glomerular basement membrane laminin in the rat. Lab Invest 1986, 54:531-542 [PubMed] [Google Scholar]
- 24.Makino H, Lelongt B, Kanwar YS: Nephritogenicity of proteoglycans: II. A model of immune complex nephritis. Kidney Int 1988, 34:195-208 [DOI] [PubMed] [Google Scholar]
- 25.Elger M, Kaissling B, Le Hir M, Kriz W: Microanatomy of the kidney: vessels, interstitium and glomerulus. Nielson EG Couser WG eds. In Immunologic Renal Diseases. 1987, :pp 15-38 Lippincott-Raven, Philadelphia [Google Scholar]
- 26.Galili U, La Temple DC, Walgenbach AW, Stone KR: Porcine and bovine cartilage transplants in cynomolgus monkey. II. Changes in anti-Gal response during chronic rejection. Transplantation 1997, 63:646-651 [DOI] [PubMed] [Google Scholar]
- 27.Galili U, Tibell A, Samelsson B, Rydberg L, Groth CG: Increased anti-Gal activity in diabetic patients transplanted with fetal porcine islet cell clusters. Transplantation 1995, 59:1549-1556 [PubMed] [Google Scholar]
- 28.Cotterell AH, Collins BH, Parker W, Harland RC, Platt JF: The humoral immune response in humans following cross-perfusion of porcine organs. Transplantation 1995, 60:861-868 [PubMed] [Google Scholar]
- 29.Vaughan HA, Loveland BE, Sandrin MS: Galα(1,3)Gal is the major xenoepitope expressed on pig endothelial cells recognized by naturally occurring cytotoxic human antibodies. Transplantation 1994, 58:879-882 [DOI] [PubMed] [Google Scholar]
- 30.Collins BH, Cotterell AH, McCurry KR, Alvarado CG, Magee JC, Parler W, Platt JL: Cardiac xenografts between primate species provide evidence for the importance of the α-Galactosyl determinant in hyperacute rejection. J Immunol 1995, 154:5500-5510 [PubMed] [Google Scholar]
- 31.Galili U, Gregory CR, Morris RE: Contribution of anti-Gal to primate and human IgG binding to porcine endothelial cells. Transplantation 1995, 60:210-213 [PubMed] [Google Scholar]
- 32.Grant DS, Kleinman HK, Leblond CP, Inque S, Chung AE, Martin GR: The basement-membrane-like matrix of the mouse EHS tumor. II. Immunohistochemical quantitation of six of its components. Am J Anat 1985, 174:387-398 [DOI] [PubMed] [Google Scholar]
- 33.Parker W, Holzknecht ZE, Song A, Blocher BA, Bustos M, Reissner KJ, Everett ML, Platt JF: The fate of antigen in xenotransplantation: implication for acute vascular rejection and accommodation. Am J Pathol 1998, 152:829-839 [PMC free article] [PubMed] [Google Scholar]
- 34.Holzknecht ZE, Platt JF: Identification of porcine endothelial cell membrane antigens recognized by human xenoreactive natural antibodies. J Immunol 1995, 154:4565-4575 [PubMed] [Google Scholar]
- 35.Kearns-Jonker M, Cramer DV, Fraiman M, Middleton TY, Shirwan A, Swensson J, Wu GD, Makowka L: Identification and characterization of monoclonal antibodies that partially block human natural antibody binding to pig endothelial cell xenoantigens. Xenotransplantation 1996, 3:287-295 [Google Scholar]
- 36.Thall A, Etienne-Decerf J, Winand RJ, Galili U: The α-galactosyl epitope on mammalian thyroid cells. Acta Endocrinologica 1991, 124:692-699 [DOI] [PubMed] [Google Scholar]
- 37.Oriol R, Ye Y, Koren E, Cooper DKC: Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-human organ xenotransplantation. Transplantation 1993, 56:1433-1442 [DOI] [PubMed] [Google Scholar]
- 38.Oriol R, Barthod F, Bergemer A-M, Ye Y, Koren E, Cooper DKC: Monomorphic and polymorphic carbohydrate antigens on pig tissues: implications for organ xenotransplantation in the pig-to-human model. Transpl Int 1994, 7:405-413 [DOI] [PubMed] [Google Scholar]
- 39.Shibata S, Peters BP, Roberts DD, Goldstein IJ, Liotta LA: Isolation of laminin by affinity chromatography on immobilized Griffonia Simplicifolia I lectin. FEBS Lett 1982, 142:194-198 [Google Scholar]
- 40.Yaar M, Foidart JM, Brown KS, Rennard SI, Martin GR, Liotta L: The Goodpasture-like syndrome in mice induced by intravenous injections of anti-type IV collagen and anti-laminin antibody. Am J Pathol 1982, 107:79-81 [PMC free article] [PubMed] [Google Scholar]
- 41.Wick G, Müller PU, Timpl R: In vivo localization and pathologic effects of passively transferred antibodies to type IV collagen and laminin in mice. Clin Immunol Immunopathol 1982, 23:656-665 [DOI] [PubMed] [Google Scholar]
- 42.Abrahmson DR, Caulfield JP: Proteinuria and structural alterations in rat glomerular basement membranes induced by intravenously injected anti-laminin immunoglobulin G. J Exp Med 1982, 156:128-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy-Ulrich JE, Oberley TD: Immune-mediated injury to basement membranes in mice immunized with murine laminin. Clin Immunol Immunopathol 1984, 31:33-43 [DOI] [PubMed] [Google Scholar]
- 44.Miettinen A, Stow JL, Mentone S, Farquhar MG: Antibodies to heparan sulfate proteoglycans bind to the laminae rarae of the glomerular basement membranes (GBM) and induce subepithelial GBM thickening. J Exp Med 1986, 163:1064-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy-Ulrich JE, Oberley TD, Mosher DF: Glomerular and vascular injury in mice following immunization with heterologus and autologous fibronectin. Virchows Arch (Cell Pathol) 1982, 39:305-321 [DOI] [PubMed] [Google Scholar]
- 46.Unanue E, Dixon FJ: Experimental glomerulonephritis: immunologic events and pathogenetic mechanisms. Adv Immunol 1967, 6:1-90 [DOI] [PubMed] [Google Scholar]
- 47.Corchrane CG: Immunologic tissue injury by neutrophilic leukocytes. Adv Immunol 1968, 68:97-162 [DOI] [PubMed] [Google Scholar]
- 48.Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH: Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation 1991, 52:214-220 [DOI] [PubMed] [Google Scholar]
- 49.Barba LM, Caldwell PRB, Downie GH, Camussi G, Brentjens JR, Andres G: Lung injury mediated by antibody to endothelium. I. In the rabbit a repeated interaction of heterolous anti-angiotensin converting enzyme antibodies with alveolar endothelium results in resistance to immune injury through antigenic modulation. J Exp Med 1983, 158:2141-2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camussi G, Biesecher G, Caldwell PRB, Biancone L, Andres G, Brentjens JR: Role of membrane attack complex of complement in lung injury mediated by antibody to endothelium. Int Arch Allergy Immunol 1993, 102:216-223 [DOI] [PubMed] [Google Scholar]
- 51.Bernaudin J-F, Bellon B, Pinchon M-C, Kuhn J, Druet P, Bignon J: Permeability of the blood-air barrier to antiperoxidase antibodies and their fragments in the normal rat. Am Rev Respir Dis 1982, 125:734-739 [DOI] [PubMed] [Google Scholar]
- 52.Maggiano N, Citterio F, Evangelista A, Pozzetto U, Castaneto M, Capelli A: Immunomicroscopical localization of human preformed natural antibodies against pig tissues in xenogeneic transplantation. Histochem J 1994, 26:553-562 [DOI] [PubMed] [Google Scholar]
- 53.Allaire E, Mandet C, Bruneval P, Bensenane S, Becquemin J-P, Michel J-B: Cell and extracellular matrix rejection in arterial concordant and discordant xenografts in the rat. Transplantation 1996, 62:794-803 [DOI] [PubMed] [Google Scholar]
- 54.Veillette CJ, Cunningham KD, Hart DA, Fritzler MJ, Frank CB: Localization and characterization of porcine patellar tendon xenograft antigens in a rabbit model of medial collateral ligament replacement. Transplantation 1998, 65:486-493 [DOI] [PubMed] [Google Scholar]
- 55.De Heer E, Davidoff A, van der Wall A, van Geest M, Paul LC: Chronic renal allograft rejection in the rat. Transplantation-induced antibodies against basement membrane antigens. Lab Invest 1994, 70:494-502 [PubMed] [Google Scholar]
- 56.Paul LC, Maralidharan J, Muzzaffar SA, Mantig EH, Valentin J-F, de Heer E, Kashgarian M: Antibodies against mesangial cells and their secretory products in chronic renal allograft rejection in the rat. Am J Path 1998, 152:1209-1223 [PMC free article] [PubMed] [Google Scholar]
- 57.Salant DJ, Natori Y, Shimizu F: Glomerular injury due to antibody alone. Immunologic Renal Diseases. 1997, :pp 359-376 editors. Philadelphia, Lippincott-Raven, EG Neilson and WG Couser [Google Scholar]
- 58.Hudson BG, Wieslander J, Wisdom BJ, Noelken ME: Biology of Disease. Goodpasture syndrome: molecular architecture and function of basement membrane antigen. Lab Invest 1989, 61:256-269 [PubMed] [Google Scholar]
- 59.Cooper NR: The classical complement pathway: activation and regulation of the first complement component. Adv Immunol 1985, 37:151-216 [DOI] [PubMed] [Google Scholar]
- 60.Laurie GW, Bing JT, Kleinman HK, Hassel JR, Aumailley M, Martin GR, Feldmann RJ: Localization of binding sites for laminin, heparan sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. J Mol Biol 1986, 189:205-216 [DOI] [PubMed] [Google Scholar]
- 61.Border WA, Noble NA: Transforming growth factor β in tissue fibrosis. N Engl J Med 1994, 331:1286-1292 [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto T, Noble NA, Miller DE, Border WA: Sustained expression of TGF-α1 underlies development of progressive renal fibrosis. Kidney Int 1994, 45:916-927 [DOI] [PubMed] [Google Scholar]
- 63.Okuda S, Languino LRR, Ruoslahti E, Border WA: Elevated expression of transforming growth factor-β and proteoglycan production in experimental glomerulonephritis: possible role in expansion of the mesangial extra-cellular matrix. J Clin Invest 1990, 86:453-462(erratum J Clin Invest 1990, 86:2175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coimbra T, Wiggins R, Noh JW, Merit S, Phan SH: Transforming growth factor-β production in anti-glomerular basement membrane disease in the rabbit. Am J Pathol 1991, 138:223-234 [PMC free article] [PubMed] [Google Scholar]
- 65.Platt JL: New directions for organ transplantation. Nature 1998, 392:11-17 [DOI] [PubMed] [Google Scholar]
- 66.Orosz CG, Sedmak DD: Concerns regarding the current paradigm for chronic allograft rejection. Transpl Immunol 1997, 5:169-172 [DOI] [PubMed] [Google Scholar]
- 67.Auchincloss A, Jr, Sachs DH: Xenogeneic transplantation. Annu Rev Immunol 1998, 16:433-470 [DOI] [PubMed] [Google Scholar]
- 68.Bracy JL, Sachs DH, Iacomini J: Inhibition of xenoreactive natural antibody production by retroviral gene therapy. Science 1998, 281:1845-1847 [DOI] [PubMed] [Google Scholar]