Abstract
Reparative process of second and third degree burns usually results in hypertrophic scar formation that can be treated by pressure. Although this method is efficient, its mechanisms of action are not known. In this work, we have studied the histological organization of hypertrophic scars submitted to pressure. Skin biopsies were performed 2 to 7 months after the onset of treatment in two adjacent regions of the scar, non-pressure- or pressure-treated and analyzed by immunohistochemistry and transmission electron microscopy for extracellular matrix organization and cellular morphology. In non-pressure-treated regions, fibrillin deposits did not present the classical candelabra-like pattern under epidermis and were reduced in dermis; in pressure-treated regions the amount was increased compared to non-pressure-treated regions but the organization was still disturbed. In non-pressure-treated regions, elastin was present in patch deposits; in pressure-treated regions elastin formed fibers, smaller than in normal dermis. Tenascin was present in the whole dermis in non-pressure-treated regions, whereas in pressure-treated regions it was observed only under epidermis and around vessels, as in normal skin. α-Smooth muscle actin-expressing myofibroblasts were absent in normal skin, present in large amounts in non-pressure-treated regions, and almost absent in pressure-treated regions. The disturbed ultrastructural organization of dermal-epidermal junction observed in non-pressure-treated regions disappeared after pressure therapy; typical features of apoptosis in fibroblastic cells and morphological aspects of collagen degradation were observed in pressure-treated regions. Our results show that, in hypertrophic scars, pressure therapy restores in part the extracellular matrix organization observed in normal scar and induces the disappearance of α-smooth muscle actin-expressing myofibroblasts, probably by apoptosis. We suggest that the pressure acts by accelerating the remission phase of the postburn reparative process.
The reparative process of second and third degree burns usually results in formation of hypertrophic scars that are clinically characterized by elevation above skin surface limited to injury borders, redness, and itching, and are frequently associated with contractures. Beyond causing bodily disfigurement, contractures may also cause disorders or even loss of the normal functions of the affected region. 1 Histologically, hypertrophic scars are characterized by α-smooth muscle (SM) actin-expressing myofibroblasts and thin, randomly organized collagen fibers, both usually arranged in nodules. 2
Myofibroblasts, which are the main cellular type observed in granulation tissue, are modified fibroblasts that present some features typical of SM cells. 3 They contain bundles of microfilaments with dense bodies similar to those found in SM cells and can express, depending on situations, specific cytoskeletal proteins including α-SM actin, desmin, and SM myosin heavy chains. 4 These features suggest that myofibroblasts are responsible for the force determining wound contraction 5 and for the pathological contractures observed in hypertrophic scars. 2,6
In the normal healing process, after re-epithelialization, the decrease in cellularity during the transition between granulation tissue and scar is mediated by apoptosis and an impressive remodeling of the extracellular matrix occurs. 7 During excessive scarring, the mechanisms involved in normal scar formation do not occur; the granulation tissue does not regress and the cells, particularly the myofibroblasts, are continually activated and producing extracellular matrix. Although some hypertrophic scars may spontaneously regress, others remain active for years. 8 Of all of the treatments available, pressure exerted with elastic bandages in such a manner that the enforced pressure (24 mm Hg) exceeds the inherent capillary pressure gives significant results. 9 Although it is an efficient method, its exact mechanisms of action are not known. Previous studies concerning pressure-treated hypertrophic scars have focused mainly on the role of hypoxia. It has been shown that granulation tissue is oxygen-poor, a condition which could stimulate fibroblast proliferation and collagen production. 10 It has been suggested that the application of pressure increases an already present condition of hypoxia, resulting in resolution of the scar. 11,12
Extracellular matrix components are involved in growth, differentiation, migration, and death of many different cellular types. During wound healing, the pattern of expression of the extracellular matrix components is different from that usually present in normal skin, 13 and various extracellular matrix components play significant roles in the different stages of wound healing. Among extracellular matrix components, some of them participate in skin resistance (eg, collagens), whereas others allow skin elasticity (eg, elastin). In the context of hypertrophic scars, which show a high tendency to develop contractures, the study of the elastic system is relevant. The elastic system is formed by three types of fibers: oxytalan, elaunin, and elastic. 14 The oxytalan fibers are formed exclusively by microfibrils, the elaunin fibers by microfibrils and patches of amorphous material (elastin), and the elastic fibers by a large amount of elastin with microfibrils. 14 In a recent study, it was shown that elastin and fibrillin (a component of microfibrils) are present 7 days after injury in human experimental full-thickness wounds, 15 showing that elastic system fibers are present in the early phases of wound healing. However, few data concerning the detailed organization of elastic system fibers in scars are available. 16-18 Among other components of extracellular matrix present in dermis, glycoproteins such as tenascin are suggested to play an important role in wound healing. 19 Tenascin is a glycoprotein sparsely distributed in normal skin, predominantly associated with basal laminas. 20 In early phases of wound healing and during the formation of granulation tissue, there is a marked increase in expression of tenascin, but tenascin returns to normal levels after the end of wound contraction. 19,21
In the present study, we investigated by histochemistry and immunohistochemistry the expression and organization of extracellular matrix components, including fibrillin, elastin, and tenascin, and the distribution of α-SM actin-expressing myofibroblasts in non-pressure-treated and pressure-treated hypertrophic scars. We also evaluated by transmission electron microscopy the dermal-epidermal junction modifications and the presence of apoptotic features during the pressure-induced scar remodeling.
Materials and Methods
Patients and Sample Processing
Nine patients were included in this study. Their ages ranged from 18 to 54 years (average 32.7 years) and they included one woman (Table 1) ▶ . All patients had hypertrophic scars that arose after burn injuries. The clinical diagnosis of hypertrophic scar was done based on the standard clinical criteria as described by Sahl and Clever, 22 such as elevation above the skin surface limited to injury borders, redness, and itching. The age of scars at the beginning of the treatment ranged from 3 to 11 months (average 7 months) and all these scars showed criteria of active hypertrophic scars. 22 The biopsies were taken 2 to 7 months (average 4.1 months) after the beginning of the pressure treatment. Each patient had two 3-mm punch biopsies, one in the pressure-treated region and the other in an adjacent, non-pressure-treated region. The distance between the two biopsies was about 7 cm and we can exclude an effect between non-pressure- and the neighboring pressure-treated biopsy site. Normal skin biopsies from three mammaplasties were used as controls. Tissue samples were fixed in Bouin’s liquid and embedded in paraffin, cryopreserved in OCT compound (Sakura, Torrance, CA) and snap-frozen in liquid nitrogen, or fixed in 2% glutaraldehyde/0.1 mol/L Na-cacodylate/HCl, pH 7.4, postfixed in 1% osmium tetroxide/0.15 mol/L Na-cacodylate/HCl, pH 7.4, and embedded in Epon. The size of the biopsies obtained from burn patients was obviously limited and did not allow the use of other analytical methods (eg, biochemical).
Table 1.
Source of Human Tissues Used in this Study
| Patient | Sex | Age (years) | Biopsy site | Age of scar (months) |
|---|---|---|---|---|
| 1 | M | 20 | leg | 11 |
| 2 | F | 35 | abdomen | 8 |
| 3 | M | 18 | arm | 9 |
| 4 | M | 54 | leg | 6 |
| 5 | M | 43 | leg | 5 |
| 6 | M | 19 | arm | 3 |
| 7 | M | 41 | shoulder | 6 |
| 8 | M | 37 | shoulder | 8 |
| 9 | M | 28 | chin | 7 |
All patients enrolled in this study gave written and informed consent to participate under protocols approved by the University of Lyon Institutional Review Board.
Histology and Immunohistochemistry
Tissue sections of material embedded in paraffin (5 μm) were stained with hematoxylin-eosin, Gomori’s silver impregnation, or orcein. The Gomori’s silver impregnation contrasted reticular collagen fibers present in the dermis and the orcein staining pointed out the elastic fibers.
Cryostat sections (6 μm) were labeled using the following primary antibodies: a mouse monoclonal anti-human fibrillin-1 (Neomarkers, Fremont, CA), a rabbit polyclonal anti-human elastin (Institut Pasteur de Lyon, Lyon, France), a mouse monoclonal anti-human tenascin (Sigma, St. Louis, MO), a rabbit polyclonal anti-human laminin (Institut Pasteur de Lyon), a rabbit polyclonal anti-human type IV collagen (Institut Pasteur de Lyon), a mouse monoclonal anti-human type VII collagen (Gibco BRL, Gaithersburg, MD), and a mouse monoclonal anti-α-SM actin. 23 These antibodies have been previously well characterized, are very specific, and have been used extensively in other experimental and clinical conditions. The secondary antibodies were cyanine 3-conjugated goat anti-mouse IgG (Jackson Immunoresearch Lab, West Grove, PA) or fluorescein-conjugated goat anti-rabbit IgG (Jackson Immunoresearch). The sections were examined in a Leitz Laborlux S microscope (Wild-Leitz, Heerbrugg, Switzerland) equipped with epi-illumination and specific filters for fluorescein and cyanine 3.
The histological grading system used for α-SM actin immunostaining was: 0, expression of α-SM actin only in vessels; 1+, discreet; 2+, moderate; and 3+, impressive amount of myofibroblasts expressing α-SM actin. The nonparametric Mann-Whitney test was used to compare the scores in non-pressure-treated and pressure-treated regions.
Morphometry
Epidermis thickness was evaluated in hematoxylin-eosin stained sections, using a computerized image analysis system (Histo 200; Biocom, Les Ullis, France). The station included an Ortoplan photomicroscope (Wild-Leitz), a CCD camera (WV-CD 52; Panasonic, Osaka, Japan), and a Pentium Biocom S/X computer (Biocom). Ten measurements were taken for each field, and five fields were analyzed in each biopsy using a 20× objective. The slides were evaluated blindly by two independent observers and no difference was found in their data. Results are presented as mean ± SD. The two different conditions (non-pressure-treated or pressure-treated) were compared using Student’s t-test, and the result was considered statistically significant when P < 0.05.
Transmission Electron Microscopy
Semithin sections were stained with toluidine blue. Thin sections were contrasted with methanolic solution of uranyl acetate and lead citrate and observed with a Philips CM120 transmission electron microscope (Philips SA, Zurich, Switzerland).
Results
Histology and Histochemistry
The presence of inflammation was not impressive in any biopsy. In non-pressure-treated regions, the epidermis thickness (130.5 ± 35.5 μm) was increased compared with pressure-treated regions (106.9 ± 26.8 μm; P < 0.01); furthermore, the epidermis in both non-pressure- and pressure-treated regions was thicker compared with normal skin (44.8 ± 17.5 μm).
In the dermis, non-pressure-treated regions showed the typical organization of hypertrophic scar with an important cellularity and numerous vessels surrounding nodule-like structures as previously described. 24 In non-pressure-treated regions, the classical dermal/epidermal interface characterized by prominent elongated rete ridge was not observed. In both non-pressure-treated and pressure-treated regions, it was not possible to define the papillary and the reticular dermis. In pressure-treated regions, the number of vessels was reduced compared with non-pressure-treated regions. Furthermore, in non-pressure-treated regions, the vessels were localized mainly in superficial dermis, whereas in pressure-treated regions most vessels were localized in the deep dermis, as in normal skin.
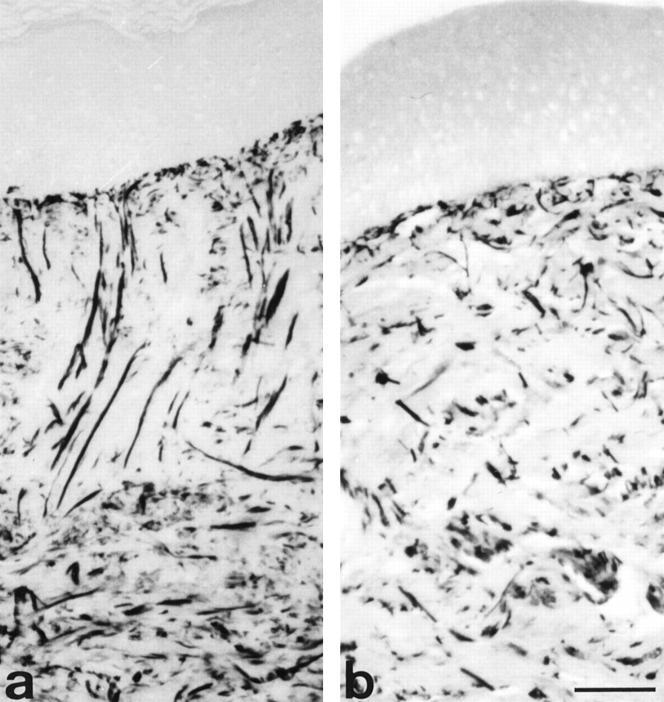
In normal skin, Gomori’s silver impregnation showed that reticular collagen fibers were randomly organized in the dermis without forming bundles (data not shown). In the non-pressure-treated regions, some bundles of reticular collagen fibers anchored perpendicular to the dermal-epidermal junction (Figure 1a) ▶ . By contrast, in the pressure-treated regions the reticular collagen fibers were thinner, with an arrangement rather parallel to skin surface (Figure 1b) ▶ , thus resembling normal skin.
Figure 1.
Gomori’s silver impregnation in non-pressure- and pressure-treated regions. In non-pressure-treated region (a), bundles of reticular collagen fibers are perpendicular to epidermis and there are a large amount of reticular collagen fibers in deep dermis. In pressure-treated region of the same patient (b), the reticular collagen fibers present a random arrangement without forming large bundles, similarly to that observed in normal skin. Scale bar, 50 μm.
In normal skin, orcein-stained elastic fibers were localized in reticular dermis, arranged, preferentially, parallel to epidermis. In non-pressure-treated regions, these fibers were absent in most cases; when present, they were located in the deep dermis, where they were short, thin, and curly. In pressure-treated regions, elastic fibers were present in superficial and deep dermis; the fibers had a random arrangement that was different from that observed in a normal dermis in that they did not form a network and had a fragmented appearance (data not shown).
Immunofluorescence Staining
The results of the immunofluorescence study are summarized in Table 2 ▶ . Using antibodies against fibrillin and elastin, the detailed organization of the elastic system fibers was studied. In normal skin, fibrillin of the papillary dermis was present in brushlike fibers inserted into the basal lamina (oxytalan fibers), showing the so-called candelabra-like configuration; more deeply, thicker fibers were observed, continuous with the superficial ones, thus forming a fibrillin network (Figure 2a) ▶ . In non-pressure-treated regions, fibrillin arrangement was disturbed. In the superficial dermis, the candelabra-like pattern disappeared and the amount of fibrillin was reduced compared with normal skin or with pressure-treated regions; in the deep dermis, discreet fibrillin deposits resembled fragmented fibers (Figure 2b) ▶ . In the pressure-treated regions, the fibers localized under the dermal-epidermal region were better organized compared with non-pressure-treated regions. However, they were thicker than normal ones and the typical candelabra-like pattern was not completely restored; in deep dermis fibrillin deposits were nearly normal although the fibers looked smaller (Figure 2c) ▶ . In normal skin, elastin was absent in the oxytalan fiber region, beneath epidermis, and was present as long aggregates forming fibers arranged mainly horizontally in the dermis (Figure 2d) ▶ . In non-pressure-treated regions, elastin was present in patch deposits, not forming fibers (Figure 2e) ▶ . In pressure-treated regions, some elastin was observed in the superficial dermis near the dermal-epidermal junction; the fibers were shorter and thinner compared with normal dermis (Figure 2f) ▶ .
Table 2.
Immunofluorescence Staining
| Proteins | Normal skin | Non-pressure-treated regions | Pressure-treated regions |
|---|---|---|---|
| Fibrillin | |||
| candelabra-like pattern | present | absent | present (disturbed organization) |
| dermis | present (fine and curly fibers) | present (fragmented fibers) | present (small fibers) |
| Elastin (dermis) | present (typical fibers) | present (patch deposits) | present (short and thin fibers) |
| Tenascin | |||
| superficial papillary dermis and around vessels | present | present | present |
| dermis | absent | present | absent |
| Laminin, type IV and type VII collagens | present | present | present |
| α-Smooth muscle actin in myofibroblasts* | − | +++ | − |
*Semiquantitative evaluation.
Figure 2.
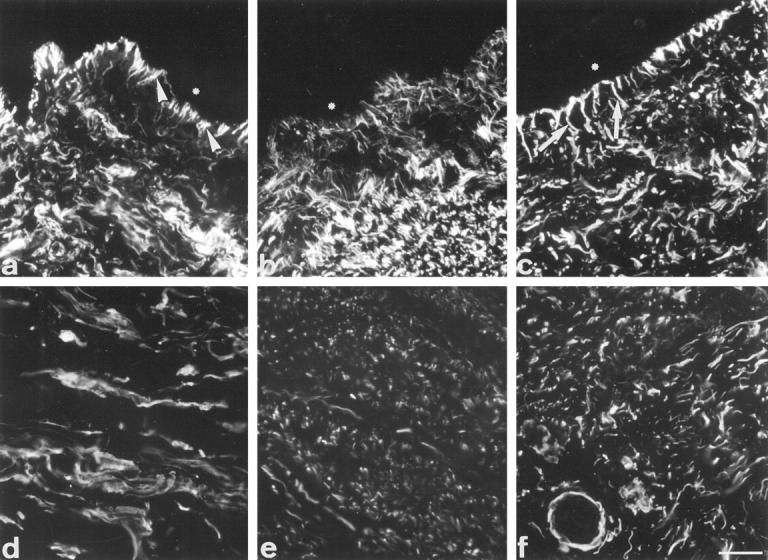
Fibrillin and elastin immunofluorescence. In normal skin (a), fibrillin labeling shows a typical candelabra-like pattern perpendicularly inserted onto the basal lamina (arrowheads); in reticular dermis, thicker fibers are visualized. In non-pressure-treated region (b), the candelabra-like pattern is not present and in deep dermis, the stained fibers show a fragmented aspect. In pressure-treated region of the same patient (c), the fibrillin-containing fibers present under the epidermis are thicker (arrows), perpendicular to the basal lamina but without showing the candelabra-like pattern; in deep dermis the stained fibers are small and arranged parallel to the epidermis, as in normal skin. In normal skin (d), elastin is observed in dermis as fibers arranged preferentially parallel to epidermis. In non-pressure-treated region (e), only sparse elastin deposits are present. In pressure-treated region (f), elastin deposits form small and thin fibers in deep dermis. Asterisks: epidermis. Scale bar, 40 μm.
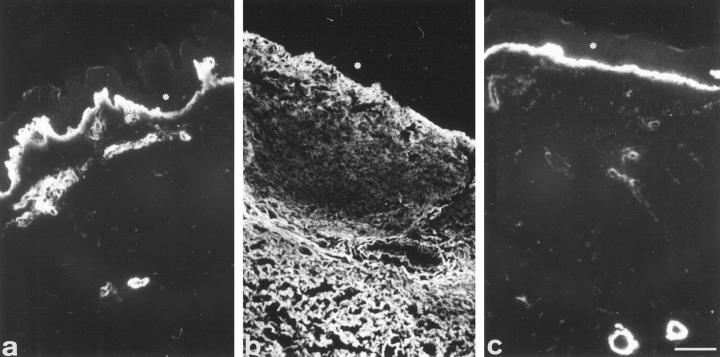
In normal skin, tenascin was observed in the superficial papillary dermis and near the basal lamina of sweat glands and vessels (Figure 3a) ▶ , as described by Lightner et al. 20 In the non-pressure-treated regions, tenascin was observed in all dermis (Figure 3b) ▶ , but the staining was not homogeneous: it was more intense under epidermis, weaker in the region just below, and then intense again (Figure 3b) ▶ . In pressure-treated regions, tenascin was restricted to a fine deposit within dermal papilla and around vessels, similar to that observed in normal skin (Figure 3c) ▶ .
Figure 3.
Tenascin immunofluorescence. In normal skin (a), tenascin is present in superficial papillary dermis and around blood vessels. In non-pressure-treated region (b), tenascin is observed in dermis with a non homogeneous distribution. In pressure-treated region of the same patient (c), tenascin is present only in dermal papilla and around blood vessels. Asterisks: epidermis. Scale bar, 30 μm.
The distribution of laminin and type IV and type VII collagens, present in dermal-epidermal junction, did not show differences when normal skin, non-pressure-treated, and pressure-treated regions were compared (data not shown).
In normal skin, α-SM actin was observed exclusively in vessels. In non-pressure-treated regions, the proportion of α-SM actin-expressing myofibroblasts was impressive (average 3+) and localized mainly in nodules (Figure 4a) ▶ , as previously described. 2 In contrast, pressure-treated regions exhibited a marked and significant decrease of α-SM actin-expressing myofibroblasts (average 0, P < 0.01, Figure 4b ▶ ).
Figure 4.
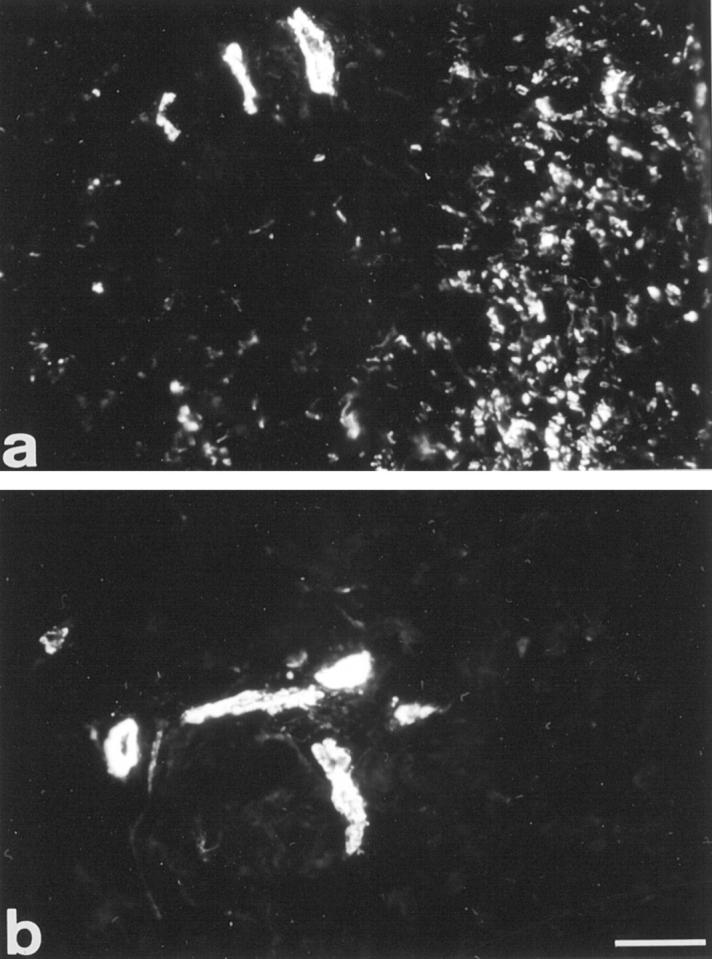
α-Smooth muscle actin immunofluorescence. In non-pressure-treated region (a), α-smooth muscle actin is present around vessels and in stromal myofibroblasts. In pressure-treated region of the same patient (b), α-smooth muscle actin is present only around vessels. Scale bar, 60 μm.
Transmission Electron Microscopy
The results of the transmission electron microscopy analysis are summarized in Table 3 ▶ . Significant morphological changes were observed within the dermal-epidermal junction. In normal skin, the typical organization of subepithelial basal lamina (lamina lucida and lamina densa) accompanying the microfoot processes of the keratinocytes, and regularly distributed hemidesmosomes and anchoring filaments and fibrils, as described by McMillan et al, 25 were observed (Figure 5a) ▶ . In non-pressure-treated regions (Figure 5b) ▶ , the dermal-epidermal junction was smooth and the keratinocyte microfoot processes were almost absent. The lamina densa was thickened and the lamina lucida was not clearly defined. The hemidesmosomes and the anchoring fibrils were not regularly distributed. Furthermore, the outer plaque of hemidesmosomes was not well delimited and the subbasal dense plate was missing; moreover, anchoring fibrils were thicker compared with those observed in normal skin or in pressure-treated regions. Collagen fibers immediately beneath the lamina densa were frequently observed. In pressure-treated regions, the dermal-epidermal junction resembled that observed in normal skin with well organized hemidesmosomes; however, the depth of keratinocyte microfoot processes was increased (Figure 5c) ▶ compared with normal skin.
Table 3.
Transmission Electron Microscopy Features
| Structures | Normal skin | Non-pressure-treated regions | Pressure-treated regions |
|---|---|---|---|
| Dermal epidermal junction | |||
| keratinocyte microfoot | typical organization | almost absent | increase of depth |
| lamina lucida | well formed | not well defined | well formed |
| lamina densa | well formed | thickened | well formed |
| hemi-desmosomes | typical organization | disturbed organization | typical organization |
| anchoring fibrils | regularly distributed | irregularly distributed and thickened | regularly distributed |
| Elastic system fibers | well organized | well organized | well organized |
| Collagen bundles | typical organization | large, with thick fibers | signs of degradation |
| Microvessels | patent | frequently occluded | frequently occluded |
| Myofibroblasts | absent | present | signs of apoptosis |
Figure 5.
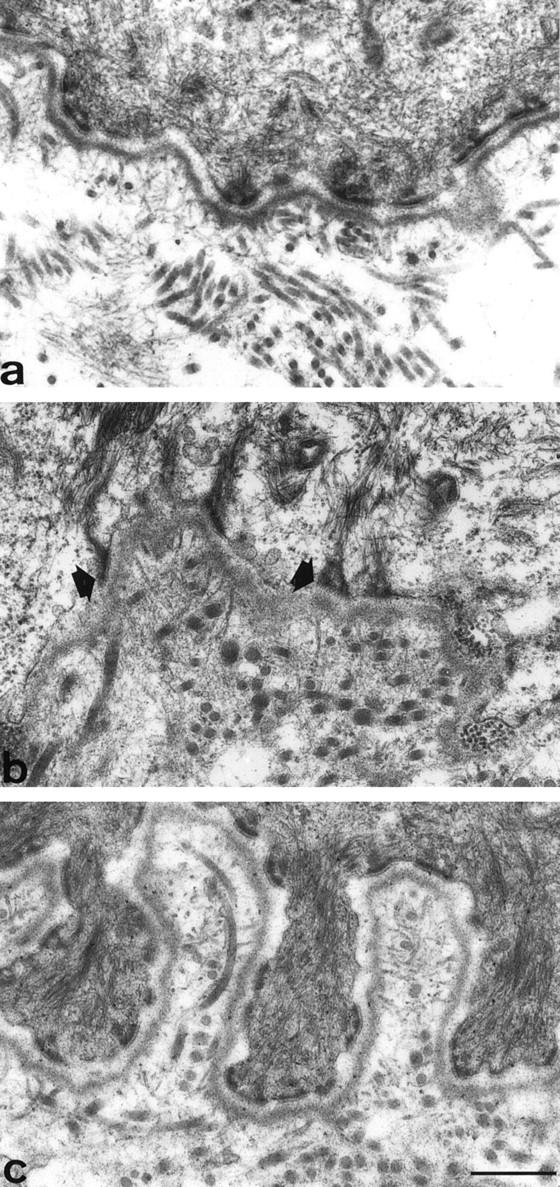
Ultrastructure of the dermal-epidermal junction. In normal skin (a), the typical architecture is represented, with keratinocyte microfoot processes, regular hemidesmosomes, lamina lucida and anchoring filaments, lamina densa and anchoring fibrils. In non-pressure-treated region (b), the keratinocytic microfeet are almost absent, the lamina lucida is indinstinct and the lamina densa is thickened. The hemidesmosomes are not regularly distributed, and their subbasal dense plate is missing (arrowheads). In pressure-treated region of the same patient (c), keratinocyte microfoot processes are elongated, while hemidesmosomes, anchoring filaments and anchoring fibrils are well organized. Scale bar, 0.5 μm.
The ultrastructural organization of the elastic system fibers was recognized in both non-pressure-treated and pressure-treated regions, with the presence of oxytalan fibers and elaunin fibers containing many microfibrils and a discrete core of elastin (Figure 6a) ▶ ; well formed elastic fibers (Figure 6b) ▶ were also observed. In non-pressure-treated regions (Figure 6a) ▶ , bundles of thick collagen fibers were present. In pressure-treated regions (Figure 6b) ▶ , collagen bundles appeared loose and numerous signs of extracellular matrix remodeling (eg, collagen fibrils with varying thickness) were observed. Moreover, a wide range in the diameter of collagen fibrils was present together with some large twisted fibrils (Figure 6b) ▶ , as usually seen during collagen breakdown. 26
Figure 6.
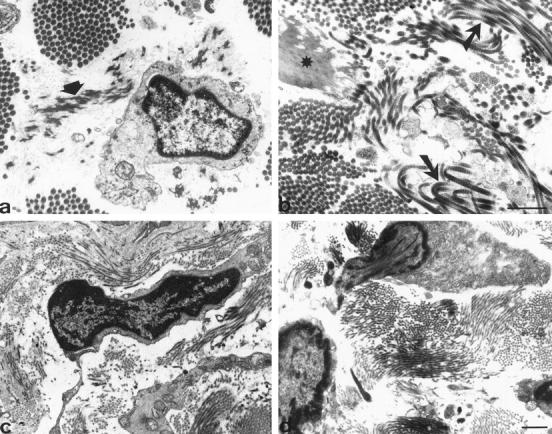
Ultrastructure of dermal components. In non-pressure-treated region (a), an elaunin fiber is observed near a fibroblast (arrowhead). In pressure-treated region (b), an elastic fiber (star) and some bundles of regular collagen fibers are observed together with collagen fibers showing typical features of degradation (arrows). In pressure-treated region (c and d), fibroblastic cells presenting typical apoptotic features such as chromatin condensation and nuclei in part extruded from the cytoplasm are observed. Scale bars, 1 μm.
Microvessels were frequently occluded (lumen size <3 μm), as described by Kischer et al, 27 without any obvious differences between non-pressure-treated and pressure-treated regions (data not shown).
Typical myofibroblasts were observed in non-pressure-treated regions, with long processes containing microfilament bundles and extending for long distances among collagen fiber bundles as previously described. 12 In pressure-treated regions, the proportion of cells showing myofibroblastic features was reduced. Furthermore, numerous fibroblastic cells presenting apoptotic features were observed (Figure 6, c and d) ▶ . The main criteria used for the identification of apoptotic cells were vesiculation, condensation and margination of the chromatin, fragmentation of the nucleus, and cytoplasmic condensation. As previously described in myofibroblasts undergoing apoptosis during granulation tissue remodeling, 7 nuclei in part extruded from the cytoplasm were frequently observed (Figure 6, c and d) ▶ .
Discussion
Little is know about the mechanisms leading to hypertrophic scar resolution in pressure-treated patients. The present study reports the structural changes in the dermis (extracellular matrix and cell components) of pressure-treated postburn hypertrophic scars and, although only descriptive, provides direct evidence for close relationships in vivo between mechanical modifications (ie, pressure treatment), cell phenotype, and matrix remodeling. A modification of the collagen fiber organization was induced by pressure. The disorganized orientation was replaced under pressure by a parallel arrangement similar to the pattern observed in normotrophic healing. This pressure-induced collagen reorganization has been observed previously by Kischer et al 11 and Baur et al. 28 In areas treated with pressure, there was a disappearance of nodular pattern. Furthermore, we observed that in hypertrophic scars, collagen fibers perpendicular to the epidermis were anchored in dermal-epidermal zone and probably participated in the impaired mechanical properties of the scar.
The elastic system fibers are not frequently considered in the studies of skin wound healing. 29 Early studies using histochemical techniques described the presence of elastic fibers only in late phases of wound healing. 17 Using similar techniques, Bhangoo et al 16 showed the presence of elastic fibers in different types of human scars (atrophic, normal, hypertrophic, and keloid) that were at least 1 year old. They observed that elastic fibers in hypertrophic scars had a disturbed arrangement and were localized mainly in superficial layers of the scar; in deep regions the distribution was patchy and some areas were entirely devoid of elastic fibers. However, more recent studies using transmission electron microscopy and immunohistochemistry showed the presence of some elastic system fiber components, fibrillin and elastin, also in early phases of wound healing in skin and liver. 15,30,31 To our knowledge the present study is the first to show the presence of fibrillin and elastin and to describe their organization in hypertrophic scars. Fleischmajer et al, 32 studying another skin fibrotic disease, scleroderma, observed an increase in deposition of microfibrils, but not of elastin, in deep dermis. In the present study we showed the presence of both components (fibrillin and elastin) in hypertrophic scars without major alterations in amount when compared with normal skin, but with important disorders in organization. Those alterations may be associated with an elastic system still immature as described by Tsuji and Sawabe. 18 The pressure treatment allowed a rearrangement of fibrillin and elastin, with acquisition of an almost normal pattern enabling those fibers to carry out their physiological functions as in normal dermis. This may explain the softness in scars acquired as a consequence of pressure treatment.
During normal wound healing tenascin is abundant in granulation tissue but disappears soon after re-epithelialization. 19 Our findings showing an impressive accumulation of tenascin in non-pressure-treated regions support the hypothesis that hypertrophic scars develop as a consequence of an excessive process of healing. In pressure-treated regions, as in normal skin, tenascin was present in dermal papilla and in basal lamina of vessels. Little is known about the function of tenascin in wound healing. However, Mackie et al 19 have suggested that the presence of tenascin allows myofibroblasts to contract the wound; in the case of hypertrophic scars, the continual presence of tenascin may contribute to the development of contractures.
Modifications of dermal-epidermal junction have been observed in different diseases, particularly in junctional forms of epidermolysis bullosa. 25 Here, we observed that hypertrophic scars presented a disorganized dermal-epidermal junction, and that after pressure treatment there is a marked improvement in this ultrastructural organization. Among the changes observed as a consequence of pressure, the hemidesmosomes were found to have the normal ultrastructure and a regular distribution; and the keratinocyte microfoot processes became more elongated forming pseudopodia-like extensions as described by Mommaas et al. 33 The presence of these long extensions probably compensates for the absence of a normal rete ridge pattern and increases the dermal-epidermal interface and attachment. Futhermore, the differences observed by transmission electron microscopy in the organization of the dermal-epidermal junction between normal skin, non-pressure-treated, and pressure-treated regions suggest subtle modifications in the organization of anchoring fibrils, although immunofluorescence did not show changes of the type IV and type VII collagen distribution.
The extracellular matrix reorganization, which also involves collagen degradation, together with the disappearance of myofibroblasts, may explain the improvements obtained with pressure in mechanical properties of the scar. We suggest that pressure induces the decrease in cellularity similar to that observed in the final stages of normal wound healing, where apoptosis is the mechanism through which vascular and fibroblastic cells are gradually eliminated. 7 We failed to demonstrate a significant proportion of apoptotic cells by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) technique, 34 probably because pressure-induced apoptosis affects target cells consecutively rather than producing a single wave of cell disappearance.
The mechanism of action of pressure is not known. Kischer et al 11 suggested that hypoxia, which causes hypertrophic scarring, is increased by pressure and causes the resolution of scar by induction of fibroblast death. Baur et al 28 disagreed and suggested that the treatment causes an increase in collagenase activity and a consequent increase in extracellular matrix degradation. It is known that mechanical forces induce modifications in extracellular matrix organization and composition in different situations such as development 35 or cholestatic fibrosis. 31 Furthermore, changes in environmental mechanical forces modulate the expression of matrix remodeling enzymes 36 and induce apoptosis in dermal fibroblasts cultured in three-dimensional collagen gels. 37 These modifications affect the mechanical properties of involved tissues as well as the cells present in these tissues.
In conclusion, in this study, we used immunofluorescence to observe the modifications of fibrillin, elastin, tenascin, and α-SM actin expression and electron microscopy to observe the changes of dermal-epidermal junction in human pressure versus non-pressure-treated hypertrophic scars. We suggest that the treatment of postburn hypertrophic scars by pressure induces extracellular matrix reorganization and apoptosis in fibrogenic (ie, myofibroblasts) and vascular cells. Further studies are necessary to clarify the mechanisms of mechanosignal transduction involved in extracellular matrix remodeling and cell death resulting in hypertrophic scar resolution.
Acknowledgments
We thank Drs. Y. N. Marduel and E. Mahjoub for their help in collection of tissue samples and Dr. S. Guerret for assistance in image analysis.
Parts of this study were presented to the Seventh Annual Meeting of the Wound Healing Society (Nashville, TN, 1997).
Footnotes
Address reprint requests to Dr. Alexis Desmoulière, GREF, INSERM E9917, Université Victor Segalen Bordeaux 2, 146, rue Léo-Saignat, 33076 Bordeaux cedex, France. E-mail: Alexis.Desmouliere@gref.u-bordeaux2.fr.
Supported in part by a Projet Hospitalier de Recherche Clinique 1995 (Centre Hospitalier Saint-Joseph et Saint-Luc, Lyon, France).
References
- 1.Linares HA: Pathophysiology of the burn scar. Herndon DN eds. Total Burn Care. 1996, :pp 383-397 Saunders, London [Google Scholar]
- 2.Ehrlich HP, Desmoulière A, Diegelman RF, Cohen IK, Compton CC, Garner WL, Kapanci Y, Gabbiani G: Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 1994, 145:105-113 [PMC free article] [PubMed] [Google Scholar]
- 3.Gabbiani G, Ryan GB, Majno G: Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27:549-550 [DOI] [PubMed] [Google Scholar]
- 4.Desmoulière A, Gabbiani G: The role of the myofibroblast in wound healing and fibrocontractive diseases. Clark RAF eds. The Molecular and Cellular Biology of Wound Repair. 1996, :pp 391-423 Plenum Press, New York [Google Scholar]
- 5.Grinnell F: Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 1994, 124:401-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baur PS, Larson DL, Stacey TR: The observation of myofibroblasts in hypertrophic scars. Surg Gynecol Obstet 1975, 141:22-26 [PubMed] [Google Scholar]
- 7.Desmoulière A, Redard M, Darby I, Gabbiani G: Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995, 146:56-66 [PMC free article] [PubMed] [Google Scholar]
- 8.Muir IFK: On the nature of keloids and hypertrophic scars. Br J Plast Surg 1990, 43:61-69 [DOI] [PubMed] [Google Scholar]
- 9.Larson DL, Abston S, Evans EB, Dobrkovsky M, Linares HA: Techniques for decreasing scar formation and contractures in the burned patient. J Trauma 1971, 11:807-823 [DOI] [PubMed] [Google Scholar]
- 10.Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, Marumo F, Hiroe M: Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res 1994, 75:426-433 [DOI] [PubMed] [Google Scholar]
- 11.Kischer CW, Shetlar MR, Shetlar CL: Alteration of hypertrophic scars induced by mechanical pressure. Arch Dermatol 1975, 111:60-64 [PubMed] [Google Scholar]
- 12.Kischer CW: Contributions of electron microscopy to the study of the hypertrophic scar and related lesions. Scanning Microsc 1993, 7:921-931 [PubMed] [Google Scholar]
- 13.Gailit J, Clark RAF: Wound repair in the context of extracellular matrix. Curr Opin Cell Biol 1994, 6:717-725 [DOI] [PubMed] [Google Scholar]
- 14.Cotta-Pereira G, Rodrigo FG, Bittencourt-Sampaio S: Oxytalan, elaunin, and elastic fibers in human skin. J Invest Dermatol 1976, 66:143-148 [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft GS, Kielty CM, Horan MA, Ferguson M: Age-related changes in the temporal and spatial distributions of fibrillin and elastin mRNAs and proteins in acute cutaneous wounds of healthy humans. J Pathol 1997, 183:80-89 [DOI] [PubMed] [Google Scholar]
- 16.Bhangoo KS, Quinlivan JK, Connely JR: Elastic fibers in scar tissue. Plastic Reconstr Surg 1976, 57:308-313 [DOI] [PubMed] [Google Scholar]
- 17.Williams G: The late phases of wound healing: histological and ultrastructural studies of collagen and elastic-tissue formation. J Pathol 1970, 102:61-68 [DOI] [PubMed] [Google Scholar]
- 18.Tsuji T, Sawabe M: Elastic fibers in scar tissue: scanning and transmission electron microscopic studies. J Cutan Pathol 1987, 14:106-113 [DOI] [PubMed] [Google Scholar]
- 19.Mackie EJ, Halfter W, Liverani D: Induction of tenascin in healing wounds. J Cell Biol 1988, 107:2757-2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lightner VA, Gumkowski F, Bigner DD, Erickson HP: Tenascin/hexabrachion in human skin: biochemical identification and localization by light and electron microscopy. J Cell Biol 1989, 108:2483-2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuong CM, Chen HM: Enhanced expression of neural adhesion molecules and tenascin (cytotactin) during wound healing. Am J Pathol 1991, 138:427-440 [PMC free article] [PubMed] [Google Scholar]
- 22.Sahl WJ, Clever H: Cutaneous scars: part 1. Int J Dermatol 1994, 33:681-691 [DOI] [PubMed] [Google Scholar]
- 23.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G: A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 1986, 103:2787-2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linares HA, Kischer CW, Dobrkovsky M, Larson DL: The histiotypic organization of the hypertrophic scars in humans. J Invest Dermatol 1972, 59:323-331 [DOI] [PubMed] [Google Scholar]
- 25.McMillan JR, Mc Grath JA, Tidman MJ, Eady RAJ: Hemidesmosomes show abnormal association wit the keratin filament network in junctional forms of epidermolysis bullosa. J Invest Dermatol 1998, 110:132-137 [DOI] [PubMed] [Google Scholar]
- 26.Garbin S, Pittet B, Montandon D, Gabbiani G, Desmoulière A: Covering by a flap induces apoptosis of granulation tissue myofibroblasts and vascular cells. Wound Rep Reg 1996, 4:244-251 [DOI] [PubMed] [Google Scholar]
- 27.Kischer CW, Thies AC, Chvapil M: Perivascular myofibroblasts and microvascular occlusion in hypertrophic scars and keloids. Hum Pathol 1982, 13:819-824 [DOI] [PubMed] [Google Scholar]
- 28.Baur PS, Larson DL, Stacey TR, Barrat GF, Dobrkovsky M: Ultrastructural analysis of pressure-treated human hypertrophic scars. J Trauma 1976, 16:958-967 [DOI] [PubMed] [Google Scholar]
- 29.Davidson JM, Giro G, Quaglino D: Elastin repair. Cohen IK Lindblad WJ Diegelman RF eds. Wound Repair: Biomedical and Clinical Aspects. 1992, :pp 223-236 WB Saunders, Philadelphia [Google Scholar]
- 30.Raghunath M, Bachi T, Meuli M, Altermatt S, Gobet R, Brucker-Tuderman L, Steinmann B: Fibrillin and elastin expression in skin regenerating from cultured keratinocytes autografts: morphogenesis of microfibrils begins at the dermo-epidermal junction and precedes elastic fiber formation. J Invest Dermatol 1996, 106:1090-1095 [DOI] [PubMed] [Google Scholar]
- 31.Desmoulière A, Darby I, Costa AMA, Raccurt M, Tuchweber B, Sommer P, Gabbiani G: Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest 1997, 76:765-778 [PubMed] [Google Scholar]
- 32.Fleischmajer R, Jacobs L, Schwartz E, Sakai LY: Extracellular microfibrils are increased in localized and systemic scleroderma in skin. Lab Invest 1991, 64:791-798 [PubMed] [Google Scholar]
- 33.Mommaas AM, Teepe RGC, Leigh IM, Mulder AA, Koebrugge EJ, Vermeer BJ: Ontogenesis of the basement membrane zone after grafting cultured human epithelium: a morphologic and immunoelectron microscopic study. J Invest Dermatol 1992, 99:71-77 [DOI] [PubMed] [Google Scholar]
- 34.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa AMA, Porto LC: Distribution of elastic system fibers in human fetal liver. J Anat 1996, 88:645-650 [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert CA, Soudant EP, Nusgens BV, Lapière CM: Pretranslational regulation of extracellular matrix macromolecules and collagenase expression in fibroblasts by mechanical forces. Lab Invest 1992, 66:444-451 [PubMed] [Google Scholar]
- 37.Fluck J, Querfeld C, Cremer A, Niland S, Krieg T, Sollberg S: Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol 1998, 110:153-157 [DOI] [PubMed] [Google Scholar]