Abstract
Defects in the newly reported gene NPHS1 in chromosome 19 cause the massive proteinuria of Finnish type congenital nephrotic syndrome (CNF). Together with its gene product, nephrin, NPHS1 is providing new understanding of the pathophysiological mechanisms of glomerular filtration. Here we show the characteristic splicing of NPHS1 mRNA in the normal and CNF kidneys and localize nephrin exclusively in the glomerulus and to the filtration slit area by light and immunoelectron microscopy. These results indicate that nephrin is a new protein of the interpodocyte filtration slit area with a profound role in the pathophysiology of the filtration barrier.
Morphological and functional evidence suggests that the glomerular visceral epithelial cells, podocytes, are crucial for maintaining the glomerular permeability barrier. 1-3 Podocytes attach to the underlying basement membrane by mechanisms involving matrix receptors capable of inside-out and outside-in signaling that is important for rapid cell shape changes. 4,5 The attachment via integrins also appears crucial for the filtration function as evidenced by recent data for mice with genetic disruption especially of α3β1 integrin. 6 The distinct structure of podocytes with primary and secondary foot processes interlinked by filtration slits appears to provide additional means for dynamic shape changes and regulation of functions. The composition and exact role of the filtration slits, however, remain to be determined. The structural complexity of podocytes is the characteristic feature of an intact glomerular filtration barrier, as contrasted with the flattening, retraction, and fusion of foot processes in human and experimental diseases with proteinuria. 1,7 Thus, various lines of evidence point to the interdependence of podocyte structure, function, and attachment for the filtration barrier.
Kestilä et al 8 recently identified NPHS1, the causative gene of Finnish type congenital nephrotic syndrome (CNF), whose protein product, nephrin, appears to be a transmembrane protein with multiple immunoglobulin-like domains. In situ hybridization results indicated that the nephrin gene is expressed exclusively in podocytes during glomerulogenesis. Furthermore, the results of Kestilä et al showed that the nephrin gene is expressed within the kidney but not in other tissues. 8 The localization of nephrin protein within the kidney or in other tissues is not known.
Here we report our studies of the expression of nephrin mRNA in the normal human kidney as well as in 28 CNF kidney samples. We used nephrin-specific antipeptide antibodies to localize nephrin within the kidney and to study its association with other proteins of the filtration slit area including occludin and ZO-1. In immunoelectron microscopy, nephrin was preferentially localized in the filtration slit area although some reactivity was also seen on the surface of podocytes. Furthermore, our results revealed a major splicing variant of nephrin that lacks the entire transmembrane domain.
Materials and Methods
Normal and Nephrotic Human Kidneys
Renal tissues of CNF patients (n = 28) were obtained at nephrectomies performed by an established treatment protocol as earlier described. 9 Diagnosis of CNF was done based on the typical clinical picture at birth (placental weight >40% of the weight of the newborn, edema, and massive proteinuria), exclusion of other types of congenital nephroses, and later the typical pathology at nephrectomy. 10,11 All procedures were approved by the ethics committee of the Helsinki University Central Hospital.
The CNF kidneys at nephrectomy were perfused with Ringer’s solution, and glomeruli were rapidly isolated as described earlier 12 and immediately processed for RNA isolation. 12,13 Also samples of CNF cortical kidney tissue were processed for RNA isolation, immunohistochemistry, and electron microscopy as previously described. 12,14,15
For normal controls, cadaver kidneys (n = 5; age of donors, 12–48 years) unsuitable for transplantation for vascular anatomical reasons (Department of Surgery, University of Helsinki) or the normal poles of kidneys removed because of Wilms’ tumor (n = 2; ages, 3 and 5 years) were used.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Cloning and Sequencing
RNA samples from isolated glomerular fractions or from cortical kidney were used as a starting material in RT-PCR analysis as earlier described. 16 The following primers fully covering the transmembrane area were used for human nephrin: sense primer 5′-CCC ATC ACT ACC CCA GGT CT corresponding to nucleotides 3094–3113 (amino acids (aa) 1033–1039) and antisense primer 5′-CTC TGT TGT GCT GAC CGT G corresponding to nucleotides 3384–3402 (aa 1130–1136). For PCR, total RNA from cortical kidney or from isolated glomeruli was DNase treated (DNase RQ1; Promega, Madison, WI) and reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Promega) as previously described. 16 cDNAs were amplified by using AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT) on an MJ Research thermal cycler (PTC-200; MJ Research Inc., Watertown, MA). The PCR product was analyzed by agarose gel electrophoresis and ethidium bromide staining, purified (Magic PCR Preps DNA purification and separation kit, Promega), and sequenced with gene-specific primers (ABIPrism 310, Perkin-Elmer Applied Biosystems, Foster City, CA) as previously described. 16
Some of the gel-purified PCR products obtained were cloned 17 to a pGEM-T vector (Promega). For each PCR product, 3 to 6 colonies from a single transformation were cultured. Plasmid DNA was isolated, 16 and clones were sequenced and screened for homology with database sequences using the BLAST search algorithm obtained via Internet (www.ncbi.nlm.nih.gov/BLAST) from the National Center for Biotechnology (Washington, DC).
Design of Synthetic Peptides
Sequence-specific intracellular (aa 1101–1126) and extracellular (aa 1039–1056) oligopeptides were selected over the human nephrin sequence (GenBank accession number AF035835) by using the PredictProtein program obtained via Internet (www.embl-heidelberg.de/predictprotein/predictprotein.html) from the European Molecular Biology Laboratory (Heidelberg, Germany). These peptides showed no homology to other known protein sequences and were synthesized and purified at a local peptide synthesis unit (Haartman Institute, University of Helsinki).
Antipeptide Antibodies
For immunizations the peptides were coupled to a multiple antigenic peptide-polylysine matrix 18,19 and injected into two rabbits each. The first immunization was with 500 μg of peptide in Freund’s complete adjuvant (Difco Laboratories, Detroit, MI), and two booster immunizations with 300 μg each in Freund’s incomplete adjuvant 4 weeks after the previous immunization. Peptide-specific fractions were immunoaffinity-purified on CNBr-Sepharose (Pharmacia, Uppsala, Sweden) coupled to the corresponding linear peptides. The specificity of the antisera was tested by immunofluorescence on kidney sections with and without free peptide competition (Figure 1A) ▶ , by immunoblotting of glomerular extracts (Figure 7) ▶ and precipitation of a full-length nephrin in an in vivo transcription and translation assay with the specific antibodies (see below).
Figure 1.
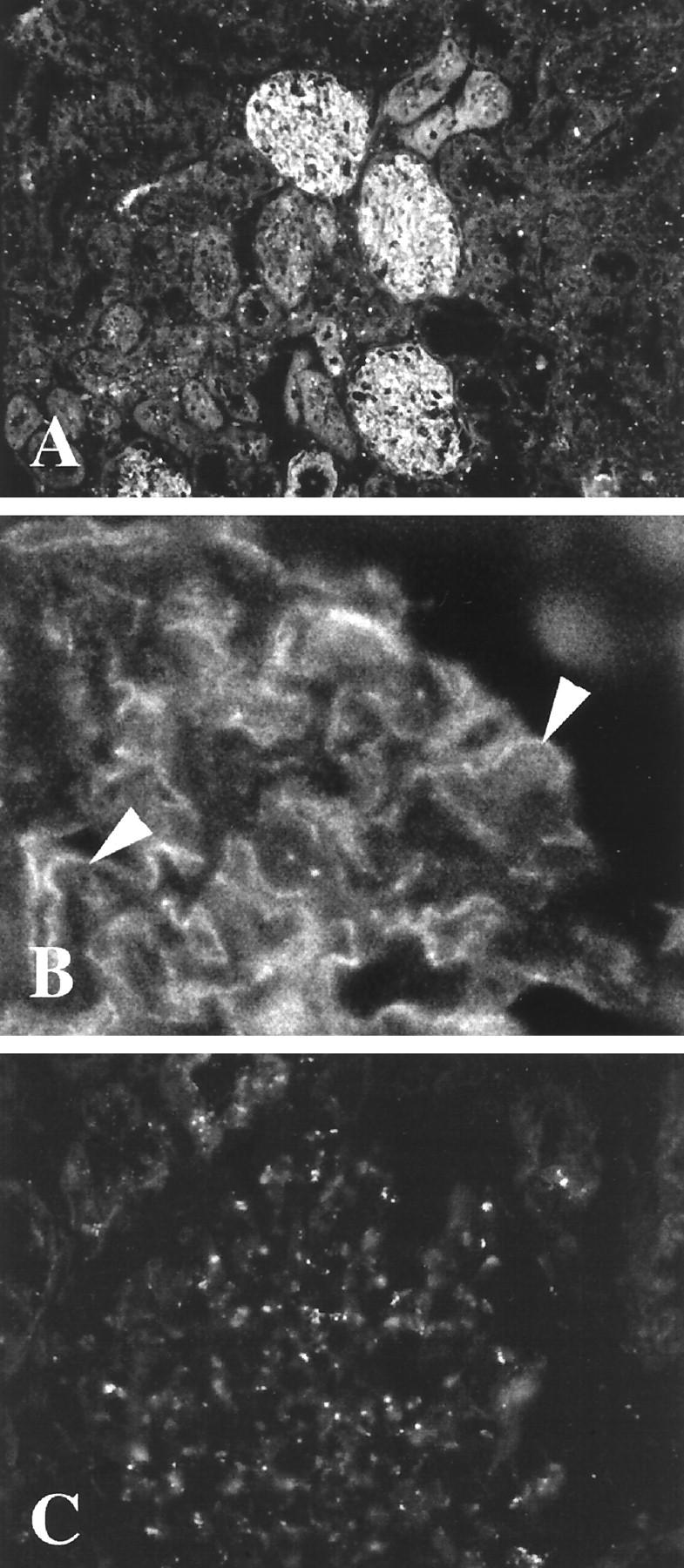
A, B: Frozen section of normal human kidney stained with antipeptide antibodies to the intracellular nephrin-specific sequence in immunofluorescence. Note the reactivity exclusively in glomerulus (A) as finely granular stretches of linear reactivity (arrows in B) in a pattern typical for podocytes. Reactivity of preimmune serum is given in C. Original magnifications, ×180 (A) and ×360 (B and C)
Figure 7.
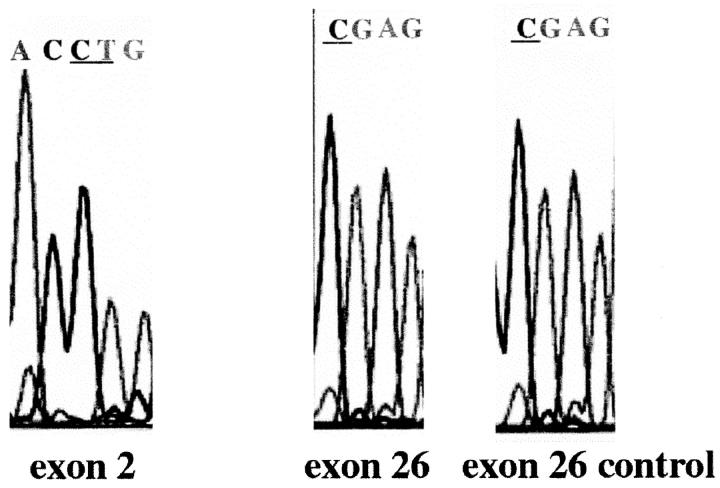
Analysis of the Finmajor (exon 2) and Finminor (exon 26) mutation sites in a patient showing positive immunoreactivity with nephrin-specific antipeptide antibodies. No mutations are found in the expected bases (underlined). For details, see Materials and Methods.
Electrophoresis and Western Blotting
For SDS-PAGE, the detergent extracts of isolated human glomeruli were suspended in the Laemmli sample buffer, boiled for 5 minutes, and run under reducing conditions using 8% gels and a Protean Minigel electrophoresis system (Bio-Rad Laboratories, Richmond, CA) as previously described. 20 The separated proteins were transferred to nitrocellulose sheets for Western blotting with a Novablot semidry blotting apparatus (Pharmacia). After blocking with 3% bovine serum albumin, the nitrocellulose strips were incubated with the respective antibodies, washed thoroughly, and further incubated with anti-rabbit IgG coupled to horseradish peroxidase. After washing, the bound antibodies were detected by using the ECL Western blotting kit (Amersham Pharmacia Biotech, Uppsala, Sweden). 20
Immunofluorescence and Immunoelectron Microscopy
Samples from normal human cortical tissue were prepared for immunofluorescence as described earlier. 12,16 Briefly, frozen cortical tissues were cut at 4 μm, fixed in acetone at −20°C for 10 minutes, and reacted with the antipeptide antibodies (1.1 mg/ml; used at 1:100 dilution in phosphate-buffered saline) for 1 hour. Fluorescein isothiocyanate-anti-rabbit IgG (Boehringer Mannheim, Mannheim, Germany) was used for second antibodies. As a control, the primary antibodies were either omitted or replaced by irrelevant antipeptide antibodies. An additional control included preincubation of the antibody with a dilution series of the oligopeptide used as the original immunogen. Postembedding electron microscopy was done as previously described, 18,21 using CNF and normal cortical kidney samples fixed in freshly prepared 4% formaldehyde in phosphate buffered saline, embedded in Lowicryl K4M (Chemische Werke LOW1, Waldkraiburg, Germany), and further incubated with the rabbit antinephrin antibodies (50 μg/ml) and the respective 10-nm gold conjugate (1:50).
In Vitro Transcription/Translation
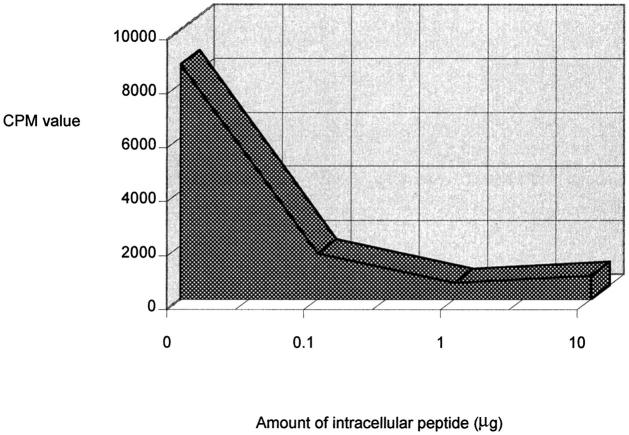
In vitro transcription/translation of the full-length nephrin sequence under the T3 promoter of pBK-cytomegalovirus (Promega) was performed according to the manufacturer’s instructions with a TNT T3-coupled reticulocyte lysate system (Promega), a single-tube modification of rabbit reticulosyte lysate translations. 22 During translation nehprin was labeled with [35S]methionine (NEN Life Science Inc., Boston, MA). Intracellular antibodies (see above) and [35S]labeled nehprin were incubated without or with increasing amounts (0.1, 1, and 10 μg) of intracellular peptide overnight at 4°C. Immunocomplexes were collected with protein A-Sepharose (Zymed Laboratories Inc., San Francisco, CA) by incubating immunocomplexes and protein A-Sepharose for 45 minutes at 4°C. After incubation the immunocomplexes were washed eight times with washing buffer (150 mmol/L NaCl, 20 mmol/L Tris, pH 7.6, 0.15% Tween 20, 0.1% bovine serum albumin, 0.02% sodium azide), and the radioactivity of precipitates was measured in scillation liquid OptiPhase SuperMix (EG&G Wallac, Turku, Finland) with 1450 MicroBeta Trilux liquid scintillation & luminescence counter (EG&G Wallac).
Immunoprecipitation
For immunoprecipitation the glomerular lysate (1 mg/ml) in radioimmunoprecipitation assay buffer was incubated with rabbit or mouse antibodies against occludin (Zymed Laboratories) or ZO-1 (Zymed), using 10 μg of IgG/200 μl of glomerular lysate at 4°C overnight. Immune complexes were collected with protein-A-Sepharose (Pharmacia LKB Biotechnology), washed, and processed for immunoblotting with antinephrin antibodies as described above.
Mutation Analysis
Analysis for the Finmajor (in exon 2) and Finminor (exon 26) mutations in the only patient sample out of 28 studied that showed immunoreactivity with the antinephrin antibodies was done as described. 8 Briefly, after DNA isolation, 17 the respective exon areas were amplified by PCR using AmpliTaq DNA polymerase (Perkin Elmer) and the following conditions: initial denaturation at 94°C for 12 minutes, 30 PCR cycles (95°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute), followed by final elongation at 72°C for 8 minutes. The primer sequences for exon 2 were 5′-GAG AAA GCC AGA CAG ACG CAG-3′ and 5′-AGC TTC CGC TGG TGG CT-3′. For exon 26 the primer sequences were 5′-CTC GGG GAG ACC CAC CC-3′ and 5′-CCT GAT GCT AAC GGC AGG GC-3′. The sequencing was done as described above, using the ABIPrism (Perkin-Elmer).
Results
Immunohistochemistry of normal human kidneys with antibodies against the intracellular nephrin peptide showed an exclusive glomerular localization within the kidney (Figure 1A) ▶ . However, a finely dotted linear reactivity giving a preferentially epithelial-like staining pattern could be seen (Figure 1B) ▶ . No appreciable reactivity of the underlying basement membrane, endothelia, mesangium, or tubuli could be observed (see Figure 2 ▶ for electron micrographs). Antibodies against the extracellular nephrin domain showed a closely similar glomerular reactivity pattern. All but one of 28 CNF samples studied failed to show reactivity in glomeruli with the extra- and intracellular nephrin-specific antibodies. Preimmune serum gave no reactivity (Figure 1C) ▶ , and preincubation of the antiserum with the respective peptide before tissue staining also decreased the reactivity to negligible levels (data not shown). Further evidence of antibody specificity was obtained by the specific immunoprecipitation and immunoblotting reactions (see Figure 6 ▶ ). Furthermore, results from in vitro transcription and translation of full-length nephrin sequences followed by immunoprecipitation with the intracellular antibodies showed specific precipitation that could be prevented by the respective peptides (Table 1) ▶ .
Figure 2.
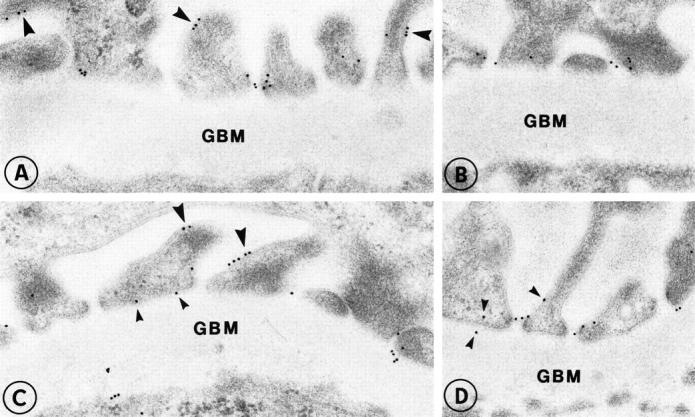
Localization of nephrin in the glomerular capillary wall of normal human kidney (A-D). Labeling is found in association with the slit diaphragms in filtration slits. In addition, small clusters of nephrin are also seen on the surface of podocytes (large arrowheads) as well as occasionally on the base of the foot processes (small arrowheads). Original magnification, ×35,000.
Figure 6.
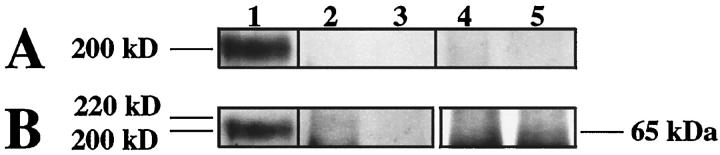
Western blotting of coimmunoprecipitations with antinephrin antibodies. Human glomerular lysate was first immunoprecipitated with anti-ZO-1 (A and B, lanes 2 and 4), antioccluding (lanes 3 and 5), or antinephrin antibodies (A and B, lane 1). Antinephrin antibodies then used for coimmunoprecipitation with ZO-1 (A, lane 2) or occludin (A, lane 3) failed to show specific coprecipitation products. The preimmune antinephrin serum (A, lanes 4 and 5) failed to precipitate any products. Control precipitation and subsequent blotting with anti-ZO-1 (B, lane 2) showed a faint band at the expected 220-kd area but no product in blots with antioccludin antibody (B, lane 3). The similar control with antioccludin immunoprecipitation showed a faint band at 65 kd (B, lane 5) but no reactivity when precipitated with anti-ZO-1 antibody (B, lane 4). Lane 1 (in A and B) shows nephrin immunoblotted with antinephrin antibodies. Subsequent blotting with the same antibody shows distinct reactivity with a 200-kd band.
Table 1.
Effect of intracellular peptide to immunoprecipitation of nephrin antibodies with in vitro translated 35S labelled nephrin and protein A-sepharose.
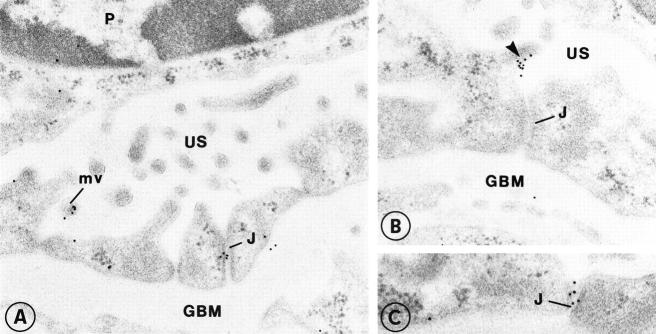
In postembedding immunoelectron microscopy, the affinity-purified anti-intracellular nephrin antibodies characteristically labeled the podocyte foot processes prominently at the level of the filtration slits in the control kidney (Figure 2, A ▶ -D). However, some immunogold particles were also seen in the plasma membrane of podocytes, preferentially in the vicinity of the filtration slits but also in clusters at the apical surface (see Figures 2 and 3 ▶ ▶ ). In the CNF kidney samples, some nephrin-specific gold particles were seen at the flattened apical surface of podocytes in association with microvilli (Figure 3, A ▶ -C) and rarely at the intercellular junctional areas (Figure 3A) ▶ .
Figure 3.
In this example of CNF, nephrin is found on the surface of flattened podocytes in association with microvilli (A-C). In occasional intercellular junctions of podocytes (J), clusters of nephrin are also found. Original magnification, ×35,000.
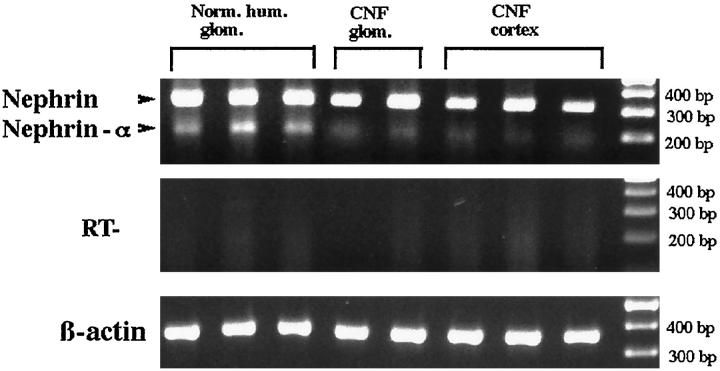
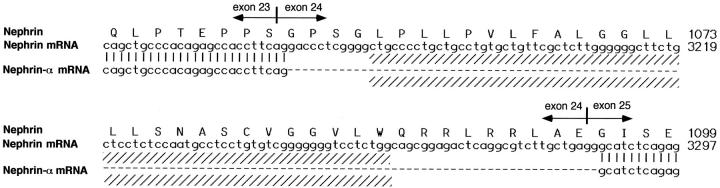
Northern blotting experiments with cortical kidney total RNA failed to show clear reactivity at the expected nephrin size. Thus, to obtain the expression pattern of nephrin, a systematic RT-PCR analysis of RNAs from normal human or CNF kidneys was performed with primers flanking the transmembrane domain. Two PCR products were seen: the dominant band at the expected size and a second PCR product, designated nephrin-α (Figure 4) ▶ with a calculated 131-kd molecular mass of the protein core (full-length nephrin, 135 kd). The authenticity of these PCR products was verified by sequencing both products directly or after cloning in an appropriate vector. The sequence of nephrin-α was identical with that of nephrin except that exon 24 (nucleotides 3167–3286) was missing (Figure 5) ▶ . This exon includes the putative transmembrane region of nephrin (nucleotides 3178–3258).
Figure 4.
Expression pattern of nephrin after PCR analysis of mRNA from normal human glomeruli, CNF glomeruli, and CNF cortical tissue. Full-length nephrin and a splicing variant, nephrin-α, missing the whole transmembrane domain, were verified by direct sequencing as described in Materials and Methods. The control of reverse transcription shows absence of reaction product in samples without reverse transcription. β-Actin was used to verify the quality of RNA.
Figure 5.
Amino acid sequence of nephrin and the respective NPHS1 nucleotides 3121–3300, including the transmembrane area (shaded) together with exon boundaries. The missing sequence of the novel nephrin-α splicing variant includes exon 24.
Associations of nephrin with other known proteins of the filtration slit area were studied with coimmunoprecipitations. For this purpose, immunoprecipitations of glomerular lysates with antibodies to ZO-1, occludin, or nephrin tail were done. Subsequent immunoblotting analysis of the precipitates with these antibodies showed the respective antigens at their molecular mass areas (Figure 6) ▶ . For nephrin, a broad band at 200 kd was seen, possibly also containing the nephrin-α variant. However, no specific reaction product with antinephrin antibodies could be seen in the material first precipitated with antioccludin or anti-ZO-1 antibodies. Neither of these proteins was detected when immunoprecipitation was done first with antinephrin antibodies.
Mutation analysis by direct sequencing of exon 2 (for Finmajor) and exon 26 (for Finminor) of the sample, showing antibody reactivity within glomeruli, was negative for both Finmajor and Finminor mutations (Figure 7) ▶ .
Discussion
Here we report the specific mRNA expression pattern in 28 CNF patient kidneys and show previously unrecognized mRNA splicing of newly cloned gene NPHS1 of CNF. The respective protein product nephrin was localized within normal human kidneys by using antipeptide antibodies against intra- or extracellular nephrin-specific domains. These antibodies both gave a typical glomerular localization suggestive of podocyte reactivity in immunofluorescence microscopy and showed a typical reactivity in immunoblotting with a 200-kd glomerular protein. Immunoelectron microscopy revealed a distinct localization at the filtration slit area of podocytes.
Congenital nephrotic syndromes are rare pediatric kidney diseases manifesting at or soon after birth with massive proteinuria. One of the best characterized congenital nephrotic syndromes is CNF with treatment-resistant proteinuria but no symptoms from other tissues. 10,11 Thus, CNF is considered a unique human model of disease to study the mechanisms maintaining the glomerular filtration barrier. Prenatal diagnosis of CNF can be made on the basis of extremely high α-fetoprotein in maternal serum or in amniotic fluid at the early second trimester of pregnancy. 23 In light of the present results, it is interesting that the centripetal morphological maturation of the glomeruli and their filtration slits occurs early during fetal development, 24 whereas little is known of the respective functional maturation of the glomerular filter. Whether nephrin has a true functional role during kidney development remains to be studied in detail.
Using PCR primers flanking the transmembrane domain of nephrin, two reaction products could be observed. These PCR products were further cloned, sequenced, and verified to be nephrin-specific. Interestingly, the novel nephrin-α variant misses the whole amino acid sequence spanning the transmembrane domain (encoded by exon 24). That such a splicing yields variants without the complete transmembrane area is interesting and could lead to secretion of the protein, with important functional consequences. Examples of such secreted transmembrane-negative splicing variants, with biologically important regulation of the respective receptor affecting the ligand binding, include interleukin-6 receptor 25 and T cell receptor. 26 The exact role of nephrin-α in proteinuric diseases is currently being studied. Our preliminary results from the rat and mouse suggest that additional splicing of nephrin homologues may also occur in these species 27 at the transmembrane domain.
Kestilä et al 8 reported two major NPHS1 gene defects responsible for CNF in the Finnish population, in exon 2 (Finmajor) and exon 26 (Finminor), whereas a variety of mutations including deletions, frameshift mutations, nonsense mutations, and early stop codons were found along nephrin genes in the CNF patients from other parts of the world, leading to various clinical pictures. 28 Our previous studies of CNF patients have suggested that biochemical variants of the disease can also be found. Thus, eg, the ratios of urinary glycosaminoglycan to creatinine and urinary heparan sulfate to chondroitin sulfate show variation among these patients. 14,29 The respective genotypes of these patients remain to be determined. Interestingly, in our study all but one CNF patient kidney failed to show reactivity with either the extra- or intracellular nephrin antipeptide antibodies. This suggests that the amount of nephrin in these patient glomeruli is below the detection limit or that no immunoreactive nephrin protein is expressed. The latter possibility is the obvious explanation in patients homozygous for Finmajor mutations with the early stop codon in exon 2 8 of NPHS1, whereas CNF patients with Finmajor Finminor heterozygosity could demonstrate negligible amounts of the protein product. Interestingly, the CNF patient with glomerular reactivity with our antibody failed to show either the Finmajor or Finminor mutation in direct sequencing. Thus, this patient most probably represents other mutations in nephrin, in the NPHS1 promoter area, or even in some nephrin-associated proteins, as suggested by Lenkkeri et al. 27
Our immunoelectron microscopic (IEM) results show that nephrin is distinctly located at the filtration slit area and may also be present at the apical plasma membrane of podocytes. This is in line with results of Tryggvason (personal communication). Our results with experimental models of glomerular diseases show a similar plasma membrane localization of nephrin in IEM analysis (Luimula P, manuscript in preparation).
However, nephrin was also seen in IEM analyses of some of the CNF samples studied, preferentially at the plasma membranes. This result appears controversial because, particularly in Finmajor, no protein should be expressed. 28 However, as discussed above, only homozygosity in respect to Finmajor mutation leads to complete lack of nephrin, whereas the Finminor mutation found in 20% of the Finnish patients causes a nonsense mutation at exon 26 in the intracellular domain. This site is downstream, beyond the recognition site of our intracellular antibodies and, if transcribed and translated, our antibodies should normally also detect this product. Thus, the heterozygotes may produce nephrin detectable only in IEM analysis but not in indirect immunofluorescence microscopy.
Little is known of the composition and molecules of the slit membrane and especially of their interactions, although, eg, synaptopodin and ZO-1 have previously been associated at this site. 30,31 Our current results suggest that, although sharing the localization at filtration slits, nephrin may not directly interact with ZO-1 or occludin, as suggested by the lack of coprecipitation of these molecules. Further double-labeling IEM studies are underway to search for the possibly interacting molecules.
Together, our results reveal the characteristic nephrin splicing and show the localization of its protein product in a key localization in the glomerulus. Thus, nephrin appears to be the first component of the podocyte slit membrane in humans with a crucial role in glomerular permeability.
Acknowledgments
We thank Dr. Christer Holmberg for invaluable help in obtaining the CNF patient samples and Ms. Riitta Väisänen for expert technical assistance.
Footnotes
Address reprint requests to Dr. Harry Holthöfer, The Haartman Institute/Division of Bacteriology and Immunology, University of Helsinki, PB 21 (Haartmaninkatu 3), Helsinki, FIN-00014 Finland. E-mail: harry.holthofer@helsinki.fi.
Supported by the Academy of Finland, a research grant from Helsinki University Hospital, the Finnish Foundation of Heart Disease, and the Sigrid Juselius Foundation.
References
- 1.Rennke HG: How does glomerular epithelial cell injury contribute to progressive glomerular damage. Kidney Int 1994, 45(Suppl.):S58-S63 [PubMed] [Google Scholar]
- 2.Kerjaschki D: Dysfunctions of cell biological mechanisms of visceral epithelial cells (podocytes) in glomerular diseases. Kidney Int 1994, 45:300-313 [DOI] [PubMed] [Google Scholar]
- 3.Mundel P, Kriz W: Structure and function of podocytes: an update. Anat Embryol 1997, 192:385-397 [DOI] [PubMed] [Google Scholar]
- 4.Smoyer WE, Mundel P: Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med 1998, 76:172-183 [DOI] [PubMed] [Google Scholar]
- 5.Shirato I, Sakai T, Kimura K, Tomiko Y, Kriz W: Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol 1996, 148:1283-1289 [PMC free article] [PubMed] [Google Scholar]
- 6.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R: Alpha3β1 integrin has a crucial role in kidney, and lung organogenesis. Development 1996, 122:3537-3547 [DOI] [PubMed] [Google Scholar]
- 7.Kanwar YS, Liu ZZ, Kashihara N, Wallner EI: Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol 1991, 11:390-413 [PubMed] [Google Scholar]
- 8.Kestilä M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Mol Cell 1998, 1:575-82 [DOI] [PubMed] [Google Scholar]
- 9.Holmberg C, Antikainen M, Rönnholm K, Ala-Houhala M, Jalanko H: Management of congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 1995, 9:87-93 [DOI] [PubMed] [Google Scholar]
- 10.Hallman N, Norio R, Kouvalainen K: Main features of the congenital nephrotic syndrome. Acta Pediatr Fenn 1967, 172:75-78 [DOI] [PubMed] [Google Scholar]
- 11.Rapola J: Congenital nephrotic syndrome. Pediatr Nephrol 1987, 1:441-446 [DOI] [PubMed] [Google Scholar]
- 12.Haltia A, Solin M-L, Jalanko H, Holmberg C, Miettinen A, Holthöfer H: Mechanisms of proteinuria: vascular permeability factor in congenital nephrotic syndrome of the Finnish type. Pediatr Res 1996, 40:652-657 [DOI] [PubMed] [Google Scholar]
- 13.Chirgwin JM, Przybyla AC, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 8:5294-5295 [DOI] [PubMed] [Google Scholar]
- 14.Ljungberg P, Haltia A, Kuusela P, Jalanko H, Holmberg C, Holthöfer H: Noncollagenous matrix components of glomeruli in congenital nephrotic syndrome of the Finnish type: evidence of abnormal splitting of nidogen. Exp Nephrol 1996, 4:286-294 [PubMed] [Google Scholar]
- 15.Miettinen A, Dekan G, Farquhar MG: Monoclonal antibodies against membrane proteins of the rat glomerulus: immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol 1990, 137:929-944 [PMC free article] [PubMed] [Google Scholar]
- 16.Holthöfer H, Kretzler M, Haltia A, Solin M-L, Taanman J-W, Schägger H, Kriz W, Kerjaschki D, Schlöndorff D: Altered gene expression and functions of mitochondria in human nephrotic syndrome. FASEB J 1999, 13:523-532 [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, ed 2. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press, 1989
- 18.Kerjaschki D, Ullrich R, Exner M, Orlando RA, Farquhar MG: Induction of passive Heymann nephritis with antibodies specific for a synthetic peptide derived from the receptor-associated protein. J Exp Med 1996, 183:2007-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam JP: Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA 1988, 85:5409-5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tissari J, Holthöfer H, Miettinen A: Novel 13A antigen is an integral protein of the basolateral membrane of rat glomerular podocytes. Lab Invest 1994, 71:519-527 [PubMed] [Google Scholar]
- 21.Kerjaschki D, Exner M, Ullrich R, Susani M, Curtiss LK, Witztum JL, Farquhar MG, Orlando RA: Pathogenic antibodies inhibit the binding of apolipoproteins to megalin/gp330 in passive Heymann nephritis. J Clin Invest 1997, 100:2303-2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelham HR, Jackson RJ: An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem 1976, 67:247-256 [DOI] [PubMed] [Google Scholar]
- 23.Seppälä M, Aula P, Rapola J, Karjalainen O, Huttunen N-P, Ruoslahti E: Congenital nephrotic syndrome: prenatal diagnosis and genetic counselling by estimation of amniotic-fluid and maternal serum α-fetoprotein. Lancet 1976, 7977:123-125 [DOI] [PubMed] [Google Scholar]
- 24.Rapola J, Sariola H, Ekblom P: Pathology of fetal congenital nephrosis: immunohistochemical and ultrastructural studies. Kidney Int 1984, 25:701-707 [DOI] [PubMed] [Google Scholar]
- 25.Säily M, Koistinen P, Pulkki K, Zheng A, Savolainen E-R: Acute myeloblastic leukemia cells produce soluble interleukin 6 receptor by a mechanism of alternative splicing. Cytokine 1998, 10:860-867 [DOI] [PubMed] [Google Scholar]
- 26.Takase K, Okazaki Y, Wakizaka K, Shevchenko A, Mann M, Saito T: Molecular cloning of pTAC12 an alternative splicing product of the CD3 uchain as a component of the pre-T cell antigen receptor complex. J Biol Chem 1998, 273:30675-30679 [DOI] [PubMed] [Google Scholar]
- 27.Ahola H, Wang S, Luimula P, Solin ML, Holzman L, Holthöfer H: Cloning and expression of the rat nephrin homolog. Am J Pathol 1999, 155:907-913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenkkeri U, Antignac C, Kashtan C, Holmberg C, Olsen A, Kestilä M, Tryggvason K: Structure of the gene for congenital nephrotic syndrome of the Finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 1999, 64:51-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungberg P: Polycosaminoglycans in urine and amniotic fluid in congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 1994, 8:531-536 [DOI] [PubMed] [Google Scholar]
- 30.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W: Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997, 139:193-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurihara H, Anderson JM, Farquhar MG: Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot podocytes. Am J Physiol 1995, 268:F514-F524 [DOI] [PubMed] [Google Scholar]